Abstract
We studied the effects of indirect allorecognition on the induction and maintenance phases of tolerance in miniature swine cotransplanted with heart and kidney allografts. MHC class I-mismatched heart and kidney grafts were cotransplanted in recipients receiving CyA for 12 days. Recipients were unimmunized or immunized with a set of donor-derived or control third-party MHC class I peptides either 21 days prior to transplantation or over 100 days after transplantation. T cell proliferation, delayed type hypersensitivity reaction (DTH) and antibody production were assessed. All animals injected with donor MHC class I peptides developed potent indirect alloresponses specific to the immunizing peptides. While untreated recipients developed stable tolerance, all animals preimmunized with donor allopeptides rejected kidney-heart transplants acutely. In contrast, when peptide immunization was delayed until over 100 days after kidney/heart transplantation, no effects were observed on graft function or in vitro measures of alloimmunity. Donor peptide immunization prevenedt tolerance when administered to recipients pretransplantation but did not abrogate tolerance when administered to long term survivors posttransplantation. This suggests that the presence of T cells activated via indirect allorecognition represent a barrier to the induction but not the maintenance of tolerance.
Keywords: indirect allorecognition, tolerance, rejection, heart transplantation, kidney transplantation
Introduction
The immunogenicity and tolerogenicity of allografts differs upon the nature of the organ and tissue transplant as well as its site of placement. Consequently, certain allotransplants are more readily accepted than others and can even exert some tolerogenic effects towards subsequent placement of other organs from the same donor. Indeed, the so-called “liver effect”, whereby a liver allograft can protect another co-transplanted organ from rejection is now a well recognized phenomenon, although the mechanisms by which it is mediated remain elusive (1–3). Likewise, some studies show that patients receiving combined heart and kidney transplants display less acute rejection than heart recipients alone (4,5).
Our laboratory has previously described a large-animal model of non-ablative cardiac allograft tolerance in which hearts and kidneys from the same donor are transplanted simultaneously. In this setting, MHC class I-disparate recipients treated with a short course of high-dose cyclosporine A become tolerant to both allografts (6). In contrast, isolated heart transplants performed under identical conditions are regularly rejected and develop vasculopathy (7). Although, the mechanisms by which heart transplants are protected from rejection by kidney co-transplantation are incompletely understood (8), this is the only protocol, to our knowledge, that induces reliable and stable tolerance in large animal recipients of cardiac allografts.
Allograft rejection is initiated by pro-inflammatory CD4+ T cells recognizing intact allo-MHC class II molecules on donor APCs (direct pathway) (9) and donor peptides presented in association with self-MHC class II on recipient APCs (indirect pathway) (10–12). Either pathway can induce CD8+ T cell-mediated cytotoxicity, DTH reactions and alloantibody production, thereby ensuring acute rejection of skin allografts (13–16). However, it is still unclear whether the CD4+ T cell indirect alloresponse is sufficient to elicit acute rejection of less immunogeic vascularized organ transplants, including kidneys and hearts. In turn, there is strong circumstantial evidence supporting the contribution of indirect alloreactivity to chronic rejection of allotransplants (17–20). It is believed that the direct alloresponse fades away rapidly after transplantation as donor MHC class II+ professional APCs become eliminated. Accordingly, short-term treatments with calcineurin inhibitors or costimulation blockade appear sufficient to abrogate direct alloreactivity and prevent early acute rejection (21). In contrast, perpetuation and amplification of the indirect alloresponse via continuous presentation of dominant and cryptic allopeptides by recipient APCs is apparently resistant to these immunosuppressive treatments (22–24). This underscores the need for inducing and maintaining tolerance to T cells recognizing donor antigen in an indirect fashion. Only by better understanding the effects of indirect allorecognition on tolerance can protocols capable of inactivating pro-inflammatory indirect alloreactivity can be developed.
In this study, we investigated the influence of an indirect alloresponse to donor MHC class I peptides in the induction and maintenance of tolerance in porcine recipients of MHC class I-mismatched heart/kidney transplants. We show that induction of an inflammatory indirect alloresponse via immunization of recipients with donor MHC class I peptides emuslified in Freund’s adjuvant prevents tolerance induction but it fails to abrogate an established state of tolerance. The implications of these findings for the design of novel tolerance strategies in allotransplantation are discussed.
Materials and methods
Animals
The MGH miniature swine pre-clinical model has been previously described (25). In this study, we used two homozygous SLA haplotypes (SLA Ic IIc and SLA Id IId) and three intra-MHC SLA recombinant haplotypes (SLA Ic IId, SLA Ia IId and SLA Ia IIc). Genotyping was controlled by strict pedigree breeding and confirmed with flow cytometric analysis using indirect allospecific antibodies. All donor-recipient pairs were SLA class II-matched (SLA class I and minor antigens mismatched). Prior to any intervention, these pairings were tested for mutual reactivity by cell mediated lympholysis, as described. The animals were 3–6 months of age at the time of transplantation. Animal care and procedures were performed in compliance with both the Principles of Laboratory Animals Care formulated by the National Society of Medical Research and the Guide for the Care and Use of Laboratory Animals published by the National Institute of Health.
Synthetic SLA Class I Peptides
There are two known SLA class I loci in the pig (PC1 and PC14), where “C” refers to the SLA class I allele “C” in our model. Most of the polymorphisms are located within the hypervariable regions of the alpha1 and alpha2 domains of the heavy chain (26). As previously describe (17), four peptides spanning the full length of the hypervariable region of the PC1 alpha 1 helix were synthesized and labeled PC1-1 (amino acid (aa) 3–27), PC1-2 (aa 35–52), PC1-3 (aa 53–73) and PC1-4 (aa 71–90). Three peptides spanning the full length of the hypervariable region of the PC14 alpha1 helix were synthesized and labeled PC14-1 (aa 3–27), PC14-2 (aa 45–59) and PC14-3 (aa 60–85). As a negative control for in vitro assays, an irrelevant rat class II MHC allopeptide was synthesized and named RT1Du (aa 68–92). Peptide immunizations were performed via subcutaneous injection of 250ug of each peptide emulsified with an equal volume of complete Freund’s adjuvant (CFA). Immunogenicity of the peptides was confirmed in vivo by delayed type hypersensitivity and in vitro by peptide proliferation assays.
Experimental Design
Heart and kidney allografts were co-transplanted into cyclosporine-treated, SLA class I mismatched recipients that 1) received no peptide immunization, 2) were preimmunized with donor-derived peptides 21 days prior to transplantation, 3) were preimmunized with third-party peptides 21 days prior transplantation, or 4) were immunized with donor-derived peptides over 100 days after transplantation.
Heart-Kidney Co-Transplantation
Heterotopic heart-kidney transplantation was performed as previously described (6,27). Cyclosporine (CyA), generously provided by Novartis (Hanover, NJ), was administered intravenously to selected recipients at 10–13 mg/kg/day beginning on the day of surgery (POD 0) and continuing until posttransplant day 11, based upon earlier results (6). Kidney function was monitored by serum creatinine levels and heart function by daily palpation and electrocardiograms (ECG). Routine biopsies were performed on all transplant recipients via an open transabdominal approach at predetermined time intervals or whenever a pathological rise in serum creatinine level or decrease in R-wave amplitude occurred. At predetermined serial time points, peripheral blood mononuclear cells (PBMC) were isolated from whole blood for in vitro testing of responsiveness to the peptides as well as donor type cells. In addition, animals were tested for in vivo responsiveness to the peptides by delayed type hypersensitivity reactions to confirm both the immunogenicity of the peptides and the in vivo relevance of their indirect presentation. Serum was evaluated for the development of anti-peptide and anti-donor antibodies at multiple time points.
Delayed Type Hypersensitivity (DTH) Reactions
DTH responses were evaluated 14 days after peptide immunization by rechallenging recipients with 200 µg of individual peptide in 0.1 ml phosphate buffered saline (PBS) intradermally into the neck of the pig. RT1Du and PBS were used as negative controls, while 100 µg of M. tuberculosis H37 RA (MTB) was used as a positive control. Width of induration was measured at 48 hours after injection by blinded observers using calipers. Positive responses were > 10 mm of induration, indeterminate responses were > 5 mm and < 10 mm and negative responses were < 5 mm.
Immunohistochemistry and Immunofluorescence
Formalin fixed tissue was stained with hematoxylin and eosin (H&E), Masson’s trichrome stain, and Verhoeff stain, and evaluated by a blinded observer. Acute interstitial rejection of heart allografts was scored from 0 to 4 was based on the ISHLT system (28). Vessel wall thickening was scored as 0 (normal artery), 1 (<10% occlusion), 2 (>10% <50% occlusion), and 3 (>50% luminal occlusion) and the average score recorded. Kidney allograft rejection was scored by standard pathologic criteria according to the Cooperative Clinical Trials in Transplantation criteria (29). Frozen tissue sections were stained with anti-α smooth muscle actin mAb (clone 1A4, Sigma Chemical) and saturating concentrations of goat anti-swine IgM-FITC and IgG-FITC (Kirkegaard and Perry Laboratories, Inc).
Cell Mediated Lympholysis Assay
CML assays were performed as previously described (30). Mixed lymphocyte cultures contained 4×106 responder PBMCs and 4×106 irradiated (2500 rad) stimulator PBMCs and were incubated in 2mL of culture medium in 24 well plates (Costar) for 6 days at 37°C and 6% CO2. Next, effector cells were harvested and tested for their cytolytic activity against 51Cr-labeled PHA targets in a 5.5 hour 51CR release assay. Supernatants were harvested using the Skatron collection system (Skatron, Sterling, VA) and 51Cr-release was determined on a gamma counter (Micromedics, Huntsville, AL). Results were expressed as the percent specific lysis representing: [(experimental release-spontaneous release) / (maximum release-spontaneous release)] × 100.
Proliferation Assays
Mixed Lymphocyte Response assays (MLRs) were performed as previously described (30). Results were expressed as stimulation indices (SI), calculated as SI = average count per million (cpm) for a responder-stimulator pair / cpm of the same responder stimulated by an autologous stimulator. Peptide-mediated T cell proliferation was measured as previously described (17). 8×105 responder PBMCs were cultured for 5 days along with donor-derived or control peptides (10ug). In all proliferation assays, 1 uCi of [3H]thymidine was added to each well, and incubated for 5 hours to allow for incorporation. [3H]-incorporation was determined in triplicate samples by β-scintillation counting. Results were expressed as stimulation indices (SI), calculated as SI = average count per million (cpm) for a responder-peptide pair / cpm of the same responder stimulated by media alone. Average maximum SI of naïve responses is 1.2 and adding 3 standard errors results in an SI of 2.2. Therefore, an SI > 2.3 was considered significant.
ELISA
Plates were coated with 50µL of peptides (2 ug/mL) or PBS and incubated overnight at 4°C. The plates were washed twice with 200uL of PBS+0.1%Tween20 and then blocked by dispensing 200uL of PBS + 0.05% Tween20 and 1% BSA with a 1 hour incubation at room temperature. The plates were then washed three times and swine serum at 1:10 dilution in PBS + 0.1% Tween20 was serially diluted. Following a 2 hour incubation at room temperature, plates were washed five more times. Rabbit anti-pig IgG (1:250) and IgM (1:500) in PBS + 0.05% Tween20 1% BSA was added to each well and incubated for 2 hours at room temperature. Following five more washes, 50 uL SAv-HRP developing solution (1:1000) was added to each well and allowed to incubate for 1 hour at room temperature and in the dark. Another five washes were performed and hydrolysis was measured adding ABTS peroxidase solutions into each well. Product absorbances were measured using a BioRad ELISA plate reader at 405nm (BioRad).
Flow Cytometry
Sera from the immunized animals were tested for the presence of anti-peptide and anti-donor IgM and IgG by indirect flow cytometry as previously described (31).
Results
DTH Responses to Allogeneic Class I MHC Peptides in Peptide-Immunized Swine
We have previously shown that following acute and chronic rejection, swine transplanted with class I mismatched hearts become sensitized to polymorphic donor class I peptides through the indirect pathway of allorecognition (15). To confirm that indirect presentation and sensitization occurred following in vivo administration of synthetic SLA peptides, DTH responses to the donor class I SLA peptides were analyzed. Animals were immunized with the PC1 and/or PC14 class Ic peptides in CFA subcutaneously in the neck and 14 days later rechallenged with the immunizing peptide or a control peptide. The level of induration was measured 48 hours later. The animals that were immunized 21 days before transplantation were injected with the PC14-1, PC14-2 and PC14-3 donor peptides. The PC14-3 peptide elicited a consistent DTH response in all animals, while PC14-2 was positive in one animal and PC14-1 failed to elicit any response (Table 1). The animals that were immunized over 100 days post-transplant, were injected with the four PC1 peptides in addition to all three PC14 peptide. Either the PC1-1, PC1-4 or PC14-3 peptides triggered DTH reactions in all animals, whereas the rest of the peptides demonstrated variable or no responses (Table 1). In all animals, the positive MTB control peptide elicited a potent DTH response while the negative RT1Du control was unreactive. These results corroborate our previous study (15) by demonstrating that certain synthetic class Ic peptides were capable of sensitizing recipients to donor antigen.
Table 1.
Delayed type hypersensitivity responses of immunized swine*
| Rx | Animal Number |
SLA class Ic peptides | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PC1 | PC14 | Controls | |||||||||
| Day −21 |
1 | 2 | 3 | 4 | 1 | 2 | 3 | MTB | Rt1 | PBS | |
| 15940 | - | - | - | - | 0 | 7 | 23 | 17 | 0 | 0 | |
| 15942 | - | - | - | - | 0 | 8 | 22 | 11 | 0 | 0 | |
| 16119 | - | - | - | - | 0 | 21 | 29 | 22 | 0 | 0 | |
| Day >100 |
14663 | 15 | 0 | 5 | 15 | 0 | 0 | 20 | 30 | 0 | 0 |
| 14779 | 17 | 11 | 0 | 13 | 7 | 0 | 22 | 37 | 0 | 0 | |
| 14781 | 13 | 0 | 13 | 9 | 0 | 0 | 17 | 33 | 0 | 0 | |
| 16946 | 7 | 5 | 0 | 16 | 0 | 8 | 15 | 31 | 0 | 0 | |
| 17033 | 12 | 8 | 0 | 16 | 11 | 6 | 11 | 41 | 0 | 0 | |
Values represent millimeters of induration with >10mm considered a positive response. MTB = Mycobacterium Tuberculosis, Rt1Du = Rat Peptide, PBS=Phosphate Buffered Saline.
Proliferative Responses against Donor Class I Peptides in Immunized Recipients
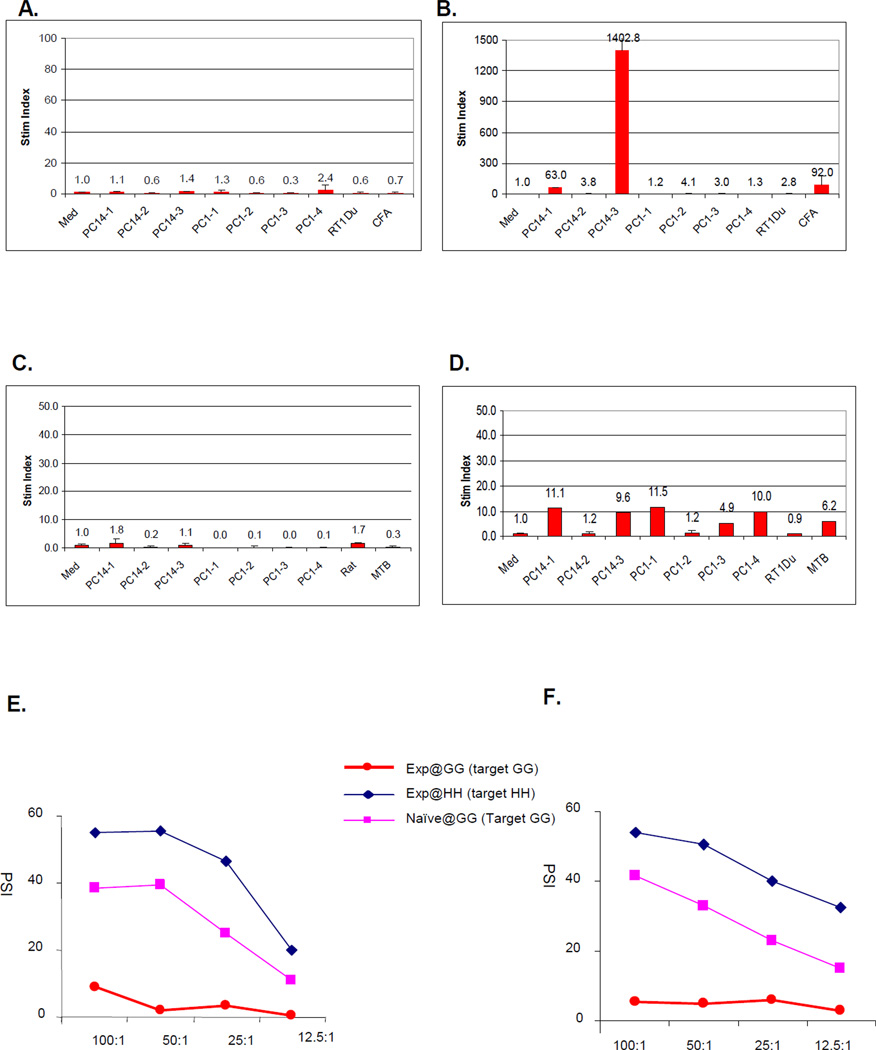
To assess in vitro reactivity to individual class I allopeptides, peptide proliferation assays were performed with PBMCs from the peptide-immunized recipients. Naïve swine did not spontaneously respond to any of the class Ic peptides (Fig 1A). However, 14 days after immunization with the three class Ic PC14 peptides, strong reactivity developed to one of the peptides (PC14-3) (Fig 1B). Similarly, before immunization, swine bearing long term heart and kidney allografts demonstrated no reactivity to any of the donor class I peptides (Fig 1C), but after immunization they responded to a number of PC1 and PC14 peptides (Fig 1D). The magnitude of the proliferative responses in the recipients bearing long term allografts, however, was markedly lower than seen in immunized naïve animals (Fig 1B). Of note, immunization of the recipients bearing long term heart and kidney allografts did not alter the donor-specific hyporesponsiveness seen in CML assays (compare Fig 1E to Fig 1F).
Figure 1.
Representative in vitro proliferation assays. (A) Peptide proliferation assays (PPA) to PC14 class Ic peptides in a naïve (unimmunized) animal, 16619 and (B) 21 days after this animal was immunized with each of the PC14 class Ic peptides. (C) PPA to PC1 and PC14 class Ic peptides in the long term heart and kidney recipient (#17033) before immunization and (D) after immunization with each of the PC1 and PC14 class Ic donor peptides. (E) Cell mediated lympholysis (CML) assays performed in at the same time in the same long term heart and kidney recipient (#17033) before immunization and (F) after immunization with each of the PC1 and PC14 class Ic donor peptides. In these assays, PBMCs from SLAdd (Id IId) experimental animals (DD) were either primed by irradiated SLAgg (Ic IId) stimulator cells (GG) and then incubated with chromium-labeled GG target cells or primed by irradiated SLAhh (Ia IId) stimulator cells (HH) and then incubated with chromium-labeled HH cells. As controls, PBMC’s from naïve DD animal were primed by irradiated GG cells then incubated with chromium-labeled GG target cells.
Immunization of heart and kidney recipients with donor MHC class I peptides 21 days prior to transplantation results in the acute rejection of heart and kidney allografts
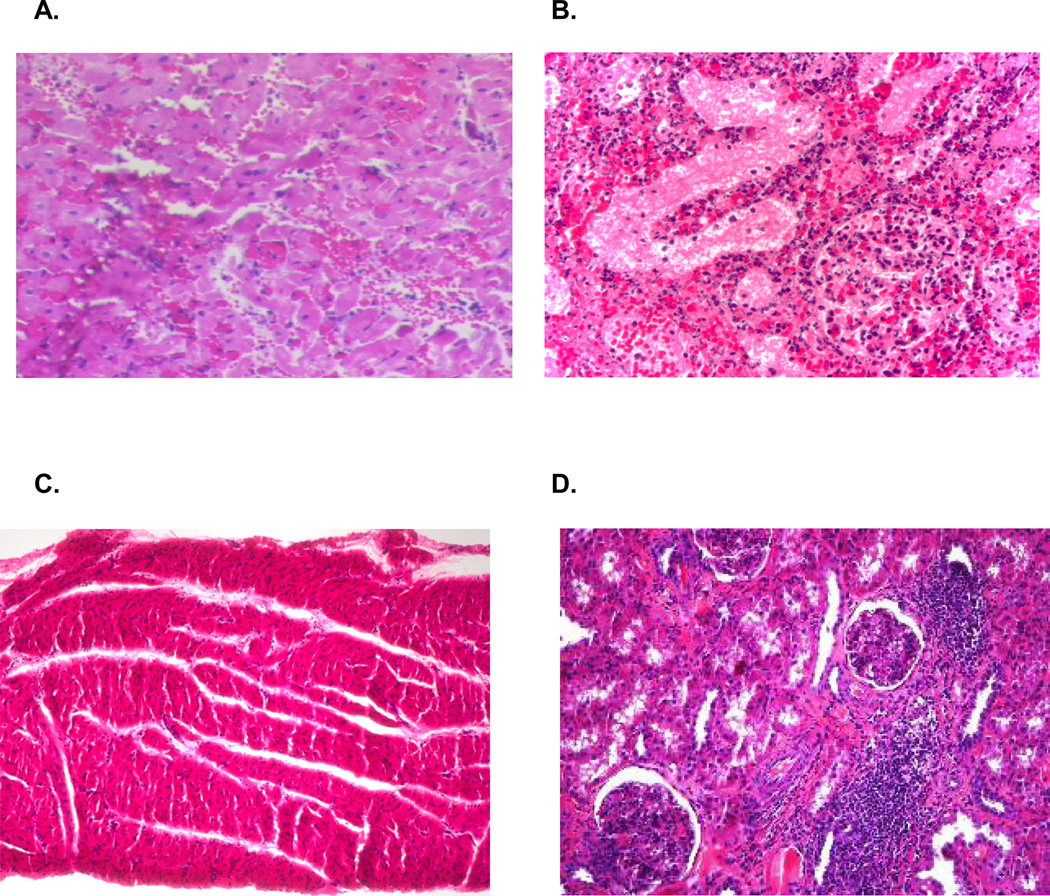
We have previously demonstrated that unimmunized recipients of class I mismatched heart and kidney grafts treated with 12 days of CyA accepted both grafts long term (6) (Table 2, group 1). Serial biopsies of these allografts revealed no heart or kidney rejection and the absence of CAV. To determine the effects of donor peptide immunization and indirect allorecognition on the induction of tolerance, three SLAdd (Id IId) swine were immunized with a mixture of all three PC14 class Ic peptides. Twenty-one days later, they were cotransplanted with class I mismatched SLAgg (Ic IId) heart and kidney allografts under the cover of a 12 day course of CyA. In stark contrast to unimmunized controls, recipients immunized with the mixture of PC14 class Ic peptides rejected their SLAgg hearts in an accelerated manner (POD 6, 8, and 10), while still receiving CyA (p<0.001, Table 2, group 2). Histological examination of the heart and kidney transplants harvested from swine immunized with donor-derived MHC peptides revealed severe acute cellular rejection of both allografts characterized by tissue necrosis and massive inflammatory cell infiltration (Fig 2A and B).To assess the specificity of this effect, two SLAdd (Id IId) swine were immunized with the same mixture of three PC14 class Ic peptides but transplanted with SLAhh (Ia IId) heart and kidney allografts with were class I mismatched to both the recipient and the peptides. These controls accepted their heart and kidney allografts long term with no histological evidence of rejection or CAV (Table 2, group 3).
Table 2.
Cardiac allograft survival in experimental and control animals
| Group | Animal # | Immunization | Peptide | Graft Survival (days) |
|---|---|---|---|---|
| 1 | 11982* | N/A | N/A | >179 |
| 12232* | N/A | N/A | >269 | |
| 12333* | N/A | N/A | >261 | |
| 12614* | N/A | N/A | >200 | |
| 2 | 15940 | Day -21 | Donor | 6 |
| 15942 | Day -21 | Donor | 8 | |
| 16119 | Day -21 | Donor | 10 | |
| 3 | 16051 | Day -21 | 3rd party | >101 |
| 16120 | Day-21 | 3rd party | >101 | |
| 4 | 14663 | Day 170 | Donor | >294 |
| 14779 | Day 101 | Donor | >199 | |
| 14781 | Day 104 | Donor | >210 | |
| 16946 | Day 150 | Donor | >390 | |
| 17033 | Day 125 | Donor | >329 | |
The results of these control animals have been previously reported (6).
Figure 2.
Representative graft histology by hematoxylin and eosin staining. (A) Necropsy specimen from the cardiac allograft in recipient #15942 showing ISHLT grade 4/4 rejection. (B) Necropsy specimen from the renal allograft in recipient #15942 showing ACR 3 rejection. (C) Biopsy specimen from the cardiac allograft of animal #17033 biopsies on POD 196 showing no evidence of cellular rejection. (D) Biopsy specimen from the renal allograft of animal #17033 biopsies on POD 196 showing a mononuclear cell infiltrate but no signs of kidney graft destruction (ACR 1).
Immunization of heart and kidney recipients with donor MHC class I peptides over 100 days following transplantation fails to break tolerance to kidney/heart allografts
To determine the effects of donor peptide immunization and indirect allorecognition on a state of tolerance that is well-established, donor peptide administration was delayed until over 100 days after transplantation. Four SLAdd (Id IId) swine received SLAgg (Ic IId) heart and kidney allografts and one SLAjj (Ia IIc) swine received a SLAcc (Ic IIc) heart-kidney transplant along with a 12 day course of CyA. As expected, these animals exhibited no signs of rejection on biopsy and no anti-donor responsiveness in CML assays (Fig 1E) by day 100. Between days 101 and 170, each recipient was immunized with a mixture of the three PC14 and the four PC1 class Ic peptides. Despite clear evidence on sensitization in DTH (Table 1) and peptide proliferation assays (Fig 1), neither the heart or kidney allografts were adversely affected by donor peptide immunization (Table 2, group 4). Each recipient was sacrificed with viable allografts. Pathologic examination of the cardiac allografts demonstrated no evidence of significant acute cellular rejection (Fig 2C) and morphometric analysis failed to reveal evidence of CAV. Pathologic examination of renal allografts revealed only low grade (ACR 1) infiltration (Fig 2D).
Alloantibody Production in Peptide-Immunized Heart and Kidney Recipients
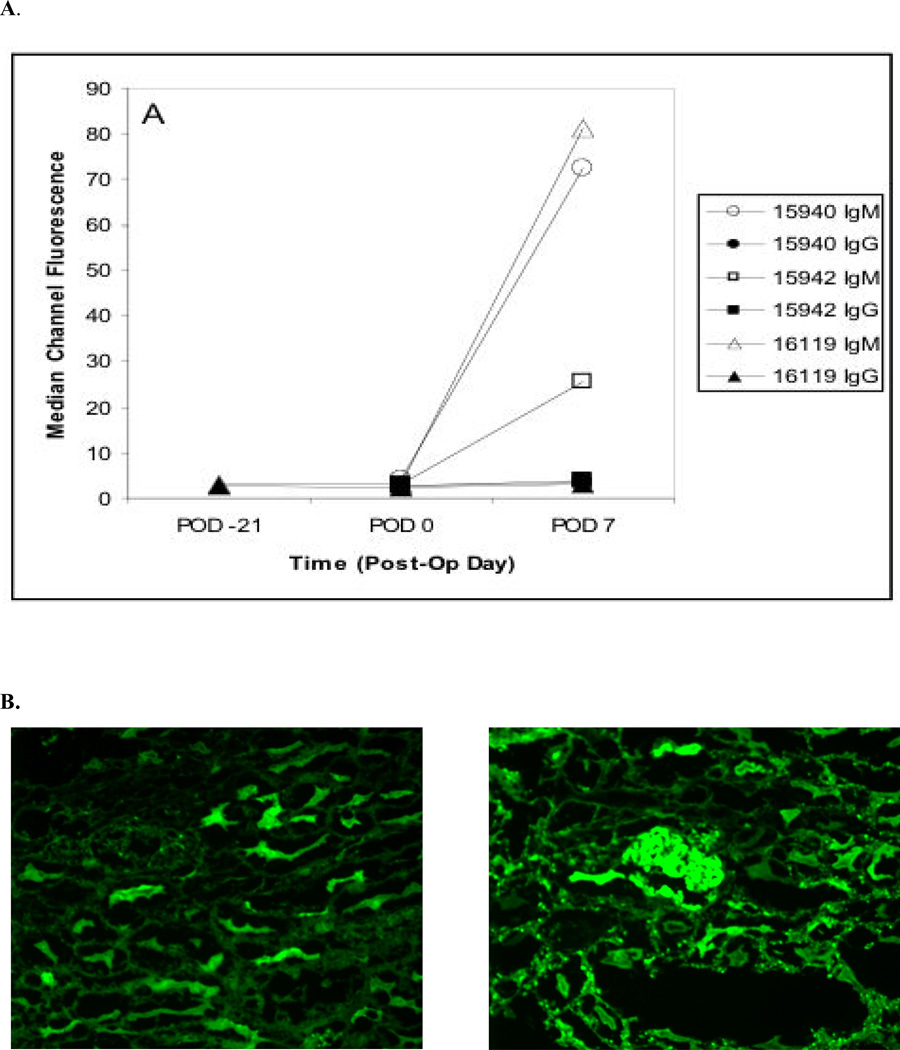
Serum samples were collected at serial time points from all transplanted swine and evaluated for the presence of donor-reactive antibodies (IgM and IgG) by flow cytometry. Figure 3A shows that allopeptide immunization did not trigger the production of anti-donor antibodies prior to transplantation. However, allospecific antibodies displaying IgM isotypes were detected as early as POD 7 in the serum of recipients transplanted 21 days after peptide immunization and rejected early (Fig 3A). Although no donor-specific IgG was found in these recipient’s sera, significant deposition of both anti-donor IgG was found on the arteriolar endothelium of both the heart and kidney by immunofluorescence (Figure 3B). In contrast, no donor-specific antibodies were detected in long term heart and kidney recipients before or after peptide immunization.
Figure 3.
Presence of alloantibodies. (A) Serum anti-donor antibody levels of pre-operatively immunized animals as assessed by flow cytometry. (B) Immunoflourescent staining shows the presence of anti-donor IgG on the endothelium of both the heart (left) and kidney (right) allografts in pig #16119.
Discussion
Fully allogeneic transplants undergo early acute rejection via a process initiated by recipient CD4+ T lymphocytes recognizing intact MHC class II molecules on donor passenger leukocytes (direct allorecognition) (32,33). In the absence of a MHC class II disparity between donors and recipients, allogeneic transplants do not elicit a direct alloresponse by CD4+ T cells (13,34). In turn, some MHC class I peptides may be endogenously processed and presented by donor APCs thus eliciting an oligoclonal direct alloresponse. Most importantly, these transplants induce an indirect CD4+ T cell-mediated alloresponse directed to MHC class I and minor histocompatibility antigen peptides presented by self-MHC class II on recipient’s APCs (14,35,36). CD4+ T cell indirect alloreactivity is thought to be sufficient to ensure the acute rejection of MHC class I disparate skin grafts presumably via the activation of CD8+ cytotoxic T cells recognizing intact donor MHC class I molecules present on transplanted cells (direct allorecognition) (14,37). However, the contribution of CD4+ T cell indirect allorecognition to the acute rejection of vascularized solid organ transplants such as kidneys and hearts is still unclear. Actually, some studies suggest that, in the absence of a direct alloresponse, some CD4+ regulatory cells (Tregs) may be activated indirectly and exert sometolerogenic effects (38–41). Supporting this view, Okumi et al. have recently shown that persistence of an indirect alloresponse is required for the maintenance of tolerance to renal allografts in swine (42). Additionally, strong circumstantial evidence has been accumulated showing that indirect allorecognition by CD4+ T cells leads to chronic type of rejection essentially by promoting the production of alloreactive (43,44) and autoreactive (45–48) antibodies. The most compelling evidence for the potential role of indirect allorecognition in chronic allograft rejection has been provided in a study showing that injection of swine with allo MHC class I peptides given along with complete Freund’s adjuvant accelerates the onset of cardiac allograft vasculopathy in heart-transplanted animals (17). These observations underscore the complex roles of indirect allorecognition in acute and chronic rejection of vascularized organ transplants.
In this paper, we studied the influence of allo-MHC class I peptide immunization on the alloimmune response to and rejection of kidney-heart cotransplants in MHC class I disparate swine. In this model, recipients treated with CyA for 12 days accept permanently MHC class I-mismatched kidneys. This suggests that, in the absence of a CD4+ T cell alloresponse, T cells activated through the indirect pathway are either ineffective at rejecting allogeneic kidneys or that they even exert some tolerogenic effect. In support of the second hypothesis is the observation that, in this setting, combined kidney-heart allografts also enjoy indefinite survival without signs of chronic rejection while isolated heart transplants are regularly rejected. Therefore, following short-term immunosuppression and abrogation of the CD8+ T cell direct alloresponse, MHC class I-mismatched kidney allografts were tolerogenic, presumably via a process involving indirectly activated regulatory T cells. It is noteworthy that removal of the kidney transplant 100 days after its placement, the animals results in the loss of donor-specific tolerance (49). This indicates that persistence of indirect antigen presentation is necessary for the maintenance of tolerance via a mechanism, which is consistent with the involvement of regulatory T cells rather than clonal deletion. Indeed, we have identified a population of CD25+ T cells in the peripheral blood of heart/kidney recipients that demonstrated in vitro suppression by preventing lysis of donor targets. This same population did not appear to exist in the peripheral blood of naïve animals (50).
First, we investigated whether induction of a pro-inflammatory Th1 indirect alloresponse could either prevent or abolish tolerance in this model. To test that, swine were immunized subcutaneously with different allo-MHC class I peptides emulsified in complete Freund’s adjuvant, a procedure known to activate a Th1 type indirect alloresponse (51). We found that swine injected pre-transplantation mounted a potent inflammatory response directed primarily to the dominant MHC class I allopeptide P14.3 corresponding to the region 60–85 of the alpha 1 domain of the donor MHC class I molecule. It is noteworthy that this region of MHC class I has been previously shown to represent the focus of indirect alloreactivity in other transplant models (52). All swine immunized with this peptide subsequently rejected both hearts and kidney transplants. This phenomenon was associated with induction of a direct alloresponse (MLR) and the differentiation of cytotoxic T cells. Therefore, pro-inflammatory CD4+ T cells activated indirectly prior to transplantation had prevented tolerance induction in this model presumably by sensitizing some CD8+ cytotoxic T cells recognizing donor MHC class I molecules in a direct fashion.
In another set of experiments, we showed that allopeptide immunization of swine, which had accepted their kidney-heart transplants for 100 days, did not cause transplant rejection. Therefore, in this model, induction of a pro-inflammatory indirect alloresponse failed to break the tolerance initially induced by the kidney. Immunization of tolerant swine with donor MHC class I peptides, although it triggered some DTH and proliferative responses (presumably by CD4+ T cells), it did not restore a CTL response in these animals. This suggests that anergic or inactivated CD8+ T cells are resistant to further stimulation by newly activated CD4+ T cells or that these CD8+ T cells have been deleted during tolerance induction. Interestingly, in contrast to what we observed in naïve swine, peptide immunization post-transplantation failed to induce potent DTH and proliferative responses following challenge with the dominant P14.3 peptide. This suggests that CD4+ T cells recognizing this dominant allodeterminant in an indirect fashion had been rendered tolerant in kidney-transplanted swine.
In summary, our results show that, following inhibition of the direct CD8+ direct alloresponse using short-term immunosuppression with CyA, MHC class I-mismatched kidney transplants are tolerogenic via a process presumably involving CD4+ T cells activated indirectly. In the presence of a pro-inflammatory indirect alloresponse pre-transplantation, tolerance cannot be induced via this mechanism. In turn, once tolerance has been established, it cannot be disrupted by oligoclonal activation of CD4+ T cells specific of donor MHC class I peptides. This implies that the lack of MHC class II disparity and the absence of MHC class I peptide-reactive memory CD4+ T cells in recipients represent two essential requirements for tolerance induction to vascularized organ transplants.
Acknowledgements
We thank Drs. Timothy M. Millington and Gregory R. Veillette for critical review of the manuscript. We also thank Novartis for their gift of cyclosporine.
Footnotes
This work was supported in part by the National Heart Lung and Blood Institute (RO1HL54211, PO1HL18646) of the National Institutes of Health and by an American College of Surgeons Resident Research Award to MJW.
Reference List
- 1.Calne R, Davies H. Organ graft tolerance: the liver effect. Lancet. 1994;343:67–68. doi: 10.1016/s0140-6736(94)90809-5. [DOI] [PubMed] [Google Scholar]
- 2.Praseedom RK, McNeil KD, Watson CJ, et al. Combined transplantation of the heart, lung, and liver. Lancet. 2001;358:812–813. doi: 10.1016/S0140-6736(01)06003-2. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen A, Davies HF, Jamieson NV, Evans DB, Calne RY. Combined transplantation of liver and kidney from the same donor protects the kidney from rejection and improves kidney graft survival. Transplantation. 1995;59:919–921. [PubMed] [Google Scholar]
- 4.Narula J, Bennett LE, DiSalvo TG, Hosenpud JD, Semigran MJ, Dec GW. Outcomes in recipients of combined heart-kidney transplantation. Multi-organ, same-donor transplant study of the ISHLT/UNOS scientific registry. Transplantation. 1997;63:861–867. doi: 10.1097/00007890-199703270-00012. [DOI] [PubMed] [Google Scholar]
- 5.Vermes E, Kirsch M, Houel R, et al. Immunologic events and long term survival after combined heart and kidney transplantation : a twelve-year single-center experience. J Heart Lung Transplant. 2001;20:247. doi: 10.1016/s1053-2498(00)00561-1. [DOI] [PubMed] [Google Scholar]
- 6.Madsen JC, Yamada K, Allan JS, et al. Transplantation tolerance prevents cardiac allograft vasculopathy in major histocompatibility complex class I-disparate miniature swine. Transplantation. 1998;65:304–313. doi: 10.1097/00007890-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 7.Madsen JC, Sachs DH, Fallon JT, Weissman NJ. Cardiac allograft vasculopathy in partially inbred miniature swine. I. Time course, pathology, and dependence on immune mechanisms. J Thorac Cardiovasc Surg. 1996;111:1230–1239. doi: 10.1016/s0022-5223(96)70226-x. [DOI] [PubMed] [Google Scholar]
- 8.Mezrich J, Yamada K, Sachs DH, Madsen JC. Regulatory T cells generated by the kidney may mediate the beneficial immune effects of combining kidney with heart transplantation. Surgery. 2004;135:473–478. doi: 10.1016/j.surg.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Lechler RI, Lombardi G, Batchelor JR, Reinsmoen NL, Bach FH. The molecular basis of alloreactivity. Immunol Today. 1990;11:83–88. doi: 10.1016/0167-5699(90)90033-6. [DOI] [PubMed] [Google Scholar]
- 10.Benham AM, Sawyer GJ, Fabre JW. Indirect T cell allorecognition of donor antigens contributes to the rejection of vascularized kidney allografts. Transplantation. 1995;59:1028–1032. doi: 10.1097/00007890-199504150-00019. [DOI] [PubMed] [Google Scholar]
- 11.Benichou G, Takizawa PA, Olson CA, McMillan A, Sercarz EE. Donor major histocompatibility complex (MHC) peptides are presented by recipient MHC molecules during graft rejection. J Exp Med. 1992;175:305–308. doi: 10.1084/jem.175.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z, Colovai AI, Tugulea S, et al. Indirect recognition of donor HLA-DR peptides in organ allograft rejection. J Clin Invest. 1996;98:1150–1157. doi: 10.1172/JCI118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auchincloss H, Lee RS, Shea S, Markowitz JS, Grusby MJ, Glimcher LH. The role of "indirect" recognition in initiating rejection of skin grafts from major histocompatibility complex class II-deficient mice. Proc Natl Acad Sci U S A. 1993;90:3373–3377. doi: 10.1073/pnas.90.8.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee RS, Grusby MJ, Glimcher LH, Winn HJ, Auchincloss H. Indirect recognition by helper cells can induce donor-specific cytotoxic T lymphocytes in vivo. J Exp Med. 1994;179:865–872. doi: 10.1084/jem.179.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, Murphy B, Waaga AM, et al. Mechanisms of indirect allorecognition in graft rejection. Transplantation. 1996;62:705–710. doi: 10.1097/00007890-199609270-00001. [DOI] [PubMed] [Google Scholar]
- 16.Steele DJ, Laufer TM, Smiley ST, et al. Two levels of help for B cell alloantibody production. J Exp Med. 1996;183:699–703. doi: 10.1084/jem.183.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee RS, Yamada K, Houser SL, et al. Indirect recognition of allopeptides promotes the development of cardiac allograft vasculopathy. Proc Natl Acad Sci U S A. 2001;98:3276–3281. doi: 10.1073/pnas.051584498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sayegh MH, Carpenter CB. Tolerance and chronic rejection. Kidney Int Suppl. 1997;58:S11–S14. [PubMed] [Google Scholar]
- 19.Shirwan H. Chronic allograft rejection Do the Th2 cells preferentially induced by indirect alloantigen recognition play a dominant role? Transplantation. 1999;68:715–726. doi: 10.1097/00007890-199909270-00001. [DOI] [PubMed] [Google Scholar]
- 20.Suciu-Foca N, Ciubotariu R, Itescu S, Rose EA, Cortesini R. Indirect allorecognition of donor HLA-DR peptides in chronic rejection of heart allografts. Transplant Proc. 1998;30:3999–4000. doi: 10.1016/s0041-1345(98)01318-9. [DOI] [PubMed] [Google Scholar]
- 21.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 22.Sawyer GJ, Dalchau R, Fabre JW. Indirect T cell allorecognition: a cyclosporin A resistant pathway for T cell help for antibody production to donor MHC antigens. Transpl Immunol. 1993;1:77–81. doi: 10.1016/0966-3274(93)90063-e. [DOI] [PubMed] [Google Scholar]
- 23.Boisgerault F, Anosova NG, Tam RC, Illigens BM, Fedoseyeva EV, Benichou G. Induction of T-cell response to cryptic MHC determinants during allograft rejection. Hum Immunol. 2000;61:1352–1362. doi: 10.1016/s0198-8859(00)00209-3. [DOI] [PubMed] [Google Scholar]
- 24.Suciu-Foca N, Harris PE, Cortesini R. Intramolecular and intermolecular spreading during the course of organ allograft rejection. Immunol Rev. 1998;164:241–246. doi: 10.1111/j.1600-065x.1998.tb01224.x. [DOI] [PubMed] [Google Scholar]
- 25.Pennington LR, Lunney JK, Sachs DH. Transplantation in miniature swine. VIII. Recombination within the major histocompatibility complex of miniature swine. Transplantation. 1981;31:66–71. [PubMed] [Google Scholar]
- 26.Sullivan JA, Oettinger HF, Sachs DH, Edge AS. Analysis of polymorphism in porcine MHC class I genes: alterations in signals recognized by human cytotoxic lymphocytes. J Immunol. 1997;159:2318–2326. [PubMed] [Google Scholar]
- 27.Madsen JC. Cardiac allograft vasculopathy in miniature swine: Utility of a large animal model. Graft. 1998;1 suppl II:41–44. [Google Scholar]
- 28.Billingham ME, Cary NR, Hammond ME, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: heart rejection study group. Journal of Heart Transplantation. 1990;9:587–593. [PubMed] [Google Scholar]
- 29.Colvin RB. The renal allograft biopsy. Kidney Int. 1996;50:1069–1082. doi: 10.1038/ki.1996.410. [DOI] [PubMed] [Google Scholar]
- 30.Leight GS, Sachs DH, Rosenberg SA. Transplantation in miniature swine. II. In vitro parameters of histocompatibility in MSLA homozygous minipigs. Transplantation. 1977;23:271–276. [PubMed] [Google Scholar]
- 31.Allan JS, Wain JC, Schwarze ML, et al. Modeling chronic lung allograft rejection in miniature swine. Transplantation. 2002;73:447–453. doi: 10.1097/00007890-200202150-00020. [DOI] [PubMed] [Google Scholar]
- 32.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990;171:307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982;155:31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Illigens BM, Yamada A, Fedoseyeva EV, et al. The relative contribution of direct and indirect antigen recognition pathways to the alloresponse and graft rejection depends upon the nature of the transplant. Hum Immunol. 2002;63:912–925. doi: 10.1016/s0198-8859(02)00449-4. [DOI] [PubMed] [Google Scholar]
- 35.Lee RS, Grusby MJ, Laufer TM, Colvin R, Glimcher LH, Auchincloss H., Jr CD8+ effector cells responding to residual class I antigens, with help from CD4+ cells stimulated indirectly, cause rejection of "major histocompatibility complex-deficient" skin grafts. Transplantation. 1997;63:1123–1133. doi: 10.1097/00007890-199704270-00012. [DOI] [PubMed] [Google Scholar]
- 36.Popov IA, Fedoseyeva EV, Orr PL, Garovoy MR, Benichou G. Direct evidence for in vivo induction of CD8+ cytotoxic T cells directed to donor MHC class I peptides following mouse allotransplantation. Transplantation. 1995;60:1621–1624. [PubMed] [Google Scholar]
- 37.Lee R, Glimcher LH, Auchincloss H., Jr Evidence that a "four-cell cluster" may prime cytotoxic T-cells during graft rejection. Transplant Proc. 1993;25:847–849. [PubMed] [Google Scholar]
- 38.Niimi M, Shirasugi N, Ikeda Y, Wood KJ. Oral antigen induces allograft survival by linked suppression via the indirect pathway. Transplant Proc. 2001;33:81. doi: 10.1016/s0041-1345(00)01913-8. [DOI] [PubMed] [Google Scholar]
- 39.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nature Reviews. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 40.Wise MP, Bemelman F, Cobbold SP, Waldmann H. Linked suppression of skin graft rejection can operate through indirect recognition. J Immunol. 1998;161:5813–5816. [PubMed] [Google Scholar]
- 41.Yamada A, Chandraker A, Laufer TM, Gerth AJ, Sayegh MH, Auchincloss H., Jr Recipient MHC class II expression is required to achieve long-term survival of murine cardiac allografts after costimulatory blockade. J Immunol. 2001;167:5522–5526. doi: 10.4049/jimmunol.167.10.5522. [DOI] [PubMed] [Google Scholar]
- 42.Okumi M, Fishbein JM, Griesemer AD, et al. Role of Persistence of Antigen and Indirect Recognition in the Maintenance of Tolerance to Renal Allografts. Transplantation. 2008;85:270–280. doi: 10.1097/TP.0b013e31815e8eed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hornick PI, Mason PD, Baker RJ, et al. Significant frequencies of T cells with indirect anti-donor specificity in heart graft recipients with chronic rejection. Circulation. 2000;101:2405–2410. doi: 10.1161/01.cir.101.20.2405. [DOI] [PubMed] [Google Scholar]
- 44.SivaSai KS, Smith MA, Poindexter NJ, et al. Indirect recognition of donor HLA class I peptides in lung transplant recipients with bronchiolitis obliterans syndrome. Transplantation. 1999;67:1094–1098. doi: 10.1097/00007890-199904270-00002. [DOI] [PubMed] [Google Scholar]
- 45.Fedoseyeva EV, Kishimoto K, Rolls HK, et al. Modulation of tissue-specific immune response to cardiac myosin can prolong survival of allogeneic heart transplants. J Immunol. 2002;169:1168–1174. doi: 10.4049/jimmunol.169.3.1168. [DOI] [PubMed] [Google Scholar]
- 46.Fedoseyeva EV, Zhang F, Orr PL, Levin D, Buncke HJ, Benichou G. De novo autoimmunity to cardiac myosin after heart transplantation and its contribution to the rejection process. J Immunol. 1999;162:6836–6842. [PubMed] [Google Scholar]
- 47.Mares DC, Heidler KM, Smith GN, et al. Type V collagen modulates alloantigen-induced pathology and immunology in the lung. Am J Respir Cell Mol Biol. 2000;23:62–70. doi: 10.1165/ajrcmb.23.1.3924. [DOI] [PubMed] [Google Scholar]
- 48.Rose ML. Anti-vimentin antibodies are an independent predictor of transplant-associated coronary artery disease following cardiac transplantation. Ital Heart J. 2001;2 Suppl 3:23S–25S. [PubMed] [Google Scholar]
- 49.Mezrich J, Yamada K, Sachs DH, Madsen JC. Regulatory T cells generated by the kidney may mediate the beneficial immune effects of combining kidney with heart transplantation. Surgery. 2004;135:473–478. doi: 10.1016/j.surg.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 50.Mezrich JD, Kesselheim JA, Johnston DR, Yamada K, Sachs DH, Madsen JC. The role of regulatory cells in miniature Swine rendered tolerant to cardiac allografts by donor kidney cotransplantation. Am J Transplant. 2003;3:1107–1115. doi: 10.1046/j.1600-6143.2003.00202.x. [DOI] [PubMed] [Google Scholar]
- 51.Benichou G, Malloy KM, Tam RC, Heeger PS, Fedoseyeva EV. The presentation of self and allogeneic MHC peptides to T lymphocytes. Hum Immunol. 1998;59:540–548. doi: 10.1016/s0198-8859(98)00059-7. [DOI] [PubMed] [Google Scholar]
- 52.Benichou G, Fedoseyeva EV. The contribution of peptides to T cell allorecognition and allograft rejection. Int Rev Immunol. 1996;13:231–243. doi: 10.3109/08830189609061750. [DOI] [PubMed] [Google Scholar]