Abstract
Malaria has had the largest impact of any infectious disease on shaping the human genome, exerting enormous selective pressure on genes that improve survival in severe malaria infections. Modern humans originated in Africa and lost skin melanization as they migrated to temperate regions of the globe. Although it is well documented that loss of melanization improved cutaneous Vitamin D synthesis, melanin plays an evolutionary ancient role in insect immunity to malaria and in some instances melanin has been implicated to play an immunoregulatory role in vertebrates. Thus, we tested the hypothesis that melanization may be protective in malaria infections using mouse models. Congenic C57BL/6 mice that differed only in the gene encoding tyrosinase, a key enzyme in the synthesis of melanin, showed no difference in the clinical course of infection by Plasmodium yoelii 17XL, that causes severe anemia, Plasmodium berghei ANKA, that causes severe cerebral malaria or Plasmodium chabaudi AS that causes uncomplicated chronic disease. Moreover, neither genetic deficiencies in vitamin D synthesis nor vitamin D supplementation had an effect on survival in cerebral malaria. Taken together, these results indicate that neither melanin nor vitamin D production improve survival in severe malaria.
Introduction
Melanins are pigments that have evolved over 500 million years and that are present in animals, microorganisms and plants. In flies and mosquitoes melanization of pathogens is part of the innate immune response and it is correlated with resistance to infection [1], [2]. In animals, melanin is produced by melanocytes, that are present in a variety of tissues, including skin, uveal tract, brain and the peritoneum [3]. The low level of melanization in the skin of humans living in temperate climates of the globe is in large part due to the fact that melanin pigments absorb and scatter the UVB wavelengths that catalyze the conversion of 7-dehydrocholesterol to pre-Vitamin D3, the precursor of Vitamin D3 in the skin [4], [5]. In fact, individuals with marked skin melanization frequently require 10–20 times longer exposure to sunlight than those of lighter pigmentation to promote adequate synthesis of vitamin D3 [4], [6]. Vitamin D deficiency can exert strong selective pressure by causing severe deformation of the pelvis in women, leading to increased perinatal morbidity and mortality, or increasing the susceptibility to infections and death in children [7]. The pressure for skin melanization in tropical regions, on the other hand, can be explained by the advantage of melanized individuals to withstand prolonged sun exposure, although a rigorous link of melanization with an increase in fitness in mammals has not been established [3]. Interestingly, dark colors are attractive to Aedes aegyptii, the mosquito vector of dengue and yellow fever, and Culex fatigans, the mosquito vector of filarial worms, but not for anopheline mosquitoes, the mosquito vectors of malaria, which find light colors more attractive [8]. In this regard skin melanization can be either advantageous or detrimental, depending on the disease.
In addition, both melanin and vitamin D have been suggested to have potential immunoregulatory functions. Melanin has been shown in some circumstances to have antioxidant [9], [10], anti-inflammatory [11], and immunoregulatory functions in innate immune responses, with melanocytes participating in phagocytosis [12] and inflammation [13], [14], [15]. This observation led to the suggestion that an additional function of melanin in the skin is to promote immune responses to infections [3], [16], [17]. In birds, melanin levels have been shown to influence the magnitude of antibody and cellular responses [18]. In addition, vitamin D deficiencies have been reported to increase [19], [20] and to decrease resistance to infection [21], [22]. Although in experiments vitamin D inhibits plasmodium growth in vitro [23], in malaria infections neither VDR gene polymorphisms or vitamin D levels correlate with infection [24]. On the other hand, vitamin D deficiency has been shown to be a risk factor for the development of autoimmune diseases including multiple sclerosis [25], [26], [27], [28] and systemic lupus erythematosus (SLE) [29], [30], [31]. SLE is eight to ten times more prevalent in women of African as compared to European descent and recently, we provided evidence in a mouse model of SLE that SLE susceptibility genes that result in hyperimmune activation are protective in cerebral malaria but not against severe anemia [32]. These results suggested that the higher incidence of SLE in African Americans may be a consequence of the beneficial effects of SLE susceptibility genes in malaria-endemic areas.
The possibility that melanin and vitamin D may play immunoregulatory roles led us to the hypothesis that melanin or vitamin D production may have been selected for in Africans in part due to a protective role in malaria. To test this hypothesis, we compared the course of malaria infections in wild type and albino B6(Cg)-Tyrc-2J/J mice (B6.Tyr−/− mice) that have mutations in the Tyr gene, that encodes tyrosinase, the same gene that is mutated in type I human oculocutaneous albinism. Tyrosinase is the key enzyme in mammalian melanin biosynthesis that catalyzes the conversion of tyrosine to dopa, the dehydrogenation of dopa to dopaquinone, and the conversion of 5,6-dihidroxyindile to melanochrome, which is the direct precursor of melanin [33]. Consequently, the absence of tyrosinase ablates completely the synthesis of the major body melanins, pheomelanin and eumelanin. We also tested whether vitamin D had an effect on the course of malaria infections in mice.
Results
Melanization does not protect against malaria-induced severe anemia
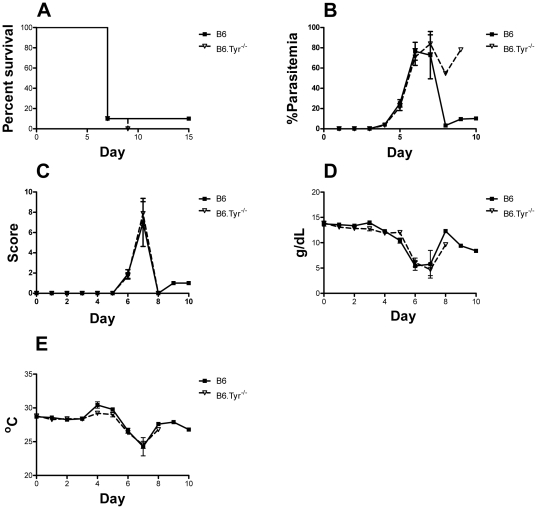
C57BL/6 (B6) mice and tyrosinase deficient B6 (B6.Tyr−/−) mice were infected with the lethal strain P. yoelii 17XL by injection of 104 infected red blood cells i.p. Mice were followed daily to evaluate parasitemia, body weight, temperature, clinical status, hemoglobin concentration and survival (Figure 1). We also determined the hematocrit, white blood cell differential count and platelets count, on day 0 and day 3 following infection (Figure S1). Infected B6.Tyr−/− mice had higher white blood cell counts, less anemia and higher lymphocyte counts on day 3 as compared to B6 mice. Despite these differences, we observed no difference in the survival of the B6 and B6.Tyr−/− mice following P. yoelii 17XL infections. The majority of B6 and B6.Tyr−/− mice died of the infection within 8–9 days and mice from the two groups showed a similar course of infection for all parameters analyzed. The levels of parasitemia appear different between the two groups after day 7 but these differences are not statistically significant as only one mouse in each group survived beyond day 7.
Figure 1. Effect of melanization in severe malaria anemia.
(A) The percent of B6.Tyr−/− and B6 P. yoelii 17XL infected mice that survived over time is given in Kaplan-Meier curves. Death was defined as a parasitemia ≥60%, moribundity or death (P = 0.64). (B) Parasitemias, determined by GIEMSA stained smears of tail blood, are given for B6.Tyr−/− and B6 infected mice over time. (C) Clinical scores determined by the modified SNAP scoring system are given. (D) Hemoglobin concentrations of B6.Tyr−/− and B6 infected mice are given. (E) Body temperature determined at the tail skin for both B6.Tyr−/− and B6 infected mice over time are given. Data showed represents the mean of 10 animals and bars represent the SEM.
Melanization does not protect against cerebral malaria
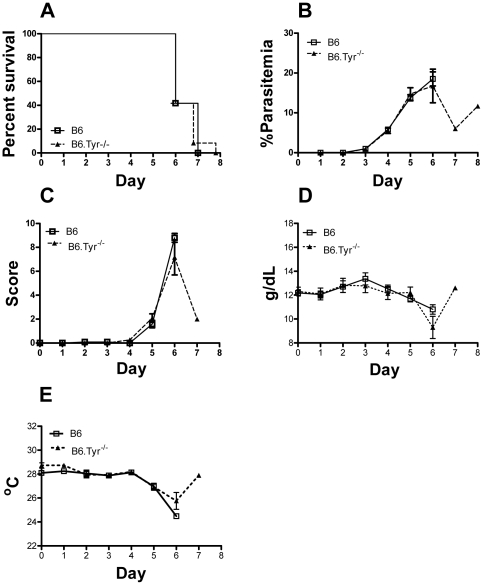
P. berghei ANKA is an established model of mouse cerebral malaria (CM) that reproduces reasonably well the pathological features of human CM. P. berghei infections are lethal in mice resulting in early death (by day 6–12) in susceptible B6 strains due to CM or, in late death (between days 14 and 21) in resistant strains due to severe anemia [34], [35]. B6 and B6.Tyr−/− mice were infected with P. berghei ANKA by injection of 106 infected red blood cells, i.p. All mice, both B6 and B6.Tyr−/−, died before day 10 and developed signs of CM (Figure 2). No differences in survival were observed between the groups. In addition, B6 and B6.Tyr−/− mice showed no significant differences in parasitemia, hemoglobin levels, body temperature or cytokine levels (Figure S2) during the course of the infection. Thus, it appears that the ability to produce melanin is not protective against severe CM.
Figure 2. Effect of melanization on cerebral malaria.
(A) The percent of B6.Tyr−/− and B6 P. berghei ANKA infected mice that survived over time is given in Kaplan-Meier curves. Death was defined as a parasitemia ≥60%, moribundity or death (P = 0.71). (B) Parasitemias, determined by GIEMSA stained smears of tail blood, are given for B6.Tyr−/− and B6 infected mice over time. (C) Clinical scores determined by the modified SNAP scoring system are given. (D) Hemoglobin concentrations of B6.Tyr−/− and B6 infected mice are given. (E) Body temperature determined at the tail skin for both B6.Tyr−/− and B6 infected mice over time are given. Data showed represents the mean of 10 animals and bars represent the SEM.
Melanization does not protect against chronic malaria infection
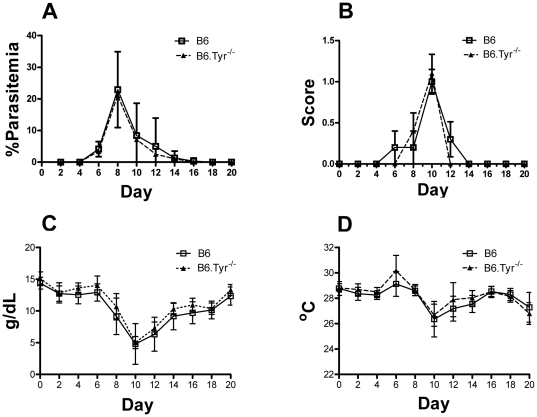
To determine the effect of melanin deficiency on the course of a chronic malarial infection we infected B6 and B6.Tyr−/− mice with P. chabaudi AS by injection of mice with 106 infected red blood cells. P. chabaudi AS causes a biphasic infection characterized initially by acute parasitemia that peaks around day 7, followed by chronic low-level infection. The immune response during the first phase is controlled both by an innate system response as well as a Th1 response [36], [37] while the control and clearance of the chronic blood infection during the second phase requires both TH1 and TH2 CD4+ cells and B cells [36], [38]. We followed the P. chabaudi infected mice for 21 days and observed no difference between B6 and B6.Tyr−/− mice in all parameters analyzed including survival, parasitemia, clinical score, hemoglobin levels, body temperature and leukocyte, neutrophil and lymphocyte numbers. (Figure 3, Figure S3). Thus, we concluded that the ability to produce melanin did not affect the course of chronic malaria infections.
Figure 3. Effect of melanization on chronic malaria infection.
(A) Parasitemias, determined by GIEMSA stained smears of tail blood, are given for B6.Tyr−/− and B6 P. chabaudi AS infected mice over time. (B) Clinical scores determined by the modified SNAP scoring system, are given. (C) Hemoglobin concentrations of B6.Tyr−/− and B6 infected mice are given. (D) Body temperature determined at the tail skin for both B6.Tyr−/− and B6 infected mice over time are given. Data showed represents the mean of 10 animals and bars represent the SEM.
Melanin treatment decreases parasitemia but does not protect against cerebral malaria
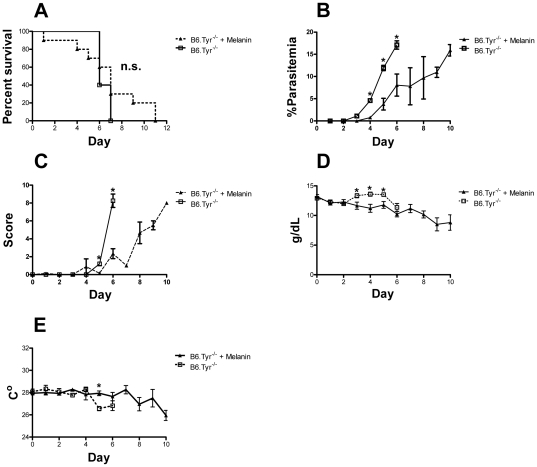
Previous studies have shown that melanin in high doses can affect the secretion of multiple cytokines, including TNF-α, IL-1β, IL-6 and GM-CSF [39]. To investigate the effects of high doses of melanin on the course of malaria CM infections, we treated P. berghei ANKA infected B6.Tyr−/− mice with daily doses of melanin (25 mg/kg) in saline i.p. or with saline alone. B6.Tyr−/− mice receiving melanin showed a delayed increased in parasitemia (P<0.05) and better clinical scores but did not better survive the infection as compared to controls B6.Tyr−/− mice receiving saline (P = 0.58) (Figure 4). On day five post-infection, melanin treated mice had higher platelet counts than controls (P<0.001) (Figure S4), and the number of platelets correlated positively with survival (Spearman Rho = 0.64, p = 0.002). On the other hand, melanin treatment caused animals to have a steeper decrease in hemoglobin levels, especially from day 3 to day 5 post-infection (P<0.05). The fact that melanin treated mice did not survive the infection any better than control B6 mice suggests that, albeit somewhat beneficial, melanin treatment is not sufficient to change the course of the disease in mice (Figure 4).
Figure 4. Effect of melanin treatment on cerebral malaria.
(A) The percent of melanin treated and untreated B6.Tyr−/− P. berghei ANKA infected mice that survived over time is given in Kaplan-Meier curves. Death was defined as a parasitemia ≥60%, moribundity or death (P = 0.33). (B) Parasitemias, determined by GIEMSA stained smears of tail blood, are given for B6.Tyr−/− treated and untreated infected mice over time. (C) Clinical scores determined by the modified SNAP scoring system are given. (D) Hemoglobin concentrations of B6. Tyr−/− treated and untreated infected mice are given. (E) Body temperature determined at the tail skin for B6.Tyr−/− treated and untreated infected mice over time are given. Data showed represents the mean of 10 animals and bars represent the SEM.
Vitamin D does not protect against cerebral malaria
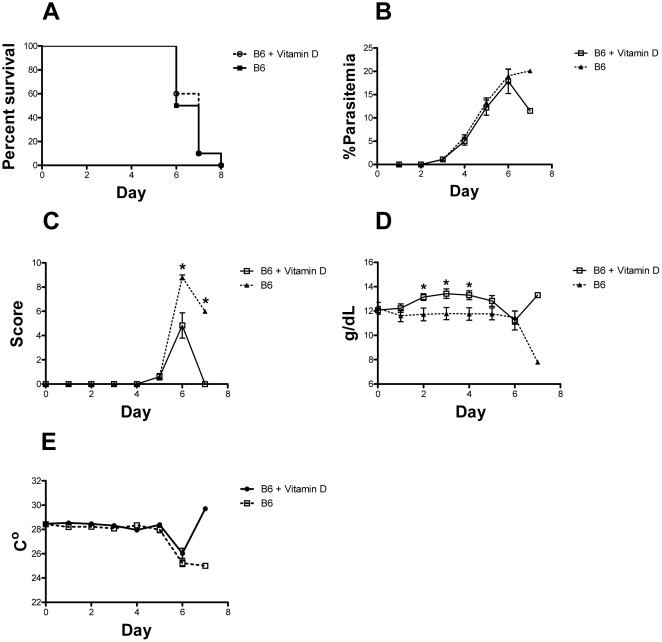
Because skin melanization is an important factor in cutaneous vitamin D biosynthesis, we tested if vitamin D deficiency influenced the pathogenesis of cerebral malaria. We also supplemented the diets of mice with vitamin D injections i.p. every other day (0.5 µg/kg), starting 3 days before infection with P. berghei ANKA. We did not observe any difference in survival between treated and untreated mice with all mice dying by day 8 (Figure 5A). Also, we did not observe any differences in parasitemia or body temperature during the course of infection suggesting that vitamin D, at the levels used in this study, does not influence the immune response to malaria (Figure 5B and 5E). Animals receiving vitamin D injections had better clinical scores at days 6 and 7 post infection and had higher hemoglobin levels than controls but this effect was apparently not enough to reduce mortality (P = 0.76).
Figure 5. Effect of vitamin D3 treatment on cerebral malaria.
(A) The percent of treated and control P. berghei ANKA infected B6 mice that survived over time is given in Kaplan-Meier curves. Death was defined as a parasitemia ≥60%, moribundity or death (P = 0.76). (B) Parasitemias, determined by GIEMSA stained smears of tail blood, are given for treated and control B6 mice over time. (C) Clinical scores determined by the modified SNAP scoring system are given. (D) Hemoglobin concentrations determined on tail blood are given. (E) Body temperature determined at the tail skin for treated and control B6 mice over time are given. Data showed represents the mean of 10 animals and bars represent the SEM.
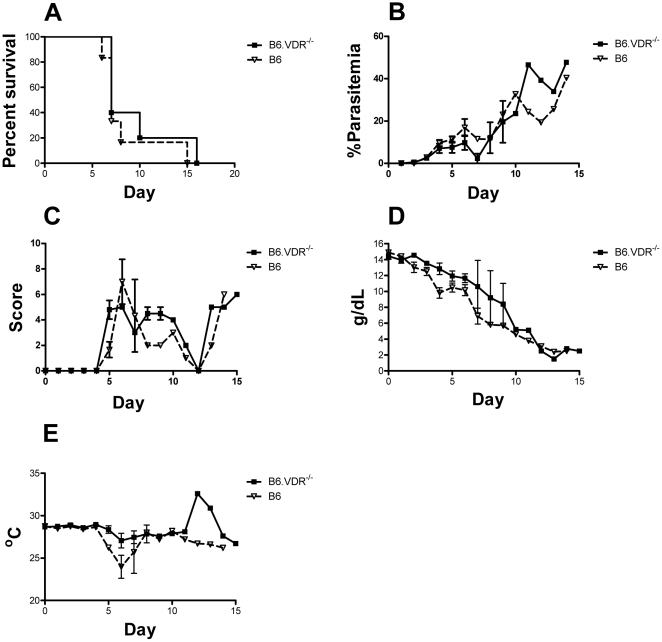
We also followed the course of P. berghei ANKA infections in wild type B6 mice and in mice deficient in the vitamin D receptor (VDR) and B6.VDR−/− mice. Because B6.VDR−/− knockout mice require a special diet to prevent severe osteoporosis and death, we kept wild type B6 mice on the same diet. We observed no difference in the course of disease in B6 and B6.VDR−/− mice (Figure 6). Most B6 and B6.VDR−/− mice died within 9–10 days of infection and showed similar parasitemias, clinical scores, hemoglobin levels and body temperatures.
Figure 6. Effect of VDR knockout on cerebral malaria.
(A) The percent of B6 and B6.VDR−/− P. berghei ANKA infected mice that survived over time is given in Kaplan-Meier curves. Death was defined as a parasitemia ≥60%, moribundity or death (P = 0.38). (B) Parasitemias, determined by GIEMSA stained smears of tail blood, are given for B6.VDR−/− and B6 infected mice over time. (C) Clinical scores determined by the modified SNAP scoring system are given. (D) Hemoglobin concentrations of B6.VDR−/− and B6 infected mice are given. (E) Body temperature determined at the tail skin for both B6.VDR−/− and B6 infected mice over time are given. Data showed represents the mean of 10 animals and bars represent the SEM.
Discussion
Our results provide evidence that melanization does not improve survival in severe malaria. Melanization did not improve survival against parasites that cause either CM or severe anemia and does not affect the course of chronic infections. Our data also suggests that neither vitamin D treatment nor vitamin D receptor deficiency affect the progression of cerebral malaria caused by P. berghei ANKA infections. Therefore the data suggest that melanin, neither directly nor through its indirect effect on vitamin D synthesis, improves survival in severe malaria in mice. High doses of vitamin D have been reported to decreased parasite growth and survival in vitro [40] but we did not observe similar effects when treating animals with lower doses. This is in agreement with previous studies in humans showing a lack of association between polymorphisms on the VDR gene and malaria infection [19]. Nevertheless, because we only tested the effects of one dose of vitamin D3 (and not of other forms) one cannot exclude completely an effect of vitamin D on malaria.
We also tested the effect of high doses of melanin on the progression of CM. Although the dose used was previously shown to reduce LPS induced TNF-α production [39] and we showed that high doses of melanin delayed increases in parasitemia and improved clinical scores, melanin treatment was not sufficient to increase survival in CM. The effect in parasitemia and clinical scores can be potentially explained by direct anti-parasite effect of melanin (as observed in mosquitoes) or by its free radical scavenging properties. CM is a complex disease caused in part by excessive free radicals generated as a response to free iron and hemoglobin [41], [42], [43] and, under certain conditions, free radical scavengers can block some of the deleterious effects of malaria [44], [45]. Interestingly, melanin treatment prevented the thrombocytopenia that was observed in B6 mice by day 5 post-infection. Changes in platelet counts are also common in human malaria infection, and the extent of reduction is a predictor of clinical outcome and severity of malarial infection in children [46]. Platelets are involved in the pathogenesis of cerebral malaria as they encourage the sequestration of infected red blood cells in the cerebral vasculature and in the innate response to the parasite [47], [48] and, in agreement with the literature, our data showed a positive correlation between platelet count and survival. Last, we have shown that melanin treatment does not affect survival in P. berghei ANKA infections, suggesting again that melanin does not affect the progression of murine CM. Taken together, our data suggests that melanization was not selected by malaria. In spite of that, one cannot exclude completely the role of melanin on the response to malaria, for the mouse and human immune system and skin are considerably different. For instance, human melanocytes are mainly found at the basal layer of the epidermis while mouse melanocytes reside in the hair follicle and the dermis [49], [50], pelage density is much higher in rodents than humans, and the stratum corneum of rodents is generally more permeable and fragile [51]. Furthermore mice are nocturnal animals with very small vitamin D needs, suggesting that melanin must play a different role in vitamin D metabolism in mice. Humans, on the other hand, are considered diurnal animals that synthesize vitamin D in the skin on exposure to UV light [52].
Methods
Ethics statement
All experiments were approved by the National Institute of Allergy and Infectious Diseases Animal Care and Use Committee.
Animals
Male 8–10 week old C57BL/6, B6.VDR[KO] (strain B6.129S4-Vdrtm1Mbd/J, stock number 006133) and B6.TYR[KO] (strain B6(Cg)-Tyrc-2J/J, stock number 000058) mice were obtained from The Jackson Laboratories and allowed to acclimate in the animal facility until the experiment was performed. B6.VDR[KO] mice were maintained constantly on a 20% lactose diet (TD 96348, Harlan) to prevent the development of osteoporosis.
Malaria infections and treatments
Mice were infected with 1×106 P. berghei ANKA, 1×106 P. chabaudi AS or 1×104 P. yoelii 17XL infected red blood cells (iRBCs) i.p. Parasites were obtained from donor mice that were infected with thawed parasite stocks. Synthetic melanin was obtained from Sigma (M0418) and it was initially dissolved in 0.1 NaOH to solubilize it. The solution pH was adjusted to 7.0, it was centrifuged and then filtered through a 0.45 µM filter before injection. Animals were injected i.p. daily with 25 mg/kg of melanin solution or saline. Vitamin D was obtained from Sigma (Supelco Cholecalciferol (D3) Cat#47763) and it was first dissolved in 10 mL of 100% ethanol to make a stock solution (10 mg/mL). The stock was further diluted in 0.02% Tween 80 to make a working solution (0.1 µg/mL). Animals were injected with 0.5 µg/kg every other day, starting 3 days before infection. A similar dose of 1,25-dihydroxivitamin D3 was previously shown not to cause hypercalcemia but to induce splenocyte apoptosis and enhance sensitivity to other parasites [53]. For the experiment investigating the role of the vitamin D receptor (VDR) on the response to cerebral malaria, VDR knockout mice were kept on a rescue diet rich in calcium, phosphorus and lactose (Harlan laboratories, stock #TD.96348) to avoid osteoporosis. 10 animals were used per group in all experiments. In all cases, parasitemia in infected mice was quantified by examining Giemsa stained thin blood smears. Hemoglobin concentration was measured daily with a HemoCue Hb 201+ using blood from the tail tip (<10 µL/day). Mice that reached 60% parasitemia or that become moribund were euthanized. All mice were evaluated daily for the presence of clinical signs of severe malaria using a simple scoring adapted from the SNAP scoring system [54]. Animals were scored by evaluating 5 categories: interactions/reflex, cage grasp, visual placing, gait/posture/appearance, and capacity to hold their body weight on a baton. Each category was divided in 3 levels, varying from 0 to 2, where 0 represented normal individuals and 2 the worst case for that particular parameter. Hematology parameters were determined on day 0 and 5 using a Hemavet 950 FS system (Drew Scientific).
Statistics
When applicable, statistical analysis of survival was by Log-rank (Mantel-Cox) test. The survival curves were calculated using the Kaplan-Meier estimates and the survival curves were compared by the log-rank test. Continuous variables were analyzed with the two-sample t-test, where the Bartlett's test for equal variances was used to determine whether the use of a pooled variance or unequal variances was appropriate. Two-sided P values were reported in the text. P values were considered significant if P<0.05.
Cytokine Quantification
The levels of cytokines in serum samples were quantified using the Q-Plex Mouse Cytokine Kit (Quansys Biosciences) according to manufacturer's instructions.
Supporting Information
Melanization does not change blood composition in response to severe malaria anemia. Animals were infected with 1×104 P. yoelii 17XL iRBCs on day 0. Hematological parameters were determined in whole blood. White blood cells (WBC), Red Blood Cells (RBC), Hemoglobin (Hb), Platelets, Neutrophils, Lymphocytes and Hematocrit are shown. Data showed represents the mean of 10 animals and bars represent the SD of the mean.
(TIF)
Melanin does not alter the cytokine profile in response to cerebral malaria. Cytokine levels before (day 0) and 5 days after infection using 1×106 P. berghei ANKA iRBCs are shown. Data showed represents the mean of 10 animals and bars represent the SD of the mean.
(TIF)
Melanization does not alter blood composition in response to chronic malaria. Animals were infected with 1×106 P. chabaudi iRBCs on day 0. White blood cells (WBC), Red Blood Cells (RBC), Hemoglobin (Hb), Platelets, Neutrophils, Lymphocytes and Hematocrit are shown. Data showed represents the mean of 10 animals and bars represent the SD of the mean.
(TIF)
Effect of melanin injections on blood composition in B6.Tyr−/− animals. Animals were infected with 1×106 P. berghei ANKA iRBCs on day 0. White blood cells (WBC), Red Blood Cells (RBC), Hemoglobin (Hb), Platelets, Lymphocytes, Neutrophils, Hematocrit and the linear regression of survival on platelet counts are given. The dashed lines represent the 95% confidence interval about the regression line. Data showed represents the mean of 10 animals and bars represent the SD of the mean.
(TIF)
Acknowledgments
The authors would like to thank Mrs. Terri Fantasia and Julie Kim for editorial and logistic support.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ayres JS, Schneider DS. A signaling protease required for melanization in Drosophila affects resistance and tolerance of infections. PLoS Biol. 2008;6:2764–2773. doi: 10.1371/journal.pbio.0060305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson JK, Rocheleau TA, Hillyer JF, Chen CC, Li J, et al. A potential role for phenylalanine hydroxylase in mosquito immune responses. Insect Biochem Mol Biol. 2003;33:345–354. doi: 10.1016/s0965-1748(02)00257-6. [DOI] [PubMed] [Google Scholar]
- 3.Burkhart CG, Burkhart CN. The mole theory: primary function of melanocytes and melanin may be antimicrobial defense and immunomodulation (not solar protection). Int J Dermatol. 2005;44:340–342. doi: 10.1111/j.1365-4632.2004.02556.x. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science. 1981;211:590–593. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]
- 5.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 6.Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000;39:57–106. doi: 10.1006/jhev.2000.0403. [DOI] [PubMed] [Google Scholar]
- 7.Muhe L, Lulseged S, Mason KE, Simoes EA. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet. 1997;349:1801–1804. doi: 10.1016/S0140-6736(96)12098-5. [DOI] [PubMed] [Google Scholar]
- 8.Brown AW. The attraction of mosquitoes to hosts. JAMA. 1966;196:249–252. [PubMed] [Google Scholar]
- 9.Herrling T, Jung K, Fuchs J. The role of melanin as protector against free radicals in skin and its role as free radical indicator in hair. Spectrochim Acta A Mol Biomol Spectrosc. 2008;69:1429–1435. doi: 10.1016/j.saa.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 10.Bustamante J, Bredeston L, Malanga G, Mordoh J. Role of melanin as a scavenger of active oxygen species. Pigment Cell Res. 1993;6:348–353. doi: 10.1111/j.1600-0749.1993.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 11.Mohagheghpour N, Waleh N, Garger SJ, Dousman L, Grill LK, et al. Synthetic melanin suppresses production of proinflammatory cytokines. Cell Immunol. 2000;199:25–36. doi: 10.1006/cimm.1999.1599. [DOI] [PubMed] [Google Scholar]
- 12.Le Poole IC, van den Wijngaard RM, Westerhof W, Verkruisen RP, Dutrieux RP, et al. Phagocytosis by normal human melanocytes in vitro. Exp Cell Res. 1993;205:388–395. doi: 10.1006/excr.1993.1102. [DOI] [PubMed] [Google Scholar]
- 13.Hu DN, Chen M, Zhang DY, Ye F, McCormick SA, et al. Interleukin-1beta increases baseline expression and secretion of interleukin-6 by human uveal melanocytes in vitro via the p38 MAPK/NF-kappaB pathway. Invest Ophthalmol Vis Sci. 2011;52:3767–3774. doi: 10.1167/iovs.10-6908. [DOI] [PubMed] [Google Scholar]
- 14.Jin SH, Kang HY. Activation of Toll-like Receptors 1, 2, 4, 5, and 7 on Human Melanocytes Modulate Pigmentation. Ann Dermatol. 2010;22:486–489. doi: 10.5021/ad.2010.22.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu N, Zhang S, Zuo F, Kang K, Guan M, et al. Cultured human melanocytes express functional toll-like receptors 2–4, 7 and 9. J Dermatol Sci. 2009;56:113–120. doi: 10.1016/j.jdermsci.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Mackintosh JA. The antimicrobial properties of melanocytes, melanosomes and melanin and the evolution of black skin. J Theor Biol. 2001;211:101–113. doi: 10.1006/jtbi.2001.2331. [DOI] [PubMed] [Google Scholar]
- 17.El-Obeid A, Al-Harbi S, Al-Jomah N, Hassib A. Herbal melanin modulates tumor necrosis factor alpha (TNF-alpha), interleukin 6 (IL-6) and vascular endothelial growth factor (VEGF) production. Phytomedicine. 2006;13:324–333. doi: 10.1016/j.phymed.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Gasparini J, Piault R, Bize P, Roulin A. Synergistic and antagonistic interaction between different branches of the immune system is related to melanin-based coloration in nestling tawny owls. J Evol Biol. 2009;22:2348–2353. doi: 10.1111/j.1420-9101.2009.01831.x. [DOI] [PubMed] [Google Scholar]
- 19.Bellamy R, Ruwende C, Corrah T, McAdam KP, Thursz M, et al. Tuberculosis and chronic hepatitis B virus infection in Africans and variation in the vitamin D receptor gene. J Infect Dis. 1999;179:721–724. doi: 10.1086/314614. [DOI] [PubMed] [Google Scholar]
- 20.Whitcomb JP, Deagostino M, Ballentine M, Fu J, Tenniswood M, et al. The Role of Vitamin D and Vitamin D Receptor in Immunity to Leishmania major Infection. J Parasitol Res. 2012;2012:134645. doi: 10.1155/2012/134645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansdottir S, Monick MM. Vitamin D effects on lung immunity and respiratory diseases. Vitam Horm. 2011;86:217–237. doi: 10.1016/B978-0-12-386960-9.00009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spector SA. Vitamin D and HIV: letting the sun shine in. Top Antivir Med. 2011;19:6–10. [PMC free article] [PubMed] [Google Scholar]
- 23.Vial HJ, Thuet MJ, Philippot JR. Inhibition of the in vitro growth of Plasmodium falciparum by D vitamins and vitamin D-3 derivatives. Mol Biochem Parasitol. 1982;5:189–198. doi: 10.1016/0166-6851(82)90020-2. [DOI] [PubMed] [Google Scholar]
- 24.Newens K, Filteau S, Tomkins A. Plasma 25-hydroxyvitamin D does not vary over the course of a malarial infection. Trans R Soc Trop Med Hyg. 2006;100:41–44. doi: 10.1016/j.trstmh.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 25.Smolders J, Thewissen M, Theunissen R, Peelen E, Knippenberg S, et al. Vitamin D-related gene expression profiles in immune cells of patients with relapsing remitting multiple sclerosis. J Neuroimmunol. 2011;235:91–97. doi: 10.1016/j.jneuroim.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Smolders J, Damoiseaux J. Vitamin D as a T-cell modulator in multiple sclerosis. Vitam Horm. 2011;86:401–428. doi: 10.1016/B978-0-12-386960-9.00018-6. [DOI] [PubMed] [Google Scholar]
- 27.Smolders J. Vitamin d and multiple sclerosis: correlation, causality, and controversy. Autoimmune Dis. 2011;2011:629538. doi: 10.4061/2011/629538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smolders J, Damoiseaux J, Menheere P, Hupperts R. Vitamin D as an immune modulator in multiple sclerosis, a review. J Neuroimmunol. 2008;194:7–17. doi: 10.1016/j.jneuroim.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Kamen D, Aranow C. Vitamin D in systemic lupus erythematosus. Curr Opin Rheumatol. 2008;20:532–537. doi: 10.1097/BOR.0b013e32830a991b. [DOI] [PubMed] [Google Scholar]
- 30.Turhanoğlu AD, Guler H, Yönden Z, Aslan F, Mansuroglu A, et al. The relationship between vitamin D and disease activity and functional health status in rheumatoid arthritis. Rheumatol Int. 2010 doi: 10.1007/s00296-010-1393-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Pelajo CF, Lopez-Benitez JM, Miller LC. Vitamin D and Autoimmune Rheumatologic Disorders. Autoimmun Rev. 2010;9:507–510. doi: 10.1016/j.autrev.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Waisberg M, Tarasenko T, Vickers BK, Scott BL, Willcocks LC, et al. Genetic susceptibility to systemic lupus erythematosus protects against cerebral malaria in mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1122–1127. doi: 10.1073/pnas.1017996108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korner A, Pawelek J. Mammalian tyrosinase catalyzes three reactions in the biosynthesis of melanin. Science. 1982;217:1163–1165. doi: 10.1126/science.6810464. [DOI] [PubMed] [Google Scholar]
- 34.Martins YC, Werneck GL, Carvalho LJ, Silva BP, Andrade BG, et al. Algorithms to predict cerebral malaria in murine models using the SHIRPA protocol. Malar J. 2010;9:85. doi: 10.1186/1475-2875-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neill AL, Hunt NH. Pathology of fatal and resolving Plasmodium berghei cerebral malaria in mice. Parasitology. 1992;105(Pt 2):165–175. doi: 10.1017/s0031182000074072. [DOI] [PubMed] [Google Scholar]
- 36.Langhorne J. The role of CD4+ T-cells in the immune response to Plasmodium chabaudi. Parasitol Today. 1989;5:362–364. doi: 10.1016/0169-4758(89)90113-0. [DOI] [PubMed] [Google Scholar]
- 37.Su Z, Stevenson MM. Central role of endogenous gamma interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infect Immun. 2000;68:4399–4406. doi: 10.1128/iai.68.8.4399-4406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor-Robinson AW, Phillips RS. B cells are required for the switch from Th1- to Th2-regulated immune responses to Plasmodium chabaudi chabaudi infection. Infect Immun. 1994;62:2490–2498. doi: 10.1128/iai.62.6.2490-2498.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohagheghpour N USPO, editor. United States: SRI International; 2001. Mediation of Cytokynes by Melanin. [Google Scholar]
- 40.Sergacheva I, Sokhanenkova TL, Soprunov FF, Lur'e AA. [Effect of vitamins D and E on the development of Plasmodium berghei infection in mice]. Med Parazitol (Mosk) 1986:15–18. [PubMed] [Google Scholar]
- 41.Gordeuk VR, Thuma PE, McLaren CE, Biemba G, Zulu S, et al. Transferrin saturation and recovery from coma in cerebral malaria. Blood. 1995;85:3297–3301. [PubMed] [Google Scholar]
- 42.Narsaria N, Mohanty C, Das BK, Mishra SP, Prasad R. Oxidative Stress in Children with Severe Malaria. J Trop Pediatr. 2011 doi: 10.1093/tropej/fmr043. [DOI] [PubMed] [Google Scholar]
- 43.Das BS, Mohanty S, Mishra SK, Patnaik JK, Satpathy SK, et al. Increased cerebrospinal fluid protein and lipid peroxidation products in patients with cerebral malaria. Trans R Soc Trop Med Hyg. 1991;85:733–734. doi: 10.1016/0035-9203(91)90436-3. [DOI] [PubMed] [Google Scholar]
- 44.Reis PA, Comim CM, Hermani F, Silva B, Barichello T, et al. Cognitive dysfunction is sustained after rescue therapy in experimental cerebral malaria, and is reduced by additive antioxidant therapy. PLoS Pathog. 2010;6:e1000963. doi: 10.1371/journal.ppat.1000963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S, Chen H, Gerhard GS. Heme synthesis increases artemisinin-induced radical formation and cytotoxicity that can be suppressed by superoxide scavengers. Chem Biol Interact. 2010;186:30–35. doi: 10.1016/j.cbi.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 46.Gerardin P, Rogier C, Ka AS, Jouvencel P, Brousse V, et al. Prognostic value of thrombocytopenia in African children with falciparum malaria. Am J Trop Med Hyg. 2002;66:686–691. doi: 10.4269/ajtmh.2002.66.686. [DOI] [PubMed] [Google Scholar]
- 47.Pleass RJ. Platelet power: sticky problems for sticky parasites? Trends Parasitol. 2009;25:296–299. doi: 10.1016/j.pt.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMorran BJ, Marshall VM, de Graaf C, Drysdale KE, Shabbar M, et al. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science. 2009;323:797–800. doi: 10.1126/science.1166296. [DOI] [PubMed] [Google Scholar]
- 49.De Luca M, D'Anna F, Bondanza S, Franzi AT, Cancedda R. Human epithelial cells induce human melanocyte growth in vitro but only skin keratinocytes regulate its proper differentiation in the absence of dermis. J Cell Biol. 1988;107:1919–1926. doi: 10.1083/jcb.107.5.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zabierowski SE, Fukunaga-Kalabis M, Li L, Herlyn M. Dermis-derived stem cells: a source of epidermal melanocytes and melanoma? Pigment Cell Melanoma Res. 2011;24:422–429. doi: 10.1111/j.1755-148X.2011.00847.x. [DOI] [PubMed] [Google Scholar]
- 51.Chilcott R. Cutaneous Anatomy and Function. In: Chilcott R, Price S, editors. Principles and Practice of Skin Toxicology. John Wiley & Sons, Ltd; 2008. 392 [Google Scholar]
- 52.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 53.Rajapakse R, Mousli M, Pfaff AW, Uring-Lambert B, Marcellin L, et al. 1,25-Dihydroxyvitamin D3 induces splenocyte apoptosis and enhances BALB/c mice sensitivity to toxoplasmosis. J Steroid Biochem Mol Biol. 2005;96:179–185. doi: 10.1016/j.jsbmb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Shelton SB, Pettigrew DB, Hermann AD, Zhou W, Sullivan PM, et al. A simple, efficient tool for assessment of mice after unilateral cortex injury. Journal of neuroscience methods. 2008;168:431–442. doi: 10.1016/j.jneumeth.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Melanization does not change blood composition in response to severe malaria anemia. Animals were infected with 1×104 P. yoelii 17XL iRBCs on day 0. Hematological parameters were determined in whole blood. White blood cells (WBC), Red Blood Cells (RBC), Hemoglobin (Hb), Platelets, Neutrophils, Lymphocytes and Hematocrit are shown. Data showed represents the mean of 10 animals and bars represent the SD of the mean.
(TIF)
Melanin does not alter the cytokine profile in response to cerebral malaria. Cytokine levels before (day 0) and 5 days after infection using 1×106 P. berghei ANKA iRBCs are shown. Data showed represents the mean of 10 animals and bars represent the SD of the mean.
(TIF)
Melanization does not alter blood composition in response to chronic malaria. Animals were infected with 1×106 P. chabaudi iRBCs on day 0. White blood cells (WBC), Red Blood Cells (RBC), Hemoglobin (Hb), Platelets, Neutrophils, Lymphocytes and Hematocrit are shown. Data showed represents the mean of 10 animals and bars represent the SD of the mean.
(TIF)
Effect of melanin injections on blood composition in B6.Tyr−/− animals. Animals were infected with 1×106 P. berghei ANKA iRBCs on day 0. White blood cells (WBC), Red Blood Cells (RBC), Hemoglobin (Hb), Platelets, Lymphocytes, Neutrophils, Hematocrit and the linear regression of survival on platelet counts are given. The dashed lines represent the 95% confidence interval about the regression line. Data showed represents the mean of 10 animals and bars represent the SD of the mean.
(TIF)