Summary
We describe behavioural changes in two generations of threespine stickleback (Gasterosteus aculeatus) exposed to environmentally relevant concentrations of perchlorate. The first generation (G0,2002) was exposed as two-year-old adults to perchlorate in experimental groups ranging in concentration from less than the method detection limit (<1.1 ppb) to 18.6 ppm for up to 22 days during their courtship, spawning, egg guarding, and first five days of fry guarding. No differences were noted in the behaviour or reproductive output of these fish that were exposed as adults. However, perchlorate exposure throughout development caused widespread effects in the second generation (G1,2003), which was spawned and raised through sexual maturity in one of four nominal experimental groups (0, 30 and 100 ppm, and a ‘variable’ treatment that progressively increased from <1.1 ppb to approximately 60 ppm perchlorate). Dose-dependent effects were found during the G1,2003’s swimming and behavioural evaluations, including higher mortality rates among treated fish following stressful events. Perchlorate-exposed fish had higher failure rates during swimming trials and failed at lower flow rates than control fish. A number of treated fish exhibited seizures. Progressively fewer males completed benchmark metrics, such as nest building, spawning, nursery formation, or fry production, in a dose-dependent manner. Fewer males from higher treatments courted females, and those that did initiated courtship later and had a reduced behavioural repertoire compared to fish from lower treatments. The lowest observed adverse effect level (LOAEL) for swimming performance, reproductive behaviour, survivorship and recruitment was 30 ppm perchlorate (our lowest G1,2003 treatment), and near complete inhibition of reproductive activity was noted among males raised in 100 ppm perchlorate. A small number of treated G1,2003 females were isolated in aquaria, and some performed reproductive behaviour typical of males, such as biting, leading and zig-zagging in the presence of gravid females. These findings have profound implications for recruitment in wild fish populations exposed to perchlorate, and suggest that perchlorate may disrupt behaviour in other vertebrates as well.
Keywords: perchlorate, endocrine disruption, masculinization, threespine stickleback, Gasterosteus aculeatus, swimming performance, reproductive behaviour, reproductive output, fitness
Introduction
Perchlorate is found in many common consumer products (such as road flares, fireworks and automobile air bags, see http://www.epa.gov/fedfac/documents/perchlorate.htm), and is used as an oxidizer in solid rocket propellant and artillery (Mendiratta et al., 1996). Perchlorate contamination is widespread across the United States. For example, as of March 2005, perchlorate contamination had been detected in 36 US states (http://www.epa.gov/fedfac/documents/detection_with_dates_03_25_05.xls). In 2005 perchlorate was reported in the Canadian Great Lakes, marking the first time that perchlorate contamination was identified outside the United States (Backus et al., 2005).
Recent testing has revealed perchlorate contamination ranging from 3.2 to 11.3 ppb (μg/l) in organic and conventional milk from 101 of 104 containers tested in 15 U.S. states (http://www.cfsan.fda.gov/~dms/clo4data.html), and in organic lettuce with levels ranging from 3200 to 6900 ng/g (http://www.ewg.org/reports_content/rocketlettuce/pdf/wecklabs.pdf). Perchlorate has also been found in tap (>4 ppb) and irrigation (>4 ppb) water derived from the Colorado River (Brechner et al., 2000), and even in human breast milk ranging from 0.6 to 92.2 ppb (mean = 10.5 ppb; Kirk et al., 2005).
Perchlorate is highly soluble in water, can persist unaltered for several decades (Urbansky, 1998), causes deleterious effects at low concentrations (Goleman et al., 2001, 2002; Baldridge et al., 2004) and lacks an enforceable water quality standard in the USA while the US Environmental Protection Agency (US EPA) attempts to establish appropriate exposure levels. The US EPA’s most recent (2005) reference dose is 24.5 ppb (http://www.epa.gov/iris/subst/1007.htm). Major sources of environmental contamination in the USA have come from military storage and disposal practices (Smith et al., 2001), from industrial contamination of the Colorado River (http://www.epa.gov/fedfac/pdf/perch_7th_mnth_rpt.pdf), and to a lesser extent from Chilean fertilizers (Urbansky, 2001).
The effects of perchlorate on thyroid histology and thyroid hormone synthesis are well established (Miranda et al., 1996; York et al., 2001a,b, 2003; Patiño et al., 2003; McNabb et al., 2004; Bradford et al., 2005; Crane et al., 2005; Park et al., 2006). Perchlorate is an endocrine disrupting toxicant known to interfere with thyroid hormone production by competitively inhibiting the ability of sodium-iodide symporters to transport iodide into thyroid follicles, which in turn reduces thyroid hormone synthesis (NRC, 2005). Many studies describe histological effects of perchlorate exposure, such as hypertrophied thyroid follicular cells (York et al., 2001a,b; Patiño et al., 2003; McNabb et al., 2004; Bradford et al., 2005; Park et al., 2006), but few have addressed perchlorate’s effects on life history characteristics, such as reproductive behaviour (York et al., 2001b, 2003; Crane et al., 2005). What few studies exist primarily document the number of mounting attempts during mice reproduction (York et al., 2001a) or fertility/fecundity parameters (York et al., 2001b; Wibe et al., 2002; Patiño et al., 2003).
Using a model fish species, the threespine stickleback (Gasterosteus aculeatus), we described that some treated females had become masculanized to the point of functional hermaphroditism (Bernhardt et al., 2006). Additionally, we found that genetic males developed enlarged testes in a dose-dependent manner. These findings suggest that perchlorate produces a variety of effects that are difficult to explain solely on the basis of impaired thyroid hormone homeostasis. Furthermore, reproductive and behavioural functions are likely to be impaired if thyroid hormone synthesis is altered or if fish are masculinized during development.
In this paper we describe how perchlorate exposure affects a variety of behavioural characteristics in stickleback. We report on swimming performance, nest-building, courtship, and parental care activities, as well as resilience to stress in two generations of fish exposed to perchlorate. We show that perchlorate affects all of these endpoints, each of which contributes significantly to the two major components of lifetime fitness, namely, survivorship until breeding age and reproductive output. Our data demonstrate that perchlorate not only has immediate effects, but that exposure during key developmental windows has delayed effects that are only expressed at reproductive maturity. Such delayed effects would not be noticed during acute toxicity experiments. Our findings make clear that this pervasive pollutant has different suites of immediate and long term effects on stickleback specifically, and likely on many other vertebrate species as well.
Material and methods
Reproductive behaviour was measured in two generations of stickleback, wild caught adults (G0,2002) and lab-reared adults (G1,2003); the swimming ability of G1,2003 fish was also tested. Details of experimental conditions and treatment groups are provided in Table 1. Exposure levels were chosen because they approximate (and, in some cases, are much less than) perchlorate concentrations at a number of contaminated sites (Smith et al., 2001; http://www.epa.gov/fedfac/documents/detection_with_dates_03_25_05.xls).
Table 1.
Experimental groups and treatment conditions for three generations of stickleback exposed to perchlorate.
| Generation | Experimental group1 | Length of exposure | Notes and developmental stage at exposure | Perchlorate source | Measured |
|---|---|---|---|---|---|
| G0,2002 | Control2 | 14–22 days | Wild-caught adults trapped May–June 20023,4; tested during nest-building, courtship and parental care | n/a | Behaviour |
| 1.5 ppm | Sodium perchlorate5 | ||||
| 12 ppm | Sodium perchlorate | ||||
| Variable (0– 18.6 ppm)6 | Solid rocket propellant | ||||
| G0,2003 | Control | 30 days | Wild-caught adults trapped May–June 20037; Parents of G18 | n/a | Behaviour not analyzed |
| 30 ppm | Sodium perchlorate | ||||
| Variable (0–33 ppm) | Sodium perchlorate | ||||
| 100 ppm | Sodium perchlorate | ||||
| G19 | Control | 1 year | Syngamy through reproductive maturity at 1 year10 | n/a | Behaviour and swimming performance |
| 30 ppm | Sodium perchlorate | ||||
| Variable 0–60 ppm11 | Sodium perchlorate | ||||
| 100 ppm | Sodium perchlorate |
For all experimental groups, adults and juveniles >2 months old were fed frozen brine shrimp (Artemia sp.) daily, while fry <2 months old were fed daily a mixture of Golden Pearls 100, Artemia food (both from Aquatic Ecosystems, Apopka, FL, USA), and ground brine shrimp.
No perchlorate added (less than the method dection limit of 1.1 ppb).
Wild-caught fish were obtained in Rabbit Slough, Alaska (61°32′12″N, 149°15′17″W) from an anadromous run.
After capture, these fish were acclimated to captivity in 400 l indoor pools (4 ppt (g/l) salinity) for approx. 2 weeks. These adults were then isolated in 38 l aquaria (also 4 ppt salinity). Males that failed to build a nest were replaced with surplus males until every aquarium (N = 80) contained a single male with a nest.
Anhydrous sodium perchlorate with a purity of 99% or greater (EM Science, Cherry Hill, NJ, USA).
Perchlorate leached from dissolving 3.70 g cores of hydroxyl-terminated polybutadiene (HTPB) solid rocket propellant, a simulation of what might occur in fresh water polluted by unburned rocket propellant.
After capture, the G0,2003 fish were housed outdoors in 1600 l pools through mid-July under ambient temperature (water temperature = 14–20°C) and photoperiod (18:6 h (light:dark) increasing to 20:4 h before decreasing to 18.5:5.5). All pools were continuously filtered and aerated through biofilters. Pools in each of the four experimental groups contained water with approximately 4 ppt salinity, filamentous algae and sand to be used as nesting material.
Nuptially colored G0,2003 males were randomly placed into separate quadrants of the 1600 l pools (four males per pool separated by netting). Gravid G0,2003 females were randomly introduced into a quadrant and allowed to spawn with males. Females were immediately removed after successfully spawning, or after 20 min if they failed to spawn. Females were introduced in succession until spawning occurred or until 7 days had passed if spawning was unsuccessful. Once a male had spawned, no additional females were provided. After caring for the embryos until hatching, and then providing five additional days of parental care (generally 12 dpf), adult males were removed from the pools, killed, and stored at −80°C.
The G1 progeny were raised outdoors in 1600 l pools under ambient temperature (20–8 °C) and photoperiod (20:4 h (light:dark) declining to 11:13 h) through October 5, 2003. At this point (15 weeks of age) 50 fish from each of the four treatments (controls, 30, 60 and 100 ppm) were transferred indoors into 400 l pools at the same concentrations in which they had been raised outdoors. This is when the only exchange of treated water occurred. With that exception, water was only added to dilute the treatments as perchlorate became more concentrated due to evaporation and to replace water removed during cleaning. Treated water lost during cleaning was replaced with water containing the appropriate mass of perchlorate crystals to restore the proper water level and perchlorate concentration in each tank. These G1 fish were maintained in 400 l tanks under simulated natural photoperiod at 17–19°C until they reached sexual maturity the following spring.
Although sexual maturity occurred at one year of age in our laboratory conditions, most stickleback from the source population reproduce at two years of age (Furin & von Hippel, unpublished data).
The variable treatment was designed to mimic the 2002 treatment when perchlorate leached from HTPB solid rocket propellant. To that end, sodium perchlorate was added to the variable treatment at a rate that matched the changing concentration in 2002 until its concentration reached approximately 60 ppm, after which the concentration was held steady.
Perchlorate measurements
Baseline perchlorate levels in the tap water used to fill all pools and aquaria were tested via ion chromatography in tandem with electrospray ionization mass spectrometry (IC-ESI-MS; Dodds et al., 2004). Temperature and perchlorate concentrations were monitored daily using an Oakton Acorn 6 perchlorate potentiometer with automatic temperature compensation (Oakton, Vernon Hills, IL, USA). The potentiometer was equipped with a perchlorateion-selective electrode (Cole Parmer, Vernon Hills, IL, USA). To avoid contaminating the negative control water, the electrode was thoroughly cleaned prior to use and only used on the negative controls on six days to take 18 readings in 2003. Dissolved oxygen, pH, and salinity were checked every 2–3 months.
Swimming trials
Swimming performance was assessed between 18 and 25 May, 2004 for 102 of the G1,2003 fish that survived to sexual maturity (N = 43, 24, 22 and 13 for controls, 30, 60 and 100 ppm fish, respectively). Two additional fish appeared moribund and were not tested during the swimming trials. Water without detectable levels of perchlorate (<1.1 ppb; 17.5–18.0°C) was used during all swimming trials. Mean body length (mbl; 47.5 ± 3.2 mm (1 sd), N = 104) did not differ significantly between treatments (ANOVA, F3,104 = 1.4, p = 0.242) when the G1,2003 were tested in the flume, so mean body length per second was determined to be an appropriate measure of flow rate.
Fish remained in the flume for up to 58 min at a time. The first 10 min allowed a fish to acclimate to slowly flowing water (flow rate = 1.6 mbl/s = 7.6 cm/s). Fish that rested or became trapped against the downstream barrier during the initial 10 min acclimation period were not considered to have failed and were immediately removed from the barrier and coaxed approximately 0.5 m upstream.
Following the acclimation period, swimming ability was quantified for up to 38 consecutive minutes (28 min for a ‘stepwise’ test, followed by 10 min for an ‘endurance’ test) by measuring the swimming performance of a single fish at a time. During the stepwise test, the flume’s flow rate was increased by increments of approximately 0.37 mbl/s (1.7 cm/s) at 2-min intervals. This stepwise process continued for a maximum of 28 min as long as the fish avoided becoming trapped against the mesh barrier at the downstream end of the swim chamber or until the flow rate reached 6.4 mbl/s (28.1 cm/s). Fish that maintained position for two minutes at each flow rate through 6.4 mbl/s were considered to have passed the stepwise swimming portion and began the endurance test. During endurance testing, the flow rate was increased to the maximum (6.5 mbl/s; 28.5 cm/s), for up to 10 additional min. If a fish successfully resisted the maximum flow rate for 10 min, it was considered to have passed the endurance test.
The final 10 min of every swim trial consisted of a ‘cool-down’ period at the original acclimation speed (1.6 mbl/s) in order to facilitate O2 exchange across the gills, reduce lactic acid buildup (Wood et al., 1983), and avoid the Root effect (massive offload of oxygen from hemoglobin at low pH and reduced overall O2 carrying capacity) in exhausted fish. Neither resting nor trapping bouts were recorded during the cool-down period; likewise success and failure were not considered.
If a fish became trapped against the downstream mesh at any point during the stepwise or endurance testing and was unable to free itself within 3 s, or if a fish rested its tail against the mesh five times at a single flow rate, it was considered to have failed the trial. At that point the flow rate was quickly reduced to the original acclimation speed (1.6 mbl/s), and the fish was freed from the mesh with a pair of forceps. Then, the velocity of failure was recorded, and the fish entered the cool-down period previously described. Fish that failed the stepwise swimming test did not participate in the endurance test.
On 22 June 2006, flow rates from three local, Alaskan streams (Anchor River (59°46′30″N, 151°51′50″W), Deep Creek (60°01′41″N, 151°41′00″W) and Rabbit Slough (61°32′12″N, 149°15′17″W)) with anadromous three-spine stickleback runs were measured. Measurements occurred approximately 1 km upstream from their confluence with Cook Inlet, using a Sigma Sport flow meter model FP101 (Global Water, Gold River, CA, USA) in order to provide a rough estimate of flow rates that returning stickleback might encounter en route to their spawning grounds.
For convenience, we divided reporting of mortality into two time frames: before reaching sexual maturity, and following sexual maturity. Flume testing represented a convenient reference point separating these periods.
Spawning protocol
The same basic protocol for introducing females to isolated males and video-taping reproductive behaviour was used in 2002 (G0,2002 wild-caught adults) and 2004 (G1,2003 lab-raised adults). For brevity, we describe how this was done for the G1,2003 fish and discuss differences in techniques for the G0,2002 following this description.
At approximately one year of age, following the conclusion of swimming trials, G1,2003 stickleback showing male nuptial coloration (N = 10, 6, 2 and 0 for the controls, 30, 60 and 100 ppm experimental groups, respectively) were assumed to be males and isolated in 38 l aquaria containing tap water without detectable levels of perchlorate. Lower survivorship among fish exposed to higher concentrations of perchlorate (Bernhardt et al., 2006) led to fewer nuptially colored fish from which to choose. Water was maintained at 4 parts per thousand (‰) salinity and continually filtered and aerated with Azoo biofilters (Aquatic Ecosystems, Apopka, FL, USA). Water was not exchanged.
Gravid females were segregated according to experimental group and collectively remained in their 400 l holding tank (controls, 30, 60, or 100 ppm). The remaining fish of unknown sex were isolated, as above, in 38 l aquaria without detectable levels of perchlorate (N = 0, 4, 8 and 10, respectively, for controls, 30, 60 and 100 ppm treatments). In this way, a total of 10 aquaria per experimental group each housed a single fish (either a nuptially colored male or a fish of unknown sex; N = 40 individually housed fish and aquaria). Extra fish were separated and later killed.
Males that died prior to the onset of mating trials were replaced as long as additional fish suspected of being males were available from the same treatment. After subtracting individually housed females and adding replacement males, a total of 10, 8, 8 and 11 males for control, 30, 60 and 100 ppm treatments, respectively, were isolated in 38 l aquaria and given the opportunity to reproduce, yet not all of these males survived to perform reproductive behaviour. Videotapes of behaviour were made for all surviving males, including: all ten control males, six of eight 30 ppm males, five of eight 60 ppm males and six of 11 100 ppm males. The remaining males died prior to making a nest.
All fish were maintained on a natural, or simulated natural, photoperiod. Nesting materials consisting of dried, filamentous algae, and a 90 mm Petri dish filled with sand were added to each of the aquaria. Females were introduced to the aquaria of individually housed presumptive-males without nests at two day intervals to stimulate nest building.
From May 29 to June 18, 2004, single gravid G1,2003 females from like experimental groups were placed into a male’s aquarium and allowed 10 min to spawn. The female was removed either after successfully spawning or at the end of the 10-min period if spawning was unsuccessful. If, however, females remained within a nest at the end of the 10-min period, they were not removed until they emerged. Gravid females continued to be introduced into each aquarium daily until either a successful spawning event occurred or three weeks of courtship activity had passed. No additional females were provided to a male after he successfully spawned. Courtship, spawning, and parental care behaviours were videotaped for analysis.
Because sample sizes were small, G1,2003 females were used for more than one spawning attempt. In vitro fertilizations were performed as described in Bernhardt et al. (2006) to compare hatching success between treatments. Following behavioural testing, the G1,2003 were killed with an overdose of tricane methane sulfonate (MS-222) anesthesia.
The G0,2002 spawning protocol differed in four respects. A plentiful supply of colorful, two-year old G0,2002 males ensured that only males were placed in each of the 80 aquaria. Nominal experimental groups differed between the G0,2002 and G1,2003 trials (Table 1). G0,2002 females were not used for more than one spawning trial, and the G0,2002 were killed in liquid nitrogen.
To test whether differences in fanning rates were related to morphological differences, we measured pectoral fin length (base to the distal end of the longest fin ray) and standard length on the G1,2003 after being killed (17–22 June 2004).
Behavioural and statistical analysis
Each fish was videotaped with a Sony (Tokyo, Japan) DCRTRV-17 or DCRTRV-25 digital video recorder for a minimum of 10 min per day. If fertilization occurred, the male was videotaped for an additional 10 min to permit analysis of his initial parental care activities. Aquaria were positioned end to end in rows, and each aquarium was surrounded with cardboard on three sides to prevent neighboring fish from interacting. To ensure filming was conducted without bias, lists of random numbers were generated daily to dictate the sequence of filming.
Behavioural events were scored by a single person (RRB) from video tapes using ‘The Observer’ behavioural analysis software (Noldus Information Technology, Wageningen, The Netherlands). Three courtship videos were randomly chosen to be re-analyzed to test for concordance between scoring attempts. Event recording was determined to be highly reliable (<1% variation in total duration of each behaviour for all three concordance analyses).
The male behaviours of interest, as described by van Iersel (1953) and Wootton (1976), included ‘carrying’ sand or vegetation to, from, or independent of the nest; ‘boring’ into the nest; ‘gluing’ at the nest; ‘fanning’ the nest; ‘creeping through’ the nest; ‘biting’ the female; ‘zig-zagging’ toward the female; ‘leading’ or attempting to lead the female; ‘dorsal pricking’, which consists of the male using his dorsal spines to prick the female’s abdomen during courtship; and ‘quivering’ along the female’s caudal peduncle to stimulate egg deposition within the nest. Finally, an ‘intermediate’ category was used to include all behaviours neither previously mentioned, nor essential to nest building, courtship, or parental care. These behavioural categories were both mutually exclusive and comprehensive.
After analyzing all of the courtship videos, the total number of behaviours performed per individual was recorded. Next, the mean number of courtship behaviours was calculated per treatment and compared across treatments. Thus, if a fish performed only two behaviours during one courtship attempt and two behaviours during a second attempt, including one new behaviour, then the individual fish was considered to have performed three distinct behaviours during its courtship testing. This number (3) was then added to the total number of courtship behaviours performed by the other males in its experimental group and divided by the number of active males to determine the mean number of courtship behaviours for a given experimental group.
To determine if perchlorate affected readiness or ability to court, the time until onset of each of the courtship behaviours was quantified. Since some males spawned on their first attempt, and others took multiple attempts, the mean time until onset of each behaviour was determined for each individual. Group means were then calculated and used for comparisons.
The Statistical Package for Social Sciences (Version 11.5; SPSS, Chicago, IL, USA) was used for all statistical procedures. Non-parametric statistics were used when data were unevenly distributed, when variances were not homogeneous, and/or when sample sizes were small (N ≤ 16 per group). Statistics were two-tailed, and values are reported as mean ± 1 standard error (SE) unless otherwise noted. Because only two 60 ppm males spawned, multiple Mann–Whitney U tests were used to compare spawning success and timing, but Kruskal–Wallis tests were used when there were between three and 16 observations (Shaw & Wheeler, 1985). One-way ANOVAs were used for normally distributed data with sample sizes above 16 observations per group and compared with non-parametric statistics for these same parameters; in all cases the results were consistent.
Results
Perchlorate measurements
Background levels of perchlorate in the source water were found to be below the method detection limit of ion chromatography in tandem with electrospray ionization mass spectrometry (<1.1 ppb). The following measures in the ppm range were performed with Acorn 6 potentiometers. In 2002, aquaria with nominal perchlorate concentrations of 1.5 and 12 ppm had measured mean perchlorate concentrations of 1.5 (N = 55, SE = 0.02) and 12.0 ppm (N = 55, SE = 0.13), respectively. In 2003, aquaria with nominal perchlorate concentrations of 30 and 100 ppm had measured mean perchlorate concentrations of 32.0 (N = 334, SE = 0.20) and 102.9 ppm (N = 334, SE = 0.57), respectively. The nominal 60 ppm treatment mimicked the increasing perchlorate concentration noted in 2002 as perchlorate leached from solid rocket propellant cores (y2002 = 0.32x – 12.04, r2 = 0.92 vs. y2003 = 0.31x – 9.44, r2 = 0.88). It reached a maximum concentration near 60 ppm about halfway through the experiment, after which the readings remained relatively stable (mean = 61.8 ppm, N = 185, SE = 0.19).
The mean perchlorate concentration of the negative control water based on the 18 Acorn 6 readings was 0.15 ppm (SE 0.01). More reliable analysis of the water in the negative control experimental group also was conducted by ion chromatography in tandem with electrospray ionization mass spectrometry (N = 21 samples). This technique revealed the water to have less than the method detection limit of 1.1 ppb perchlorate (Dodds et al., 2004).
G0,2002 reproductive benchmarks
Neither the success rate (Kruskal–Wallis, χ2 = 0.61, df = 3, p = 0.893) nor the timing (ANOVA, F3,76 = 1.94, p = 0.131) of wild-caught adult males (G0,2002) making nests varied across experimental groups in 2002. Likewise, all 80 of the G0,2002 males were recorded fertilizing a clutch of eggs within 7 days of one another. Thus, there were no differences between experimental groups in spawning success (Kruskal–Wallis, χ2 = 3.05, df = 3, p = 0.384) or in the timing of spawning events (ANOVA, F3,76 = 0.93, p = 0.430). Of the fish that spawned (N = 20 per treatment), fewer made nurseries (hole torn into nest that exposes newly hatched fry and enhances the exchange of gasses; N = 17, 19, 20, and 18 for controls, 1.5 ppm, 12 ppm, and the variable treatment, respectively). Experimental group did not affect nursery making success (Kruskal–Wallis, χ2 = 3.44, df = 3, p = 0.329) or timing of nursery production (Kruskal–Wallis, χ2 = 6.99, df = 3 p = 0.072). Most males that made nurseries also produced fry (N = 16, 19, 20, and 17 for controls, 1.5, 12, and the variable treatment, respectively), and whether or not fry were produced was not influenced by experimental group (Kruskal–Wallis, χ2 = 5.31, df = 3, p = 0.150); nor was the timing of fry production affected (ANOVA, F3,68 = 1.31, p = 0.280).
G1,2003 swimming performance
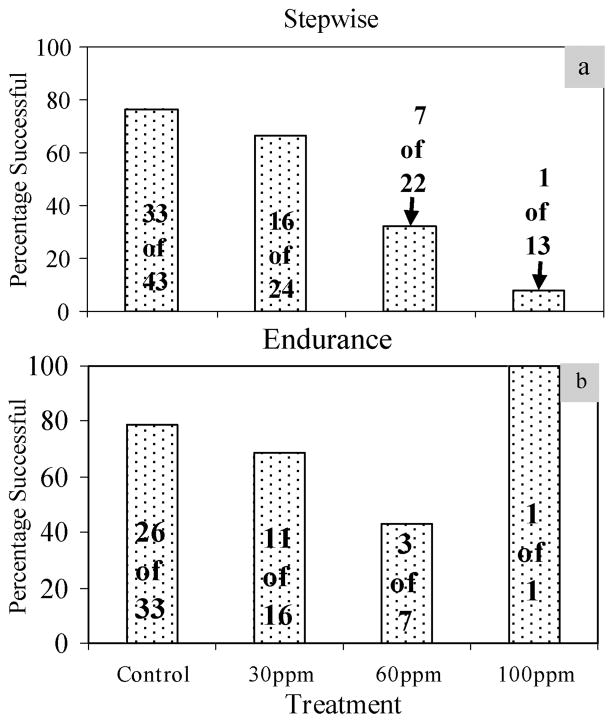
A clear dose-dependent relationship was evident in the swimming trials as fish from higher perchlorate treatments experienced progressively lower success rates than those from lower treatments and control fish (Figure 1): 76% of control fish successfully completed the stepwise swimming trials, compared to 67%, 32% and 8% of the 30, 60 and 100 ppm fish, respectively. The velocity at which fish failed the stepwise trials reflected a similar dose-dependent relationship. Control, 30, 60 and 100 ppm fish that failed did so at significantly different (Kruskal–Wallis, χ2 = 16.59, df = 3, p < 0.001) mean velocities (± 1 SE) of: 5.8 (± 1.5), 5.3 (± 1.6), 4.8 (± 0.23) and 3.7 (± 1.4) mbl/s, respectively. The same dose-dependent relationship was generally noted among the fish that passed the stepwise trials and participated in the endurance trials with 79% of control fish successfully completing the endurance trials compared to 69%, 43%, and 100% (N = 1) of the 30, 60 and 100 ppm fish, respectively. Differences in success rates between treatments were significant in the stepwise test (χ2 = 26.14, df = 3, p < 0.001). Overall, differences for the endurance tests were not significant (Kruskal–Wallis, χ2 = 3.66, df = 2, N = 56, p = 0.160), but post-hoc comparisons approached significance between the control and 60 ppm groups (Mann–Whitney U(control – 60 ppm), N = 40, Z = −1.91, p = 0.056).
Figure 1.
Swim trial results. (a) Stepwise increment testing. Swimming performance degraded in a dose-dependent manner during the stepwise swim trials. (b) Endurance testing. Swimming performance also degraded in a dose-dependent manner during endurance testing with the exception of the 100 ppm treatment, which shows the results of the sole 100 ppm fish that passed both the stepwise and endurance trials.
In field studies, mean flow rates of 15, 46 and 110 cm/s were recorded in Rabbit Slough, Anchor River and Deep Creek, respectively. These flow rates equate to 3.2, 9.7 and 21.3 mbl/s, respectively, illustrating that the flow rates used in the swimming trials were below those recorded at two of the three stickleback-bearing streams. However, flow rates taken from the field should be viewed with caution as both seasonal and geospatial variations occur. These flow rates provide an index of resistance that fish may encounter during their upstream migration, though stickleback are likely to find more favorable flow rates as they negotiate the stream.
Flume success rates alone are an incomplete measure of swimming performance since mortality rates following the swimming trials were quite different between experimental groups (Kruskal–Wallis, χ2 = 11.33, df = 3, N = 39 males, p = 0.010). Post-flume mortalities, 0–22 days later, included zero control males, four 30 ppm males, six 60 ppm males and seven 100 ppm males. Tetany occurred in at least one of these fish within 24 h of flume testing, with ruptured blood vessels apparent at the base of its pectoral fins.
Although standard lengths were similar across treatments (see Methods), lengths of pectoral fins were significantly different (Kruskal–Wallis, χ2 = 18.80, df = 3, N = 26, p < 0.001). Bonferroni tests showed that the pectoral fins of the controls and 30 ppm fish were similar in size (means 16.44 mm (± 0.47), N = 7 and 15.34 mm (± 0.66), N = 6, respectively) but significantly larger than the pectoral fins of the 60 and 100 ppm fish (means 11.72 mm (± 0.63), N = 6 and 12.61 mm (± 0.45), N = 7, respectively), which were also similar in size to one another.
G1,2003 courtship
Unlike control males, several treated males displayed brief erratic, twitchy and seemingly uncontrolled swimming patterns that might be characterized as seizures. Many ‘seizures’ preceded or accompanied fleeing behaviour from the female during courtship and often ended when the male swam into the glass. Fish with aberrant swimming patterns include: zero of ten control fish, four of eight 30 ppm fish, three of seven 60 ppm fish and two of eight 100 ppm fish. Only one of the fish that had these unusual swimming patterns died prior to the end of the experiment.
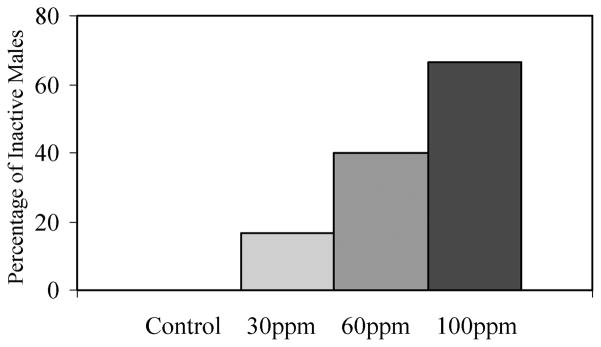
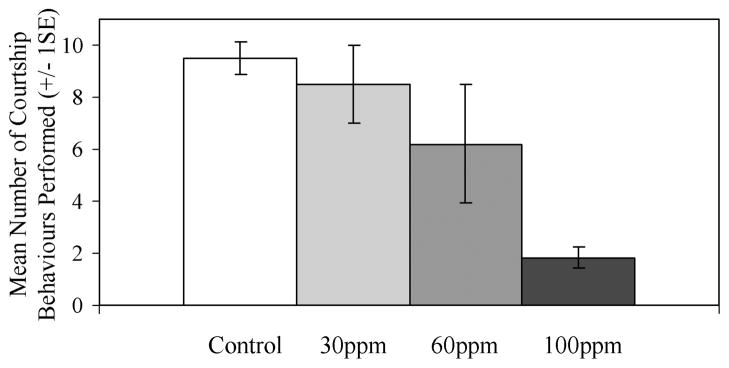
Failure to perform any courtship behaviours during at least one spawning attempt increased in frequency in a dose-dependent manner (Kruskal–Wallis, χ2 = 9.12, df = 3, N = 27, p = 0.028; Figure 2). Additionally, treated males that participated in courtship displayed a smaller behavioural repertoire (performed fewer behaviours) in a dose-dependent manner (Figure 3).
Figure 2.
Inactive males during courtship. Males from higher treatments failed to court introduced females during at least one of their courtship trials more frequently than males from lower treatments. These data exclude males that died prior to courtship trials.
Figure 3.
The behavioural repertoire of courting males (± 1 SE) shows statistically significant differences between treatments (Kruskal–Wallis, df = 3, N = 27 fish, χ2 = 10.77, p = 0.013). Behaviours must have occurred at least once during an individual’s spawning trials.
The length of time between a female’s introduction and the onset of 11 male-typical behaviours was analyzed for those fish that performed courtship displays (Table 2). Of these, only biting differed significantly between treatments, with control males initiating courtship by biting females sooner than treated males in a dose-dependent manner (N = 26 first biting means, χ2 = 8.24, df = 3, p = 0.041). Although differences in the onset of other behaviours were not found between treatments, it should be noted that fewer 100 ppm males courted (Figure 2), and those that did performed few courtship activities (Figure 3, Table 2). Hence, the 100 ppm fish were different by virtue of their omission of behaviours.
Table 2.
Mean (± 1 SE) duration until onset of each male’s behaviour after a female’s introduction (in s).
| Control | 30 ppm | 60 ppm | 100 ppm | Statistics (K–W) | |
|---|---|---|---|---|---|
| Biting | 46.0 (21.1) | 54.7 (19.5) | 99.2 (57.7) | 165.3 (35.1) | χ2 = 8.24, df =3, p = 0.041 |
| Leading | 134.9 (31.2) | 133.6 (40.8) | 133.8 (60.6) | 276.1 (126.6) | χ2 = 1.26, df = 3, p = 0.747 |
| Zig-zagging | 218.5 (44.6) | 167.5 (44.4) | 195.5 (42.5) | 318.6 (288.9) | χ2 = 0.80, df = 3, p = 0.849 |
| Dorsal pricking | 471.5 (174.8) | 165.6 (93.1) | 291.5 (59.6) | χ2 = 1.39, df = 2, p = 0.498 | |
| Boring | 330.9 (43.5) | 201.8 (55.0) | 254.8 (36.5) | χ2 = 3.10, df = 2, p = 0.213 | |
| Fanning | 275.7 (46.6) | 248.8 (73.1) | 218.7 (7.2) | χ2 = 0.03, df = 2, p = 0.984 | |
| Gluing | 555.9 (125.0) | 298.9 (83.9) | 269.8 (242.2) | χ2 = 1.74, df = 2, p = 0.419 | |
| Creeping through | 294.6 (71.6) | 292.2 (116.3) | 368.7 (87.1) | χ2 = 1.06, df = 2, p = 0.590 | |
| Quivering | 461.9 (97.8) | 394.8 (90.7) | 285.5 (86.4) | χ2 = 0.63, df = 2, p = 0.730 | |
| Fertilizing | 890.8 (172.7) | 791.9 (298.8) | 518.5 (360.6) | χ2 = 0.89, df = 2, p = 0.640 |
Kruskal–Wallis (K–W) tests were used since sample sizes were small (N ≤ 10 fish per treatment), and only biting differed significantly between groups. Individual fish means were used to calculate experimental group means.
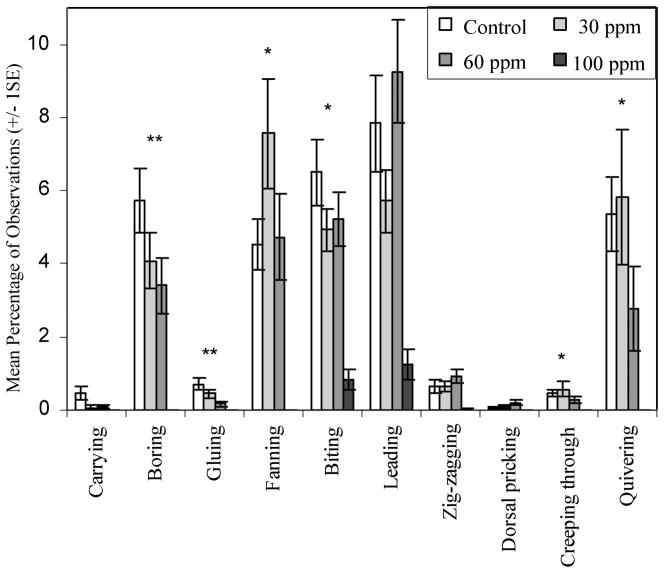
Control fish spent the greatest percentage of time carrying, boring, gluing, and biting during courtship compared to all treated fish (Figure 4), and the percentage of time spent conducting these behaviours decreased in a dose-dependent manner, although differences were not statistically significant after the 100 ppm males were excluded (Kruskal–Wallis, df = 2, N = 21, p > 0.05 for all). The 100 ppm fish always spent the least percentage of time engaged in courtship behaviours and none managed to spawn successfully. Control males spent the lowest percentage of time conducting non-courtship-related intermediate behaviours (72%), followed by the 30 and 60 ppm males (77% each). The 100 ppm fish spent the greatest percentage of time conducting intermediate behaviours (98%). Variations in the proportion of time spent in the intermediate category were statistically significant (Kruskal–Wallis test, χ2 = 9.324, df = 3, N = 27, p = 0.025) when all four experimental groups were compared, but not significant when the 100 ppm males were excluded (Kruskal–Wallis test, χ2 = 0.16, df = 2, N = 21, p = 0.699).
Figure 4.
Mean percentage of time spent engaged in each behaviour during courtship. Time spent in the ‘intermediate’ category is not depicted in this figure in order to highlight the other categories. Statistically significant differences (Kruskal–Wallis test, df = 3, N = 27) between treatments are depicted with asterisks (*p < 0.05, **p < 0.01).
G1,2003 parental care
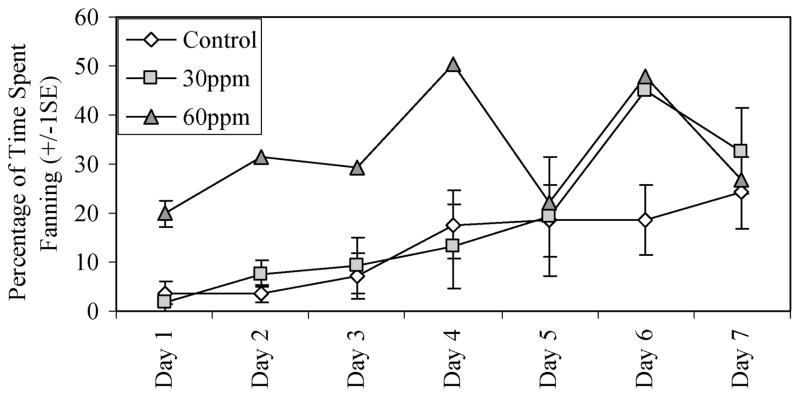
Because none of the 100 ppm males spawned, they conducted no parental care and have been excluded from these analyses. Fry normally hatched after 6 days post fertilization (dpf; range 5–7) in 2002 at a mean water temperature of 18°C and after 7 dpf (range 6–8) in 2004 at a mean water temperature of 14°C. On parental care days one through seven, the two 60 ppm males provided the most parental care (total percent of time spent fanning, boring, carrying, and gluing) and spent the least amount of time conducting non-parental care-related ‘intermediate’ activities compared to control (Mann–Whitney U(60 ppm – control), N = 66, Z = −2.89, p = 0.004) and 30 ppm males (Mann–Whitney U(60 ppm – 30 ppm), N = 32, Z = −2.58, p = 0.008). On parental care days 1–6, the 60 ppm fish always spent the greatest percentage of time fanning (Figure 5).
Figure 5.
Seven day parental care analysis shows the mean duration spent fanning from parental care day 1 (the day fertilization occurred) through day 8 (generally 1 day post-hatch). Although two 60 ppm males spawned, only one was recorded per day after the first day.
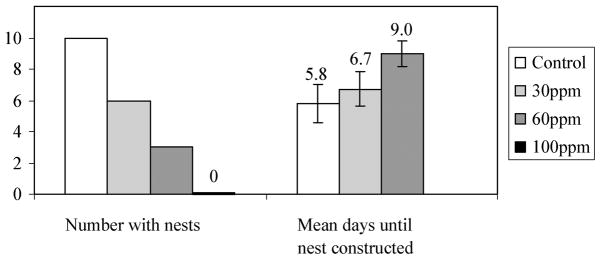
G1,2003 fish exposed to perchlorate showed delays and sequentially worse success rates for nest building (Figure 6). Perchlorate-exposed fish also showed progressively lower success for spawning, nursery production, and fry production compared with controls (Table 3). Although not statistically significant (Kruskal–Wallis, df = 2, N = 19, χ2 = 2.065, p = 0.356), there was a trend for perchlorate-treated fish to build nests later than control fish (Figure 6). The timing of fertilization events was not significantly different between treatments (Mann–Whitney U(control – 30 ppm), N = 14, Z = −0.81, p = 0.419; Mann–Whitney U(control – 60 ppm), N = 11, Z = −1.32, p = 0.188; and Mann–Whitney U(30 – 60 ppm), N = 7, Z = 1.37, p = 0.171). A total of zero, two, three and five males from the control, 30, 60 and 100 ppm experimental groups died following the swim tests, bringing the number of control, 30, 60 and 100 ppm males that survived through the end of the spawning trials to ten, six, five and six, respectively. All but one of these mortalities occurred prior to a fertilization event. Although the remaining male successfully attracted a mate and fertilized her eggs, he died three dpf. His lack of parental care resulted in the majority of his eggs becoming infected with fungus and dying prior to hatching. Two of the eggs hatched 7 dpf, but both fry died one day later.
Figure 6.
Nest-building analysis. Fewer treated males produced nests in a dose-dependent manner, and those that did took longer to create their nests. Shown are means (± 1 SE).
Table 3.
2004 benchmark completion.
| Control (% of 10) | 30 ppm (% of 8) | 60 ppm (% of 8) | 100 ppm (% of 11) | |
|---|---|---|---|---|
| Nest building | 100 | 75 | 38 | 0 |
| Successful spawning | 80 | 50 | 25 | 0 |
| Nursery production | 70 | 25 | 13 | 0 |
| Fry production | 60 | 25 | 13 | 0 |
Values reflect the percentage of individually housed males that succeeded at completing each benchmark metric. When the G1 matured in 2004, marked differences were apparent in their abilities to complete benchmark reproductive metrics. Control males had the greatest success for each metric, and there was a clear dose–response relationship with fish in higher treatments always performing worse than controls and those in lower treatments.
Fecundity rates in terms of the number of eggs produced per female did not differ between females exposed to 30, 60 or 100 mg/l perchlorate. However, control females produced more eggs than treated females (Kruskal–Wallis test, χ2 = 11.645, df = 3, p = 0.009), even though their mean standard length and mass did not differ at sexual maturity. Half of the treated males that managed to spawn (30 and 60 ppm only) eventually produced fry, compared to 75% of control fish (Table 3). Differences in hatching success, based on in vitro fertilizations, were statistically significant (Kruskal–Wallis test, χ2 = 13.462, df = 3, p = 0.004), but perhaps not biologically significant since control, wild, and 100 ppm females had similar hatching success while 30 and 60 ppm females had significantly lower hatching success.
G1,2003 behaviour of hermaphrodites
DNA sex markers were used to determine the genetic sex of the G1,2003 fish suspected of being hermaphrodites, as well as of a sample of presumptive males and females. Two genetically-female 100 ppm fish were confirmed to be functional hermaphrodites via in vitro fertilizations of their eggs and sperm and through histological examination of their gonads (Bernhardt et al., 2006); these two fish also performed male courtship behaviours in the presence of gravid females (Table 4). In addition to these known hermaphrodites, two 30 ppm genetic females were suspected hermaphrodites because they also performed male courtship behaviours when exposed to gravid females (Table 4). None of the genetically female hermaphrodites made nests, even though they performed ‘male’ courtship displays. Introduced females often responded to these ‘male’ courtship displays by assuming the head-up posture typical of receptive females and by following the hermaphrodite as it attempted to lead. Two of the 60 ppm females died before encountering a gravid female, and the third remained inactive throughout the encounter. This third 60 ppm female died before encountering another gravid female; therefore, it is unknown if any of the 60 ppm females were hermaphroditic.
Table 4.
Repertoire of isolated genetic females.
| Fish ID | Head down | Biting | Leading | Zig-zagging | % Intermediate | Comments |
|---|---|---|---|---|---|---|
| 30-2 | X | X | X | 89.6 | Died after one courtship trial | |
| 30-7 | X | X | X | 94.2 (2.0) | Killed | |
| 60-1 | n/a | n/a | n/a | n/a | n/a | Died before courting |
| 60-3 | 100 | Died after one courtship trial | ||||
| 60-4 | n/a | n/a | n/a | n/a | n/a | Died before courting |
| 100-4 | X | X | X | X | 99.8 (0.2) | Died after seven courtships trials |
| 100-5 | X | X | X | X | 95.3 (1.9) | Killed |
Individually housed females from the 30 and 100 ppm treatments performed male-typical courtship displays when a gravid female was introduced into their aquaria. X indicates that a given masculine courtship behaviour was performed by the female on at least one courtship trial. n/a indicates that a fish could not have performed the activity because it was already dead. Head down represents the hermaphrodites that assumed a masculine head-down position, and % Intermediate represents the mean percent (± 1 SE) of time that each female spent conducting non-courtship-related activities. Killed indicates a fish was killed after the spawning trials. 100-4 and 100-5 were confirmed to be functionally hermaphroditic.
While hermaphroditic females (presumed and confirmed) showed a limited male courtship repertoire, their levels of inactivity were comparable to those for males exposed to 100 ppm perchlorate. Differences between the time spent engaged in male courtship behaviour by hermaphrodites and 100 ppm males were not statistically significant (Mann–Whitney U -tests, all p > 0.05). However, the trend was for the hermaphrodites to spend slightly more time engaged in a given courtship state while courting introduced females (means(100 ppm males vs. hermaphroditic females); for leading = 1.1 s vs. 1.4 s; and for zig-zagging = 0.9 s vs. 2.1 s).
Discussion
Our study demonstrates that chronic perchlorate exposure during development is capable of altering the behaviour of threespine stickleback. Sub-chronic perchlorate exposure (<22 days) of adult stickleback did not significantly alter any of the measured variables (i.e., survivorship, reproductive behaviour, fertility, or spawning success). Conversely, chronic exposure (approx. 1 year) from syngamy through sexual maturity impaired survivor-ship and caused numerous reproductive behavioural abnormalities, which increased in frequency with higher exposure concentrations of perchlorate and ultimately reduced reproductive success. A no adverse effect level was not determined because significant behavioural effects were found even among fish in the lowest treatment level of 30 ppm. These chronically exposed G1,2003 fish will be the focus of the discussion.
Swimming performance
Swimming performance was impaired in a dose-dependent manner (Figure 1), suggesting that fish exposed to perchlorate would be less capable of capturing prey, evading predators, and returning to their spawning grounds. Anadromous stickleback have been shown to rely on pectoral fin rowing for several hours at a time at velocities exceeding five body lengths per second (Taylor & McPhail, 1986). The flow rates used in this experiment are well below those measured in two of three local streams with anadromous stickleback runs. Therefore, the flow rates (≤ 6.5 mbl/s) and test durations (≤ 58 min) chosen to assess swimming performance are ecologically relevant. This suggests fish exposed to perchlorate would be less successful at returning to suitable spawning habitat. Furthermore, Mesa et al. (1994) demonstrated that poor fast-start swimming ability and reduced endurance among fish leads to increased susceptibility to predation, which further reduces the likelihood of survival to reproductive age and would expose predators to dietary perchlorate.
Perchlorate-exposed fish suffered higher mortality following stressors such as exhaustive exercise, relocation and handling while out of the water for <10 s (Bernhardt et al., 2006). Exhaustive exercise causes plasma concentrations of lactate, Na+, Cl−, plasma proteins and hemoglobin to rise as plasma hydrates white muscle tissue to offset intracellular lactic acid production (Wood et al., 1983). Recovery depends on lipid oxidation (Richards et al., 2002). Since THs affect osmoregulation (Leatherland, 1982) and lipid catabolism (Marieb, 2004), perchlorate-treated fish would be expected to display reduced recovery rates. Moreover, LaRoche et al. (1966) demonstrated that radiothyroidectomized fish had marked hemolysis attributable to erythrocyte fragility compared with control fish. Such results may help to explain higher mortality rates among treated fish following swimming trials. In addition to the higher mortality among perchlorate-treated fish prior to sexual maturity, mortality rates were elevated among males in a dose-dependent manner during their 33 days of isolation for courtship and parental care.
Both thyroid hormones and serotonin can have a direct stimulatory effect on spontaneous locomotion, and prenatal reductions of thyroid hormone can lead to lower levels of serotonin in the adult (Leatherland, 1982; Castonguay & Cyr, 1998; Weis et al., 2000). Reduced thyroid hormone levels during development have also been shown to affect spontaneous activity via reduced production of seratonergic neurotransmitters, which in turn leads to sluggishness (Castonguay & Cyr, 1998; Weis et al., 2000). Mummichogs (Fundulus heteroclitus) living in PCB-, DDT- and heavy metal-contaminated water had reduced thyroid hormone production, and demonstrated sluggish swimming performance, poor prey capture and poor predator avoidance abilities (Smith & Weis, 1997). These factors are all likely to contribute to the production of lethargic adults with impaired swimming ability.
Chemicals, such as perchlorate, that inhibit thyroid hormone production can damage or impair nervous system development, since thyroid hormones have critical roles during neurogenesis (Porterfield, 2000). Neuronal out-growth and migration are dependent upon microtubule synthesis, which is regulated by thyroid hormones (Nunez et al., 1991). Thyroid hormones also regulate nerve growth factor production (Oh et al., 1991). Animals with experimentally induced hypothyroidism show decreased axonal and dendritic arborization, fewer nerve terminals, and a variety of other neural impairments (Porterfield & Hendrich, 1993) and hence might be expected to have altered neurons associated with behaviour. Deficiencies in proper neuromuscular development and function may explain the impaired swimming performance and reproductive behaviour noted in the 30, 60 and 100 ppm perchlorate-treated fish.
LaRoche et al. (1966) noted that radiothyroidectomized rainbow trout had neuromuscular anomalies as indicated by seizures, which confer a poor degree of coordination. These fish often displayed differences in swimming performance and “inefficient swimming motions resembled those of tadpoles”. These inefficient swimming movements appeared to be related to improper neuromuscular coordination and occasional and unpredictable seizures, which involved violent flexions of the body (LaRoche et al., 1966). Such seizures were observed among perchlorate-treated stickleback, possibly implicating impaired neuronal development in the aberrant swimming and reproductive behaviours.
Reproductive behaviour
Whoriskey & FitzGerald (1994) categorized male stickleback reproductive success into three major components: nest building; the number of females the male can attract, and hence the number of eggs he can acquire; and the ability to provide appropriate parental care. All three of these components were impaired among stickleback chronically exposed to perchlorate, resulting in a dose-dependent reduction in reproductive success: 60% of controls produced fry, while 20% of 30 ppm, 10% of 60 ppm and none of the 100 ppm fish produced fry. Although behavioural inhibition was noted among 30 ppm and 60 ppm fish during courtship (Figure 3), there appears to be a point over which exposed fish are unable to produce nests or perform appropriate reproductive behaviours. This point for complete inhibition of reproductive behaviour was between 60 and 100 ppm.
The courtship phase in threespine stickleback normally begins when the male creeps through his nest (Wootton, 1976). Yet, nest-building was delayed in perchlorate-treated fish in a dose-dependent manner (Figure 6), which would shorten the effective breeding season for wild stickleback. Delayed nest building can translate into the male attracting fewer or no females, leading to reduced reproductive success (Mori, 1993). Douthwaite et al. (1981) determined that exposure to endosulfan (a xenoestrogenic chemical) caused Tilapia rendalli to build fewer nests than unexposed fish, thereby reducing reproductive output.
Chronic perchlorate exposure also caused G1,2003 males to court less often (Figure 2) and perform fewer courtship behaviours in a dose-dependent manner (Figure 3). These factors contributed to the poor spawning success and reduced reproductive output of treated males (Table 3). When treated males performed courtship behaviours, the timing to onset of a given behaviour after a female’s introduction differed only for biting (Table 2), which initiates courtship in this population. Furthermore, G1,2003 males treated with ≥60 ppm perchlorate (and some treated with 30 ppm) bit females with less vigor/intensity than control fish (Bernhardt, pers. obs.).
The O2 consumption and CO2 production of embryos following fertilization is initially minimal (van Iersel, 1953). At this time, the water and gasses within the nest are rapidly exchanged, and the O2 demands of the zygotes are quickly met after only a few moments of fanning (van Iersel, 1953). The initial bout of fanning following fertilization may help to distribute sperm throughout the eggs to ensure their fertilization. Metabolic demands steadily rise as the embryos mature, and such as elevated CO2 levels have been shown to increase fanning activity in stickleback (Sevenster, 1961). Consequently, the male typically devotes more time and energy toward fanning the nest through at least day five post fertilization at 20°C (van Iersel, 1953; von Hippel, 2000; Pall et al., 2002, 2005). After day 5 or 6, less fanning is needed because the male tears a hole into the top of his nest to make a nursery that further promotes gas exchange, before the embryos hatch into fry (van Iersel, 1953). Thus, prolonged bouts spent fanning the nest are normally unnecessary immediately following fertilization, but males devote more time toward fanning as the embryos mature, until he makes a nursery.
This pattern of increased fanning activity during embryonic development was noted among control and treated fish (Figure 5), but fish exposed to 60 ppm perchlorate spent significantly more time fanning until the eggs began to hatch on day 7. The most parsimonious explanation may be that the smaller pectoral fins of the 60 ppm males displaced less water within the nest and, therefore, required more fanning effort to adequately ventilate the nest. This would have caused the 60 ppm males to fan more in response to elevated CO2 levels within the nest. However, the plethora of behavioural differences makes it difficult to discount the possibility that perchlorate exposure interfered with parental behaviour via altered thyroid hormone levels and impaired neural development/function (Porterfield & Hendrich, 1993; Porterfield, 2000), via altered brain aromatase levels, or by interference with hormonal metabolic pathways. It may also be possible that increased fanning among 60 ppm males was an artifact of small sample size.
While a degree of parental care is necessary to remove dead eggs and those covered with fungus (van Iersel, 1953) and to deliver O2 and remove CO2 from the nest, the control fish clearly demonstrated that they are capable of caring for the developing embryos with less energy investment in terms of fanning (Figure 5), and possibly boring, gluing and carrying. Less energy expenditure in parental care not only conserves energy that can be devoted toward future courtship and spawning, but also reduces the conspicuousness of the nest to nest predators. Stickleback males that are better able to conceal their nests are more likely to be undetected by roving groups of cannibalistic stickleback and are more likely to hatch their eggs (FitzGerald, 1993).
Several hormones normally regulate sexual development, sexual physiology and sexual behaviour, and prodigious cross-talk occurs between various endocrine axes. Interference with one is likely to cause concomitant effects among others (Matthiessen, 2003), yet individual hormones have been linked to specific reproductive behaviours. For example, prolactin (PRL) has been implicated in the control of parental care in vertebrates, including fishes (Slijkhuis et al., 1984). A functional role for PRL in the control of fanning behaviour in sticklebacks was confirmed by Pall et al. (2004), who demonstrated that PRL administration increased fanning behaviour in nesting males. Bell (2001) determined that gonadal steroids have contrasting effects on conspecific-oriented and nest-oriented behaviours during stickleback courtship. Specifically, high plasma 11-ketotestosterone (11-KT) levels were positively correlated with conspecific-oriented behaviours (e.g., aggression toward males and courtship toward females) and negatively correlated with nest-oriented behaviours. Conversely, elevated estradiol levels increase nest-oriented activity at the expense of courtship or defense. Pall et al. (2002) also found contrasting behavioural responses to 11-KT levels between the courtship and parental care phases of threespine stickleback, with levels of 11-KT falling during the transition from the courtship phase into the parental care phase.
Perchlorate exposure masculinized developing stickleback in terms of producing male fish with hypertrophied testes and genetically-female hermaphrodites (Bernhardt et al., 2006). This masculinization of male and female gonads likely occurred during early development when sexual differentiation was underway, but concomitantly altered hormone production may affect reproductive behaviour at sexual maturity. We found that control males were the most active courters while 60 ppm males were the most active during the parental care phase. In other words, perchlorate-treated fish showed less conspecific-oriented behaviour during courtship and more nest-oriented activity during parental care. These findings would be characteristic of fish with lower 11-KT levels and/or higher prolactin or estradiol levels. Therefore, perchlorate’s role(s) as a masculinizing agent are not easily reconciled with the reduced courtship activity and increased parental care among the 60 ppm males, suggesting perchlorate does not mimic endogenous androgens. Smaller pectoral fins that are less efficient at water and gas exchange may have incidentally increased the fanning activity of 60 ppm males, though this does not exclude the possibility of altered hormonal and neural function.
Perchlorate exposure may also have induced a stress response that contributed to differential mortality and the inhibition of courtship behaviours. Stress effects were most clearly evident among 100 ppm fish following disturbance. Stress leads to elevated levels of circulating glucocorticoids, depressed levels of circulating gonadotropins and sex steroids, as well as increased mineralocorticoids (Carr & Norris, 2006). Since thyroid hormones and gonadotropic hormones are known to act together during gonadal formation, impaired production of either or both of these hormones is likely to impair sexual development. Virtually every process influenced by gonadotropic hormones, from courtship behaviour to fertilization, was inhibited by perchlorate exposure. Inhibition may have resulted from organizational rather than activational effects, since subchronically-exposed G0,2002 adults showed no behavioural inhibition (though exposure levels only ranged up to 18.6 ppm) while G1,2003 fish that were chronically exposed to 30, 60 and 100 ppm perchlorate from early development through sexual maturity showed marked behavioural inhibition. The hormonal bases of perchlorate’s actions are likely to be a productive avenue for future research.
Behaviour of hermaphrodites
Individually housed intersex females (genetically-female hermaphrodites in the 30 and 100 ppm treatments) courted introduced, gravid females. However, 30 ppm and 60 ppm females that were housed collectively by treatment in female pools managed to spawn naturally when introduced to territorial males from the same treatment. Some or all of these treated females may have been hermaphrodites; this was not tested. In the two instances when gamete viability of hermaphroditic fish was tested (Bernhardt et al., 2006), the eggs fertilized by sperm from a hermaphrodite died prior to hatching. This raises the question of whether a treated, hermaphroditic female may have fertilized a portion of her own eggs before the male fertilized the rest. If so, it would have contributed to the poor hatching success noted among the 30 and 60 ppm clutches that arose from natural spawning events since the results of in vitro fertilization testing revealed that eggs fertilized by a hermaphrodite’s sperm die before hatching. Whether perchlorate-exposed hermaphroditic females would be more likely to establish a territory in the wild and attempt to court other females, respond to male courtship displays, or both, remains unclear.
The presence of motile sperm in female stickleback exposed to perchlorate (Bernhardt et al., 2006) suggests that the steroidogenic lobule-boundary cells (homologous with Interstitial cells of Leydig) may have been functional and producing enough testosterone to support sperm development, or that perchlorate altered the metabolism of testosterone (either directly or indirectly) from the gonads and/or from precursors made in the adrenal cortex. Elevated testosterone levels, altered hormone metabolism and/or altered brain aromatase activity in genetically-female hermaphrodites may help to explain their ‘masculine’ courtship displays. Yet, none of the females exposed to perchlorate produced nests, suggesting that if testosterone levels were changed, the degree was limited.
Female stickleback exposed to testosterone are capable of making spiggin (the male glue protein produced by the kidneys and used for nest building; Katsiadaki et al., 2002), and a small proportion of gonadectomised female stickleback treated with the synthetic androgen 17α-methyltestosterone build rudimentary nests (Wai & Hoar, 1963). The females that built nests in Wai & Hoar’s (1963) study showed most of the relevant behavioural patterns associated with nest building, but the organization of these patterns was in-sufficient to produce a proper nest. Their nest pit was shallow; there was little boring; and although gluing occurred, the vegetation was not neatly glued together so that the end product was merely a flat mass of vegetation lying in a shallow pit. It is, therefore, unlikely that the intersex gonads were capable of producing sufficient testosterone to induce complete behavioural sex reversal, or capable of completely transforming the kidneys into a spiggin-producing organ (Guderly, 1994).
Administration of precise concentrations of exogenous sex steroid hormones during specific developmental windows is a common procedure in fish aquaculture to produce monosex populations (Donaldson & Hunter, 1982; Matty, 1985). The resultant sex-reversed fish are fertile and function as the phenotypic sex, including the display of behaviours typical of their phenotypic sex (Hunter & Donaldson, 1983; Pandian & Sheela, 1995; Piferrer, 2001). Complete sex reversal (fish of one genotypic sex with only the gonads of the other sex), however, has not been reported among wild fish exposed to xenobiotics (Kime, 1998). Rather, such fish develop intersex gonads (ovotestes), which is consistent with the effects of chronic perchlorate exposure (Bernhardt et al., 2006).
Mukhi et al. (2007) demonstrated that administration of perchlorate to zebrafish (Danio rerio) during the larval-juvenile developmental period produced a female-biased sex ratio, and supplemental thyroid hormone administration produced a male-biased sex ratio. This finding demonstrates that thyroid hormones play a key role in the sexual development of at least some fish. However, comparisons between the sexual development of zebrafish and stickleback should be made with caution, since a sex chromosome has not been identified for zebrafish, and their sexual development appears to be labile and influenced by a number of environmental factors including dissolved oxygen content (Shang et al., 2006), temperature (Uchida et al., 2004) and others (Strussmann & Nakamura, 2002).
It is often assumed, and the majority of available evidence supports the conclusion, that genetic males are typically feminized into hermaphrodites by contaminants rather than genetic females being masculinized. However, this can only be confirmed by determining the genetic sex of intersex individuals, and DNA sex markers have only been developed for a few species, including the stickleback. Nevertheless, based on current knowledge, the endocrine-disrupting effects of perchlorate are rare in that perchlorate exposure is capable of invoking masculinization during development (Bernhardt et al., 2006).
This study demonstrates that there are delayed costs of perchlorate exposure that would not be detected in standard acute or subchronic toxicity testing and that perchlorate exposure during early development poses risks that may only become apparent at sexual maturity. Poor swimming ability (Figure 1) would decrease the likelihood of avoiding predators, catching prey, and reaching suitable spawning habitat. Inhibition of nest construction and courtship behaviour (Figures 2, 3 and 6) and failure to complete reproductive benchmark metrics (Table 3) suggest that fish capable of reaching their spawning grounds would be unlikely to build a nest, attract mates, or produce fry. Diminished resilience in the face of disturbance would further reduce survivorship and contribute to lower recruitment among contaminated fish. The effects of perchlorate on the two major components of lifetime fitness, namely survivorship until breeding age and reproductive output, are unlikely to be limited to the stickleback.
Acknowledgments
We thank Jeff Jones and Heidi Weigner for their invaluable laboratory assistance. Todd O’Hara, Loren Buck, Jennifer Burns and Bill Cresko offered many thought-provoking discussions and generously provided helpful feedback on the manuscript. Orson Smith provided the flume, which allowed us to conduct the swimming analysis. This work was partially supported by the US Air Force Space and Missile Systems Center and The Aerospace Corporation. This work was also supported by Grant Number 5P20RR016466 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. Fish were collected under Alaska Department of Fish and Game permit numbers SF-2002-002 and SF-2003-019, and all research protocols were approved by the University of Alaska Anchorage Institutional Animal Care and Use Committee.
References
- Backus SM, Klawuun P, Brown S, D’sa I, Sharp S, Surette C, Williams DJ. Determination of perchlorate in selected surface waters in the Great Lakes Basin by HPLC/MS/MS. Chemosphere. 2005;61:834–843. doi: 10.1016/j.chemosphere.2005.04.054. [DOI] [PubMed] [Google Scholar]
- Baldridge MG, Stahl RL, Gerstenberger SL, Tripoli V, Hutz RJ. In utero and lactational exposure of Long-Evans rats to ammonium perchlorate (AP) disrupts ovarian follicle maturation. Reprod Toxicol. 2004;19:155–161. doi: 10.1016/j.reprotox.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Bell AM. Effects of an endocrine disrupter on courtship and aggressive behaviour of male three-spined stickleback, Gasterosteus aculeatus. Anim Behav. 2001;62:775–780. [Google Scholar]
- Bernhardt RR, von Hippel FA, Cresko WA. Perchlorate induces hermaphroditism in threespine sticklebacks. Environ Toxicol Chem. 2006;25:2087–2096. doi: 10.1897/05-454r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford CM, Rinchard J, Carr JA, Theodorakis C. Perchlorate affects thyroid function in eastern mosquitofish (Gambusia holbrooki) at environmentally relevant concentrations. Environ Sci Technol. 2005;39:5190–5195. doi: 10.1021/es0484505. [DOI] [PubMed] [Google Scholar]
- Brechner RJ, Parkhurst GD, Humble WO, Brown MB, Herman WH. Ammonium perchlorate contamination of Colorado River drinking water is associated with abnormal thyroid function in newborns in Arizona. J Occup Environ Med. 2000;42:777–782. doi: 10.1097/00043764-200008000-00002. [DOI] [PubMed] [Google Scholar]
- Carr JA, Norris DO. The adrenal glands. In: Norris DO, Carr JA, editors. Endocrine disruption. Oxford University Press; New York, NY: 2006. pp. 111–134. [Google Scholar]
- Castonguay M, Cyr DG. Effects of temperature on spontaneous and thyroxine-stimulated locomotor activity of Atlantic cod. J Fish Biol. 1998;53:303–315. [Google Scholar]
- Crane HM, Pickford DB, Hutchinson TH, Brown JA. Effects of ammonium perchlorate on thyroid function in developing fathead minnows, Pimephales promelas. Environ Health Perspect. 2005;113:396–401. doi: 10.1289/ehp.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds ED, Kennish JM, von Hippel FA, Bernhardt R, Hines ME. Quantitative analysis of perchlorate in extracts of whole-fish homogenates by ion chromatography: comparison of suppressed conductivity detection and electrospray ionization mass spectrometry. Anal Bioanal Chem. 2004;379:881–887. doi: 10.1007/s00216-004-2660-8. [DOI] [PubMed] [Google Scholar]
- Donaldson EM, Hunter GA. Sex control in fish with particular reference to Salmonids. Can J Fish Aquat Sci. 1982;39:99–110. [Google Scholar]
- Douthwaite RJ, Fox PJ, Matthiessen P, Russell-Smith A. The environmental impact of aerosols of endosulfan applied for tsetse fly control in the Okavango Delta, Botswana. Overseas Development Administration; London: 1981. [Google Scholar]
- FitzGerald GJ. The reproductive behaviour of stickleback. Sci Am. 1993;268:80–85. [Google Scholar]
- Goleman WL, Carr JA, Anderson TA. Environmentally relevant concentrations of ammonium perchlorate inhibit thyroid function and alter sex ratios in developing Xenopus laevis. Environ Toxicol Chem. 2001;21:590–597. [PubMed] [Google Scholar]
- Goleman WL, Urquidi LJ, Anderson TA, Smith EE, Kendall RJ, Carr JA. Environmentally relevant concentrations of ammonium perchlorate inhibit development and metamorphosis in Xenopus laevis. Environ Toxicol Chem. 2002;21:424–430. [PubMed] [Google Scholar]
- Guderly HE. Physiological ecology and evolution of the threespine stickleback. In: Bell MA, Foster SA, editors. The evolutionary biology of the threespine stickleback. Oxford University Press; New York, NY: 1994. pp. 85–113. [Google Scholar]
- von Hippel FA. Vigorously courting male sticklebacks are poor fathers. Acta Ethol. 2000;2:83–89. [Google Scholar]
- Hunter GA, Donaldson EM. Fish physiology: reproduction. Academic Press; New York, NY: 1983. Hormonal sex control and its application to fish culture; pp. 223–303. [Google Scholar]
- van Iersel JJA. An analysis of the parental behaviour of the male three-spined stickleback (Gasterosteus aculeatus L.) Behaviour. 1953;Suppl III:1–159. [Google Scholar]
- Katsiadaki I, Scott AP, Mayer I. The potential of the three-spined stickleback (Gasterosteus aculeatus L.) as a combined biomarker for oestrogens and androgens in European waters. Mar Environ Res. 2002;54:725–728. doi: 10.1016/s0141-1136(02)00110-1. [DOI] [PubMed] [Google Scholar]
- Kime DE. Endocrine disruption in fish. Kluwer; Boston, MA: 1998. p. 127. [Google Scholar]
- Kirk AB, Martinelango PK, Tian K, Dutta A, Smith EE, Dasgupta PK. Perchlorate and iodide in dairy and breast milk. Environ Sci Technol. 2005;39:2011–2017. doi: 10.1021/es048118t. [DOI] [PubMed] [Google Scholar]
- LaRoche G, Woodall AN, Johnson CL, Halver JE. Thyroid function in the rainbow trout (Salmo gairdnerii Rich) II Effects of thyroidectomy on the development of young fish. Gen Comp Endocrinol. 1966;6:246–266. doi: 10.1016/s0016-6480(66)80013-8. [DOI] [PubMed] [Google Scholar]
- Leatherland JF. Environmental physiology of the teleostean thyroid gland: a review. Environ Biol Fish. 1982;7:83–110. [Google Scholar]
- Marieb EN. Human anatomy & physiology. Pearson Benjamin Cummings; San Francisco, CA: 2004. The endocrine system; pp. 603–643. [Google Scholar]
- Matthiessen P. Endocrine disruption in marine fish. Pure Appl Chem. 2003;75:2249–2261. [Google Scholar]
- Matty AB. Fish endocrinology. Timber Press; Portland, OR: 1985. [Google Scholar]
- McNabb FMA, Jang DA, Larsen CT. Does thyroid function in developing birds adapt to sustained ammonium perchlorate exposure? Toxicol Sci. 2004;82:106–113. doi: 10.1093/toxsci/kfh247. [DOI] [PubMed] [Google Scholar]
- Mendiratta SK, Dotson RL, Brooker RT. Perchloric acid and perchlorates. In: Kroschwitz JI, Howe-Grant M, editors. Kirk–Othmer Encyclopedia of chemical technology. 4. Vol. 18. Wiley; New York, NY: 1996. pp. 157–170. [Google Scholar]
- Mesa MG, Poe TP, Gadomski DM, Petersen JH. Are all prey created equal? A review and synthesis of differential predation on prey in substandard condition. J Fish Biol. 1994;45:81–96. [Google Scholar]
- Miranda LA, Pisano A, Casco V. Ultrastructural study on the thyroid glands of Bufo arenarum larvae kept in potassium perchlorate solution. Biocell. 1996;20:147–153. [PubMed] [Google Scholar]
- Mori S. The breeding system of the threespine stickleback Gasterosteus aculeatus (forma Leiura) with reference to spatial and temporal patterns of nesting activity. Behaviour. 1993;126:97–124. [Google Scholar]
- Mukhi S, Torres L, Patiño R. Effects of larval-juvenile treatment with perchlorate and co-treatment with thyroxine on zebrafish sex ratios. Gen Comp Endocrinol. 2007;150:486–494. doi: 10.1016/j.ygcen.2006.11.013. [DOI] [PubMed] [Google Scholar]
- National Research Council of the National Academies. Health implications of perchlorate ingestion. National Academies Press; Washington, DC: 2005. The thyroid and disruption of thyroid function in humans; pp. 35–74. [Google Scholar]
- Nunez J, Couchie D, Aniello F, Bridoux AM. Regulation by thyroid hormone of microtubule assembly and neuronal differentiation. Neurochem Res. 1991;16:975–982. doi: 10.1007/BF00965840. [DOI] [PubMed] [Google Scholar]
- Oh JD, Butcher LL, Woolf NJ. Thyroid hormone modulates the development of cholinergic terminal fields in the rat forebrain: relation to nerve growth factor receptor. Dev Brain Res. 1991;59:133–142. doi: 10.1016/0165-3806(91)90093-x. [DOI] [PubMed] [Google Scholar]
- Pall MK, Mayer I, Borg B. Androgen and behaviour in the male three-spined stickleback, Gasterosteus aculeatus II. Castration and 11-ketoandrostenedione effects on courtship and parental care during the nesting cycle. Horm Behav. 2002;42:337–344. doi: 10.1006/hbeh.2002.1820. [DOI] [PubMed] [Google Scholar]
- Pall MK, Liljander M, Borg B. Prolactin diminishes courtship behaviour and stimulates fanning in nesting male three-spined sticklebacks, Gasterosteus aculeatus. Behaviour. 2004;141:1511–1519. [Google Scholar]
- Pall MK, Hellqvist A, Schmitz M, Olsson PE, Mayer I, Borg B. Changes in reproductive physiology and behaviour over the nesting cycle in male three-spined sticklebacks. J Fish Biol. 2005;6:1400–1410. [Google Scholar]
- Pandian TJ, Sheela SG. Hormonal induction of sex reversal in fish. Aquaculture. 1995;138:1–22. [Google Scholar]
- Park JW, Rinchard J, Liu F, Anderson TA, Kendall RJ, Theodorakis CW. The thyroid endocrine disruptor perchlorate affects reproduction, growth, and survival of mosquitofish. Ecotoxicol Environ Safe. 2006;63:343–352. doi: 10.1016/j.ecoenv.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Patiño R, Wainscott MR, Cruz-Li EI, Balakrishnan S, McMurry C, Blazer VS, Anderson TA. Effects of ammonium perchlorate on the reproductive performance and thyroid follicle histology of zebrafish. Environ Toxicol Chem. 2003;22:1115–1121. [PubMed] [Google Scholar]
- Piferrer F. Endocrine sex control strategies for the feminization of teleosts fish. Aquaculture. 2001;197:229–281. [Google Scholar]
- Porterfield SP. Thyroidal dysfunction and environmental chemicals — potential impact on brain development. Environ Health Perspect. 2000;108 (Suppl):433–438. doi: 10.1289/ehp.00108s3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield SP, Hendrich CE. The role of thyroid hormones in prenatal and neonatal neurological development — current perspectives. Endocr Rev. 1993;14:94–106. doi: 10.1210/edrv-14-1-94. [DOI] [PubMed] [Google Scholar]
- Richards JG, Heigenhauser JF, Wood CM. Lipid oxidation fuels recovery from exhaustive exercise in white muscle of rainbow trout. Am J Physiol. 2002;282:R89–R99. doi: 10.1152/ajpregu.00238.2001. [DOI] [PubMed] [Google Scholar]
- Sevenster P. A causal analysis of a displacement activity (fanning in Gasterosteus aculeatus L.) Behaviour. 1961;Suppl IX:1–170. [Google Scholar]
- Shang EH, Yu RM, Wu RS. Hypoxia affects sex differentiation and development, leading to a male-dominated population in zebrafish (Danio rerio) Environ Sci Technol. 2006;40:3118–3122. doi: 10.1021/es0522579. [DOI] [PubMed] [Google Scholar]
- Shaw G, Wheeler D. Statistical techniques in geographical analysis. Wiley; New York, NY: 1985. [Google Scholar]
- Slijkhuis H, de Ruiter AJH, Baggerman B, Wendelaar-Bonga SE. The effect of prolactin on fanning behaviour in the male three-spined stickleback, Gasterosteus aculeatus L. Gen Comp Endocrinol. 1984;64:273–283. doi: 10.1016/0016-6480(86)90014-6. [DOI] [PubMed] [Google Scholar]
- Smith GM, Weis JS. Predator-prey relationships in mummichogs (Fundulus heteroclitus (L.)): Effects of living in a polluted environment. J Exp Mar Biol Ecol. 1997;209:75–87. [Google Scholar]
- Smith PN, Theodorakis CW, Anderson TA, Kendall RJ. Preliminary assessment of perchlorate in ecological receptors at the Longhorn Army Ammunition Plant (LHAAP), Karnack, Texas. Ecotoxicology. 2001;10:305–313. doi: 10.1023/a:1016715502717. [DOI] [PubMed] [Google Scholar]
- Strussmann CA, Nakamura M. Morphology, endocrinology, and environmental modulation of gonadal sex differentiation in teleost fishes. Fish Physiol Biochem. 2002;26:13–29. [Google Scholar]
- Taylor EB, McPhail JD. Prolonged and burst swimming in anadromous and freshwater threespine stickleback, Gasterosteus aculeatus. Can J Zool. 1986;64:416–420. [Google Scholar]
- Uchida D, Yamashita M, Kitano T, Iguchi T. An aromatase inhibitor or high water temperature induce oocytes apoptosis and depletion of P450 aromatase activity in the gonads of genetic female zebrafish during sex-reversal. Comp Biochem Physiol A: Mol Integr Physiol. 2004;137:11–20. doi: 10.1016/s1095-6433(03)00178-8. [DOI] [PubMed] [Google Scholar]
- Urbansky ET. Perchlorate chemistry: implications for analysis and remediation. Bioremediation J. 1998;2:81–95. [Google Scholar]
- Urbansky ET, Brown SK, Magnuson ML, Kelty CL. Perchlorate levels in samples of sodium nitrate fertilizer derived from Chilean caliche. Environ Pollut. 2001;112:299–302. doi: 10.1016/s0269-7491(00)00132-9. [DOI] [PubMed] [Google Scholar]
- Wai EH, Hoar WS. The secondary sex characters and reproductive behaviour of gonadectomised sticklebacks treated with methyltestosterone. Can J Zool. 1963;41:611–628. [Google Scholar]
- Weis JS, Smith G, Zhou T, Santiago-Bass C, Weis P. Effects of contaminants on behaviour: biochemical mechanisms and ecological consequences. BioScience. 2001;51:209–217. [Google Scholar]
- Wibe AE, Rosenqvist G, Jenssen BM. Disruption of male reproductive behaviour in threespine stickleback (Gasterosteus aculeatus) exposed to 17β estradiol. Environ Res Sec A. 2002;90:136–141. doi: 10.1006/enrs.2002.4392. [DOI] [PubMed] [Google Scholar]
- Whoriskey FG, FitzGerald GJ. Ecology of the threespine stickleback on the breeding grounds. In: Bell MA, Foster SA, editors. The evolutionary biology of the threespine stickleback. Oxford University Press; Oxford: 1994. pp. 188–206. [Google Scholar]
- Wood CM, Turner JD, Graham MS. Why do fish die after severe exercise? J Fish Biol. 1983;22:189–201. [Google Scholar]
- Wootton RJ. The biology of the sticklebacks. Academic Press; London: 1976. [Google Scholar]
- York RG, Brown WR, Girard MF, Dollarhide JS. Two-generation reproduction study of ammonium perchlorate in drinking water in rats evaluates thyroid toxicity. Int J Toxicol. 2001a;20:183–197. doi: 10.1080/109158101750408019. [DOI] [PubMed] [Google Scholar]
- York RG, Brown WR, Girard MF, Dollarhide JS. Oral (drinking water) toxicity study of ammonium perchlorate in New Zealand white rabbits. Int J Toxicol. 2001b;20:199–205. doi: 10.1080/109158101750408028. [DOI] [PubMed] [Google Scholar]
- York RG, Funk KA, Girard MF, Mattie D, Strawson JE. Oral (drinking water) developmental toxicity study of ammonium perchlorate in Sprague-Dawley rats. Int J Toxicol. 2003;22:453–464. doi: 10.1177/109158180302200606. [DOI] [PubMed] [Google Scholar]