Abstract
The electrical activity pattern of endocrine pituitary cells regulates their basal secretion level. Rat somatotrophs and lactotrophs exhibit spontaneous bursting and have high basal levels of hormone secretion, while gonadotrophs exhibit spontaneous spiking and have low basal hormone secretion. It has been proposed that the difference in electrical activity between bursting somatotrophs and spiking gonadotrophs is due to the presence of large conductance potassium (BK) channels on somatotrophs but not on gonadotrophs. This is one example where the role of an ion channel type may be clearly established. We demonstrate here that BK channels indeed promote bursting activity in pituitary cells. Blocking BK channels in bursting lacto-somatotroph GH4C1 cells changes their firing activity to spiking, while further adding an artificial BK conductance via dynamic clamp restores bursting. Importantly, this burst-promoting effect requires a relatively fast BK activation/deactivation, as predicted by computational models. We also show that adding a fast-activating BK conductance to spiking gonadotrophs converts the activity of these cells to bursting. Together, our results suggest that differences in BK channel expression may underlie the differences in electrical activity and basal hormone secretion levels among pituitary cell types and that the rapid rate of BK channel activation is key to its role in burst promotion.
Introduction
Excitable cells generate patterns of electrical activity that regulate diverse functional characteristics such as transmitter release, hormone secretion, and contractibility. This electrical activity is shaped by the combination of ion channels present in the cellular membrane. Experimental and computational approaches have revealed the dynamic mechanisms underlying excitability and oscillations in neurons, cardiac cells and endocrine cells (Hodgkin and Huxley, 1952; Rinzel and Ermentrout, 1998; Izhikevich, 2000). However, determining the role of a particular type of ion channel on the pattern of electrical activity remains a challenge, in part because many of them are involved, so multiple channel types could contribute to a given electrophysiological property (Goaillard et al., 2009). One example where a single-channel type is thought to play a clear role in a cell's electrical pattern is in the spiking/bursting activity of pituitary cells. It has been proposed that large conductance potassium (BK) channels (Latorre and Brauchi, 2006) primarily determine whether a pituitary cell spikes continuously or bursts (Stojilkovic et al., 2005). Here, we use a hybrid computational/experimental strategy to determine the influence of BK channels on the electrical activity of pituitary cells.
BK channels produce a voltage- and calcium-dependent outward current that usually limits spike duration, extracellular calcium influx, and transmitter and hormone release (Lang and Ritchie, 1990; Robitaille and Charlton, 1992; Sah and Faber, 2002; Miranda et al., 2003; Raffaelli et al., 2004; Vandael et al., 2010). In pituitary cells, some steroids and hypothalamic factors that inhibit hormone release activate BK channels (White et al., 1991; Shipston et al., 1996; Kanyicska et al., 1997). These observations suggest that BK channel activation may help shorten electrical events in pituitary cells. However, blockade of BK channels in pituitary somatotrophs can switch the activity pattern of these cells from bursting to spiking, greatly reducing the amplitude of [Ca2+]i oscillations (Van Goor et al., 2001b). Thus, in these cells BK channels may have a stimulatory effect. Mathematical models suggest that the stimulatory effect of BK channels on somatotrophs is due to their fast activation. This limits spike amplitude, preventing full activation of the repolarizing delayed rectifier K+ current, so membrane potential weakly oscillates around a depolarized level (Van Goor et al., 2001b; Tabak et al., 2007; Tsaneva-Atanasova et al., 2007) before falling back toward rest, resulting in a burst.
While channel blockage is effective for demonstrating that the associated ionic current is important in a behavior like bursting, it does not allow for an examination of the properties of the current that are crucial for the behavior. To do this, we first block the BK channels of lacto-somatotroph GH4C1 cells and then add back a BK-like current using the dynamic clamp technique (Sharp et al., 1993). This allows us to set the kinetic properties of the added current and determine constraints on the conductance and activation rate required for a burst-promoting role of the current.
Unlike somatotrophs, unstimulated pituitary gonadotrophs generally exhibit spiking instead of bursting (Van Goor et al., 2001c), and, interestingly, they express very little BK conductance (Van Goor et al., 2001a). To test the hypothesis that spiking gonadotrophs would burst if they had larger BK conductance, we added an artificial BK conductance to these cells through dynamic clamp. This indeed changed their activity pattern from spiking to bursting. Altogether, our results demonstrate that fast-activating BK channels promote bursting in anterior pituitary cells.
Materials and Methods
Cell preparations and perforated patch recordings.
GH4C1 cells were obtained from ATCC and maintained in culture conditions in supplemented F10 medium (Sigma-Aldrich) according to established procedures (Tashjian et al., 1968). Primary pituitary cells were obtained from diestrous female rats (Sprague Dawley, aged 3–6 months) using enzymatic dispersion of pituitary fragments (Tabak et al., 2010). Animal procedures were approved by the Florida State University Animal Care and Use Committee. Cells were cultured in supplemented M199 medium (Invitrogen) for 1 d before being used for patch-clamp experiments. Gonadotrophs were identified by their larger size and by their typical rhythmic hyperpolarizations in response to 1 nm gonadotropin-releasing hormone (Bachem) applied at the end of the experiment (Tse and Hille, 1992).
During the experiment, cells were superfused with HEPES-buffered saline (138 mm NaCl, 5 mm KCl, 10 mm α-d-glucose, 25 mm HEPES, 0.7 mm Na2HPO4, 1 mm MgCl2, 2 mm CaCl2) at room temperature. Fire-polished pipettes (resistance 6–9 MΩ) were filled with solution containing 90 mm KAsp, 60 mm KCl, 10 mm HEPES, and 1 mm MgCl2 with the addition of 120 μg/ml amphotericin B. Usually, access resistance decreased below 50 MΩ within 10 min following seal (>5 GΩ) formation. A junction potential of 6.5 mV was not corrected; an undischarged Donnan potential also exists across the perforated membrane, but it is assumed to be negligible. BK channels were blocked by bath application of 1 μm paxilline (Tocris Bioscience) or 100 nm iberiotoxin (Tocris Bioscience).
Membrane potential time courses were analyzed to evaluate the degree of burstiness of each cell under different conditions. To measure the duration of each event (i.e., spikes or bursts), we obtained the times of event onset and termination using a threshold-crossing detection algorithm. The algorithm first normalized the voltage values relative to the minimum and maximum membrane potentials of each sequence to minimize the differences in event durations that could result from differences in voltage offset and event amplitude between cells. Events were then classified as spikes or bursts according to their position in the histogram of event duration (see Results).
Dynamic clamp.
Membrane potential was monitored in current-clamp (bridge mode) and acquired from the patch amplifier (Multiclamp 700B, Molecular Devices) through an analog-to-digital acquisition card (DAQ) on a separate PC running the software QuB with a dynamic clamp module (Milescu et al., 2008). Membrane potential (V) was used to compute the BK current using the same mathematical expression as for the model simulations, IBK = gBK f (VK − V), with f obtained by integrating Equation 3.
The calculated BK current was injected back into the cell through the same DAQ. Parameter values were chosen to qualitatively match current–voltage plots obtained using voltage steps applied from a holding potential of −40 mV (to minimize the inactivating currents) before and after BK blocker application. The parameter values were as follows: gBK = 0.5 nS; τBK = 5 ms; vf = −15 mV; sf = 1 mV, unless otherwise noted. Equation 3 was evaluated in real time using the forward Euler method (Milescu et al., 2008), with dt average = 54 μs, dt maximum = 100 μs, and coefficient of variation (CV) = 1.2. Using an integration time step as long as 100 μs did not significantly alter the “burstiness factor” in our model simulations.
The calculated current qualitatively matches recorded BK currents in voltage-clamp, although in some cells BK currents also had a slower, Ca2+-dependent component. This late component was not modeled because our hypothesis is that it is the fast component that is responsible for bursting. Slow-activating outward currents do not promote bursting, as we show later on, and in many cases inhibit bursting (see Results). Also note that, apart from minor quantitative differences such as maximum conductance, we have injected the same artificial BK current in all cells tested, despite cell-to-cell differences in BK currents. The goal was to evaluate the effect of a generic, fast-activating BK current, without having to adjust parameters to promote bursting.
Model.
The pituitary cell model used in this article is based on a previous lactotroph model (Tabak et al., 2007). It incorporates three voltage-gated currents (ICa, IK, IBK), one Ca2+-gated current (ISK), one leak current, and a stochastic current reflecting channel noise. Although BK channels are both voltage gated and Ca2+ gated, BK channels are typically adjacent to Ca2+ channels and are gated by Ca2+ nanodomains (Fakler and Adelman, 2008). For this reason, the Ca2+ concentration felt by the BK channel reaches equilibrium in microseconds, and the activation can be modeled as a purely voltage-dependent process (Simon and Llinás, 1985; Sherman et al., 1990). The following equations describe the dynamics of the four model variables V (membrane potential), n (activation of IK), f (activation of IBK), and [Ca] (intracellular Ca2+ concentration):
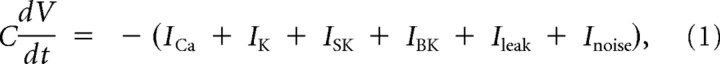 |
 |
 |
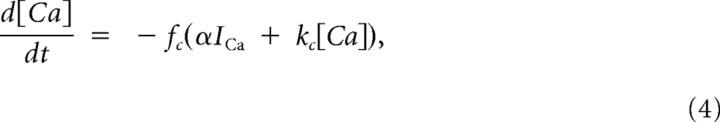 |
with
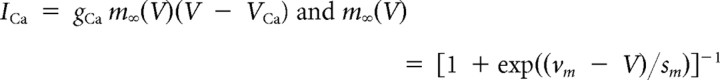 |
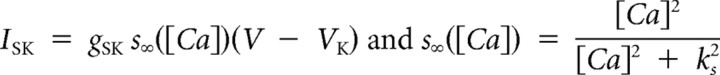 |
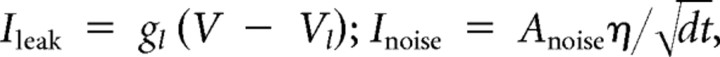 |
where η is a random process drawn from a normal distribution. Parameter values and their definitions are given in Table 1.
Table 1.
Parameter values used in the simulations unless otherwise noted
| Parameter | Value | Definition |
|---|---|---|
| C | 10 pF | Membrane capacitance |
| gCa | 2 nS | Maximal conductance of Ca2+ channels |
| VCa | 60 mV | Reversal potential for Ca2+ |
| vm | −20 mV | Voltage value at midpoint of m∞ |
| sm | 12 mV | Slope parameter of m∞ |
| gK | 3.2 nS | Maximal conductance of K channels |
| VK | −75 mV | Reversal potential for K+ |
| vn | −5 mV | Voltage value at midpoint of n∞ |
| sn | 10 mV | Slope parameter of n∞ |
| τn | 30 ms | Time constant of n |
| gSK | 2 nS | Maximal conductance of SK channels |
| ks | 0.4 μm | [Ca] at midpoint of s∞ |
| gBK | 0–1 nS | Maximal conductance of BK channels |
| vf | −20 mV | Voltage value at midpoint of f∞ |
| sf | 2 mV | Slope parameter of f∞ |
| τBK | 2–10 ms | Time constant of f |
| gl | 0.2 nS | Leak conductance |
| Vl | −50 mV | Reversal potential for the leak current |
| Anoise | 4 pA | Amplitude of noise current |
| fc | 0.01 | Fraction of free Ca2+ ions in cytoplasm |
| α | 0.0015 μm fC−1 | Conversion from charges to molar concentration |
| kc | 0.12 ms−1 | Rate of Ca2+ extrusion |
The simulation package XPP (Ermentrout, 2002) was used to run the simulations (forward Euler method, dt = 0.01 ms). The code for this model is freely available at www.math.fsu.edu/∼bertram/software/pituitary. For large numbers of simulations (512 parameter sets), we used custom developed software that runs on Graphics Processing Units (CUDA parallel computing architecture, NVIDIA).
Results
Fast-activating BK channels promote bursting in a pituitary cell model
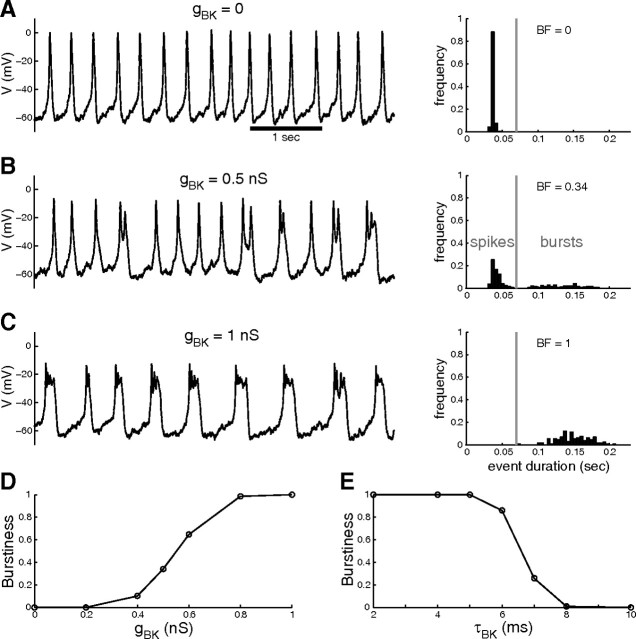
We first illustrate how BK channels promote bursting behavior in a mathematical model of a pituitary cell, and develop a measure of the “burstiness” of the activity. The Hodgkin–Huxley-type model incorporates Ca2+ (L-type) and K+ (delayed rectifier) voltage-dependent conductances and a slow, Ca2+-activated K+ conductance representing small conductance (SK) channels. The model also includes a small leak conductance and a stochastic current representing channel noise to create irregular voltage time courses as observed in pituitary cells. We assume that the BK channels are located near Ca2+ channels, within the Ca2+ nanodomains created by Ca2+ influx through these channels (Van Goor et al., 2001b; Fakler and Adelman, 2008). Because the Ca2+ concentration in nanodomains equilibrates within microseconds (Simon and Llinás, 1985), the gating of BK channels is effectively driven by the membrane potential (Van Goor et al., 2001b), and we can model the BK conductance as purely voltage dependent. Parameters are set so that in the absence of BK channels the model generates continuous spiking, as shown in Figure 1A, left. If we add a fast, voltage-dependent BK conductance, the cell model produces a mix of spikes and bursts for moderate values of the conductance (Fig. 1B, left) (gBK = 0.5 nS) and mostly bursts for larger BK conductance values (Fig. 1C) (gBK = 1 nS).
Figure 1.
Model predictions for the burst-promoting action of BK channels. A, spiking activity obtained with the pituitary cell model in the absence of BK conductance. Left, Membrane potential time course. Right, Distribution of event duration. All the events are spikes, so the burstiness is null (BF = 0). B, For gBK = 0.5 nS, spike amplitude is reduced and a few large duration events (i.e., bursts) are produced; burstiness has increased to 0.34. The vertical line separates events between spikes and bursts. C, For gBK = 1 nS, the model produces only bursts with small amplitude spikes on top of a plateau, typical of pituitary cells (BF = 1). D, Burstiness increases with gBK (τBK = 5 ms). E, Burstiness decreases with τBK (gBK = 1 nS). BF, Burstiness factor.
We quantify the degree of burstiness in each case using the distribution of event (spikes and bursts) durations. For purely spiking activity, event durations are tightly clustered around 50 ms (Fig. 1A, right). As gBK is increased, bursts appear and the event duration distribution becomes bimodal (Fig. 1B, right), with one mode around 50 ms (spikes) and a wider mode covering the 100–400 ms range (bursts). For large gBK values, only bursts remain, and the distribution is mostly unimodal (Fig. 1C, right) over the 100–400 ms range. We separate events into spikes and bursts according to their duration and define the fraction of events that are bursts as the burstiness factor (BF). This factor goes from 0 (only spikes, as in Fig. 1A) to ∼1 (mostly bursts, as in Fig. 1C). Figure 1D shows how the burstiness factor increases with gBK.
These results were obtained with a time constant for τBK of 5 ms. To evaluate how this time constant affects the burst-promoting action of the BK conductance, we vary τBK and compute the resulting burstiness factor of the model activity (for gBK = 1 nS). As shown in Figure 1E, burstiness is close to 1 for τBK ≤ 5 ms, but it decreases quickly as τBK is increased to >5 ms. Thus, BK channels must activate quickly to promote bursting.
To demonstrate the robustness of these results to parameter choices, we ran 512 simulations for which the values of the conductances gK, gSK, gCa, and gl where chosen randomly between −50% and +50% of their default values. The distribution of burstiness across the active models is shown in Figure 2 for gBK = 0, 0.5, and 1 nS. Increasing gBK raised the burstiness in 70% of the cases for which the model was active but not already bursting in the absence of BK conductance. Interestingly, 66% of the parameter combinations produced activity with burstiness factor <0.3 in the absence of BK conductance. With gBK increased to 1 nS, only 20% of parameter combinations resulted in burstiness <0.3. Thus, while the presence of a BK conductance is not necessary for bursting, for a majority of models the presence/absence of BK conductance predicts the occurrence/absence of bursting. Also, as in Figure 1E, the burstiness decreased when τBK was increased from 5 to 10 ms for 80% of parameter combinations. This decreased burstiness with increased activation time constant included 17% of the cases for which the model was already bursting in the absence of BK conductance. These observations indicate that when BK channel activation becomes too slow, BK channels lose their stimulatory effect on bursting and can become inhibitory.
Figure 2.
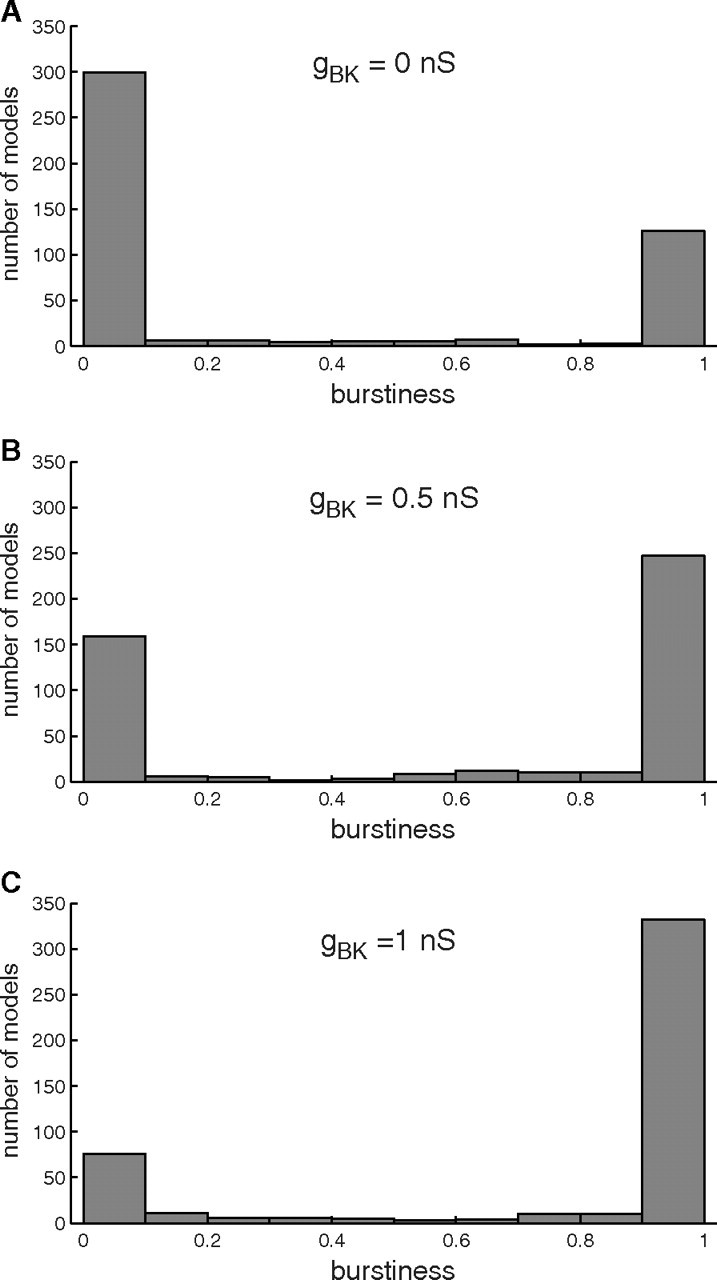
Robustness of the burst-promoting effect of gBK with respect to model parameters. Conductance parameters were varied (see Results) to produce 512 models, of which 463 produced rhythmic activity with a difference between maximum and minimum voltage of at least 30 mV. A–C, The distribution of burstiness for the active models is shown for gBK = 0 nS (A), 0.5 nS (B), and 1 nS (C). A, In the absence of BK conductance, the distribution of burstiness is heavily biased to the left (skewness = 0.82), with 66% of all active models being spikers. B, For gBK = 0.5 nS the proportion of spikers is already <50% (skewness = −0.44). C, For gBK = 1 nS, the distribution of burstiness is heavily biased to the right (skewness = −1.32), and spikers make up only 20% of the population.
Artificial BK conductance restores bursting under pharmacological BK blockage
If a fast voltage-dependent BK conductance is responsible for bursting in pituitary cells, then blocking BK channels should abolish bursting. Moreover, bursting should be restored by adding back an artificial, fast voltage-dependent outward conductance via dynamic clamp.
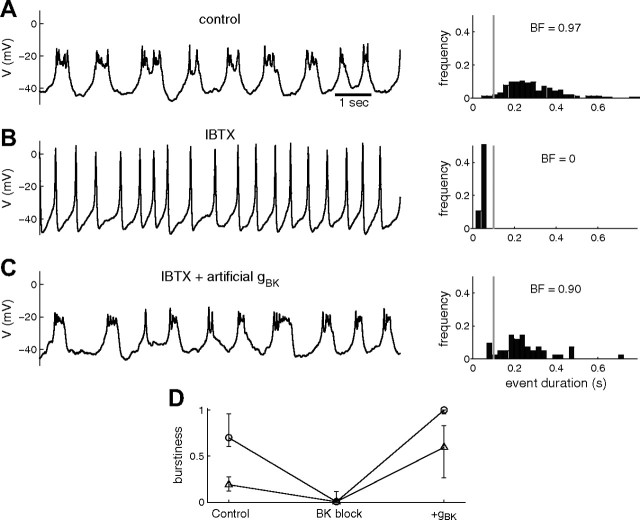
We examined the electrical activity of lacto-somatotroph GH4C1 cells, which generated spiking, bursting, or mixed firing patterns. Blocking BK channels with either paxilline (1 μm) or iberiotoxin (100 nm) irreversibly converted bursting cells and weakly bursting cells to cells that primarily spiked (11 of 13 cells), as shown in Figure 3, A and B, but did not affect purely spiking cells (2 of 2 cells). The decrease in burstiness due to blockage of BK channels is illustrated in Figure 3D. In control conditions, the burstiness is widely distributed with a subpopulation of cells having high burstiness (“bursters”; BF >0.5; Fig. 3D, circle) and a subpopulation having low burstiness (“spikers”; BF <0.3; Fig. 3D, triangle). The BF drops to a value close to 0 under BK blockage, reflecting purely spiking cells. These results show that removing BK current impairs bursting in these cells. To test whether fast-activating BK channels have a direct burst-promoting effect, we used dynamic clamp to add back a fast-activating BK conductance to the GH4C1 cells under pharmacological BK blockage conditions. Adding the artificial BK conductance immediately switched the pattern of electrical activity from spiking to bursting. The bursts obtained under these conditions are characterized by slow plateau oscillations with low-amplitude fluctuations on top of the plateaus (Fig. 3C), like the bursts observed under control conditions (Fig. 3A). The BK conductance added to the cells was identical to the one used in our model, with parameters chosen to approximately match current–voltage curves obtained in voltage-clamp recordings. Parameter tuning was rarely needed to obtain bursting. Most cells required a maximal BK conductance of ≤0.5 nS to switch to a bursting pattern. Increasing the conductance of the injected BK current also increased burst duration.
Figure 3.
BK conductance promotes bursting in GH4C1 cells. A, Left, Time course of the membrane potential of a GH4C1 cell in control conditions. Right, Histogram of event duration; the vertical line separates events between spikes and bursts. B, Voltage time course and event distribution for the same cell after iberiotoxin (IBTX, 100 nm) application. All events have shorter duration and higher amplitude (spikes). C, After adding an artificial fast-activating BK conductance (gBK = 0.5 nS) via dynamic clamp, the cell exhibits bursting with amplitude and duration similar to control conditions. D, Median and interquartile range of burstiness factor for spikers (BF ≤ 0.3, triangles) and bursters (BF > 0.3, circles) in control conditions (n = 5 and 8, respectively), BK block (with either 1 μm paxilline or 100 nm IBTX), and after adding an artificial BK conductance in the presence of a BK blocker (gBK = 0.5 nS; n = 4 and 4). Addition of gBK (0.5 nS) restored burstiness to control or above control level in seven of eight cells [in the remaining cell, a larger BK conductance (gBK = 1nS) could also bring burstiness back to control level].
Figure 3D summarizes the burstiness in two groups of cells (spikers with BF <0.3; triangles; and bursters with BF >0.3; circles) in control conditions, in the presence of BK blocker, and after addition of 0.5 nS BK conductance via dynamic clamp (with BK blocker still present). After BK channels were blocked with paxilline or iberiotoxin, most cells were spiking, so the median BF is near 0 for both groups. Only 2 of 13 cells were still bursting in the presence of BK channel blocker. In seven of eight cells (four of each group) with BK blocker present, the addition of 0.5 nS BK conductance with dynamic clamp converted spikers to bursters. The remaining cell was converted by a larger conductance of 1.0 nS. The consistent conversion of spikers to bursters after receiving artificial BK conductance suggests that the difference in activity pattern between spikers and bursters is explained, at least in part, by differences in BK conductance.
If this is true, then artificially subtracting a fast BK conductance from bursting cells in control conditions should switch the activity of these cells to spiking. A decrease in burstiness was indeed observed after artificially subtracting a fast BK conductance via dynamic clamp (Fig. 4A–C) in six of seven cells, similarly to pharmacological BK blockage. Finally, addition of a fast BK conductance in control conditions increased burstiness (Fig. 4D) to higher than control values in the combined (high plus low burstiness) cell population (12 of 12 cells). These effects were usually more pronounced with higher levels of gBK injection. These results suggest that higher density of fast-activating BK conductance leads to higher burstiness.
Figure 4.
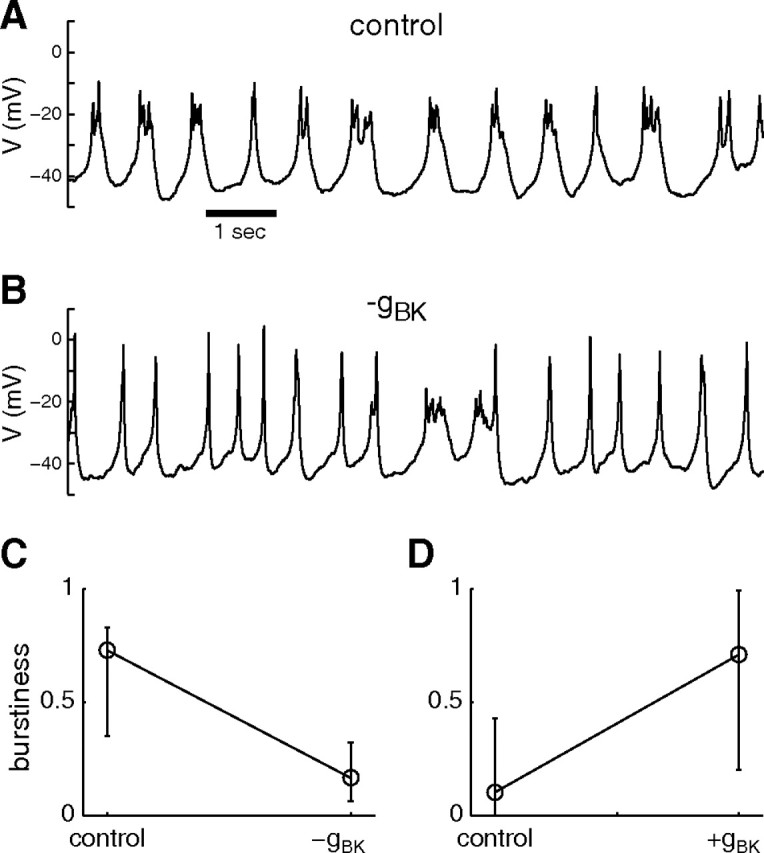
Subtracting/adding BK current decreases/increases burstiness. A, Bursting activity of a GH4C1 cell in control conditions. B, During application of a negative BK conductance via dynamic clamp (gBK = −1 nS), the electrical activity switches to mostly spiking. C, Summary of the effect of negative artificial BK conductance on burstiness (gBK = −0.5 nS, p < 0.02, n = 7). D, Summary of the effect of positive artificial BK conductance to mostly spiking cells (8 of 12 had BF ≤ 0.3) in control saline (gBK = 0.5 nS, p < 0.02). Data are summarized using the median and interquartile range, and significance was assessed using the nonparametric Wilcoxon test.
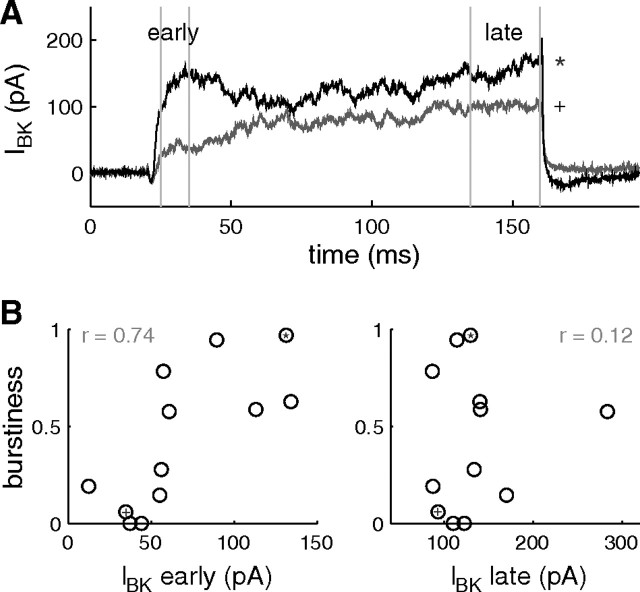
To test the hypothesis that the activity pattern (spiking vs bursting) of a cell is influenced by the amount of BK conductance in the cell, we measured the BK current in response to depolarizing voltage steps, by subtracting the currents obtained before and after pharmacological BK blockage. We computed the average current measured over a short period just after the onset of the depolarization (Fig. 5A, early) and over a period near 150 ms after onset (Fig. 5A, late). The early component of the current was positively correlated with the burstiness factor (Fig. 5B, left) (r = 0.74, p < 0.01). This indicates that >50% of the variance in burstiness across cells can be explained by the variability in the amount of fast activating BK current—the rest of the variance being explained by the differences in the expression of other ion channels. In contrast, the amplitude of the current after 150 ms was not correlated with burstiness (Fig. 5B, right) (r = 0.12, p = 0.7). This supports the hypothesis that bursters have higher levels of fast-activating BK conductance than spikers. These results also indicate that the fast component of the activated BK current is important for bursting, while the total amount of BK current that slowly increases with time is not.
Figure 5.
Amplitude of fast-activating BK current correlates with burstiness. A, BK currents recorded in response to voltage steps, averaged over four steps from a holding V = −40 to 0, 10, 20, and 30 mV. The voltage steps were performed before and after pharmacological BK block, and the difference current was computed. The two traces correspond to two different cells, one bursting in control conditions (*), one spiking (+). B, Scatter plot of the burstiness in control conditions and the BK current measured (in the same cells) as illustrated in A and averaged over the periods of time shown (early, late). Burstiness and amplitude of the early component of the BK current are positively correlated (left, r = 0.74, p < 0.01). In some cells, a slow component of BK current is observed that contributes significantly to the late part of the current. The amplitude of the late part of the current is not correlated with burstiness (right). The * and + signs correspond to the cells whose currents are shown in A.
BK conductance must activate quickly to promote bursting
Our model predicts that the fast activation of the BK current is crucial for its burst-promoting effect (Fig. 1E) (see also Van Goor et al., 2001b; Tabak et al., 2007). The fast activation of BK channels limits spike amplitude and prevents full activation of delayed-rectifier K+ current, preventing full repolarization and allowing membrane potential to remain at a depolarized level, until the event is terminated by activation of SK channels. If this is indeed how BK channels promote bursting in pituitary cells, then adding an artificial BK conductance with a slower time constant to cells with low burstiness should not increase burstiness as efficiently as a faster-activating BK conductance. To test this, we varied the time constant of the artificial BK conductance injected into cells with low burstiness (spikers, or bursters under pharmacological BK blockage). Figure 6A–D shows that, as the time constant of the BK current is increased past 5 ms, the burst-promoting effect of the current weakens. In the example shown, addition of an artificial BK current with an activation time constant of 5 ms switched the cell activity from spiking to bursting (Fig. 6B). Increasing the BK activation time constant from 5 to 10 ms decreased burstiness from 1 to <0.5 (Fig. 6B,C, right), with a further drop in burstiness to the control level at τBK = 20 ms (Fig. 6D, right). A similar drop in burstiness due to slower BK activation was observed in all cells tested (n = 8), even though the critical value of τBK varied from cell to cell (Fig. 6E,F, summary). In some cells, burstiness was lower than in control conditions for high values of τBK, indicating that slow BK current can inhibit bursting, as predicted by the model. Altogether, these results (Figs. 3–6) demonstrate that BK channels have a robust burst-promoting effect in pituitary cells, due to their fast activation.
Figure 6.
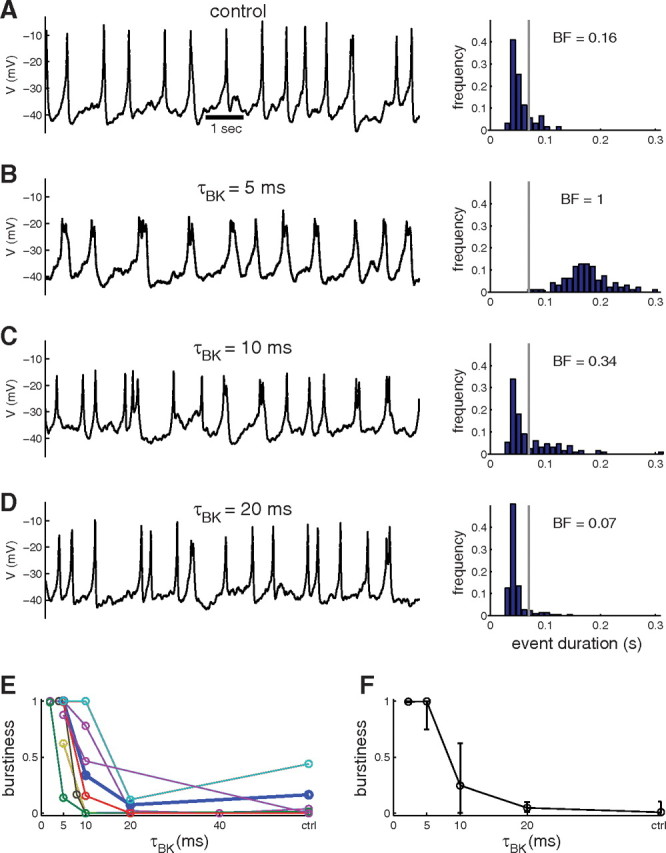
The burst-promoting effect of BK channels depends on their activation time constant. A, Voltage time course of a mostly spiking GH4 cell in control conditions (left). Very few events are bursts as seen in the histogram of event durations (right). B, Injecting via dynamic clamp an artificial BK conductance (gBK = 0.5 nS) with τBK = 5 ms switches the electrical activity to bursting. C, Slowing BK conductance activation using τBK = 10 ms decreases burstiness. The activity is now a mix of spikes and bursts. D, Slower BK activation (τBK = 20 ms) prevents the burst-promoting effect of the BK conductance. The activity is mostly spiking as in control. E, The effects of activation time constant (τBK) on burstiness in eight different cells (the bold curve corresponds to the cell illustrated in A–D). F, Summary of the time constant effect over the eight cells.
Artificial BK conductance transforms spiking gonadotrophs into bursters
Spontaneous bursting behavior has been described in pituitary somatotrophs, which have a substantial BK channel conductance. In contrast, unstimulated pituitary gonadotrophs usually exhibit sharper spikes, and these cells possess very little BK conductance (Van Goor et al., 2001a,c). Can the difference in the spontaneous electrical activity between these two cell types be explained by their different BK channel density, as was suggested by Van Goor et al. (2001b)? To answer this question, we injected an artificial BK conductance into female rat pituitary gonadotrophs. About half of the cells tested exhibited spontaneous spiking (Fig. 7A). In these cells, adding an artificial BK conductance switched the activity to bursting (n = 6/6), as shown in Figure 7, B and D. If this conversion from spiking to bursting by BK channels operates through the same mechanism as discussed above, it should depend strongly on the BK activation time constant. Slowing down the activation of the BK conductance decreased this effect (Fig. 7C,E), again demonstrating that the burst-promoting effect of BK channels requires fast activation. These results suggest that the difference in spontaneous activity between pituitary somatotrophs (bursting) and gonadotrophs (spiking) is due to their difference in BK channel density.
Figure 7.
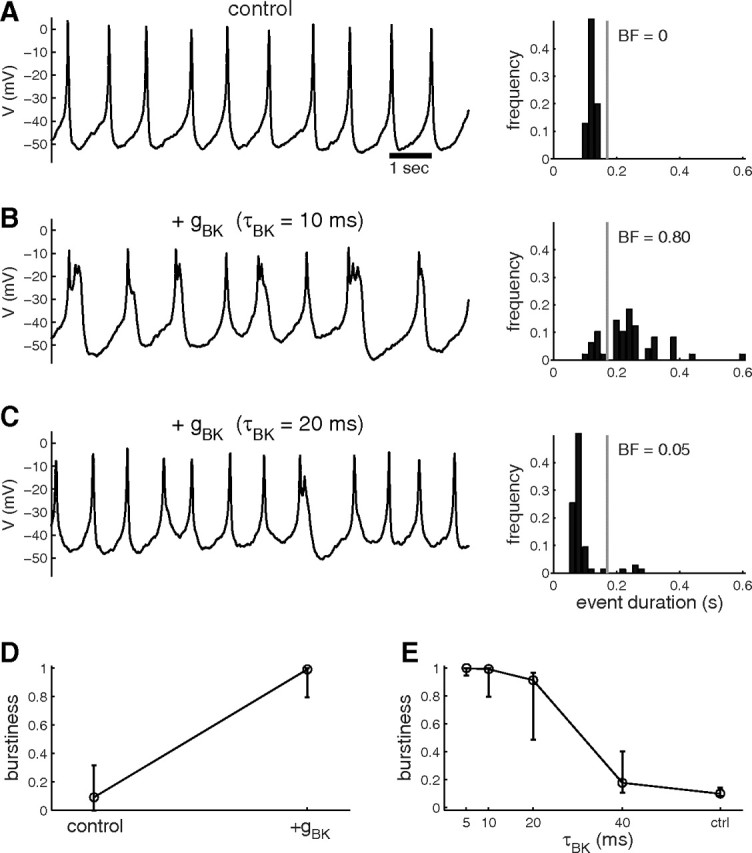
Artificial fast-activating BK current promotes bursting in gonadotrophs. A, Voltage time course recorded from a gonadotroph under control conditions. The cell is spiking, and the distribution of event duration is sharp (right). B, Addition via dynamic clamp of an artificial BK current (gBK = 0.5 nS) with activation time constant of 10 ms switches the cell activity to mostly bursting. C, Increasing the BK activation time constant decreases the burstiness. Very few bursts are present, as shown by the voltage time course (left) and the event duration distribution (right). D, Summary of the effect of adding an artificial BK current (τBK = 10 ms) on burstiness in six gonadotrophs. E, Summary of the effect of increasing BK activation time constant (τBK) on burstiness (n = 6). Gonadotrophs were identified by their larger size and the characteristic hyperpolarizations recorded in response to 1 nm gonadotropin-releasing hormone.
Discussion
It is generally difficult to clearly establish the role played by an ion channel in shaping the electrical activity pattern in a given cell type. Van Goor et al. (2001b) have suggested that the difference between the bursting activity pattern of rat pituitary somatotrophs and the spiking activity pattern of gonadotrophs (Van Goor et al., 2001c) could be explained by the fact that somatotrophs express BK channels, whereas gonadotrophs do not (Van Goor et al., 2001a). To demonstrate the role played by BK channels in promoting bursting, they pharmacologically blocked BK channels in somatotrophs, which switched the activity of these cells from bursting to spiking (Van Goor et al., 2001b). They also showed that including BK channels in a mathematical model of gonadotroph electrical activity could switch this activity from spiking to bursting. These results were in contrast to a number of studies showing opposite effects of BK channels on electrical activity, reducing spike duration in several cell types, including pituitary cells (Lang and Ritchie, 1990; Miranda et al., 2003; Vandael et al., 2010). In this study, we provide direct evidence that BK channels are in fact a key element of bursting in pituitary cells. Using dynamic clamp, we identify the features of the current important for bursting.
Addition of an artificial BK conductance via dynamic clamp to spiking GH4C1 lacto-somatotrophs and gonadotrophs immediately switched their activity to bursting. Mathematical modeling predicted that the activation time constant of the BK conductance is important. BK activation must be fast to promote bursting; if too slow, then the BK current does not promote bursting and takes on its traditional inhibitory role on electrical activity by speeding up the repolarization (negative feedback). We verified this prediction by varying the kinetic properties of the artificial BK conductance injected via dynamic clamp. This agreement with the mathematical model demonstrates that the BK current does not simply put the cell into the operational range where bursting occurs, but actually plays an active role in burst generation. The results also suggest that the rate of BK channel activation can determine the type of feedback provided by these channels. Too slow and they shorten spikes, limiting Ca2+ entry, thus acting as a negative-feedback process. With faster activation speeds, they promote bursting and Ca2+ entry, providing positive feedback by limiting spike amplitude and reducing the activation of the delayed rectifier current (Van Goor et al., 2001b).
Our results lead to the hypothesis that the BK channels closely associated with Ca2+ channels will promote bursting and thus increase the amplitude of intracellular Ca2+ oscillations, while those located further away or associated with different Ca2+ channel types (Prakriya and Lingle, 2000; Tsaneva-Atanasova et al., 2007; Berkefeld and Fakler, 2008; Fakler and Adelman, 2008) will activate more slowly and have an opposite effect. This hypothesis could reconcile the present results describing BK channels acting as burst promoter with those that showed BK channels decreasing action potential duration (Lang and Ritchie, 1990; Miranda et al., 2003). It is also possible that BK channels have different kinetic properties in different cells as a result of differences in their phosphorylation state (Reinhart et al., 1991) or association with β-subunits (Solaro et al., 1995; Brenner et al., 2005). In pituitary cells, these differences could be mediated in a time-dependent manner by steroids and hypothalamic factors. Thus, BK channels may have different effects on electrical activity and pituitary hormone secretion, depending on the physiological conditions.
These observations suggest that culture conditions may also help to explain why different laboratories observe different effects of BK channels on spike duration in pituitary cells (Lang and Ritchie, 1990; Van Goor et al., 2001b). Three key components that define one particular culture condition are the cell dissociation procedure, the culture media, and the substrate. Given that proteases used for cell dissociation and isolation can differentially modify ion channels (Spreadbury et al., 2004), that culture media may differentially regulate the expression of ion channel accessory subunits (Martin et al., 2002), and that cell substrate changes may induce differences in current amplitude and dynamics (Romanova et al., 2004), it is possible that culture conditions influence the activation kinetics of BK channels, which in turn determines the global effect of these channels. Here, GH4C1 cells and gonadotroph cells were cultured in different conditions, but this was not an issue because we added the same BK conductance to these different cells. Thus, by sharing models, dynamic clamp may allow different laboratories to overcome the differences in BK channel properties caused by different culture conditions. However, culture conditions may also affect the expression of other ion channels, possibly changing how BK channels shape electrical activity.
The effect of any ionic current on the pattern of electrical activity depends on the other ion channel types and their densities. Because different cells may express different combinations of ion channels, the effect of BK current can vary among cells (Lledo et al., 1991; Van Goor et al., 2001a). In addition, stimulatory and inhibitory hormones can dynamically modulate the properties of channels (Stojilkovic et al., 2010). Therefore, the mix of ion channels and hormonal effects could also determine whether the BK current promotes or inhibits bursting in vivo. Theoretically, bursting arises from the interactions between at least three types of channels: fast inward and outward voltage-dependent conductances to produce spikes, and a slow inhibitory current to switch between periods of spiking and quiescence (Rinzel and Ermentrout, 1998). Numerous combinations of channel conductances that include this minimal set of three conductance types can produce bursting oscillations (Prinz et al., 2003). In addition, pituitary cells are very heterogeneous, so bursting might arise in these cells through a wide variety of conductance densities. Since fast-activating BK channels are not part of the minimum set of channels necessary for bursting in our model, it is surprising that BK channel block reliably prevents bursting in somatotrophs (Van Goor et al., 2001b) and GH4C1 lacto-somatotrophs (this study). In other words, a majority of these cells have ion channel distributions that prevent bursting in the absence of BK channels, or if BK channels become slowly activating. This strengthens the view that BK channels are likely targets of hormonal modulation, which can profoundly affect the activity of pituitary cells.
The dynamic clamp, in conjunction with mathematical modeling, is a potentially powerful tool for directly determining the functional properties of a current within the context of its native mix of ion channels (Goaillard and Marder, 2006; Olypher et al., 2006; Putzier et al., 2009; Economo et al., 2010; Milescu et al., 2010). Here, we used these approaches to directly show that fast-activating BK channels promote bursting activity in anterior pituitary cells. Our results imply that differences in BK channel expression level may underlie the differences in electrical activity and hormone release among anterior pituitary cell types. In addition, whether BK channels promote bursts or shorten spike duration is determined by their activation kinetics and their proximity to L-type Ca2+ channels, and depends on the properties of the other ion channels on the cell membrane.
Footnotes
This work was supported by NIH Grant DK43200 and National Science Foundation Grant DMS0917664.
References
- Berkefeld H, Fakler B. Repolarizing responses of BKCa–Cav complexes are distinctly shaped by their Cav subunits. J Neurosci. 2008;28:8238–8245. doi: 10.1523/JNEUROSCI.2274-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci. 2005;8:1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- Economo MN, Fernandez FR, White JA. Dynamic clamp: alteration of response properties and creation of virtual realities in neurophysiology. J Neurosci. 2010;30:2407–2413. doi: 10.1523/JNEUROSCI.5954-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermentrout B. Philadelphia, PA: Society for Industrial and Applied Mathematics; 2002. Simulating, analyzing, and animating dynamical systems: a guide to XPPAUT for researchers and students. [Google Scholar]
- Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/microdomains. Neuron. 2008;59:873–881. doi: 10.1016/j.neuron.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Goaillard JM, Marder E. Dynamic clamp analyses of cardiac, endocrine, and neural function. Physiology (Bethesda) 2006;21:197–207. doi: 10.1152/physiol.00063.2005. [DOI] [PubMed] [Google Scholar]
- Goaillard JM, Taylor AL, Schulz DJ, Marder E. Functional consequences of animal-to-animal variation in circuit parameters. Nat Neurosci. 2009;12:1424–1430. doi: 10.1038/nn.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhikevich EM. Neural excitability, spiking and bursting. Int J Bifurcat Chaos. 2000;10:1171–1266. [Google Scholar]
- Kanyicska B, Freeman ME, Dryer SE. Endothelin activates large-conductance K+ channels in rat lactotrophs: reversal by long-term exposure to dopamine agonist. Endocrinology. 1997;138:3141–3153. doi: 10.1210/endo.138.8.5299. [DOI] [PubMed] [Google Scholar]
- Lang DG, Ritchie AK. Tetraethylammonium blockade of apamin-sensitive and insensitive Ca2+-activated K+ channels in a pituitary cell line. J Physiol. 1990;425:117–132. doi: 10.1113/jphysiol.1990.sp018095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R, Brauchi S. Large conductance Ca2+-activated K+ (BK) channel: activation by Ca2+ and voltage. Biol Res. 2006;39:385–401. doi: 10.4067/s0716-97602006000300003. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Guerineau N, Mollard P, Vincent JD, Israel JM. Physiological characterization of 2 functional-states in subpopulations of prolactin cells from lactating rats. J Physiol. 1991;437:477–494. doi: 10.1113/jphysiol.1991.sp018607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DJ, McClelland D, Herd MB, Sutton KG, Hall MD, Lee K, Pinnock RD, Scott RH. Gabapentin-mediated inhibition of voltage-activated Ca2+ channel currents in cultured sensory neurones is dependent on culture conditions and channel subunit expression. Neuropharmacology. 2002;42:353–366. doi: 10.1016/s0028-3908(01)00181-2. [DOI] [PubMed] [Google Scholar]
- Milescu LS, Yamanishi T, Ptak K, Mogri MZ, Smith JC. Real-time kinetic modeling of voltage-gated ion channels using dynamic clamp. Biophys J. 2008;95:66–87. doi: 10.1529/biophysj.107.118190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milescu LS, Yamanishi T, Ptak K, Smith JC. Kinetic properties and functional dynamics of sodium channels during repetitive spiking in a slow pacemaker neuron. J Neurosci. 2010;30:12113–12127. doi: 10.1523/JNEUROSCI.0445-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda P, de la Peña P, Gómez-Varela D, Barros F. Role of BK potassium channels shaping action potentials and the associated Ca2+i oscillations in GH3 rat anterior pituitary cells. Neuroendocrinology. 2003;77:162–176. doi: 10.1159/000069509. [DOI] [PubMed] [Google Scholar]
- Olypher A, Cymbalyuk G, Calabrese RL. Hybrid systems analysis of the control of burst duration by low-voltage-activated calcium current in leech heart interneurons. J Neurophysiol. 2006;96:2857–2867. doi: 10.1152/jn.00582.2006. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lingle CJ. Activation of BK channels in rat chromaffin cells requires summation of Ca2+ influx from multiple Ca2+ channels. J Neurophysiol. 2000;84:1123–1135. doi: 10.1152/jn.2000.84.3.1123. [DOI] [PubMed] [Google Scholar]
- Prinz AA, Billimoria CP, Marder E. Alternative to hand-tuning conductance-based models: construction and analysis of databases of model neurons. J Neurophysiol. 2003;90:3998–4015. doi: 10.1152/jn.00641.2003. [DOI] [PubMed] [Google Scholar]
- Putzier I, Kullmann PH, Horn JP, Levitan ES. Cav1.3 channel voltage dependence, not Ca2+ selectivity, drives pacemaker activity and amplifies bursts in nigral dopamine neurons. J Neurosci. 2009;29:15414–15419. doi: 10.1523/JNEUROSCI.4742-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaelli G, Saviane C, Mohajerani MH, Pedarzani P, Cherubini E. BK potassium channels control transmitter release at CA3-CA3 synapses in the rat hippocampus. J Physiol. 2004;557:147–157. doi: 10.1113/jphysiol.2004.062661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart PH, Chung S, Martin BL, Brautigan DL, Levitan IB. Modulation of calcium-activated potassium channels from rat brain by protein kinase A and phosphatase 2A. J Neurosci. 1991;11:1627–1635. doi: 10.1523/JNEUROSCI.11-06-01627.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinzel J, Ermentrout GB. Analysis of neural excitability and oscillations. In: Koch C, Segev I, editors. Methods in neuronal modeling. Cambridge, MA: MIT; 1998. [Google Scholar]
- Robitaille R, Charlton MP. Presynaptic calcium signals and transmitter release are modulated by calcium-activated potassium channels. J Neurosci. 1992;12:297–305. doi: 10.1523/JNEUROSCI.12-01-00297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanova EV, Fosser KA, Rubakhin SS, Nuzzo RG, Sweedler JV. Engineering the morphology and electrophysiological parameters of cultured neurons by microfluidic surface patterning. FASEB J. 2004;18:1267–1269. doi: 10.1096/fj.03-1368fje. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol. 2002;66:345–353. doi: 10.1016/s0301-0082(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Sharp AA, O'Neil MB, Abbott LF, Marder E. Dynamic clamp—computer-generated conductances in real neurons. J Neurophysiol. 1993;69:992–995. doi: 10.1152/jn.1993.69.3.992. [DOI] [PubMed] [Google Scholar]
- Sherman A, Keizer J, Rinzel J. Domain model for Ca2+-inactivation of Ca2+ channels at low channel density. Biophys J. 1990;58:985–995. doi: 10.1016/S0006-3495(90)82443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipston MJ, Kelly JS, Antoni FA. Glucocorticoids block protein kinase A inhibition of calcium-activated potassium channels. J Biol Chem. 1996;271:9197–9200. doi: 10.1074/jbc.271.16.9197. [DOI] [PubMed] [Google Scholar]
- Simon SM, Llinás RR. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985;48:485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro CR, Prakriya M, Ding JP, Lingle CJ. Inactivating and noninactivating Ca2+- and voltage-dependent K+ current in rat adrenal chromaffin cells. J Neurosci. 1995;15:6110–6123. doi: 10.1523/JNEUROSCI.15-09-06110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreadbury IC, Kros CJ, Meech RW. Effects of trypsin on large-conductance Ca2+-activated K+ channels of guinea-pig outer hair cells. Hear Res. 2004;190:115–127. doi: 10.1016/S0378-5955(03)00376-9. [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS, Zemkova H, Van Goor F. Biophysical basis of pituitary cell type-specific Ca2+ signaling-secretion coupling. Trends Endocrinol Metab. 2005;16:152–159. doi: 10.1016/j.tem.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS, Tabak J, Bertram R. Ion channels and signaling in the pituitary gland. Endocr Rev. 2010;31:845–915. doi: 10.1210/er.2010-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak J, Toporikova N, Freeman ME, Bertram R. Low dose of dopamine may stimulate prolactin secretion by increasing fast potassium currents. J Comput Neurosci. 2007;22:211–222. doi: 10.1007/s10827-006-0008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak J, Gonzalez-Iglesias AE, Toporikova N, Bertram R, Freeman ME. Variations in the response of pituitary lactotrophs to oxytocin during the rat estrous cycle. Endocrinology. 2010;151:1806–1813. doi: 10.1210/en.2009-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian AH, Jr, Yasumura Y, Levine L, Sato GH, Parker ML. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968;82:342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- Tsaneva-Atanasova K, Sherman A, van Goor F, Stojilkovic SS. Mechanism of spontaneous and receptor-controlled electrical activity in pituitary somatotrophs: experiments and theory. J Neurophysiol. 2007;98:131–144. doi: 10.1152/jn.00872.2006. [DOI] [PubMed] [Google Scholar]
- Tse A, Hille B. GnRH-induced Ca2+ oscillations and rhythmic hyperpolarizations of pituitary gonadotropes. Science. 1992;255:462–464. doi: 10.1126/science.1734523. [DOI] [PubMed] [Google Scholar]
- Van Goor F, Zivadinovic D, Stojilkovic SS. Differential expression of ionic channels in rat anterior pituitary cells. Mol Endocrinol. 2001a;15:1222–1236. doi: 10.1210/mend.15.7.0668. [DOI] [PubMed] [Google Scholar]
- Van Goor F, Li YX, Stojilkovic SS. Paradoxical role of large-conductance calcium-activated K+ (BK) channels in controlling action potential-driven Ca2+ entry in anterior pituitary cells. J Neurosci. 2001b;21:5902–5915. doi: 10.1523/JNEUROSCI.21-16-05902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goor F, Zivadinovic D, Martinez-Fuentes AJ, Stojilkovic SS. Dependence of pituitary hormone secretion on the pattern of spontaneus voltage-gated calcium influx-Cell type-specific action potential secretion coupling. J Biol Chem. 2001c;276:33840–33846. doi: 10.1074/jbc.M105386200. [DOI] [PubMed] [Google Scholar]
- Vandael DH, Marcantoni A, Mahapatra S, Caro A, Ruth P, Zuccotti A, Knipper M, Carbone E. Cav 1.3 and BK channels for timing and regulating cell firing. Mol Neurobiol. 2010;42:185–198. doi: 10.1007/s12035-010-8151-3. [DOI] [PubMed] [Google Scholar]
- White RE, Schonbrunn A, Armstrong DL. Somatostatin stimulates Ca2+-activated K+ channels through protein dephosphorylation. Nature. 1991;351:570–573. doi: 10.1038/351570a0. [DOI] [PubMed] [Google Scholar]