Abstract
Recently, concern regarding perchlorate contamination has arisen in many contexts. Perchlorate has many military, commercial, and domestic applications, and it has been found in milk, drinking and irrigation water, and produce. Perchlorate is harmful at low levels, yet it remains unregulated in the United States while the U.S. Environmental Protection Agency attempts to establish acceptable exposure levels. The present study investigated potential reproductive effects on vertebrates using a model fish species, the threespine stickleback (Gasterosteus aculeatus). Sticklebacks were raised from syngamy through sexual maturity in untreated water and in three target concentrations of sodium perchlorate–treated water. Perchlorate was found to interfere with the expression of nuptial coloration, courtship behavior, and normal sexual development. Genetic testing revealed that some females were masculinized to the extent that they produced both sperm and eggs, and histological analysis showed that these individuals had intersexual gonads (ovotestes) containing both oocytes and cells undergoing spermatogenesis. In vitro fertilizations revealed that those gametes were capable of self- and cross-fertilization. However, crosses using sperm derived from genetic females died either during the blastula phase or near the onset of organogenesis. Sperm derived from genetic males produced viable fry when crossed with eggs derived from genetic females from all treatments. To our knowledge, the present study provides the first evidence that perchlorate produces androgenic effects and is capable of inducing functional hermaphroditism in a nonhermaphroditic vertebrate.
Keywords: Perchlorate, Hermaphroditism, Threespine stickleback, Gasterosteus aculeatus, Endocrine disruption
INTRODUCTION
As of March 2005, perchlorate contamination had been detected in 36 U.S. states (http://www.epa.gov/fedfac/documents/detection_with_dates_03_25_05.xls). Perchlorate is used in many common household and industrial products (http://www.epa.gov/fedfac/documents/perchlorate.htm) and by the U.S. Department of Defense as an oxidizer in solid rocket propellant and artillery. Recent testing has revealed perchlorate contamination ranging from 3.16 to 11.3 μg/L (ppb) in organic and conventional milk from 101 of 104 containers tested in 15 states (http://www.cfsan.fda.gov/~dms/clo4data.html) and from 3,200 to 6,900 ng/g in organic lettuce (http://www.ewg.org/reports_content/rocketlettuce/pdf/wecklabs.pdf). Perchlorate also has been found in tap water (>4 μg/L) and irrigation water (>4 μg/L) derived from the Colorado River [1] and even in human breast milk (0.6–92.2 μg/L; mean, 10.5 μg/L) [2]. Perchlorate is highly soluble in water, can persist unaltered for several decades [3], causes deleterious effects at low concentrations [4–6], and lacks an enforceable water-quality standard while the U.S. Environmental Protection Agency (U.S. EPA) attempts to establish appropriate exposure levels. The most recent (2005) U.S. EPA reference dose is 24.5 μg/L (http://www.epa.gov/iris/subst/1007.htm). Major sources of environmental contamination have come from military storage and disposal practices [7], from an industrial spill into the Colorado River (http://www.epa.gov/fedfac/pdf/perch_7th_mnth_rpt.pdf), and to a lesser extent, from Chilean fertilizers [8].
To determine potential effects of perchlorate on vertebrates, we conducted experiments over a three-year period (2002–2004) on a model fish species, the threespine stickleback (Gasterosteus aculeatus). The 2002 experiment was designed to understand the effects of perchlorate on morphological development (to be published separately) and provided pilot data to establish the concentrations used in the 2003 and 2004 experiments. These included a study concerning the effects of perchlorate on reproduction, during which hermaphroditism was serendipitously discovered. The present paper describes the fertility, gamete viability, behavior, and histology of genetically female, intersex fish and the fertility, gamete viability, and nuptial coloration of “overmasculinized,” genetically male fish exposed to perchlorate. Perchlorate is a known disrupter of thyroid function, inhibiting the uptake of iodide during the synthesis of triiodothyronine and thyroxine [7,8]. Although it has been shown to skew sex ratios with a female bias among developing Xenopus laevis [5], its androgenic effects have not been noted previously.
Functionally hermaphroditic (i.e., containing both eggs and sperm capable of fertilization) threespine sticklebacks have not been described unequivocally in more than 150 years of intense scientific research in Europe, North America, and Asia. In 1959, however, a female stickleback with eyed embryos in her ovaries was photographed and described by a U.S. Fish and Wildlife Service employee at Karluk Lake (AK, USA) [9]. In this description of their life history, threespine sticklebacks were described as hermaphroditic. However, testes were mis-identified as ovaries, and subsequent histological examination revealed what was described as ovaries (in nongravid fish) to be fatty tissue [10]. Stenger [10] concluded that the stickleback population in Karluk Lake was not, in fact, hermaphroditic, but since then, the original publication occasionally has been cited in the absence of Stenger’s follow-up. The eyed embryos were located in the posterior portion of the ovaries, and they may have been fertilized by sperm that entered the cloaca with water during a failed spawning attempt [10].
In 2001, Gercken and Sordyl [11] described intersex sticklebacks in northeastern Germany in waters considered to be both heavily and mildly polluted, but no attempts were made to determine the functionality of their gametes. More recently, sex determination in sticklebacks has been shown to be determined genetically [12–15]. Thus, it is accepted that the threespine stickleback is not a hermaphroditic species.
Endocrine-disrupting chemicals have been used to produce intersex fishes in the laboratory [16,17] and by aquaculturists [18]. However, relatively few studies have described masculinized females [19]. Nonetheless, Hahlbeck et al. [16] produced juvenile intersex sticklebacks (that were genetically determined to be females) by exposing fry at various developmental stages to the synthetic androgen 17α-methyltestosterone. Because Hahlbeck et al. [16] did not raise these fish to sexual maturity, functional hermaphroditism was not determined. In the present paper, we document what is to our knowledge the first identification of perchlorate as a presumptive endocrine disrupter with androgenic properties and the first unequivocal account of functional hermaphroditism among threespine sticklebacks.
MATERIALS AND METHODS
The goal of the present examination was to study potential reproductive effects associated with development in perchlorate-contaminated water. To accomplish this, we exposed wild-caught fish to different target concentrations of perchlorate (negative controls and 30, 60, and 100 mg/L) during reproduction, raised their offspring (F1) in those same treatments until sexual maturity, and then studied their reproduction as described below.
Animals and husbandry
Wild-caught, adult, anadromous sticklebacks were trapped from Rabbit Slough (AK, USA) during May and June 2003. They were housed outdoors in 1,600-L pools through mid-July under ambient temperature (14–20°C) and photoperiod (18:6-h light:dark, increasing to 20:4-h light:dark before decreasing to 18.5:5.5-h light:dark). All pools were continuously filtered and aerated through biofilters. Each pool contained water with a salinity of approximately 4 g/L, filamentous algae, and sand to be used as nesting material. Adults and juveniles older than two months were fed frozen brine shrimp daily, whereas fry (age, <2 months) were fed a mixture of Golden Pearls 100, Artemia food (both from Aquatic Ecosystems, Apopka, FL, USA), and ground brine shrimp daily.
Experimental groups
Fish from four experimental groups were raised to sexual maturity for reproductive analyses. These experimental groups were distributed among eleven 1,600-L pools and included the following: Negative controls (less than the method detection limit of 1.1 μg/L; three replicates), three replicates with 30 mg/L of anhydrous sodium perchlorate (purity, ≥99%; EM Science, Cherry Hill, NJ, USA), two replicates with 100 mg/L of sodium perchlorate, and three replicates of a variable treatment. The variable treatment was designed to mimic a 2002 treatment in which perchlorate leached from 3.70-g cores of hydroxyl-terminated polybutyrate solid rocket propellant (as might occur in natural waters exposed to rocket propellant). To that end, sodium perchlorate was added over time until the concentration reached approximately 60 mg/L, after which the concentration was held steady. The only exchange of treated water occurred on October 5, 2003, when the F1 generation (see below) were moved indoors and placed into 400-L tanks at the same concentrations in which they had been raised outdoors. With that exception, water was only added to dilute the treatments as perchlorate became more concentrated because of evaporation and to replace water removed during cleaning. These exposure levels were chosen because they approximate (and, in some cases, are much less than) the perchlorate concentrations occurring at a number of contaminated sites [7] (http://www.epa.gov/fedfac/documents/detection_with_dates_03_25_05.xls).
Baseline perchlorate levels of the tap water used to fill control and treated pools were tested via ion chromatography in tandem with electrospray ionization mass spectrometry (IC-ESI-MS). Following this, temperature and perchlorate concentrations in pools treated with perchlorate were monitored daily using an Acorn 6 perchlorate potentiometer (Oakton, Vernon Hills, IL, USA) with automatic temperature compensation. The potentiometer was equipped with a perchlorate ion–selective electrode (Cole Parmer, Vernon Hills, IL, USA). To avoid contaminating the negative control water with residual perchlorate, the electrode was thoroughly cleaned before use in the negative controls and was only used in the negative controls for 6 d to take 18 readings. Dissolved oxygen, pH, and salinity were checked every two to three months.
Parental spawning protocol
Male nuptial coloration in the Rabbit Slough population is expressed as iridescent blue in the iris and/or reddish throats, mouths, or sides and bluish-gray backs. Nuptially colored males were randomly placed into separate quadrants of the 1,600-L pools (four males per pool; isolated by netting). Gravid females were randomly introduced into a quadrant and allowed to spawn with the males. Females were immediately removed after successfully spawning or after 20 min if they failed to spawn. Females were introduced in succession until spawning occurred or until 7 d had passed if spawning was unsuccessful. Once a male had spawned, no additional females were provided. After providing 5 d of parental care (12 d postfertilization), adult males were removed from the pools, killed, and stored at −80°C.
F1 husbandry and reproduction
The offspring (F1 generation) continued to be raised outdoors under ambient temperature (20° declining to 8°C) and photoperiod (20:4-h light:dark, declining to 11:13-h light:dark) through October 5, 2003. At this point (age, 15 weeks), 50 fish from each of the four treatments (controls and 30, 60, and 100 mg/L) were transferred indoors. To assess the effects of perchlorate on fertility and reproductive behavior, these F1 offspring were maintained in 400-L tanks under a simulated natural photoperiod at 17 to 19°C until they reached sexual maturity the following spring. Although sexual maturity occurred at one year of age in our laboratory conditions, most sticklebacks from the source population reproduce at two years of age.
At approximately one year of age, sticklebacks showing male nuptial coloration (n = 10, 6, 2, and 0 for the controls, 30, 60, and 100 mg/L experimental groups, respectively) were assumed to be males and isolated in individual 40-L aquaria containing perchlorate-free water. Gravid females were segregated according to experimental group and collectively remained in their 400-L holding tank (controls, 30, 60, or 100 mg/L). The remaining fish of unknown sex were isolated in 40-L aquaria without perchlorate (n = 0, 4, 8, and 10 for the controls and the 30, 60, and 100 mg/L treatments, respectively), until a total of 10 aquaria per experimental group each housed a single fish (either a nuptially colored male or a fish of unknown sex; n = 40 isolated fish and aquaria). Lower survivorship among fish exposed to higher concentrations of perchlorate (see Results) led to fewer nuptially colored fish from which to choose. Extra fish were isolated and later killed.
Nesting material consisting of dried, filamentous algae, and sand was added to each of the 40 aquaria. Biofilters were placed in each aquarium, and the water was continually filtered and aerated. The water in the aquaria was maintained at a salinity of 4 g/L and was not exchanged. Each fish isolated in a 40-L aquarium was scored daily for body and eye color.
From May 29 to June 18, 2004, females from like treatments were placed individually into one of the 40-L aquaria and allowed 10 min to spawn. The female was immediately removed after successfully spawning or at the end of the 10-min period if spawning was unsuccessful. Gravid females continued to be introduced into each aquarium daily until either a successful spawning occurred or three weeks of courtship activity had passed. Once a male had successfully spawned, no additional females were provided. Courtship, spawning, and parental care behaviors were videotaped for analysis.
Gamete viability
To determine whether males and females from any or all experimental groups were capable of producing viable gametes, we conducted in vitro fertilizations. Three males from a given experimental group were dissected; their testes were macerated, mixed homogeneously with water in a Petri dish, and stored on ice for 10 to 50 min until this procedure had been completed for males from each of five experimental groups (controls; 30, 60, and 100 mg/L; and wild-caught Rabbit Slough fish, the source population for the present study). Next, the eggs from three females per experimental group (controls; 30, 60, and 100 mg/L; and wild-caught Rabbit Slough females) were removed, mixed homogeneously, and evenly distributed between five Petri dishes per treatment (n = 25 dishes). Two to three droplets of sperm per treatment were added and mixed with the eggs from each treatment so that all combinations of sperm and eggs were achieved in the 25 Petri dishes. The eggs were housed in a low-temperature incubator at 20°C (Fisher Scientific, Hampton, NH, USA) and monitored at least daily for evidence of micropyle formation, blastula formation, organogenesis, and hatching.
Three fish from two treatments (one fish from the 30 mg/L treatment and two fish from the 100 mg/L treatment) displayed both a gravid appearance and characteristic male courtship behavior (see Results). The two fish from the 100 mg/L treatment were dissected, and evaluation by light microscopy revealed the presence of both eggs and motile sperm. In vitro fertilizations were conducted to assess their gamete viability. In each case, testicular material was removed, macerated, and mixed in approximately 10 drops of water. Next, the eggs were removed and separated into two Petri dishes. Eggs and sperm also were removed from a wild-caught male and female from Rabbit Slough in a similar manner. By adding the sperm to the eggs, three crosses were made for each hermaphrodite: Between hermaphrodite eggs and wild male sperm (H♀ × RS♂), between hermaphrodite sperm and wild female eggs (H♂ × RS♀), and self-fertilization with hermaphrodite sperm and eggs (H♂ × H♀). Five randomly selected females from each experimental group (n = 20 total) also were dissected for evidence of structural hermaphroditism.
Genotypic sex determination
To establish if the hermaphrodites where masculinized females or feminized males, we examined each fish using a polymerase chain reaction (PCR) analysis to determine their genotypic sex. A total of five control males that exhibited male nuptial coloration and courtship behavior and six control females that became gravid were tested along with the three hermaphrodites. We also tested additional fish from each experimental group (n = 43 total). Pectoral fins were taken from each fish and digested with ProteinaseK (Acros Organics, Somerville, NJ, USA) overnight, and DNA was extracted using standard phenol–chlorform–isoamyl techniques. The PCR was performed using the Ga1 forward and reverse primers (Ga1F, 5′-CTTCTTTCCTCTCACCATACTCA-3′; Ga1R, 5′-AGAT-GACGGGTTGATAAACAG-3′) as reported by Griffiths et al. [14]. The PCR reactions were carried out in 20-μl volumes containing 20 pmol of each primer, 200 μM of each deoxyribonucleotide triphosphate, 100 to 150 ng of target DNA, 0.5 U of Taq DNA polymerase, 2.5 mM MgCl2, 50 mM KCl, and 10 mM Tris-HCl (pH 8.0). The thermocycler conditions were 94°C for 6 min, followed by 37 cycles of 94°C for 40 sec, 44°C for 40 sec, and 72°C for 50 sec, followed by a final extension at 72°C for 10 min. These primers produce fragments of two sizes, approximately 370 and 600 bp, with characteristic male and female XY (both bands) and XX (only the 600-bp band) genotypes, respectively. Genotypes were visualized by electrophoresis on a 2% agarose gel. The female-specific band is larger than the male-specific, and it serves as an internal control for the bias of PCR for amplification of smaller products. In addition, we ran the PCR independently three times on each fish, and the results of the control fish were compared with their known sexual phenotype (ovaries or testes).
Histological analysis
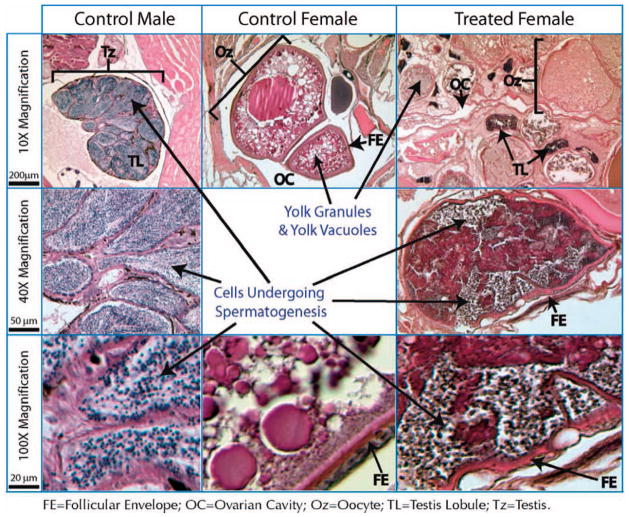
The gonads of both untreated male and female fish were sectioned and compared to a genotypically female, but apparently hermaphroditic stickleback from the highest perchlorate treatment (100 mg/L) to determine if perchlorate affected gonadal structure. The control fish were from the same population (Rabbit Slough) as the parents of the treated fish. Sticklebacks were fixed for 36 h in Bouin-Hollande solution (4.0% acetic acid, 4.0% formaldehyde, 4.0% picric acid, 2.5% copper(II) acetate, and 1% distilled water), then rinsed and stored in 70.0% ethanol. The samples were then dehydrated in pure ethanol and embedded in paraffin for sectioning. Transverse sections (thickness, 5 μm) were cut through the trunk portion of each fish, starting approximately at the pectoral fin and moving posteriorly to the anal fin. The sections were stained in hematoxylin and eosin. Slides were examined by light microscopy under a variety of magnifications, and digital images of sections were taken using a SPOT camera (Diagnostic Instruments, Sterling Heights, MI, USA) mounted to the scope. Analysis of the gonads from the control male involved identification of spermatogenic cells in lobules, and analysis of those from the control female involved identification of the follicular envelope and oocytes, with characteristic yolk granules and vacuoles. In the sections of the treated genotypic female, intersexual gonads were identified by the presence of several oocytes as well as cells that appeared to be similar to those undergoing spermatogenesis in the lobules of the control male.
Statistics
The Statistical Package for Social Sciences (Ver 11.5; SPSS, Chicago, IL, USA) was used for all statistical procedures. Differences between groups were analyzed using a Kruskall–Wallis test; statistics were two-tailed.
RESULTS
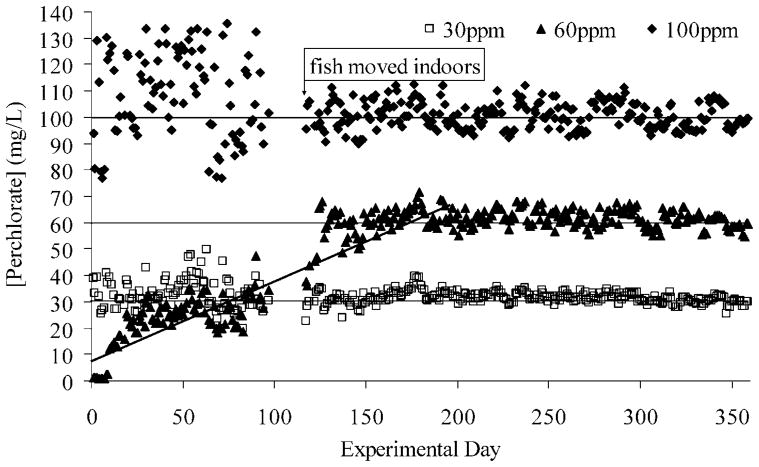
The results of the daily perchlorate readings from the Acorn 6 perchlorate potentiometer are shown in Figure 1. The mean perchlorate concentration of the negative control water based on the 18 Acorn 6 readings was 0.15 mg/L (SE = 0.01). The more precise perchlorate measurements of the negative control water from the IC-ESI-MS (n = 21 samples) revealed the perchlorate concentration to be less than the method detection limit of 1.1 μg/L [19].
Fig. 1.
Daily perchlorate readings using the Acorn 6 perchlorate potentiometer (Oakton, Vernon Hills, IL, USA). Readings were more variable when the fish were housed outdoors, where temperatures fluctuated more than indoors. At day 100, the potentiometer broke, and it was replaced three weeks later with the same model. This period is indicated by a gap in readings. The entire data set revealed that the nominal 30 mg/L treatment had a mean perchlorate concentration of 32.00 mg/L (n = 334, SE = 0.20), and the 100 mg/L treatment had a mean perchlorate concentration of 102.92 mg/L (n = 334, SE = 0.57). The nominal 60 mg/L treatment, which mimicked the increasing perchlorate concentration noted in the year 2002, as perchlorate leached from solid rocket propellant cores (y2002 = 0.32x + 12.04, r2 = 0.92 vs y2003 = 0.31x + 9.44, r2 = 0.88), reached a maximum concentration near 60 mg/L about halfway through the experiment, after which the readings remained relatively stable (mean, 61.80 mg/L; n = 185; SE = 0.19). Values for the target dose of zero (negative controls) were less than the method detection limit of the ion chromatograph in tandem with electrospray ionization mass spectroscopy (1.1 μg/L) [19].
Perchlorate becomes deposited in whole-body stickleback homogenates in proportion to their levels of exposure [19]. Adult, wild-caught, male sticklebacks were analyzed in the year 2002 after they had been exposed to perchlorate for up to 22 d. Perchlorate was never detected at or above the IC-ESI-MS limits of quantification (1.1 μg/L) in any of the 19 control tissue samples tested [19]. Fish housed in perchlorate-treated water with a mean concentration of 1.59 mg/L (n = 21 daily potentiometer readings, SE = 0.04 mg/L) had a mean tissue concentration of 0.66 mg/L (IC-ESI-MS; n = 17 fish exposed on average for 17.7 d, SE = 0.06 mg/L). Fish in a mean concentration of 12.15 mg/L (n = 22 daily potentiometer readings, SE = 0.28 mg/L) had a mean tissue concentration of 4.27 mg/L (IC-ESI-MS; n = 15 fish exposed on average for 18.0 d, SE = 0.44 mg/L). Those in a treatment with an increasing perchlorate concentration that began at zero and reached 18.4 mg/L at harvest (potentiometer; y = 0.69x + 3.96, r2 = 0.90) had a mean tissue concentration of 5.03 mg/L (IC-ESI-MS; n = 17 fish exposed on average for 19.0 d, SE = 0.40 mg/L).
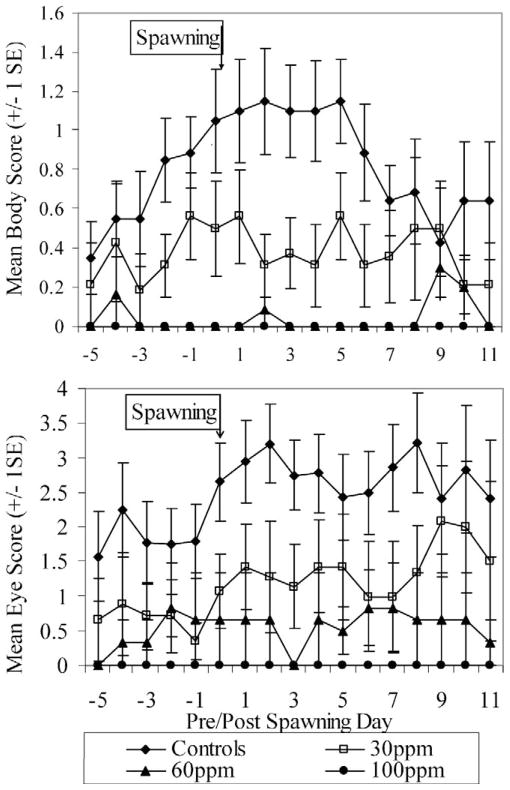
Perchlorate exposure was found to interfere with the expression of male nuptial coloration among fish raised in per-chlorate (F1 generation) (Fig. 2). Nuptial coloration was only rarely and weakly expressed among perchlorate-exposed fish; only 5 of 24, 2 of 22, and 0 of 13 fish in the 30, 60, and 100 mg/L treatments, respectively, showed nuptial coloration. Therefore, the sex of most treated fish could not reliably be determined before genetic testing, which was conducted postmortem. Because of the ambiguity of their sex and impaired survival among treated fish (Fig. 3), fewer treated males were available from which to select for behavioral analysis. These factors led to a small number of genetic females being isolated into 40-L aquaria as if they were males (n = 0, 2, 3, and 2 for the controls and the 30, 60, and 100 mg/L treatments, respectively).
Fig. 2.
Body and eye color cycling of nuptial males. Mean color scores show that few treated males expressed nuptial coloration, and those that did lacked the color intensity shown by control fish. The 100 mg/L males failed to develop any nuptial coloration. Body scoring criteria were as follows: 0 = no nuptial coloration; 1 = light-orange coloration in/around the mouth or throat (ventrally); 2 = orange remains on mouth and is present on the operculum, but not posterior to it; 3 = light-orange coloration along body posterior to the operculum (easily seen). Light or patchy bluish-gray coloration on back. Eye scoring criteria were as follows: 0 = no color; 1 = light blue on dorsal side only; 2 = light blue on dorsal and ventral sides only; 3 = dark blue on dorsal and ventral sides, with light blue on anterior and posterior; 4 = eyes completely the same shade of blue; 5 = iridescent or glowing appearance to entire eye. Eye still completely blue.
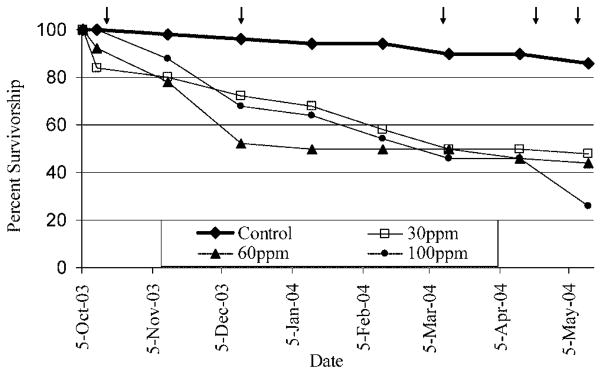
Fig. 3.
Survivorship curves by experimental group. Arrows represent handling/stressful events, such as periodic measuring or relocation.
All control males (n = 10) expressed normal nuptial coloration and displayed normal courtship behavior (e.g., nest building, territoriality, biting introduced females, zigzagging, leading, and fanning), and 8 of 10 control males ultimately spawned. Most treated males ignored gravid females and failed to display appropriate courtship behavior, and spawning success among treated fish was much lower than that for controls: Four of eight males, two of seven males, and none of eight males successfully spawned in the 30, 60, and 100 mg/L treatments, respectively. All 10 control males built nests, but only six of eight, three of seven, and none of eight males in the 30, 60, and 100 mg/L treatments, respectively, built nests.
The combined onset of their ripe appearance and display of male-typical behavior gave the initial indication of potential hermaphroditism among three of the treated fish (one fish in the 30 mg/L treatment and two fish in the 100 mg/L treatment). Although all three failed to make nests, each became territorial and performed male courtship displays, such as biting, zigzagging, and assuming aggressive, head-down postures when females were introduced into their aquaria. They also attempted to lead females, just as courting males do. Introduced females responded to these “male” courtship displays by assuming the head-up posture typical of receptive females and by following the hermaphrodite as it attempted to lead.
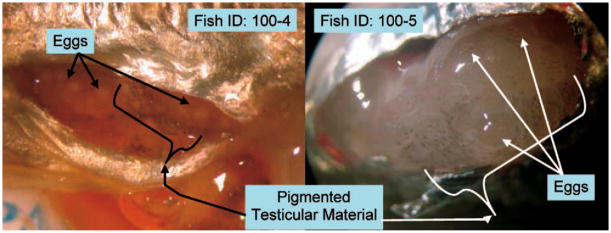
Genotypic testing of fish from all experimental groups revealed that fish with discrete testes were genotypically male and fish that developed discrete ovaries were genotypically female. The genotypic sex also matched the phenotypic sex in each of the 11 fish tested from the control group. The three known hermaphrodites were found to be genetic females. Dissections provided further evidence of their masculinization. Hermaphrodites lacked discrete testes but had a thin layer of macroscopically amorphous testicular tissue with sparse melanophore deposition (Fig. 4), but their eggs did not become fertilized in vivo.
Fig. 4.
Pigmented, sperm-producing testicular material located distally from ripe ovaries. Discrete testes were not produced.
Histological sections of a genotypically female hermaphrodite in the 100 mg/L treatment provided evidence for intersexual gonads. Comparisons between sections of the control male and female and the treated genotypic female showed the treated female contained gonads that were a chimera of ovarian and testicular tissues (ovotestes) (Fig. 5). In particular, the gonads of the treated female clearly contained oocytes, as evidenced by yolk granules and vacuoles, follicular envelopes, and ovarian cavities around the oocytes. We would not have expected to find mature eggs, because they had been stripped from the hermaphroditic females for in vitro fertilizations before the fish were sectioned for histology. Structures similar to individual testis lobules also were present. Under higher magnification, these structures had morphological and staining patterns consistent with the presence of spermatogenic cells. This is particularly evident when comparing the higher-magnification sections from the control male to those from the treated female (Fig. 5).
Fig. 5.
Hematoxylin and eosin–stained sections through untreated, control male and female fish along with a treated, hermaphroditic female. The left three panels show male histological features, including the entire testis (Tz), testis lobules (TL), and sperm-producing cells, stained dark blue. The center panel shows a section of a developing oocyte (Oz) in a control female, including dark red–staining yolk granules and clear yolk vacuoles. The right panels show intersexual gonads of a genotypically female fish, which appears to contain a mixture of both types of tissues, including oocytes, ovarian cavity (OC), and what appear to be sperm lobules. The higher-magnification image in the bottom right corner exhibits darkly staining sperm-producing cells, similar to what is seen in the control male testis (lower left). The histological work indicates that the intersexual gonad is a chimera of ovarial and testicular tissue.
The putative hermaphrodite in the 30 mg/L treatment was the first to become gravid, and in vitro fertilizations were not performed on this fish. When the testicular material from the two hermaphrodites in the 100 mg/L treatment was removed and macerated in a Petri dish with water, motile sperm became evident. The two freshly killed hermaphrodites in the 100 mg/L treatment had testicular tissue with motile sperm. However, dissections of other fish that had been stored at −80°C (including the putative hermaphrodite in the 30 mg/L treatment) for months after death failed to reveal clearly the number of females that had become structurally hermaphroditic. These fish had been thawed and refrozen repeatedly for analysis during their storage, which led to poor preservation of their gonadal tissue and a lack of shimmering, motile sperm. Therefore, dissections could not be used as a reliable, stand-alone indicator of hermaphroditism.
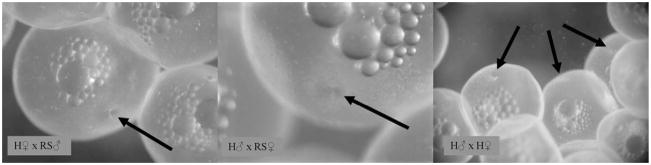
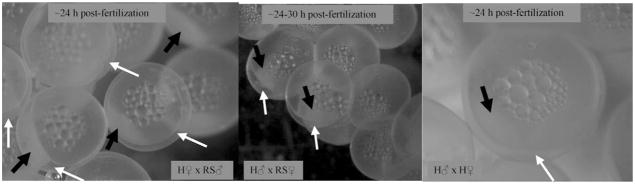
The in vitro fertilizations of the two fish in the 100 mg/L treatment provided the final evidence of their functional hermaphroditism. In all three crosses from both hermaphrodites in the 100 mg/L treatment, identification of the micropyle (Fig. 6) and separation of the chorion from the vitelline membrane (Fig. 7) revealed that motile sperm had fertilized the eggs.
Fig. 6.
Penetration of eggs. Formation of the micropyle (arrows) provided the first evidence that fertilization occurred for all three crosses with both hermaphrodites tested. H ♂ = hermaphrodite sperm; H ♀ = hermaphrodite eggs; RS ♂ = sperm from wild Rabbit Slough (AK, USA) male; RS ♀ = eggs from wild Rabbit Slough female.
Fig. 7.
Chorion separation and cleavage. After fertilization, separation of the chorion from the vitelline membrane (white arrows) and initial cleavage (black arrows) occurred in all three crosses for each hermaphrodite. H ♂ = hermaphrodite sperm; H ♀ = hermaphrodite eggs; RS ♂ = sperm from wild Rabbit Slough (AK, USA) male; RS ♀ = eggs from wild Rabbit Slough female.
Development continued in all three crosses for both hermaphrodites to at least the blastula phase, beyond which organogenesis concluded only for crosses between hermaphrodite eggs and wild male sperm. Thus, each cross using hermaphrodite sperm resulted in embryonic death during the blastula phase or near the onset of organogenesis. Embryonic development continued to progress in the crosses between hermaphrodite eggs and wild male sperm, and fry hatched in both cases.
In vitro fertilizations using gametes from randomly chosen fish not suspected of being hermaphroditic revealed that fish of both sexes from all experimental groups were capable of producing viable gametes. Twenty-four of these 25 in vitro fertilizations resulted in fry successfully hatching from their eggs. The only exception involved a cross between a control female and a male in the 30 mg/L treatment. During the initial series of crosses, none of the eggs from control females became fertilized because they recently had spawned naturally with isolated control males and only contained immature oocytes. When they reclutched two weeks later, a second series of in vitro fertilizations were performed using mature eggs from the control females and sperm from control males, males in the 60 and 100 mg/L treatments, and Rabbit Slough males. No males in the 30 mg/L treatment remained alive from which to acquire sperm, but all other combinations were successful. It therefore was determined that males and females from each experimental group were capable of producing viable gametes and that sperm from each experimental group could fertilize eggs from any group.
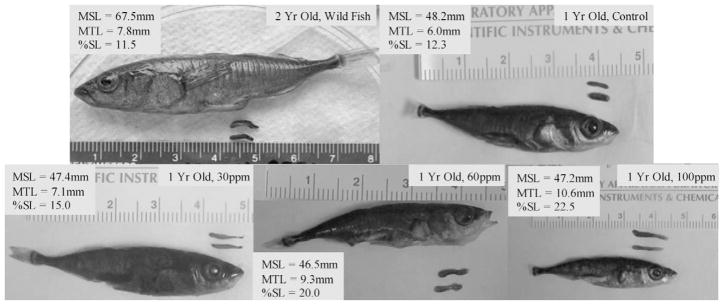
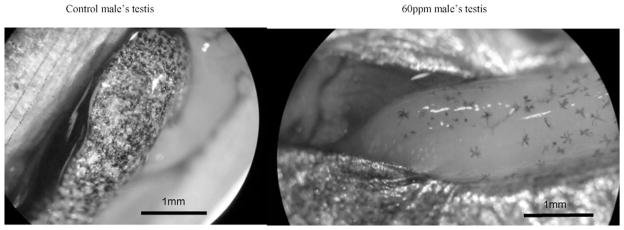
Perchlorate exposure also produced an overmasculinizing effect among fish that were genetically determined to be males (Fig. 8). A dose–response relationship became evident as fish exposed to higher perchlorate concentrations grew larger testes than controls or those exposed to lower perchlorate concentrations (Kruskall–Wallis test: χ2 = 16.249, df = 3, p = 0.001). Melanophore deposition on the testes was much sparser in males exposed to higher concentrations of perchlorate (Fig. 9).
Fig. 8.
Testicular length by experimental group. As the perchlorate concentration increased, so did the length of testes. Mean testicular length (MTL) for two-year-old, wild-caught males was 7.8 mm (n = 8; SE = 0.24; top left). One-year-old control fish had an MTL of 6.0 mm (n = 7, SE = 0.54; top right). One-year-old fish in the 30 mg/L treatment had an MTL of 7.1 mm (n = 7, SE = 0.35; bottom left). One-year-old fish in the 60 mg/L teratment had an MTL of 9.3 mm (n = 7, SE = 0.21; bottom center), and one-year-old fish in the 100 mg/L treatment had an MTL of 10.6 mm (n = 6, SE = 0.22; bottom right). MSL = mean standard length; %SL = MTL/MSL).
Fig. 9.
Testicular melanophore density. As the perchlorate concentration increased, the density of testicular melanophores decreased.
DISCUSSION
All developing vertebrates pass through a stage with undifferentiated tissue capable of producing either ovaries or testes, but teleost fish have no spatial distinction between these types of tissues. With the correct dose of estrogens or androgens at the correct time and for the correct duration, these intermingled, undifferentiated gonadal primordia can be induced to form either distinct ovaries or testes, despite the genotype of the fish [16,20]. Endocrine-disrupting chemicals can lead to atypical gonadal formations [11,16,21]. For sticklebacks, exogenous hormones have the strongest influence on gonadal development during their first 14 d posthatch. Gonads are less responsive to exogenous hormonal treatment beyond 14 d, but a lower occurrence of intersexuality can be induced with estrogenic, but perhaps not androgenic, hormones administered between 14 and 60 d posthatch [16,20]. Therefore, briefly exposing adult sticklebacks to perchlorate would not be expected to produce the same type of hermaphroditic changes as observed in our research.
Those who have studied the reproductive effects of perchlorate on fish [22,23] generally have exposed adults to perchlorate and then analyzed the mass or volume of eggs produced and/or the gonadosomatic index (GSI) of females. Patiño et al. [22] found no effects on the spawned egg volume at 18 mg/L, but spawned egg volume became negligible after four weeks of exposure to 677 mg/L. Conversely, Park et al. [23] found that perchlorate had a stimulatory effect on GSI, egg/ embryo mass, and fecundity in some of the females exposed to 1, 10, and 100 mg/L. To our knowledge, the present study is the first to analyze the gonadal histopathology of fish that were exposed to perchlorate and the effects of perchlorate exposure during early development on gonads. Our findings suggest that gonadal histopathology as well as male GSI may be more sensitive biomarkers of effect for perchlorate exposure than previous reproductive endpoints focused on female fecundity and female GSI.
In the present study, chronic exposure to sodium perchlorate at nominal concentrations of 30, 60, and 100 mg/L from syngamy through sexual maturity induced functional hermaphroditism in a vertebrate with genetically controlled sex determination [15]. Perchlorate produced functional hermaphrodites by masculinizing genetically female threespine sticklebacks. Histological examination revealed hermaphrodite gonads to be a chimera of ovarian and testicular material. Treated male sticklebacks exhibited marked testicular enlargement in a dose-dependent manner, with fish exposed to higher perchlorate concentrations developing larger testes. Thus, these findings give the initial indication that perchlorate produces androgenic effects (either directly or indirectly) and that perchlorate is capable of inducing functional hermaphroditism in a nonhermaphroditic vertebrate. Those findings raise the possibility that the androgenic effects of perchlorate could occur in other species of vertebrates as well.
Spawning success also was reduced in a dose-dependent manner. The differences in spawning success between treated and control fish appeared to be related, in part, to the lack of proper behavior, poor expression of nuptial coloration (Fig. 2) among treated males, and apparent inability of hermaphroditic sticklebacks to build nests. These reproductive anomalies would pose a formidable barrier to recruitment in wild fish exposed to perchlorate.
Gamete viability was uncompromised among sperm-producing males or egg-producing females for all treatments. However, all four crosses using sperm derived from genetic females died either during the blastula stage or near the onset of organogenesis, whereas crosses using the hermaphrodite’s eggs and sperm derived from genetic males produced viable fry. Because cell division beyond the blastula stage is regulated by the developing embryo, perchlorate may have produced lethal mutations in the hermaphrodite’s sperm that led to early embryonic death. Alternatively, the sperm, although capable of fertilizing the eggs, may have incompletely contributed DNA to the egg, thus making an aneuploid or even haploid embryo, as has been produced using protocols developed for zebrafish (Danio rerio) [24]. Stickleback eggs also can be induced to develop haploid embryos when fertilized with sperm that has been irradiated to cross-link male DNA (W. Cresko, University of Oregon, Eugene, OR, USA, unpublished data).
Overall, survivorship was poor among treated fish (Fig. 3), suggesting that many fish are incapable of tolerating the stresses induced by chronic perchlorate exposure. Two perchlorate median lethal concentration (LC50) estimates have been made for zebrafish embryos/larvae. Patiño et al. [22] cited unpublished data corresponding to a 5-d LC50 for ammonium perchlorate of 529 mg/L. Liu et al. [25] determined the 96-h LC50 for sodium perchlorate to be 1,401.2 mg/L and suggested that the higher lethality reported by Patiño et al. [22] may have been the result of ammonium toxicity. Park et al. [23] determined the 5-d LC50 for sodium perchlorate to be 404 mg/L using mosquitofish (Gambusia holbrooki) larvae. Additional 96-h LC50 estimates have been calculated for rainbow trout (Onchorynchus mykiss), fathead minnow (Pimephales pro-melas), and bluegill sunfish (Lepomis macrochirus), corresponding to 2,100, 1,655, and 1,470 mg/L, respectively [26,27] (http://www.epa.gov/ncea/perchlorate/references2/documents/44908.pdf).
If a dose–response relationship exists between exposure concentrations and the range and severity of deleterious effects, as the present study indicates, then only a small fraction of those exposed to low doses would display pathological conditions (i.e., those that are more vulnerable) [28]. Additionally, limited sample sizes may mask potential outcomes at the margins of normalcy when using lower doses. Therefore, doses up to 100 mg/L were chosen to explore the range of deleterious effects that may occur among more vulnerable individuals at lower contaminant levels and among less vulnerable individuals at higher levels. The experimental levels of perchlorate used in the present study are far above the U.S. EPA 2005 reference dose of 24.5 μg/L. However, our concentrations are not only environmentally relevant but also below levels that have been detected in the groundwater of seven U.S. states (AL, AR, CA, MD, MO, NV, and TX; http://www.epa.gov/fedfac/documents/detection_with_dates_03_25_05.xls).
The present study demonstrates that the negative effects of perchlorate are not limited to thyroid function, and its androgenic effects may apply to humans as well. We would not expect hermaphroditism to be induced in any mammal, including humans, but other disruptions of reproductive function are possible. The primary sex-determination gene at the head of the pathway seems to vary across species, but many of the downstream genes and pathways in sex determination, such as Sox9 and Doublesex- and MAB3-Related Transcription Factor (DMRT) genes, seem to be conserved across species, including sticklebacks [29]. Thus, if the androgenic mode of action for perchlorate in sticklebacks is downstream in the pathway, mechanistic studies in this species may well inform us about possible effects in humans and other mammals [30].
The threespine stickleback is uniquely suited for research concerning the disruption of sex steroids, because it has both male- and female-specific biomarkers that can be easily and rapidly measured by enzyme-linked immunosorbent assays [17,31]. Reproductive males produce a glue protein called spiggin from the kidneys, which is used to glue together nesting material, and the reproductive female produces the egg-yolk protein vitellogenin. Exposure to androgenic compounds can cause the female kidney to produce spiggin, whereas estrogenic compounds can cause the male to produce vitellogenin [17].
The mechanisms of action that induced hermaphroditism in the present study remain unclear. However, hermaphroditism among treated females and oversized testes among treated males suggest that perchlorate has an androgenic endocrine-disrupting role in addition to its known thyroid-disrupting properties. With the knowledge that perchlorate is capable of inducing hermaphroditism, a sensitive assay, such as that described by Katsiadaki et al. [17], could be combined with structural studies of reproductive tissues and genetic testing to explore perchlorate’s mode of action. Studies should examine whether the masculinization response is mediated through direct activation of androgen receptors or by indirect means, such as alteration of steroid metabolism. In vitro examination of female or immature male stickleback kidney cell cultures after exposure to perchlorate may reveal whether perchlorate is capable of directly activating androgen receptors and, hence, inducing transformation of the stickleback kidney from an osmoregulatory organ into a spiggin-producing organ for nest construction (see, e.g., [32]). The impaired ability of treated males to build nests may have been caused, in part, by a lack of spiggin production, which warrants further investigation. A replication of the present study should include histological analysis of the overmasculinized testes to determine if spermatogenesis and testicular morphology (e.g., sperm ducts or unusual cavities as described in Hahlbeck et al. [16]) have been affected. Sperm analysis could reveal whether quantity, viability, and motility have been affected. Because the viability of any species ultimately depends on its ability to reproduce, the significance of perchlorate contamination should not be downplayed.
Acknowledgments
We thank Jeff Jones, Christoff Furin, Anjali Karve, Heidi Weigner, and Rajit Patankar for their invaluable assistance in the lab. Todd O’Hara and Christoff Furin also offered many thought-provoking discussions. Ruth BreMiller provided excellent assistance by performing the histological work, including sectioning and staining of the fish. The manuscript was greatly improved by the input of David Mount and three anonymous reviewers. The project described was supported by National Institutes of Health (NIH) grant RR-020010-01 from the Research Facilities Improvement Program of the National Center for Research Resources (NCRR; RRB), NIH grant 1P20RR020010-01 from the Biomedical Research Idea Network/Idea Network of Biomedical Research Excellence program of the NCRR (RRB), National Science Foundation Integrative Biology and Neuroscience grant 0236239 (W. A. Cresko), and NIH 5 F32 GM020892 (W. A. Cresko). Fish were collected under Alaska Department of Fish and Game permit number SF-2003-019, and all research protocols were approved by the University of Alaska Anchorage Institutional Animal Care and Use Committee.
References
- 1.Brechner RJ, Parkhurst GD, Humble WO, Brown MB, Herman WH. Ammonium perchlorate contamination of Colorado River drinking water is associated with abnormal thyroid function in newborns in Arizona. J Occup Environ Med. 2000;42:777–782. doi: 10.1097/00043764-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kirk AB, Martinelango PK, Tian K, Dutta A, Smith EE, Dasgupta PK. Perchlorate and iodide in dairy and breast milk. Environ Sci Technol. 2005;39:2011–2017. doi: 10.1021/es048118t. [DOI] [PubMed] [Google Scholar]
- 3.Urbansky ET. Perchlorate chemistry: Implications for analysis and remediation. Bioremediat J. 1998;2:81–95. [Google Scholar]
- 4.Goleman WL, Urquidi LJ, Anderson TA, Smith EE, Kendall RJ, Carr JA. Environmentally relevant concentrations of ammonium perchlorate inhibit development and metamorphosis in Xenopus laevis. Environ Toxicol Chem. 2002;21:424–430. [PubMed] [Google Scholar]
- 5.Goleman WL, Carr JA, Anderson TA. Environmentally relevant concentrations of ammonium perchlorate inhibit thyroid function and alter sex ratios in developing Xenopus laevis. Environ Toxicol Chem. 2001;21:590–597. [PubMed] [Google Scholar]
- 6.Baldridge MG, Stahl RL, Gerstenberger SL, Tripoli V, Hutz RJ. In utero and lactational exposure of Long-Evans rats to ammonium perchlorate (AP) disrupts ovarian follicle maturation. Reprod Toxicol. 2004;19:155–161. doi: 10.1016/j.reprotox.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Smith PN, Theodorakis CW, Anderson TA, Kendall RJ. Preliminary assessment of perchlorate in ecological receptors at the Longhorn Army Ammunition Plant (LHAAP), Karnack, Texas. Ecotoxicology. 2001;10:305–313. doi: 10.1023/a:1016715502717. [DOI] [PubMed] [Google Scholar]
- 8.Urbansky ET, Brown SK, Magnuson ML, Kelty CL. Perchlorate levels in samples of sodium nitrate fertilizer derived from Chilean caliche. Environ Pollut. 2001;112:299–302. doi: 10.1016/s0269-7491(00)00132-9. [DOI] [PubMed] [Google Scholar]
- 9.Greenbank J, Nelson P. Life history of the three-spine stickleback Gasterosteus aculeatus Linnaeus in Karluk Lake and Bare Lake Kodiak Island, Alaska. U S Fish and Wildlife Service Bulletin. 1959;153:537–559. [Google Scholar]
- 10.Stenger AH. An apparent error in a report of structural hermaphroditism in an Alaskan threespine stickleback, Gasterosteus aculeatus. Copeia. 1963;1963:454–455. [Google Scholar]
- 11.Gercken J, Sordyl H. Intersex in feral marine and freshwater fish from northeastern Germany. Mar Environ Res. 2002;54:651–655. doi: 10.1016/s0141-1136(02)00156-3. [DOI] [PubMed] [Google Scholar]
- 12.Avise JC. Genetics of plate morphology in an unusual population of threespine sticklebacks (Gasterosteus aculeatus) Genet Res. 1976;27:33–46. [Google Scholar]
- 13.Withler RE, McPhail JD. Genetic variability in freshwater and anadromous sticklebacks (Gasterosteus aculeatus) of southern British Columbia. Can J Zool. 1985;63:528–533. [Google Scholar]
- 14.Griffiths R, Orr K, Adam A, Barber I. DNA sex identification in the three-spined stickleback. J Fish Biol. 2000;57:1331–1334. [Google Scholar]
- 15.Peichel CL, Ross JA, Matson CK, Dickson M, Grimwood J, Schmutz J, Myers RM, Mori S, Schluter D, Kingsley DM. The master sex-determination locus in threespine stickleback is on a nascent Y chromosome. Curr Biol. 2004;14:1416–1424. doi: 10.1016/j.cub.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Hahlbeck E, Griffiths R, Bengtsson BE. The juvenile three-spined stickleback (Gasterosteus aculeatus L.) as a model organism for endocrine disruption I. Sexual differentiation. Aquat Toxicol. 2004;70:287–310. doi: 10.1016/j.aquatox.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Katsiadaki I, Scott AP, Hurst MR, Matthiessen P, Mayer I. Detection of environmental androgens: A novel method based on enzyme-linked immunosorbent assay of spiggin, the stickleback (Gasterosteus aculeatus) glue protein. Environ Toxicol Chem. 2002;21:1946–1954. [PubMed] [Google Scholar]
- 18.Matty AJ. Fish Endocrinology. Timber; Portland, OR, USA: 1985. [Google Scholar]
- 19.Dodds ED, Kennish JM, von Hippel FA, Bernhardt R, Hines ME. Quantitative analysis of perchlorate in extracts of whole-fish homogenates by ion chromatography: Comparison of suppressed conductivity detection and electrospray ionization mass spectrometry. Anal Bioanal Chem. 2004;379:881–887. doi: 10.1007/s00216-004-2660-8. [DOI] [PubMed] [Google Scholar]
- 20.Matty AB. Fish Endocrinology. Timber; Portland, OR, USA: 1985. [Google Scholar]
- 21.Patiño R. Manipulations of the reproductive system of fishes by means of exogenous chemicals. Prog Fish-Cult. 1997;59:118–128. [Google Scholar]
- 22.Patiño R, Wainscott MR, Cruz-Li EI, Balakrishnan S, McMurry C, Blazer V, Anderson TA. Effects of ammonium perchlorate on the reproductive performance and thyroid follicle histology of zebrafish. Environ Toxicol Chem. 2003;22:1115–1121. [PubMed] [Google Scholar]
- 23.Park J-W, Rinchard J, Fujun Liu, Anderson TA, Kendall RJ, Theodorakis CW. The thyroid endocrine disruptor perchlorate affects reproduction, growth, and survival of mosquitofish. Ecotoxicol Environ Saf. 2006;63:343–352. doi: 10.1016/j.ecoenv.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Streisinger G, Walker C, Dower N, Knauber D, Singer F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio) Nature. 1981;291:293–296. doi: 10.1038/291293a0. [DOI] [PubMed] [Google Scholar]
- 25.Liu F, Kendall RJ, Theodorakis CW. Joint toxicity of sodium arsenate and sodium perchlorate to zebrafish Danio rerio larvae. Environ Toxicol Chem. 2005;24:1505–1507. doi: 10.1897/04-313r.1. [DOI] [PubMed] [Google Scholar]
- 26.Dean KE, Palachek RM, Noel JM, Warbritton R, Aufderheide J, Wireman J. Development of freshwater water-quality criteria for perchlorate. Environ Toxicol Chem. 2004;23:1441–1451. doi: 10.1897/02-648. [DOI] [PubMed] [Google Scholar]
- 27.EA Engineering, Science, and Technology. Report. Brooks Air Force Base; TX, USA: 1998. Results of acute and chronic toxicity testing with sodium perchlorate; p. 2900. [Google Scholar]
- 28.Klaasen CD. Casarett and Doull’s Toxicology The Basic Science of Poisons. 6. McGraw Hill; New York, NY, USA: 2001. [Google Scholar]
- 29.Cresko WA, Yan Y-L, Baltrus DA, Amores A, Singer A, Rodriguez-Mari A, Postlethwait JH. Genome duplication, sub-function partitioning, and lineage divergence: Sox9 in stickleback and zebrafish. Dev Dyn. 2003;228:480–489. doi: 10.1002/dvdy.10424. [DOI] [PubMed] [Google Scholar]
- 30.Marshall Graves JA, Shetty S. Sex from W to Z: Evolution of vertebrate sex chromosomes and sex-determining genes. J Exp Zool. 2001;290:449–462. doi: 10.1002/jez.1088. [DOI] [PubMed] [Google Scholar]
- 31.Nilsen BM, Berg K, Eidem J, Kristiansen S-I, Brion F, Porcher J-M, Goksoyr A. Development of quantitative vitellogenin ELISAs for fish test species used in endocrine disruptor screening. Anal Bioanal Chem. 378:621–633. doi: 10.1007/s00216-003-2241-2. [DOI] [PubMed] [Google Scholar]
- 32.De Ruiter AJH, Wendelaar Bonga SE. Consequences of nestbuilding behavior for osmoregulation in male three-spined sticklebacks. Behavior. 1985;93:8–20. [Google Scholar]