Abstract
The hypotensive and hypoglycaemic effects of Ficus exasperata (Vahl) (family: Moraceae) leaf aqueous extract (FEE) were investigated in experimental rat models. In this study, spontaneously-hypertensive rats (SHR) (type 1 diabetes), obese Zucker (type 2 diabetes) and Wistar rats were used. Three (A, B and C) groups of rats, each group consisting of 10 rats, were used. Group A Wistar rats received distilled water in quantities equivalent to the volume of streptozotocin (STZ) and FEE administered intraperitoneally to treated rats. Diabetes mellitus was induced in the SHR group B rats by multiple low-dose (MLD) intraperitoneal injections of STZ (40 mg/kg body weight) to induce type 1 diabetes. The animals in group C were the obese Zucker rats with non-insulin-independent diabetes mellitus (NDDM) (type 2 diabetes) on genetic basis. F. exasperata leaf aqueous extract (FEE, 100 mg/kg/day p.o.) was administered orally by orogastric intubation to fasted Groups B and C rats. In groups B and C rats, administration of FEE commenced 4 weeks post STZ injection, and continued for the next 4 consecutive weeks. Group A rats gave normal biochemical and morphological findings. Group B rats exhibited pronounced polyuria, hypoinsulinaemia, hyperlipidaemia and hyperglycaemia. These findings were also observed in group C rats, except that there was hyperinsilinaemia. Histopathological study of the aortic blood vessels showed extensive collagen fiber formation as well as perivascular fibrosis in both groups B and C rats. Four weeks of oral administration of F. exasperata leaf aqueous extract to diabetic groups of rats decreased blood glucose, blood pressure and lipid profiles. Administration of FEE (100 mg/kg p.o.) also restored the microanatomy of the blood vessels to almost normal levels. The findings of this study suggest that F. exasperata leaf aqueous extract possesses hypoglycaemic, hypotensive and hypolipidaemic properties. These findings lend biomedical and pharmacological support to the folkloric, ethnomedical uses of the plant in the management and/or control of diabetes and hypertension among the Yoruba-speaking people of Western Nigeria.
Keywords: Ficus exasperata (Vahl) leaf aqueous extract, streptozotocin, rats, hypoglycaemic, hypotensive and hypolipidaemic properties
Introduction
It is known that streptozotocin (STZ)-induced diabetes mellitus causes functional and structural changes in some tissues and organs of mammalian animals. However, the mechanism of the cytotoxic action of STZ is not yet fully understood. Experimental evidence has demonstrated that some of the toxic, deleterious effects of STZ are attributable to induction of metabolic processes, which lead on to an increase in the generation of reactive oxygen species (ROS) (Chen et al; 1990). Apart from production of ROS, STZ also inhibits free radical scavenger-enzymes (Kröncke et al; 1995). The superoxide radical has been implicated in lipid peroxidation, DNA damage, and sulphydryl oxidation (Matkovics et al; 1998).
Diabetes mellitus is a complex and multifarious group of disorders characterized by hyperglycaemia that has reached epidemic proportions in the present century. Infection is a leading cause of morbidity and mortality among the diabetic population (Hostetter, 1990). Diabetes is also associated with vascular and renal dysfunctions characterized by hypertension, dyslipidaemia and arteriosclerosis (Freener and King, 1997). Numerous studies have provided convincing evidence for the presence of oxidative stress, and its role in the pathogenesis of the complications of diabetes (Ceriello et al., 2001). Reactive oxygen species (ROS) react with and modify lipids, carbohydrates, proteins and DNA, resulting in cytotoxicity and dysfunction (Rosen et al., 2001). In addition, ROS avidly react with nitric oxide (NO), which is a major signaling molecule with diverse biological functions (Gryglewski et al., 1986). This can lead to functional NO deficiency and formation of highly reactive nitrogen species such as peroxynitrite (Squadrito and Pryor, 1995) and/or peroxynitrous acid (Beckman et al., 1990. In turn, the latter agents can attack, denature or modify various structural and functional molecules (Beckman et al., 1990). For instance, peroxynitrite can react with tyrosine or cysteine residues of proteins to produce nitrotyrosine and/or nitrocysteine, which are considered as footprints of ROS interaction with nitrires.
Hypertension and diabetes mellitus are known to frequently co-exist simultaneously, with resultant cardiovascular and renal damages (Yamori, 1994). Because both conditions frequently occur concurrently, much interest has developed in the fields of hypertension and diabetes research concerning the backgrounds, relevance and sequelae of their combined occurrence, both in animal models and in patients (Van Zwieten et al., 1996). Streptozotocin (STZ)-treated, spontaneously-hypertensive rats (STZ-treated SHR) usually develop hyperglycaemic syndrome, associated with other biochemical and morphological changes that, to some extent, approach insulin-dependent diabetes mellitus (type I diabetes), combined with hypertension (Chappel and Chappel, 1983). The obese Zucker rats are characterized by simultaneous occurrence of obesity, hyperglycaemia, hyperinsulinaemia, hyperlipidaemia and moderate hypertension. As such, the obese Zucker rat model approaches a patient with non-insulindependent diabetes mellitus (type 2 diabetes) who is simultaneously hypertensive (Factor et al., 1981).
Ficus. exasperata (Vahl), commonly known by the local name of “sand paper tree”, is a deciduous shrub or tree of up to 20 m high, with smooth grey bark and very rough leaves; viscid non-milky sap; and coppice shoots with lobed leaves. F. exasperata is widespread in tropical Africa; and is also recorded in the Arabian Gulf (Berg, 1991). Phytochemical and toxicological analyses of the leaf and stem extracts of F. exasperata have revealed the presence of flavonoids, tannis, saponins, alkaloids and cyanogenic glycosides (Ijeh and Ukweni, 2007). It is used medicinally for treating different human diseases. For example, the viscid, non-milky sap is used for treating sores and stomach pains in Ivory Coast (Burkill, 1997). The sap is also used to arrest bleeding in Ghana (Abbiw, 1990). In South Africa, scrapings of the bark are used in an embrocation for body pains and also as a stimulant (Burkill, 1997). In Upper Ivory Coast, its sap is applied to leprous sores (Bouquet, 1969).
The Yoruba-speaking people of Western Nigeria often employ decoctions and infusions of F. exasperata leaves traditionally for the management, control and/or treatment of an array of human diseases, including diabetes mellitus, hypertension and certain cardiovascular dysfunctions. The present study was prompted by the paucity of ethnomedical and pharmacological information on F. exasperata (Vahl) extracts in biomedical literature. The core aim of the present study were, therefore, to examine the effects of F. exasperata leaf aqueous extract on lipid profiles, hyperglycaemia, hypertensive and microanatomical changes of arteries of both type 1 and type 2 diabetic and hypertensive rat models.
Material and Methods
All experiments were conducted in strict compliance with the humane animal care standards outlined in the “Guide to the Care and Use of Laboratory Animals in Research and Teaching” prepared by the National Institutes of Health (NIH), publication 86-23 (revised in 1985).
Animals
This study was carried out in male and female Wistar rats (230–250 g), spontaneously-hypertensive rats (SHR) 230–260 g treated with multiple low dose streptozotocin (40 mg/kg body weight) and obese Zucker rats (240–480 g). In the STZ-treated SHR and obese Zucker rat models, hypertension and diabetes co-existed simultaneously. The animals were housed under standard laboratory conditions of light, temperature and humidity. The animals were given standard rat chow and drinking tap water ad libitum. The rats were randomly divided into three (A, B and C) experimental groups, comprising Group A (distilled water-treated ‘control’), Group B (STZ-treated SHR model) and Group C (obese Zucker model) rats. Each group consisted of 10 rats.
Plant material
Fresh leaves of Ficus exasperata Vahl [family: Moraceae], known by the local name as “sand paper tree” or “Ipin” in Yoruba language of Western Nigeria, were collected from suburban villages of Ile-Ife metropolis in Osun State of Nigeria, in March 2009. The leaves were botanically identified by the taxonomist/curator of the Department of Botany, Obafemi Awolowo University, Ile-Ife, Nigeria. A voucher specimen of the plant has been deposited in the University's Botany Departmental Herbarium.
Preparation of F. exasperata leaf aqueous extract
Fresh leaves of F. exasperata were air-dried at room temperature. One kilogram (1 kg) of the air-dried leaves of the plant was milled into fine powder in a Waring commercial blender. The powdered leaves were macerated in distilled water and extracted twice, on each occasion with 2.5 1itres of distilled water at room temperature for 48 h. The combined aqueous extract solubles were concentrated to dryness under reduced pressure at 60±1°C in a rotary evaporator. The resulting aqueous extract was freeze-dried; finally giving 46.18 g (i.e., 4.618% yield) of a dark-green, powdery, crude aqueous extract of F. exasperata leaf (FEE). Aliquot portions of the crude extract residue were weighed and dissolved in distilled water for use on each day of our experiment.
Induction of experimental diabetes
Diabetes mellitus was induced in (Group B) STZ-treated SHR rats by multiple low dose intraperitoneal injections of STZ (40 mg/kg body weight), freshly dissolved in 0.1mol/l citrate buffer, daily for consecutive five days (Rossini et al; 1978). Group A ‘control’ rats were injected with volumes of distilled water equivalent to the volume of FEE administered intraperitoneally. The ‘test’ rats in group B became diabetic within 48 h after STZ administration. Diabetes was allowed to develop and stabilize in these STZ-treated SHR rats over a period of 3–5 days. Group C rats were genetically diabetic. All the animals in groups A, B and C were kept and maintained under laboratory conditions of light, humidity and temperature. Before the commencement of our experiments, the control, normal (normoglycemic and normotensive), STZ-treated SHR, diabetic (hyperglycemic) ‘test’ rats and obese Zucker rats were fasted for 16-h, but still allowed free access to drinking tap water throughout. At the end of the 16-h fasting period - taken as 0 time (i.e., 0 h) - blood glucose levels (initial glycemia, G0) of the fasted normal, STZ-treated SHR and obese Zucker diabetic rats were determined and recorded.
Blood glucose and serum insulin estimations
Blood samples (0.02ml) were obtained from each rat by repeated needle puncture of the same tail tip vein. Blood samples were taken 1 day before STZ- and FEE-treatments, and also on various days after the induction of diabetes mellitus and the commencement of our experiments. Blood glucose concentrations were determined by means of Bayer's Elite® Glucometer and compatible blood glucose test strips (Atkin et al., 1991). Fasted STZ-treated rats with blood glucose concentrations ≥18 mmol l−1 were considered to be diabetic, and used in this study. Serum insulin concentrations were determined by an enzyme-linked immunosorbent assay (ELISA), using a commercial kit (Crystal Chem, Illinois, Chicago, USA). The test compound [i. e., Ficus exasperata leaf aqueous extract (FEE, 100 mg kg−1 day−1 p.o.)] was administered orally by intragastric intubation to fasted Groups B and C rats. In both groups, administration of FEE (100 mg kg−1) commenced as from the 30th day post STZ injection, and continued for the next consecutive 4 weeks.
Determination of serum cholesterol, lipoproteins and triglyceride
Blood samples were collected from tail veins of the rats after 16 h of fasting, and transferred to sterilized centrifuge tubes at room temperature. The blood samples were centrifuged for 10 min at 4000 × g to obtain serum. The serum was stored in a freezer at 0°C for later analysis of total cholesterol (TC) and triglyceride (TG), high- and low-density lipoprotein (HDL and LDL)-cholesterols. Aliquots of serum were taken for determination of total cholesterol by enzymatic colorimetric method of Allain et al (1974), and triglycerides determined by enzymatic glycerol phosphate oxidase/peroxidase method of Cheng et al (1988). Autoanalyzer (Express Plus, Ciba Corning, USA) and Elitech kit were used. Serum high density lipoprotein (HDL)-cholesterol was assayed by precipitation of chylomicrons, while very low-density lipoproteins (VLDL) and low-density lipoproteins (LDL) were determined with sodium phosphotungstic acid and magnesium chloride (Rainwater et al, 1995). Centrifugation of the mixture left only the HDL in the supernatant; their cholesterol content was determined by the method of Virella-Lopes et al; (1977). Estimation of low density lipoprotein (LDL)-cholesterol was done by using the empirical formula of Friedewald et al; (1972) for samples with TG levels <4.5 mmol l−1. [LDL-chol] = [Total chol] − [HDL-chol] − ([TG]/2.2); where all concentrations are given in mmol l−1.
Haemodynamic characteristics of hypertensive rats
Induction of diabetes in SHR experimental group B rats further caused elevation in the arterial blood pressure of the animals in group B. Mean arterial blood pressures in groups B and C rats rose to between 160/110 and 180/110 mmHg. Animals with arterial blood pressures ≥ 170/110 mmHg were considered to be hypertensive and used in this study. Conscious rats were placed in a restrainer and allowed to rest inside the cage for 15 minutes before blood pressure measurements. Rat tails were placed inside a tail cuff, and the cuff was inflated and released a few times to allow the animal to be conditioned to the procedure. Mean blood pressure values (four consecutive readings) were taken by a rat tail plethysmograph coupled to a computer system (Power Lab, AD Instruments, Australia) Gonick et al., 1997).
Nitric Oxide (NO)
Aortic blood vessel samples were harvested from each experimental rat and immediately homogenized with cold phosphate-buffered saline (PBS) on ice, which inhibited the activity of nitric oxide sythase ex-vivo. The homogenate was centrifuged (3000 × g, 5 min) and the supernatant was collected. The supernatant obtained from tissues was passed through a 1.2 µm multiscreen filter plate. Serum nitrite/nitrate levels was determined by converting the nitrate to nitrite, using enzyme nitrate reductase followed by addition of Griess reagent to colorimetrically quantify the nitrite concentration (Green et al; 1982). The serum was diluted 1:5 in PSB before a 25-µl aliquot was added to a mixture of 25 µl nitrate reductase (1.5 U ml−1) and 25 µl of NADPH (0.134 mg ml−1), both prepared in 40 mM Tris, pH 7.6. The samples were thereafter incubated at room temperature for 3 hours. Following this period, 100 µl of Griess reagent (1:1 mixture of 1% sulphanilamide in 5% phosphoric acid and 0.1% naphyly-ethylenediamine) was added and incubated for a further 10 min at room temperature; the absorbency of the samples was measured at 540 nm with a 650 nm reference. The concentration of nitrite/nitrate was determined from a standard curve of sodium nitrate and calculated as µmol g−1 protein in tissue. Protein in the supernatant obtained from sample was determined using standard Lowry et al., (1951) method.
Histological procedures
Post euthanisation, aortic artery and left ventricular tissues were excised on days 30 and 60 in Groups A, B and C rats. The tissues were fixed in aqueous Bouin's solution for 48 h and were sequentially embedded in paraffin wax blocks according to the standard procedure of Stevens (1982). Five micron sections were cut onto poly-L-lysine coated slides and stained for elastic fibre with Verhoeff Van-Gieson stain. Sections were analysed on an Olympus BX-50 photomicroscope interfaced with Olympus DR10 digital camera system.
Statistical Analysis
Data obtained from ‘control’ and ‘test’ (treated) rats were pooled and expressed as means (±SEM), and analyzed using repeated measures of variance. The differences between the means were analyzed statistically with one-way analysis of variance (ANOVA; 95% confidence interval), followed by Scheffe's multiple-range comparison test. Values of p=0.05 were taken to imply statistical significance.
Results
General characteristics of the animals
All the ‘control’ rats were moving freely in their individual cages throughout the study period. The diabetic rats appeared to be lethargic and displayed restricted movements. The blood glucose levels of the diabetic animals were significantly elevated (p<0.05) (Table 2), and their body weights were significantly reduced (p<0.05) (Fig 1). Although, there was no sign or evidence of infection or motor disorder in any of the rats studied, the STZ-treated SHR diabetic rats displayed significant polyuria, hypoinsulinaemia and weight loss.
Table 2.
Changes in blood glucose concentrations, serum insulin and aortic nitric oxide contents of ‘control’, STZ-treated SHR and obese Zucker rat groups during the study period.
| Blood glucose concentrations (mmol L−1) | ||||||||
| Before FEE Treatment | After FEE-Treatment | |||||||
| Parameters/Days | 0 | 7 | 15 | 22 | 30 | 40 | 50 | 60 |
| ‘Control’ | 4.2±0.2 | 4.5±0.4 | 4.0±0.6 | 4.3±0.5 | 4.1±0.2 | 4.2±0.1 | 3.9±0.3 | 4.0±0.4 |
| STZ-treated SHR | 4.8±0.6 | 18.8±0.2a | 20.2±0.4a | 21.4±0.3a | 22.6±0.1a | 10.2±0.4a | 8.4±0.3a | 6.8±0.1a |
| Obese Zucker | 18.3±0.2 | 17.6±0.4b | 18.2±0.2b | 18.6±0.3b | 18.9±0.4b | 8.2±0.2b | 7.6±0.3b | 5.9±0.4b |
| Serum insulin concentrations (µU ml−1) | ||||||||
| ‘Control’ | 12.7±1.2 | 12.9±1.0 | 12.9±1.3 | 12.9±1.7 | 13.0±1.2 | 12.9±1.3 | 12.9±1.7 | 13.0±1.2 |
| STZ-treated SHR | 12.8±1.5 | 8.7±1.4c | 6.3±1.4c | 5.9±1.3c | 5.7±2.3c | 8.3±1.4c | 10.9±1.3c | 10.7±2.3c |
| Obese Zucker | 54.8±4.3 | 53.3±6.2d | 53.9±7.1d | 56.4±5.7d | 55.5±3.2d | 45.7±5.1d | 36.3±5.7d | 30.5±3.2d |
| Aortic nitric oxide contents (µmol g−1) | ||||||||
| ‘Control’ | 56.7±1.2 | 55.9±1.0 | 57.2±1.3 | 56.9±1.7 | 58.0±1.2 | 57.2±1.3 | 56.9±1.7 | 58.0±1.2 |
| STZ-treated SHR | 55.8±1.5 | 88.7±1.4x | 92.3±1.4x | 95.9±1.3x | 95.7±2.6x | 83.3±2.4x | 75.9±1.3x | 66.5±1.6x |
| Obese Zucker | 110.2±4.3y | 112.3±6.2y | 115.9±7.1y | 116.4±5.7y | 115.5±3.2y | 95.9±7.1y | 86.4±5.7y | 78.5±3.2y |
| Values are expressed as means (±SEM) of 10 rats. a,b,x Significant increase (p<0.05) between STZ-treated SHR groups and ‘control’. d,y Significant difference (p<0.05) between STZ-treated SHR and obese Zucke rat groups. Values for ‘control’ group being value for 0 day of our experiment. | ||||||||
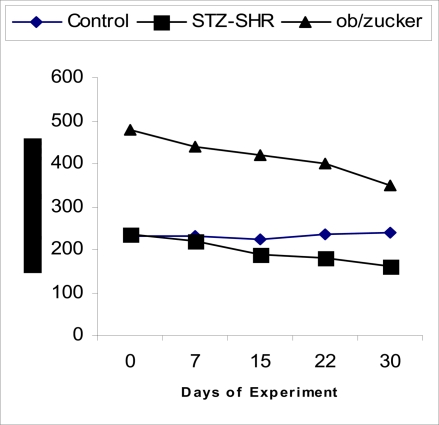
Fig. 1.
Body weights of experimental animals before FEE treatment. ‘Control’ rats virtually maintained normal baseline weight, while STZ-treated SHR and obese Zucker diabetic rats significantly reduced in body weight.
As shown in Table 1, all blood pressure parameters (systolic, diastolic and mean arterial blood pressures) were moderately to significantly higher (p<0.05) in obese Zucker than in STZ-treated SHR rats. However, heart rates proved significantly lower (p<0.05) in the obese Zucker than STZ-treated SHR rats. It can, therefore, be inferred that obese Zucker rats suffer from the syndrome of obesity-hypertension-insulin resistance-hyperlipoproteinaemia. Other Biochemical variables were deranged in diabetic rats compared to normal ‘control’ rats.
Table 1.
General characteristics of ‘control’, STZ-treated SHR and obese Zucker diabetic rats (n=6; *p<0.05 vs control rats).
| Parameter | Control rats | STZ-treated SHR rats | Obese Zucker rats |
| Body weight (g) | 230.0±3.9 | 240.2±5.2 | 480.5±6.5 |
| Blood glucose (mmol l−1) | 4.8±0.2 | 22.6±1.4 | 17.4±2.1 |
| Heart weight (g) | 0.624±3.0 | 0.91±1.0 | 2.16 ±1.3 |
| Aortic weight (g) | 0.042±1.2 | 0.053±0.9 | 0.084±1.2 |
| Plasma insulin (µU l−1) | 12.7±2 | 7.8±1.2 | 54.8±4.3 |
| Aortic NO (µmol g−1) | 65.2±2 | 88.6±4 | 135.3±7 |
| Cholesterol (mmol l−1) | 1.28±0.02 | 1.82±0.06 | 4.25±0.82 |
| HDL (mmol l−1) | 0.84±0.04 | 1.08±0.02 | 2.98±0.35 |
| LDL (mmol l−1) | 0.36±0.05 | 0.44±0.03 | 0.97±0.19 |
| Triglycerides (mmol l−1) | 0.42±0.03 | 0.52±0.01 | 1.28±0.23 |
| Systolic BP (mmHg) | 118.1±2.2 | 166.2±4.3 | 172.6±3.1 |
| Diastolic BP (mmHg) | 98.4±4.0 | 101.7±2.6 | 109.4±5.2 |
| Mean ABP (mmHg) | 94.6±5.0 | 126.5±3.5 | 135.7±2.3 |
| Heart rate (beat/min) | 342.3±15.2 | 382.4±12.4 | 298.9±17.9 |
Blood glucose and serum insulin concentrations
The mean blood glucose concentrations and serum insulin levels of the STZ-treated SHR and obese Zucker animals are shown in Table 2. In our ‘control’ set of experiments, pretreatment of the rats with distilled water alone did not significantly modify (p>0.05) the serum insulin and blood glucose concentrations. As shown in Table 2, induction of diabetes with STZ resulted in significant increases in the blood glucose levels of the rats. There was a gradual rise in the blood glucose concentrations of the animals as from day 1 following injection of STZ, and the values were significantly higher (p<0.05) than those of the ‘control’ animals (Table 2). Furthermore, high levels of blood glucose concentrations of the obese Zucker rats were persistently observed throughout the study period (Table 2). FEE treatment significantly reduced (p<0.05-0.001) the blood glucose concentrations of both groups B and C animals, but specifically more in the obese Zucker than in the STZ-treated SHR rats (Fig. 2). FEE treatment also significantly increased (p<0.05) serum insulin levels in Group B rats, but without any significant (p>0.05) effect on the serum insulin of obese Zucker rats (Table 2). Nitric oxide contents of the aortic tissues were significantly higher (p<0.05) in both Groups B and C diabetic animals after FEE treatments (Table 2).
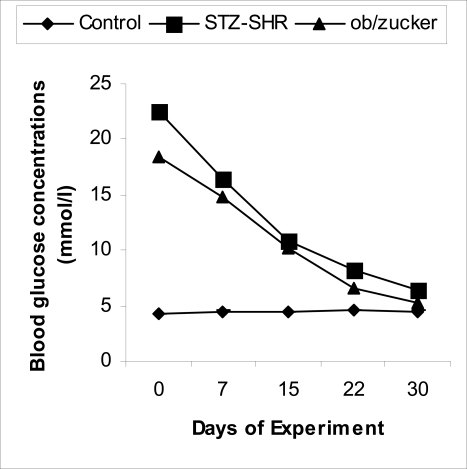
Fig. 2.
Blood glucose concentrations of the animals after FEE treatment. ‘Control’ rats remained normoglycaemic while STZ-treated SHR and obese Zucker diabetic rats showed significantly decreased blood glucose concentrations.
Biochemical findings
Serum total cholesterol, triglycerides, HDL and LDL cholesterols and (T-chol/HDL-chol) in the ‘control’, STZ-treated SHR and obese Zucker rats are shown in Table 3. Serum total cholesterol, triglycerides, LDL cholesterol and (T-chol/HDL-chol) were significantly elevated (p<0.05) in STZ-treated SHR group B diabetic rats as well as in the Group C obese Zucker rats when compared with the ‘control’ Group A rats. Similarly, HDL cholesterol significantly reduced (p<0.05) in STZ-treated SHR group B diabetic rats (Table 3). All the lipid parameters tested were significantly improved (p<0.05) towards normal values after FEE treatment in groups B and C rats (Table 3).
Table 3.
Serum lipid profiles (Total cholesterol, Triglyceride, High and Low Density cholesterol (mmol l-1) in ‘control’, STZ-treated SHR and obese Zucker rats.
| Experimental groups |
HDL | LDL | TC | TRIG | HDL | LDL | TC | TRIG |
| Before FEE treatment | After FEE treatment | |||||||
| ‘Control’ | 0.84±0.4 | 0.36±0.5 | 0.98±0.2 | 0.92±0.3 | 0.82±0.3 | 0.38±0.6 | 0.89±0.7 | 0.97±0.4 |
| STZ-treated SHR | 0.52±0.3 | 2.64±0.4 | 2.82±0.6 | 2.52±0.7 | 0.76±0.2* | 0.98±0.4** | 1.02±0.6** | 1.05±0.7** |
| Obese Zucker | 0.48±0.5 | 3.27±0.9 | 4.25±0.8 | 2.78±0.3 | 0.69±0.6* | 1.20±0.9** | 1.35±0.8** | 1.27±0.3** |
| Values are expressed as means (±SEM) of 10 rats for all groups. * Significantly increased (p<0.05) and **Significantly decreased (p<0.05) when compared with the experimental groups in the same row. | ||||||||
Hypotensive effect of FEE in STZ-treated SHR and obese Zucker rats
Induction of STZ diabetes in spontaneously-hypertensive rats significantly increased (p<0.05) both blood pressures and heart rates when compared with the ‘control’ rats, but only slightly reduced when compared with the obese Zucker rats. This might possibly be as a result of hypovolaemia, provoked by osmotic polyuria. Administration of FEE into groups B and C rats caused significant reductions (p<0.05) in systolic, diastolic and mean arterial blood pressures and heart rates of the rats (Table 4, Fig. 3). It was observed that the depressor/hypotensive effects of FEE were always followed by transient, reflex tachycardia before the blood pressures and heart rates returned to baseline levels. The hypotensive effect of FEE on systemic arterial blood pressures and heart rates persisted throughout the period of our study (Fig. 3).
Table 4.
Hypotensive effects (systolic, mean and diastolic BP (mmHg) and heart rate (beats/min) ) of FEE in STZ-treated SHR and obese Zucker rats
| Experimental groups |
SBP | DBP | MABP | HR | SBP | DBP | MABP | HR |
| Before FEE treatment | After FEE treatment | |||||||
| Control | 118.1±4 | 98.6±2 | 94.5±2 | 224.3±3 | 116.2±3 | 92.7±6 | 95.9±7 | 229.6±4 |
| STZ-treated SHR | 176.2±3 | 119.7±4 | 126.5±6 | 398.4±7 | 135.3±7* | 98±0.4* | 76.2±6* | 312.5±7* |
| Obese Zucker | 182.6±5 | 121.4±9 | 135.7±8 | 382.9±3 | 149.5±6** | 108.3±9** | 95.4±8** | 307±0.3** |
| Values are expressed as means (±SEM) of 10 rats for all groups. *, **Significantly decreased (p<0.05) when compared with the experimental groups in the same row. | ||||||||
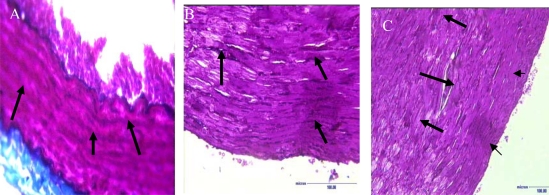
Fig. 3.
Verhoeff Van-Gieson stain of the aortic artery sections. Arrows show perivascular collagen contents. (A) Control group: showing normal collagen fiber distribution within the tunica media. (B) STZ-treated SHR: abundant collagen fibers with thick tunica media; and (C) obese Zucker rats: abundant overstretched collagen fibers, decreased cellular density and perivascular fibrosis (Short arrow) x100.
Histological findings
The tunica media of the aortic blood vessels were found to be significantly increased (p<0.05) in STZ-treated SHR and obese Zucker rats when compared with the ‘control’ rats (Fig. 3). The tunica media thickness was noticed to be higher in STZ-treated rats, probably suggesting vascular hypertrophy of the myocardium. It seems likely that the vascular change was a compensatory response to STZ-induced diabetes. The pattern of interstitial collagen distribution was similar in both groups B and C rats. However, quantitative collagen contents showed that perivascular fibrosis was significantly higher (p<0.05) in the obese Zucker rats, probably suggesting that it was due to long-standing hyperglycaemia, and not the hypertensive state, which caused the alterations.
Discussion
All civilizations have had traditions of using herbs to promote healing. Plants still remain the pillar for the development of modern drugs, and medicinal plants have been used for centuries to treat diseases all over the world. According to Ayitey-Smith (1989), traditional medicine evolved from environmental resources which the people of a community adapted in desperation for survival from diseases. In general, the use of synthetic pharmaceutical products as hypoglycaemic or hypotensive agents is usually accompanied by some serious adverse effects. There is, therefore, a dire need for the development of cheap, effective and safe hypoglycaemic and hypotensive agents from plants and other natural sources.
The result of the present study indicates that F. exasperata leaf aqueous extract caused significant reduction in the blood glucose levels of diabetic animals. Although the precise mechanism of hypoglycaemic action of FEE remains speculative at present, previous phytochemical studies on some Moraceae family of plants have indicated the presence of several chemical compounds, including alkaloids, flavonoids, saponins, tannins, glycosides and so forth (Obatomi et al; 1996). Our preliminary phytochemical screening of FEE suggests that the extract used in this study is rich in alkaloids, flavonoids, saponins and tannins. It has been proposed that the medicinal properties of F. exasperata could be attributed, at least in part, to the antioxidant, antimicrobial and established pharmacological effects of its constituent phytochemicals (Obatomi et al; 1996). However, experimental evidence obtained in this laboratory animal study indicates that F. exasperata leaf aqueous extract possesses hypoglycaemic and hypotensive properties.
An observation in this study corroborates the previous findings of Yomori (1994) who indicated that blood glucose levels significantly increased in STZ-treated SHR and obese Zucker rats. The STZ-treated SHR animals showed significant rise (p<0.05) in blood glucose and nitric oxide concentrations, and significant decrease (p<0.05) in serum insulin (Table 2). Although the blood glucose levels of both groups of diabetic animals were significantly elevated (p<0.05), and the body weights were significantly reduced, the STZ-treated SHR rat group showed more weight loss than the obese Zucker rats (Fig. 1). In the present study, continuous treatment of STZ-treated SHR and obese Zucker diabetic rats with FEE for a period of 4 weeks caused significant decrease (p<0.05) in blood glucose levels of the FEE treated diabetic rats. The possible mechanism by which the aqueous leaf extract of F. exasperata brings about its hypoglycaemic action may be by potentiating insulin effect, either by increasing pancreatic secretion of insulin from the β-cells of islet of Langerhans, or its release from bound insulin (Peri, 2004). Furthermore, we noticed elevated serum lipids in STZ-treated SHR and obese Zucker diabetic rats. Diabetes is usually marked by characteristic alterations in lipoprotein levels, including elevation of triglycerides and very low density lipoproteins (VLDL), and decreased HDL concentration (Howard, 1987). This observation is important because of its association with increased risk of developing cardiovascular diseases. Diabetes-induced hyperlipidaemia is attributable to excess mobilization of fat from adipose tissue due to under-utilization of glucose (Krishnakumar et al, 2000).
The simultaneous occurrence of hypertension and diabetes is a relevant problem that deserves intensive studies, including biochemical, histological and immunocytochemical investigations. In both groups of diabetic animals, the fur was discoloured, and the skin showed erythema. Other typical symptoms were polyuria, dehydration, proteinuria, glycosuria, diarrhoea, swollen intestine (caecum in particular), and pronounced loss of abdominal adipose tissue. Hypertension and diabetes can now be evoked separately or in combination in at least two types of experimental animal models, which reasonably resemble and approach the two major categories of diabetes in humans (IDDM and NIDDM), combined with hypertension. The STZ-treated SHR model has been characterized by occurrence of hyperglycaemic syndrome, hypoinsulinaemia associated with other biochemical and morphological changes that, to some extent, approach IDDM (type 1 diabetes) combined with hypertension as occurred in humans (Van Zwieten et al, 1996). The obese Zucker rat model is characterized by simultaneous occurrence of hyperglycaemia, hyperinsulinaemia, hyperlipidaemia, obesity and moderate hypertension. As such, this model approaches patients with NIDDM (type 2 diabetes) who are simultaneously hypertensive (Van Zwieten et al, 1996).
The present study shows that FEE significantly reduced (p<0.05) systemic arterial blood pressures and heart rates of hypertensive rats. Therefore, the observation that the plant's extract reduced or annulled pressor effects could be due to one of the following plausible mechanisms of hypotensive action of FEE in rats: (i) vasodilation; (ii) central nervous system depressant activity; (iii) peripheral, autonomic a-adrenoceptor blockade; (iv) direct myocardial depression; (v) activation and/or stimulation of nitric oxide synthase (NOS); (vi) non-specific spasmolytic activity, (vii) ganglion blockade, and so on.
The data obtained in the present study do not allow definite conclusions to be drawn on the mechanisms of action of FEE in the experimental animal paradigms used. However, a number of investigators have shown that tannins and other polyphenolic compounds (e.g., coumarins, flavonoids, triterpenoids, saponins) and a host of other plant secondary metabolites possess hypoglycaemic, hypotensive, anti-inflammatory, and other pharmacological properties in various experimental animal models (Ojewole, 2005, Akah and Okafor, 1992). F. exasperata is known to contain tannins, flavonoids, polyphenolic compounds, β-sitosterol and catechins (Alldrick, 1986). Therefore, it is not unreasonable to speculate that some of these chemical compounds, especially the coumarins and flavonoids, are probably responsible for recovery in the altered biochemical and pharmacological variables.
Based on our findings, we conclude that diabetes and hypertension have deleterious effects on body organs. Nevertheless, F. exasperata leaf aqueous extract exhibited remarkable potential for the treatment of diabetes mellitus and effective lipid lowering properties in diabetic and hypertensive rats, and is able to diminish and/or prevent, histological and biochemical derangements produced by hyperglycaemia and hypertention in rats.
References
- 1.Abbiw T. Study of tropical shrubs and plants. J Biogeorge. 1990;23:591–602. [Google Scholar]
- 2.Adewole S O, Ojewole J AO. Effect of insulin on pancreatic β-cells of streptozotocin-treated diabetic rats: Experimental observations on immunohistochemical and morphological changes. Pharmacologyonline. 2006;1:120–134. [Google Scholar]
- 3.Allain C C, Poon L S, Chon C S G, Richmond W, Fu P C. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 4.Alldrick A J. Effects of plant-derived flavonoids and polyphenolic acids on the activity of mutagens from cooked food. Motet Res. 1986;163(3):225–232. doi: 10.1016/0027-5107(86)90020-5. [DOI] [PubMed] [Google Scholar]
- 5.Akah P A, Okafor C L. Blood sugar lowering effect of Vernonia amygdalina (Del) in an experimental rabbit model. Phytother Res. 1992;6:171–173. [Google Scholar]
- 6.Atkin S, Jaker M A, Chorost M I, Reddy S. Fingerstick glucose determination in shock. Annals Int Med. 1991;144:1020–1024. doi: 10.7326/0003-4819-114-12-1020. [DOI] [PubMed] [Google Scholar]
- 7.Ayitey-Smith E. Prospects and Scope of Plant Medicine in Healthcare. Accra: Ghana University Press; 1989. pp. 1–2. [Google Scholar]
- 8.Beckman J S, Beckman T W, Chen J, et al. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg C C. Flora of West Tropical Africa. 1991;9(6) 13p. [Google Scholar]
- 10.Bouquet A J. Natural products as alternative remedy. 4th Ed. Kew: Royal Botanic Gardens; 1969. pp. 166–179. [Google Scholar]
- 11.Burkill H M. The useful plants of tropical West Africa. 3rd Ed. Kew: Royal Botanic Gardens; 1997. pp. 166–179. [Google Scholar]
- 12.Chappel C L, Chappel W R. The discovery and development of the BB rat colony: an animal model of spontaneous diabetes mellitus. Metabolism. 1983;32:8–10. doi: 10.1016/s0026-0495(83)80004-3. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y T, Zheng R L, Jia Z J, Ju Y. Flavonoids as superoxide scavengers and antioxidants. Free Radic Biol Med. 1990;9:19–20. doi: 10.1016/0891-5849(90)90045-k. [DOI] [PubMed] [Google Scholar]
- 14.Cheng M L, Kammerer C M, Lowe W F. Method for quantitating cholesterol in subfractions of serum lipoproteins separated by gradient gel electrophoresis. Biochem Genet. 1988;26:657–681. doi: 10.1007/BF02395514. [DOI] [PubMed] [Google Scholar]
- 15.Ceriello A, Mercuri F, Quagliaro L. Detection of nitrotyrosine in the diabetic plasma: Evidence of oxidative stress. Diabetologia. 2001;44:834–838. doi: 10.1007/s001250100529. [DOI] [PubMed] [Google Scholar]
- 16.Factor S M, Bhan R A J, Minase T, Wolinsky H, Sonnenblick E H. Hypertensive-diabetic cardiomyopathy in the rat, an experimental model of human disease. Am J Pathol. 1981;102:219–228. [PMC free article] [PubMed] [Google Scholar]
- 17.Freener E P, King G L. Vascular dysfunction in diabetes mellitus. Lancet. 1997;350(Suppl 1):S19–S113. doi: 10.1016/s0140-6736(97)90022-2. [DOI] [PubMed] [Google Scholar]
- 18.Friedewald W T, Levy R I, Fredickson D S. Estimation of the concentration of low density lipoprotein cholesterol in plasma without the use of preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Gonick H C, Ding Y, Bondy S C, Ni Z, Vaziri N D. Lead-induced hypertension. Interplay of nitric oxide and reactive oxygen species. Hypertension. 1997;30:1487–1492. doi: 10.1161/01.hyp.30.6.1487. [DOI] [PubMed] [Google Scholar]
- 20.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 21.Gryglewski R J, Palmer R M, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 22.Hostetter M K. Handicaps to host defence: effects of hyperglycaemia on C3 and Candida albicans. Diabetes. 1990;39:271–275. doi: 10.2337/diab.39.3.271. [DOI] [PubMed] [Google Scholar]
- 23.Howard B V. Lipoprotein metabolism in diabetes mellitus. J Lipid Res. 1987;28:613–628. [PubMed] [Google Scholar]
- 24.Ijeh I I, Agbo C A. Body organ weight changes following administration of aqueous extracts of Ficus exasperata. Vahl J Aminal and Vet Adv. 2006;5:277–279. [Google Scholar]
- 25.Ijeh I I, Ukweni A I. Acute effect of administration of ethanol extacts of F. exasperata vahl on kidney function in albino rats. J Med Plant Res. 2007;1(2):27–29. [Google Scholar]
- 26.Krishnakumar K, Augustti K T, Vijayammal P L. Hypolipidaemic effect of Salacia oblonga Wall root-bark in streptozotocin diabetic rats. Med Science. 2000;28:65–67. [Google Scholar]
- 27.Kröncke K D, Fehsel K, Sommer A, Rodriguez M L, Kolb-Bachofen V. Nitric oxide generation during cellular metabolization of diabetogenic N-methyl nitroso-urea: Streptozotocin contributes to islet cell DNA damage. Biol Chem Hoppe-Seyler. 1995;376:179–185. doi: 10.1515/bchm3.1995.376.3.179. [DOI] [PubMed] [Google Scholar]
- 28.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 29.Matkovics B, Kotorman M, Varga I S, Hai D Q, Varga C. Oxidative stress in experimental diabetes induced by streptozotocin. Acta Physiol Hung. 1998;85:29–38. [PubMed] [Google Scholar]
- 30.Obatomi D K, Aina V O, Temple V J. Effects of African mistoletoe on blood pressure in spontaneously-hypertensive rats. Int J Pharmacol. 1996;34:124–127. [Google Scholar]
- 31.Ojewole J A O. Antinociceptive, anti-inflammatory and antidiabetic effects of Bryophyllum pinnatum (Crassulaceae) leaf aqueous extract. J Ethnopharmacol. 2005;99:13–19. doi: 10.1016/j.jep.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 32.Peri L A S. Antidiabetic activity of Boerhaavia diffusa L.: effect on hepatic enzymes in experimental diabetes. J Ethnopharmacol. 2004;91:109–113. doi: 10.1016/j.jep.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Rainwater D L, Ludwig M J, Haffner S M, VandeBerg J L. Lipid and lipoprotein factors associated with variation in Lp(a) density. Arterioscler Thromb Vasc Biol. 1995;15:313–319. doi: 10.1161/01.atv.15.3.313. [DOI] [PubMed] [Google Scholar]
- 34.Rosen P, Nawroth P P, King G, et al. The role of oxidative stress in the onset and progression of diabetes and its complications: A summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 35.Rossini A A, Williams R M, Appel M C, Like A A. Complete protection from low-dose streptozotocin-induced diabetes in mice. Nature. 1978;276:182–184. doi: 10.1038/276182a0. [DOI] [PubMed] [Google Scholar]
- 36.Squadrito G L, Pryor W A. Formation of peroxynitrite in vivo from nitric oxide and superoxide. Chem Biol Interact. 1995;96:203–206. doi: 10.1016/0009-2797(94)03591-u. [DOI] [PubMed] [Google Scholar]
- 37.Stevens A. The haematoxylins. In: Bancroft JD, Stevens A, editors. Theory and practice of Histological Techniques. London: Longman Group; 1982. pp. 109–122. [Google Scholar]
- 38.Van Zwieten P A, Kam K L, Pijl A J, Hendriks M G C, Beenen O H M, Pfaffendorf M. Hypertensive diabetic rats in pharmacological studies. Pharmacol Res. 1996;33(2):95–105. doi: 10.1006/phrs.1996.0015. [DOI] [PubMed] [Google Scholar]
- 39.Virella-Lopes M F L, Stone P G, Colwel J A. Serum High Density Lipoprotein in diabetic patients. Diabetologia. 1977;13:285–291. doi: 10.1007/BF01223267. [DOI] [PubMed] [Google Scholar]
- 40.Yamori Y. Development of spontaneously-hypertensive rat (SHR) and various spontaneous rat models, and their implications. In: de Jong W, editor. Handbook of hypertension. Vol. 4. Amsterdam: Elsevier; 1994. pp. 224–239. [Google Scholar]