Abstract
Bone morphogenetic protein 11 (BMP11) is a key regulatory protein in skeletal development. BMP11 propeptide has been shown to antagonize GDF11 activity in vitro. To explore the role of BMP11 propeptide in skeletal formation in vivo, we generated transgenic mice with skeleton-specific overexpression of BMP11 propeptide cDNA. The mice showed a transformation of the seventh cervical vertebra into a thoracic vertebra in our previous report. Presently, further characterizations of the transgenic mice indicated that ossification in calvatia was dramatically enhanced in transgenic fetuses at 16.5 dpc in comparison with their wild-type littermates. At 10 weeks of age, bone mineral content and bone mineral density were significantly (P<0.05) higher in transgenic mice than that in their wild-type littermates based on dual energy X-ray absorptiometry analysis. The relative trabecular bone volume measured by histological analysis was dramatically increased in transgenic mice compared with their wild- type littermates. The enhanced bone formations in the transgenic mice appear to result from increase osteoblast activities as the expressions of four osteoblast markers-α 1 type 1 collagen, osteocalcin, alkaline phosphatase and phex were significantly higher in transgenic fetuses than that in their wild-type littermates. These results suggest that over-expression of BMP11 propeptide stimulates bone formation by increasing osteoblast cell functions.
Keywords: BMP11 propeptide, GDF11, skeleton, osteoblast: transgenic mice
Introduction
Bone morphogenetic proteins (BMPs) are a group of regulatory proteins that participate in various biological processes such as embryonic skeletal patterning, postnatal bone formation and metabolism [1–3]. BMP11, also known as growth differentiation factor 11 (GDF11), was initially identified as a BMP member highly expressed in mesodermal tissue during embryogenesis [4, 5]. It was demonstrated that BMP11 sregulates axial skeletal patterning as well as limb skeletal formation [6–8]. BMP11 is initially translated as a precursor protein that undergoes a proteolytic cleavage to generate the mature BMP11 protein and the BMP11 propeptide, correspond respectively to the C-terminal and the N-terminal peptide [9]. BMP11 propeptide has been shown to effectively antagonize BMP11 activity in vitro by forming a latent complex with BMP 11 [9]. To evaluate the roles of BMP11 propeptide in skeletal development in vivo, we generated transgenic mice that over-express BMP11 propeptide under the control of a bone-specific regulatory element, the 2.3 kb α1 type 1 collagen promoter. These transgenic mice were viable and fertile, but exhibited abnormal patterning of axial skeleton with transformation of the seventh cervical vertebra into a thoracic vertebra by forming ectopic ribs on the seventh cervical vertebra [10]. The transgene mRNA was detectable in tail tissue at 12.5 dpc and later times (14.5 and 16.5 dpc). The transgene mRNA expression was detected in bone and several other tissues. A high level of transgene mRNA was detected in calvaria bone compared with muscle, fat, brain, lung, heart, liver and kidney [10]. A further characterization of the transgenic mice in the present study shows that skeletal- specific expression of the BMP11 propeptide cDNA significantly increased skeletal formation and expression levels of several osteoblast marker genes.
Materials and Methods
Generation of the transgenic mice
The transgene construct with osteoblast-specific 2.3kb α1 type 1 collagen promoter, mouse BMP11 propeptide cDNA (encoding for 1-296 AA) and mouse protamine 1 poly A signal sequence, and production of the transgenic mice were previously reported[10]. The transgenic mice and their wild-type littermates that were used for analyses in the present study were from the high transgene expression line [10].
Alizarin red and alcian blue staining of the skeletons
Mouse embryos at 16.5 d.p.c. were collected from pregnant wild-type B6SJL F1 female mice that were mated with transgenic males. A piece of tail tissue was taken from each embryo to isolate DNA for PCR genotyping. The skeletons of embryos were stained with alizarin red and alcian blue as previously described [10]. Stained skeletons were compared between the transgenic embryos and wild-type littermates.
X-ray analyses of total bone mineral content, bone area and bone mineral density
Ten week-old transgenic mice and their wild-type littermates were euthanized and scanned by dual energy X-ray absorptiometry (DXA). The DXA instrument used in this study was the PIXImus small animal DXA system (Lunar Corporation). Total bone mineral content, bone area and bone mineral density were measured as described by Mitchell and Wall [11].
Histological analysis of bone volume
The femora were dissected from euthanized 10 week-old mice, fixed in 4% paraformaldehyde at 4°C for 24h, washed with PBS, stored in 70% ethanol, embedded in glycol methacrylate and 7–10 μm sections were cut by a microtome. The sections were stained by Von Kossa method which is used to detect the deposits of calcium or calcium salt. Stained sections were observed under the microscopy and images of the sections were captured with a camera connected to the microscope. Section images were then analyzed by the BioQuant image analysis system. Relative trabecular bone volume (trabecular bone volume/tissue volume, %) was measured in the bone marrow cavity area between 0.5 mm to 2 mm away from the growth plates.
Expression analysis of osteoblast marker genes by quantitative real-time PCR
The calvarial skeletal tissue was dissected from 17.5 dpc wild-type fetuses and their transgenic littermates. Total RNA was isolated from calvaria and then was reverse transcribed into cDNA, followed by DNase I treatment to eliminate any contaminating genomic DNA. Expression levels of 4 osteogenic marker genes, including α 1 type 1 collagen, osteocalcin, alkaline phosphatase and phex, were measured by quantitative real-time PCR as previously described [12]. The primers for the quantitative real-time PCR analyses of 4 osteoblast markers are as follow: α1 type 1 collagen-F: 5′-GGGCGAGTGCTGTGCTTT-3′; α1 type 1 collagen-R: 5′-CCCTCGACTCCTACATCTTCTGA-3′; Osteocalcin-F: 5′-GAGGACCATCTTTCTGCTCACTCT-3′; Osteocalcin-R: 5′-GAACCCGGAGGACACATACCT-3′; Alkaline phosphatase-F: 5′-GGTATGGGCGTCTCCACAGT-3′; Alkaline phosphatase-R: 5′-GCCCGTGTTGTGGTGTAGCT-3′; Phex-F: 5′-GACATTGGTCCCTCGGAGAA-3′; Phex-R: 5′-GGCACCTAATATCCTAAACAAATCTTTAA-3′. GAPDH gene was used as internal control in quantitative real-time PCR and it was amplified by the following set of primers: GAPDH-F: 5′-GTGCTGAGTATGTCGTGGAG-3′; GAPDH-R: 5′-GTCTTCTGGGTGGCAGTGAT-3′.
Statistical analysis
Data were analyzed by SAS 9.2 program (SAS Institute, Cary, NC), and the two samples T-test for means program was used for mean comparisons. Data are present as mean ± standard error of mean (SEM). Significant difference of means between two different groups was determined at P < 0.05 (*) or P < 0.01 (**).
Results
Ossification was enhanced in transgenic mice
BMP11 propeptide transgene were expressed at 12.5 dpc in transgenic mice. To examine the effects of the transgene on bone development during fetal development, we set up the mating of three wild-type B6SJL F1 females with BMP11 propeptide transgenic males, and analyzed the skeletons of 16.5 dpc-old wild-type mice and their transgenic littermates by alizarin red and alcian blue staining. As demonstrated in Figure 1, ossification was enhanced in the calvaria of transgenic fetuses in comparison with their wild-type littermates. The mineralized area was wide-spread in the skull of transgenic mice while it was not seen in the skull of their wild-type littermates (Figure 1). This result suggests that transgenic over-expression of BMP11 propeptide enhances bone mineralization
Figure 1. Comparison of ossification status at 16.5 dpc between transgenic fetuses and mice and their wild-type littermates.
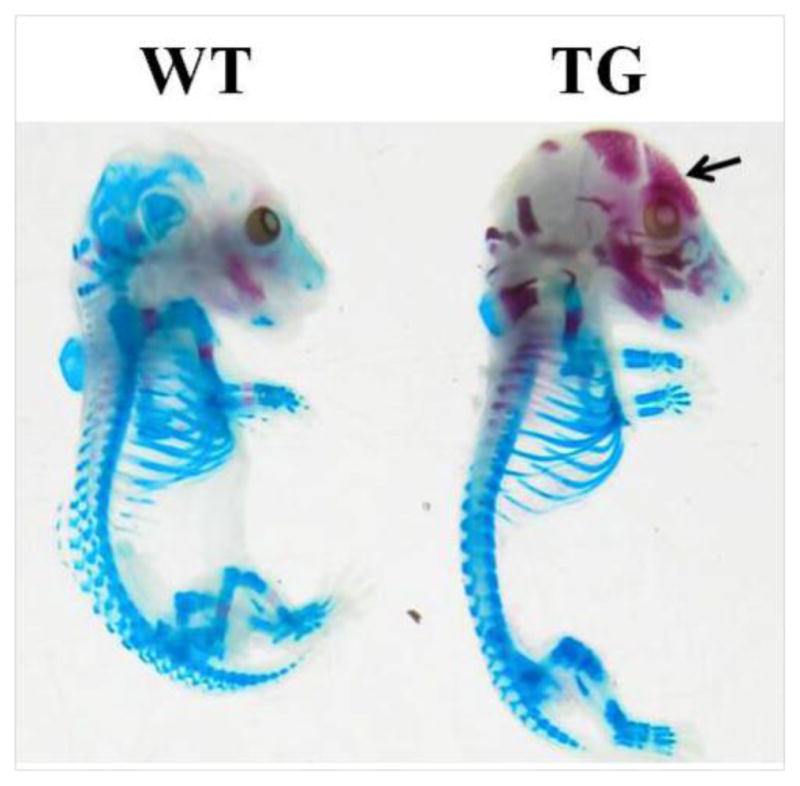
Ossification center, the red area pointed by an arrow, was formed in calvarial bones of transgenic (TG) fetuses but not in calvarial bones of their wild-type (WT) littermates.
Bone mineral content and mineral density were significantly increased in transgenic mice
To analyze the effects of skeleton-specific expression of BMP11 propeptide on postnatal bone formation, we measured the bone mineral content (BMC), bone area (BA) and bone mineral density (BMD) of 10 week-old transgenic mice and their wild-type littermates by DXA analysis. As shown in Table 1, BMC was significantly elevated by 11.12% and 14.96% in transgenic males and transgenic females, respectively; BMD was significantly increased in transgenic male mice and transgenic female mice by 9.68% and 8.69%, respectively, in comparison with their sex-matched wild-type littermates. There was no difference in BA between transgenic mice and their wild-type littermates. BMD also was significantly increased in transgenic male mice and transgenic female mice by 9.68% and 8.69%, respectively (Table 1).
Table 1.
Total bone mineral content, bone area and bone mineral density of wild-type mice and their transgenic littermates at 10 weeks of age*
| Male
|
Female
|
|||||
|---|---|---|---|---|---|---|
| Wild-type (n=12) | Transgenic (n=9) | Increase (%) | Wild-type (n=16) | Transgenic (n=14) | Increase (%) | |
| BMC (g) | 0.350±0.016 | 0.389±0.006* | 11.12 | 0.297±0.017 | 0.341±0.011* | 14.96 |
| BA (cm2) | 7.262±0.199 | 7.412±0.181ns | --- | 6.712±0.259 | 7.119±0.114ns | --- |
| BMD (g/cm2) | 0.480±0.001 | 0.526±0.001* | 9.68 | 0.438±0.001 | 0.476±0.001* | 8.69 |
BMC=bone mineral content; BA= bone area; BMD=bone mineral density. Values are presented as means ± SEM. The means between wild-type group and transgenic group in males or in females differ at P<0.05 (*).
not significant at P>0.05.
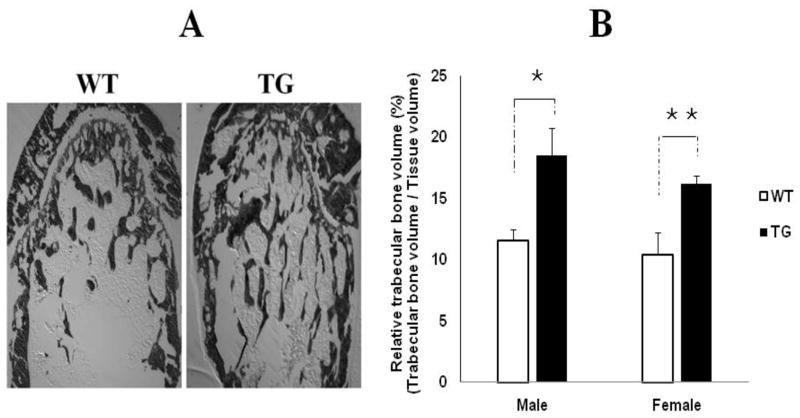
Bone volume was significantly increased in transgenic mice
The impacts of over-expression of BMP11 propeptide on postnatal bone growth were also studied by histological analysis. The results showed that transgenic mice have more trabecular bone in the bone marrow cavity than their wild-type littermates (Figure 2A). Relative trabecular bone volume (RTBV) was dramatically increased by 60.9% [(18.5% - 11.5%)/11.5%] and 57.7% [ (16.4%-10.4%)/10.4% ] in transgenic males and transgenic females, respectively, in comparison with their sex-matched wild-type littermates (Figure 2B).
Figure 2. Comparison of trabecular bone volume at 10 weeks of age wild-type mice and their transgenic littermates.
WT = wild-type, TG = transgenic. A: Von Kossa-stained femur bone sections from WT mice and their sex-matched TG littermates, showing TG mice have more trabecular bone in bone marrow cavity than WT mice. B: Comparison of relative trabecular bone volume between WT mice and their TG littermates, showing the relative trabecular bone volume was significantly increased in male and female transgenic mice. n=7 in both male and female WT group, n=8 in both male and female TG group. Values are presented as mean ± SEM. The means between WT male group and TG male group, and the means between WT female group and TG female group differ at P<0.05 (*) and P<0.01(**).
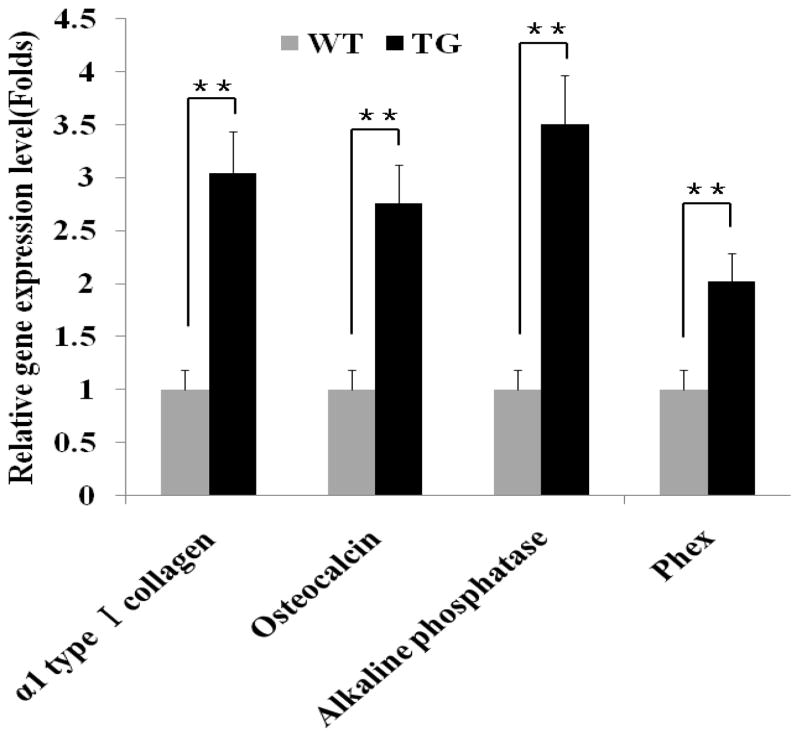
Expressions of osteoblast marker genes were significantly up-regulated in transgenic mice
The results of fetal skeletal staining and postnatal bone measurement demonstrated that skeleton-specific over-expression of BMP11 propeptide enhances bone formation. To evaluate whether this is related to alteration in osteoblast activity in the transgenic mice, we employed quantitative real time PCR to analyze the expression levels of four osteoblast marker genes in the calvaria of 17.5 dpc transgenic mice and their wild-type littermates. These four osteoblast indicators are α 1 type 1 collagen, osteocalcin, alkaline phosphatase and phex [13, 14], which represent a main component of osteoid matrix, a secretary protein only from osteoblasts, an enzyme produced by bone tissue, and a phosphate-regulating endopeptidase predominantly expressed in osteoblasts, respectively. As shown in Figure 3, the expression levels of four osteoblast markers were all significantly increased by 2–3 folds in transgenic mice compared to that in their wild-type littermates.
Figure 3. Comparisons of osteoblast marker expressions in skeleton of transgenic fetuses and their wild-type littermates.
WT = wild-type, TG = transgenic. N=10 in both WT and TG group. Fetal tissues were collected at 17.5 dpc. The expression levels of osteoblast marker genes in TG fetuses are presented relatively to that in WT fetuses, which are arbitrarily defined as 1 fold. Values are presented as mean ± SEM. The means between WT group and TG group differ at P<0.01(**).
Discussion
In cell culture studies, BMP11 propeptide regulates several aspects of BMP11 biology, including the synthesis, extracellular localization and activity [18]. In the transgenic mouse model with skeletal specific expression of BMP11 propeptide, we demonstrated its roles in axial skeletal patterning and effects on Hoxa-4 and Hoxa-5 mRNA [10]. In this report, we further studied the effects of BMP11 protpetide transgene on bone formation in both fetal and postnatal developmental stages. The transgenic mice have enhanced ossification in fetal calvaria and elevated bone mineral content and density, and relative trabecular bone volume. These results strongly suggest the enhanced bone formation in the transgenic mice as a result of the skeletal-specific expression of BMP11 propeptide. Compared with BMP11 knock-out mice, the expressed BMP11 propeptide protein by the transgene is generally believed to partially depress BMP protein function. As the BMP11−/ − mice died before birth, The BMP11 propeptide trangeneis mice is an important animal model as an alternate to the knockout mice for the further understanding of BMP11 function in vivo. To our knowledge, this is the first report to demonstrate that BMP11 propeptide promote bone formation in live animals.
Bone formation and growth is mainly determined by the cell activity of osteoblast as well as the resorbing cell-osteoclast. BMP11 has been shown to inhibit the elongation of chicken limb bones by suppressing the capacity of chondrogenesis and myogenesis [7]. Presumably, BMP11 limits bone growth via negative regulation on osteogenesis. Given that the expression of BMP11 propeptide transgene in transgenic mice is driven by an osteoblast-specific promoter, the expression of BMP11 propeptide transgene in the transgenic mice is probably in a high level in osteoblasts. Therefore, the increased levels of osteoblast activity, demonstrated by the increased expression levels of four osteoblast markers in calvaria of 17.5 dpc transgenic mice in this report, likely result from the depressed function of BMP11 by its propeptide. The results provide in vivo evidence that BMP11 inhibits osteoblast cell activity. The results from the BMP11 proepetide transgenic mice indicate that a partial depression of BMP11 function by its propeptide has positive effects on bone formation and growth.
BMP11 amino acid sequence is 90% identical to myostatin [6]. Skeletal muscle-specific transgenic over-expression of myosatin propeptide results in significant growth performances and muscle mass increase [19–21]. Eliminations of the functions of both BMP11 and myostatin, caused serious defects in the axial skeleton and limb skeletons, further indicating that BMP11 regulates both vertebral and limb formation [8]. The enhanced bone formation and elevated expressions of osteoblast cell markers in the transgenic mice may be also caused by inactivation of myostatin in the musculoskeletal tissue in utero and postnatal stages. Research data have indicated tht BMP11 propeptide antagonizes the activity of BMP11 and myostatin [9]. Mice with null-myosation function have increased osteoblast activity [15], bone mineral content and bone mineral density [16]. Suppression of myostatin function by its propeptide in animals also was shown to increase bone formation [11, 17]. Therefore, transgenic over-expression of BMP11 propeptide in skeleton may antagonize myostatin function, thereby promoting osteoblast activity and bone growth. The transgenic mouse model will be useful for further understanding of both BMP11 and myostatin functions in skeletal formation and growth.
In summary, skeletal-specific overexpression of BMP11 propeptide resulted in enhanced bone formation, along with increased expressions of four osteoblast markers. The transgenic mouse model provides important animal model for a further understanding of BMP11 function and its roles in bone formation and growth.
Highlights.
Over-expression of BMP11 propeptide promotes fetal ossification.
Over-expression of BMP11 propeptide increases bone mineral content and density during the postnatal growth period.
BMP11 propetide transgene results in elevated osteoblast marker gene expressions.
Acknowledgments
This work was supported by grants from NIH COBRE Grant (5P20RR024206-01A1), USDA-TSTAR programs (Grant # 2008-34135-19322), and Wuhan “3551 Talent Program”. We thank Fanming Meng for his assistances in preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 2.Zhao GQ. Consequences of knocking out bmp signaling in the mouse. Genesis. 2003;35:43–56. doi: 10.1002/gene.10167. [DOI] [PubMed] [Google Scholar]
- 3.Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A. Bone morphogenetic proteins: a critical review. Cell Signal. 2011;23:609–620. doi: 10.1016/j.cellsig.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Gamer LW, Wolfman NM, Celeste AJ, Hattersley G, Hewick R, Rosen V. A novel bmp expressed in developing mouse limb, spinal cord, and tail bud is a potent mesoderm inducer in xenopus embryos. Dev Biol. 1999;208:222–232. doi: 10.1006/dbio.1998.9191. [DOI] [PubMed] [Google Scholar]
- 5.Nakashima M, Toyono T, Akamine A, Joyner A. Expression of growth/differentiation factor 11, a new member of the bmp/tgfbeta superfamily during mouse embryogenesis. Mech Dev. 1999;80:185–189. doi: 10.1016/s0925-4773(98)00205-6. [DOI] [PubMed] [Google Scholar]
- 6.McPherron AC, Lawler AM, Lee SJ. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat Genet. 1999;22:260–264. doi: 10.1038/10320. [DOI] [PubMed] [Google Scholar]
- 7.Gamer LW, Cox KA, Small C, Rosen V. Gdf11 is a negative regulator of chondrogenesis and myogenesis in the developing chick limb. Dev Biol. 2001;229:407–420. doi: 10.1006/dbio.2000.9981. [DOI] [PubMed] [Google Scholar]
- 8.McPherron AC, Huynh TV, Lee SJ. Redundancy of myostatin and growth/differentiation factor 11 function. BMC Dev Biol. 2009;9:24. doi: 10.1186/1471-213X-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge G, Hopkins DR, Ho WB, Greenspan DS. Gdf11 forms a bone morphogenetic protein 1-activated latent complex that can modulate nerve growth factor-induced differentiation of pc12 cells. Mol Cell Biol. 2005;25:5846–5858. doi: 10.1128/MCB.25.14.5846-5858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Kawasumi M, Zhao B, Moisyadi S, Yang J. Transgenic over-expression of growth differentiation factor 11 propeptide in skeleton results in transformation of the seventh cervical vertebra into a thoracic vertebra. Mol Reprod Dev. 2010;77:990–997. doi: 10.1002/mrd.21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell AD, Wall RJ. In vivo evaluation of changes in body composition of transgenic mice expressing the myostatin pro domain using dual energy x-ray absorptiometry. Growth Dev Aging. 2007;70:25–37. [PubMed] [Google Scholar]
- 12.Li Z, Cao B, Zhao B, Yang X, Fan MZ, Yang J. Decreased expression of calpain and calpastatin mrna during development is highly correlated with muscle protein accumulation in neonatal pigs. Comp Biochem Physiol A Mol Integr Physiol. 2009;152:498–503. doi: 10.1016/j.cbpa.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Ecarot B, Desbarats M. 1,25-(oh)2d3 down-regulates expression of phex, a marker of the mature osteoblast. Endocrinology. 1999;140:1192–1199. doi: 10.1210/endo.140.3.6593. [DOI] [PubMed] [Google Scholar]
- 14.Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Dev Dyn. 2006;235:176–190. doi: 10.1002/dvdy.20603. [DOI] [PubMed] [Google Scholar]
- 15.Hamrick MW, Shi X, Zhang W, Pennington C, Thakore H, Haque M, Kang B, Isales CM, Fulzele S, Wenger KH. Loss of myostatin (gdf8) function increases osteogenic differentiation of bone marrow-derived mesenchymal stem cells but the osteogenic effect is ablated with unloading. Bone. 2007;40:1544–1553. doi: 10.1016/j.bone.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamrick MW, McPherron AC, Lovejoy CO. Bone mineral content and density in the humerus of adult myostatin-deficient mice. Calcif Tissue Int. 2002;71:63–68. doi: 10.1007/s00223-001-1109-8. [DOI] [PubMed] [Google Scholar]
- 17.Hamrick MW, Arounleut P, Kellum E, Cain M, Immel D, Liang LF. Recombinant myostatin (GDF-8) propeptide enhances the repair and regeneration of both muscle and bone in a model of deep penetrant musculoskeletal injury. J Trauma. 2010;69:579–583. doi: 10.1097/TA.0b013e3181c451f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison CA, Al-Musawi SL, Walton KL. Prodomains regulate the synthesis, extracellular localisation and activity of TGF-β superfamily ligands. Growth Factors. 2011;29:174–186. doi: 10.3109/08977194.2011.608666. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Ratovitski T, Brady JP, Solomon MB, Wells KD, Wall RJ. Expression of myostatin pro domain results in muscular transgenic mice. Mol Reprod Dev. 2001;60:351–361. doi: 10.1002/mrd.1097. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Zhao B. Postnatal expression of myostatin propeptide cDNA maintained high muscle growth and normal adipose tissue mass in transgenic mice fed a high-fat diet. Mol Reprod Dev. 2006;73:462–469. doi: 10.1002/mrd.20452. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Zhao ZB, Kim YS, Hu CY, Yang J. Administration of a mutated myostatin propeptide to neonatal mice significantly enhances skeletal muscle growth. Mol Reprod Dev. 2009;77:76–82. doi: 10.1002/mrd.21111. [DOI] [PubMed] [Google Scholar]