Introduction
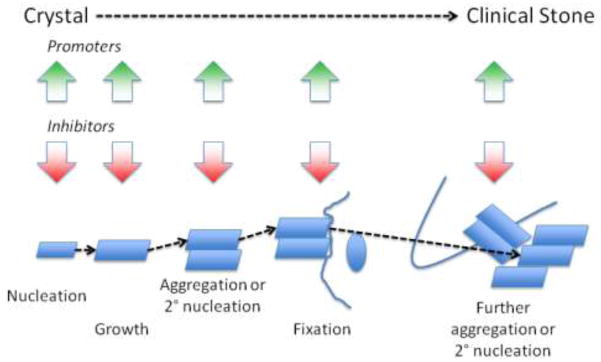
The mechanism of stone formation include nucleation of stone constituent crystals, their growth or aggregation to a size that can interact with some intrarenal structure, their retention within the kidney or renal collecting system and further aggregation and/or secondary nucleation to form the clinical stone. This sequence is depicted in Figure 1. The crystals form either in renal tubular fluid or in the renal interstitial fluid that is supersaturated with respect to these constituents, which in turn may be a consequence of increased excretion of stone constituent molecules, reduced urine volume, an alteration in urine pH, or a combination of these factors.[1] The urine and, presumably, the tubular fluid of stone formers is often more highly supersaturated than that of normal healthy adults, which favors nucleation and growth of crystals.[2] Long-term accretion of additional elements, crystalline as well as organic matrix, produces the clinical stone.
Figure 1.
The mechanisms responsible for the greater degree of supersaturation of stone chemical constituents in stone-forming individuals may differ depending on the type of stone; however, excessively low urine volume is often present. However, despite current understanding of the physical chemistry of stone formation, no single abnormality has served to distinguish stone formers from normal individuals, and many stone formers have no identifiable abnormality that explains the propensity of their urine (or tubular fluid) to nucleate stone constituents at lower levels of supersaturation, to support accelerated growth of the crystals formed, or to permit crystals to adhere to each other (aggregate) or to some intrarenal structure. For the sake of brevity, when urine is referred to in this context, it is understood to encompass tubular fluid at some critical point in the nephron where the processes under discussion are occurring.
Saturation and Crystallization
Concept of saturation (minimum activity product to support crystallization) and metastability
A solution is saturated when the activity product of solutes supports neither growth nor dissolution of crystals composed of those solutes. However, spontaneous crystallization often does not occur when the activity product is higher than this value. Such a solution is said to be supersaturated with respect to these moieties or metastable. Urine is virtually always metastable with respect to calcium oxalate in most individuals, whether they are stone formers or not, and it is metastable at least some of the time with respect to other stone constituents, such as uric acid, other urates, and calcium phosphate. Tubular fluid supersaturation estimates based on rat data have been made, which suggest that supersaturation for calcium phosphate regularly occurs in the loop of Henle.[3] If true for humans, it could provide the driving force for the development of interstitial calcium phosphate deposits known as Randall’s plaques. The role of these deposits in stone disease is discussed later.
Although the urine of calcium stone formers is often more supersaturated with respect to calcium oxalate and calcium phosphate than normal individuals, most of the latter are also supersaturated. However, nucleation of calcium oxalate or phosphates usually does not occur and, even if it does, the crystals produced do not grow or aggregate to a sufficient size to be retained in the kidney on the basis of size alone.[4] Consequently, urine or, more properly, tubular fluid likely contains inhibitors of crystal formation, specifically, nucleation, growth, or aggregation. Inhibition of all of these aspects of crystallization has been observed in vitro with urine itself, with some low molecular weight components of urine, and with some macromolecules isolated from urine.[5–7] This inhibitory property has been reported to be defective in some stone formers.
Supersaturation is expressed as the ratio of urinary calcium oxalate or calcium phosphate concentration to its solubility, which is the driving force in stone formation. Supersaturation is generally higher in patients with recurrent kidney stones than in those without, and the type of stone that is formed correlates with urinary supersaturation. At supersaturation levels above 1, crystals can nucleate and grow, promoting stone formation, while they dissolve at levels below 1. Calcium oxalate supersaturation is independent of urine pH; however calcium phosphate supersaturation increases rapidly as urine pH rises from 6 to 7. Since calcium oxalate stones may form over an initial calcium phosphate layer, treatment optimally should lower the supersaturation of both chemical species.[8]
Concept of formation product (activity product that forces crystallization)
Even in metastable solutions, nucleation will occur if the supersaturation rises high enough. Consequently this is an upper limit of metastability. This has lead to the concept of formation product or the upper limit of metastability; which denote the activity product that induces crystallization in urine or artificial solutions made to simulate urine.[5;9] This is thought to be the point where there is formation of solid crystal phase in solution and nucleation occurs.
The point at which spontaneous crystallization occurs can be influenced by the presence of particulates, such as some components of cells or other crystals; much in the way that a piece of string or a sugar crystal will induce crystallization in a metastable sugar solution to produce rock candy. [10] Soluble promoters of crystallization will reduce the formation product or upper limit of metastability and inhibitors will raise it.
Modes of Stone Growth
The modes and putative sites of stone formation are depicted in Figure 2. The authors hasten to point out that much of this scheme is speculative and based on indirect evidence.
Figure 2.
Nucleation
Nucleation is the process by which free ions in solution associate into microscopic particles. Crystallization can occur in solution micro-environments, such as may be present in certain points in the nephron [11], as well as on surfaces, such as those of cells and on extracellular matrix [12]. There is considerable dispute about the importance of free solution crystallization versus crystallization at other sites, in renal tubules or on bladder walls, on normal or damaged cells, on areas denuded of cells by certain forms of injury, or at interstitial sites.[13]
Aggregation
Aggregation is a process by which there is agglomeration of crystals that form in free solution into larger multicomponent particles. It may also encompass the phenomenon of secondary nucleation of new crystals on the surface of those already formed. The structure of stones suggests that one or other of these processes must occur for the stone to grow to a clinically significant size.[14;15] Kidney stones can be thought of as being similar to concrete, a mixture of a binding agent (cement), and particulates such as sand, pebbles, or glass. Stones are an aggregation of crystals and an organic matrix, the latter serving as the binding agent. The organic matrix contains proteins, lipids, polysaccharides, and other cell-derived material. [16–18].
Crystal growth
Growth of microscopic crystals is accomplished by movement of ions out of solution onto the growing crystal. While some growth of nuclear crystals must occur by movement of ions from solution, this is clearly a limited process, as giant single crystals of stone constituents are not generally observed. It is more likely that stone growth is accomplished through aggregation of preformed crystals or secondary nucleation of crystal on the matrix coated surface of another. It has been proposed that the growth of these microscopic crystals to the extent that they can be retained in the kidney on the basis of size alone cannot occur without aggregation or attachment to specific intrarenal structures.[4]
Sites of Stone Growth
Randall’s plaques
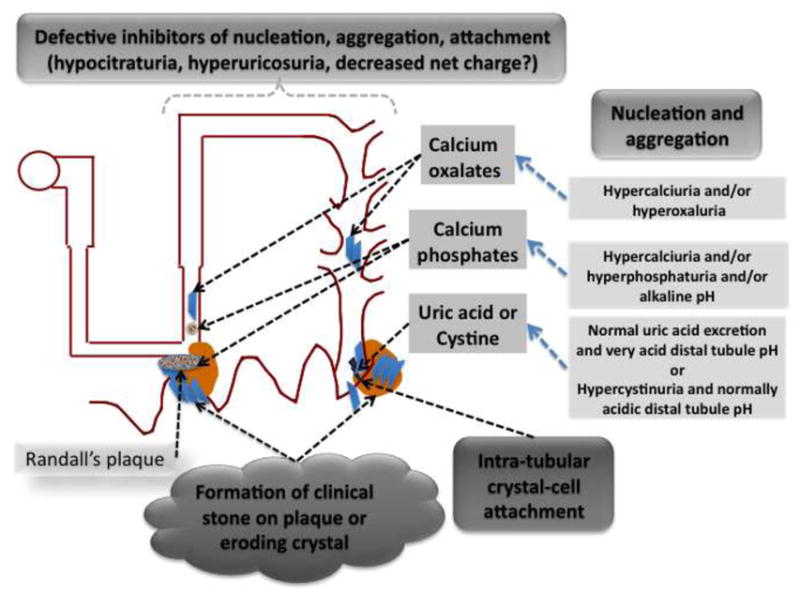
Although urine is not generally supersaturated with respect to calcium phosphate such conditions may exist in the loop of Henle.[19] This may lead to precipitation of calcium phosphate in interstitial sites in the inner medulla. These deposits often become extensive enough to be visible macroscopically in the form of Randall’s Plaques. These deposits have been proposed to act as a nidus for development of the most usual variety of calcium oxalate stones.[13] Some studies have demonstrated stones that appear to have been attached directly to the Randall’s plaque which has eroded through the overlying uroepithelium at the surface of a renal papilla. [8] While Randall’s plaques appear to be a risk factor for stone formation it still is unclear whether they are necessary in every stone that is formed, as both intratubular crystals as well as prominent crystalluria are features of stone disease.
Calcium oxalate “receptors” in collecting duct epithelium
Crystalluria is commonly observed in hyperoxaluric patients, in animals made hyperoxaluric, as well as in patients with the usual sort of calcium oxalate nephrolithiasis where intratubular crystals of calcium oxalate has also been demonstrated.[20] Physical interaction between crystals and tubular epithelium has also been reported. This interaction has been studied extensively by examining the interaction of preformed calcium oxalate crystals (usually the monohydrate) with cultured renal tubule cells.
Cells exposed to crystals may internalize them, where their fate may involve dissolution or they may undergo transcytosis through the epithelial layer.[21;22] This process may have consequences for cell function; initiating mitogenesis, activation of arachidonic acid and other signaling pathways. Additional consequences include membrane lysis, necrosis of cells, apoptosis, and production of reactive oxygen species.[23] The interaction of stone crystals of various types with primary cultures of inner medullary collecting duct cells demonstrates saturability and inhibition to some degree of one crystal type by others. [24–27]. Thus, it seems like there are some receptor-like features of cells to which stone crystals adhere.
Maneuvers that lead to loss of cell polarity lead to increased adherence of calcium oxalate crystals, suggesting that the basolateral membranes of tubule cells have components to which the crystals can attach.[28] Enrichment of cell membranes with phosphatidylserine also leads to enhanced calcium oxalate adherence.[29] Also, proteins and glycosaminoglycan expressed at the cell surface have also been implicated as attachment sites for calcium oxalate at least. These include hyaluronan, nucleolin, annexin II, and osteopontin.[30–32] It is likely that a number of different structure or molecular components are responsible for crystal attachment. As noted above, these may include phosphatidylserine component of the lipid bilayer, the acidic side chains of proteins (carboxyl groups of amino acids or sialic acid-containing glycosidic side chains). Atomic force microscopy has been used to measure the force of attraction of carboxyl and amidinium groups to the surface of crystals. [33]
Promoters of Stone Formation
Promoters can increase crystallization of stone constituents or their growth by a number of mechanisms. The saturation can be raised by increases in concentration of the reactants. Substances in urine may be present that lower the formation product, but the formation product may also be lowered by a lack of endogenous inhibitors or opposition to their effects by defects in their structure or other interfering substances. Excessively low or high urinary pH may induce the formation of heterogeneous nucleating substances.
Uric acid or urate
Monosodium urate appears to directly reduce the formation product for calcium oxalate. [34] The mechanism for this effect is likely through its antagonistic effect to substances in the urine that raise the formation product, specifically that attributable to mucopolysaccharides. [35;36] In addition to this effect there may be promotion of heterogeneous nucleation by uric acid or monosodium urate and enhancement of the attachment of calcium oxalate to cells.[37]
Urine pH
Highly acid urine leading to precipitation of uric acid crystals may not only lead to uric acid stone disease but may also enhance calcium oxalate crystallization due to heterogeneous crystallization, in which one type of crystal acts as a template, thereby promoting crystallization of a second type of crystal, as noted above.[38] Highly alkaline urine may also promote secondary nucleation of calcium oxalate by precipitation of calcium phosphate.[39]
Low pH cannot really be said to promote crystallization of cystine, as the solubility of this substance is minimal at most usual urinary pH values. On the other hand, the solubility of this substance, increases significantly at pH values in excess of 7.5.[40] Commercial laboratories that market packages of urine stone chemistries consider stone risk to be minimal when urine pH is between 5.8–6.2, in one instance, or between 5.5–7.0 in another.
Inhibitors of Stone Formation
There are at least four types of inhibitors in urine: small organic anions such as citrate, small inorganic anions such as pyrophosphates, multivalent metallic cations such as magnesium, or macromolecules such as osteopontin and Tamm-Horsfall protein. The table is a listing of substances generally considered to inhibit stone formation and the stone formation process they appear to inhibit.
Alkaline pH
Alkaline pH cannot be said to be an inhibitor of stone formation, as it has both beneficial and deleterious effects depending on the stone constituent under consideration, however alkaline pH inhibits cystine and uric acid stone formation, which tend to form in acidic urine, as noted above.
Citrate
Citrate can be said to be an inhibitor of stone formation. It has several effects. It will lower saturation of calcium oxalate by virtue of forming complexes with calcium. When studies are performed in which free calcium is controlled, it appears to have an independent effect of nucleation and growth (unpublished observations). However, it has also been shown to inhibit aggregation of preformed crystals as well as attachment of crystals to urinary epithelium.[41;42]
Pyrophosphate
Pyrophosphate, a naturally occurring substance in urine, has been demonstrated to inhibit both calcium oxalate and calcium phosphate crystallization. It was found that the average urine pyrophosphate concentration was sufficient to significantly inhibit crystal growth. [43;44] This agent has given discordant results on tests of aggregation and crystal attachment to epithelia. [45]
Phytate
Phytate (myo-inositol hexakisphosphate), a natural compound formed during maturation of plant seeds and grains is a common constituent of plant-derived foods. In an animal model, calcification in renal tissue was induced in hypercalcemic, hypertensive, male Wistar rats that were fed a purified phytate-free diet.[46] On this diet rats developed significant calcium deposits in kidneys and papillae, as well as in kidney tubules and vessels, whereas calcium deposits were absent in control and phytate treated rats. Fragments of hydroxyapatite (HAP) calculi exhibited the capacity to induce the growth of calcium salts on their surfaces, however 1.5 mg/L phytate in the synthetic urine utilized in the study inhibited the formation of calcium oxalate monohydrate on HAP renal calculi fragments under when calcium concentration were in the range considered normal. These findings show that the action of phytate as a crystallization inhibitor takes place both in the intrapapillary tissue and urine.
Magnesium
Magnesium has also been demonstrated to inhibit stone formation by inhibition of growth (and presumably nucleation) of crystals as well as aggregation.[6] Inhibition of crystal attachment of calcium oxalate appears to require supra-physiologic concentrations.[47] Stone formation in vitamin B6-deficient animals has been attributed to magnesium depletion, as it is ameliorated by magnesium repletion.[48] However, magnesium supplementation for stone prevention in humans has had disappointing results. [49]
Glycoproteins
The effects of the small amount of proteins and glycosaminoglycans present in urine are more complex. A number of them are found preferentially in stone matrix, specifically, osteopontin/uropontin, Tamm-Horsfall protein, urinary prothrombin fragment 1, and some subunits of the serum inter-α-inhibitor. As noted earlier, some of these substances may act as attachment sites—hence promoters—when expressed on the surface of cells. As will be explained below, the physico-chemical state of these substances may also determine whether they act as inhibitors of stone formation processes or promoters. Finally, it is to be expected that proteomic analysis of stone matrix may reveal other stone matrix components involved in stone formation, however, to date there are little published data.
• Osteopontin/uropontin
Osteopontin/uropontin inhibits spontaneous nucleation from metastable solutions as well as the growth of preformed crystals in a seed growth assay.[50;51] The intact molecule and several regions associated with both acidic amino acid residues and phosphorylation slows crystal growth in seeded assay systems and the intact molecule inhibits attachment to cells, at least in some reports.[52] Others have provided evidence that osteopontin/uropontin bound to the surface of cells may enhance attachment.[53;54] And finally, calcium oxalate aggregation inhibition by osteopontin in vitro can be switched to aggregation promotion by neutralization of its net negative charge by poly-arginine.[55]
• Tamm-Horsfall protein
Tamm-Horsfall protein is the most abundant of the urinary proteins under normal circumstances. Its excretion rate is approximately 100 mg day. [56] It has not been demonstrated to affect nucleation or growth of most crystals, but exerts a powerful effect as an inhibitor of crystal aggregation. Abnormalities in stone former Tamm-Horsfall protein have been difficult to identify. Early on a very few patients have been described with reduced Tamm-Horsfall inhibition of crystal aggregation.[57] In more recent studies, it has been shown that this substance can act as an inhibitor of crystal aggregation or a promoter.[58] Tamm-Horsfall protein coats calcium oxalate crystals and prevents adhesion to cultured epithelia, but how it would affect attachment when anchored to epithelia in vivo is not known. [59]
Removal of sialic acid residues from glycosylated proteins proposed as stone inhibitors interferes with inhibitory activity.[60;61] Desialylated THP has been isolated from stone matrix as well as the urine of stone formers.[62] A recent study of chemically desialylated THP isolated from a normal individual has demonstrated calcium oxalate crystal aggregation promotion compared with the unmodified molecule.[58] Aggregation promotion was relatively independent of pH but reduced at low solution ionic strength, a condition where protein aggregation was also reduced. Formation of protein aggregates with a multiplicity of urinary proteins was demonstrated.
• Urinary prothrombin fragment 1
Another moiety that is produced by thrombin cleavage of the serum protein is called urinary prothrombin fragment 1 which has been isolated from the matrix of crystals formed by addition of oxalate to urine[63] This is an effective inhibitor of both calcium oxalate crystal growth and aggregation. It is unclear at this time if the urinary prothrombin fragment 1 derives from the serum protein prothrombin. It was noted that there was no difference in the ability to inhibit crystal growth in the urine from patients on warfarin to that of normal individuals.[64] Urinary prothrombin fragment 1 also inhibits calcium oxalate attachment to cultured cells.[59]
• Inter-α-inhibitor related proteins
Another protein which has sequence identity to serum inter-α-inhibitor is known to inhibit crystal growth in urine. This molecule is composed of two heavy chains of ~900 residues linked residues linked covalently to the light chain bikunin by chondroitin sulfate. Bikunin is a uronic-acid-rich protein with calcium oxalate growth inhibitory activity that has been isolated from human urine [65].
It has been proposed that the inhibitor referred to as nephrocalcin may also be a portion of the light chain of from serum inter-α-inhibitor, either identical or closely related to bikunin. [66]. All these inhibitors are acidic proteins. They contain rather large numbers of aspartic or glutamic acid residues, often in clusters; their peptide backbones contain many sulfated or phosphorylated amino acids; or their post-translational modifications include terminal glycosidic sialic acids or heavily sulfated glycosaminoglycans. Bikunin also inhibits calcium oxalate attachment to cultured cells.[59]
Control of crystal formation by inhibitors has been proposed to play a role in the normal defense against the development of stones, and abnormalities of these inhibitors may permit stone formation and growth. Although they may act as inhibitors, their activity may be diminished or counteracted by their physico-chemical state. Loss of charged moieties may lead to protein aggregation and, secondarily, crystal aggregation. Alternatively, those that are capable of attachment to cell surfaces rather than being free in solution may actually mediate crystal attachment thereby fixing the stone nidus within the kidney.
Urine Chemical Risk Factors for Calcium Stone Formation
Increased crystalloid concentration
• Low urine volume
Whatever the type of stone, low urine volume is often present. Unselected first time stone formers have lower 24-h urine volume than age and sex-matched controls.[67] Increased fluid intake was successful at significantly reducing the stone recurrence rate in this group. With urine volumes of less than 2 liters/day, the supersaturation of urine with respect to calcium oxalate increased in an exponential manner.[2] The proportion of individuals with such low urine volumes has been reported to be in the neighborhood of 70%.[68] Low urine volume has also been felt to be an important contributing factor to the formation of uric acid stones in patients with intestinal disorders.[69] Together with low urine volume, Hypercalciuria, hyperoxaluria, or hyperphosphaturia will tend to result in supersaturation of stone forming constituents, thereby promoting nucleation if the upper limit of metastability for that species is exceeded or the continued growth of stones that have already formed.
• Hypercalciuria
Although there is considerable overlap with non-stone formers, the urine of stone formers is often more highly supersaturated with respect to stone forming constituents. Hypercalciuria, present in 25–60% of stone formers, if not offset by increased urine volume or citrate excretion, will lead to increased supersaturation for calcium oxalate or phosphate. Among these individuals with hypercalciuria is a minority with unrecognized metabolic causes of increased urinary Ca excretion. These include primary hyperparathyroidism, granulomatous diseases, primarily sarcoidosis, Vitamin D intoxication, milk-alkali syndrome, and the use of carbonic acid inhibitors.
Many hypercalciuric stone formers have what has been referred to as idiopathic hypercalciuria. This subject will be reviewed in detail in another article. Briefly, hypercalciuria is the most common metabolic abnormality found in patients with recurrent calcium stones. It is most often familial and idiopathic and is strongly influenced by diet.[70] Patients typically have excessive intestinal calcium absorption and may also have decreased renal tubular calcium reabsorption and decreased bone mineralization. The etiology of this systemic disorder in calcium transport has, in hypercalciuric stone-forming rats and in humans, been linked to an excessive number of receptors for vitamin D by some but not others. [71;72] There is also evidence for association with base substitutions in a soluble adenylate cyclase on human chromosome 2 in humans with this disorder related to increased intestinal absorption of calcium.[73]
• Hyperoxaluria
A study suggests that a diet characterized by normal calcium, low animal protein, and low salt levels is more effective than the traditional low-calcium diet for the prevention of recurrent stones in men with idiopathic hypercalciuria.[74] This appears to be due to a salutary effect of calcium in the diet on oxalate absorption. Hyperoxaluria promotes stone disease, either by virtue of its pronounced effect on calcium oxalate supersaturation or because of injurious effects of oxalate on the renal epithelium.[75–78] Hyperoxaluria is noted among patients with recurrent calcium stones more often than among those without the condition, possibly due of increased oxalate absorption in the gut.[79] The intake of a high level of protein may increase oxalate production.[80]
Hyperoxaluria may be due to genetic overproduction or increased absorption due to ingestion of foods high in oxalate or its precursors, intestinal disorders or bowel resection (including gastric bypass surgery).[81–84] Mice deficient for SL626, an intestinal oxalate transporter, hyper-absorb oxalate & develop calcium oxalate deposits in their kidneys.[85] To date, no human analog of this abnormality has been described.
• Phosphaturia
There have been several recent investigations looking at phosphaturia in subjects with nephrolithiasis.[86;87] A study has described a mutation in the NHERF1 gene responsible for decreased renal phosphate reabsorption.[88] It has been postulated that the associated hypophosphaturia causes increased 1, 25- dihydroxy-vitamin D production, which causes increased intestinal phosphate and calcium absorption. This combined hypercalciuria and hyperphosphaturia favor the formation of calcium phosphate precipitates that can result in nephrolithiasis, as described earlier.
Increased promoter concentration
• Hyperuricosuria
Hyperuricosuria is a risk factor both for the development of stones composed of uric acid or its various salts, sodium urate and ammonium urate, as well as the development of calcium oxalate or calcium phosphate stones. Often from high dietary intake of purines, is thought to promote the formation of calcium stones by reducing the solubility of calcium oxalate.[89;90]
Even stones that are composed primarily of uric acid frequently have components of the Ca salts. The factors that lead to hyperuricosuria and the solution conditions that result in uric acid stones are several, depending on the clinical circumstances, as discussed earlier. Low urine volume has been implicated in the uric acid stones that occur in chronic diarrhea and that result from excessive exercise or exposure to very warm ambient conditions.[69;91;92]
• Alkaline urine pH
Urine pH that is alkaline more of the time is likely responsible for the minority of calcium stone formers whose stone are composed predominantly of calcium phosphate.[93] Other conditions where acidification of the urine is compromised, such as in medullary sponge kidney, hyperparathyroidism, use of carbonic anhydrase inhibitors or carbonic anhydrase deficiency and in hereditary and acquired forms of renal tubular acidosis.[94–96] Of course, some of these conditions induce nephrocalcinosis as well as nephrolithiasis, and the highest urinary pH values are associated with struvite, magnesium ammonium phosphate, rather than predominantly calcium stone formation, although carbonate apatite may be formed as well.[97]
Reduced inhibitor concentration
• Hypocitraturia
Hypocitraturia has a wide range in reported prevalence which may be due to differences in the populations studied, differences in dietary background, and differences in the laboratory definition of hypocitraturia. Generally, it is between 30–40% of stone formers, but values as low as 8% to almost 70% have been reported.[98;99] In only a small proportion of patients can this abnormality be ascribed to renal tubular acidosis and chronic diarrhea syndromes.[100] Most appear to be dietary in origin; the different proportions among stone patients likely being explained by ethnic variations in food intake, specifically fruit.[99] Hypocitraturia as an isolated abnormality is not common among stone formers but is often accompanied by other defects such as hypercalciuria and hyperoxaluria. [100]
In general Hypocitraturia is found in conditions that acidify the proximal tubule cell by one means or other, perhaps related to high protein diets.[101] These diets, while not resulting in frank metabolic acidosis, may lower the serum bicarbonate to a small degree and, thus, induce hypocitraturia. The transported responsible for proximal tubule citrate reabsorption is stimulated by metabolic acidosis, which may be the proximate cause of hypocitraturia.[102] Occasionally, no underlying defect can be uncovered and the patient is diagnosed to have idiopathic hypocitraturia.[100]
Summary and Conclusions
Stone formation is a complex process involving crystal nucleation, aggregation and/or secondary nucleation, fixation within the kidney, and more aggregation and secondary nucleation. These steps are heavily modulated by the balance of amounts of stone constituents appearing in tubular fluid, their concentration as affected by water excretion, the pH of tubular fluid and/or urine, and the balance of promoters and inhibitors that are not major components themselves of the clinical stones. While these factors appear to explain stone formation, none of them clearly separate the stone forming population from non-stone formers, with the possible exception of those individuals with genetic cystinuria, and there is a small proportion in which no abnormality can be identified. Nevertheless, success strategies for diminishing stone recurrence rate have been based on manipulating these processes.
Table 1.
Effects of Inhibitors on stone formation
| INHIBITOR | Nucleation Inhibitor | Growth Inhibitor | Aggregation inhibitor | Attachment inhibitor |
|---|---|---|---|---|
| Citrate | I | I | I | I |
| Pyrophosphate | I | I | I/NI | ND |
| Phytate | * | I | ND | ND |
| Magnesium | I | I | I | I (high conc.) |
| Osteopontin | I | I | I/NI | I |
| THP | NI | NI | I/NI | I |
| UPF- 1 | * | I | I | I |
| Bikunin | I | I | I | I |
property was not explicitly tested but is likely to be present, as nucleation appears to always track with growth inhibition; I, inhibitory activity demonstrated; NI, inhibitory activity not demonstrated, ND, Inhibitory activity not determined; I/NI, both inhibitory and promoter activity noted
Reference List
- 1.Coe FL, Parks JH, Asplin JR. The pathogenesis and treatment of kidney stones. N Engl J Med. 1992;327:1141–1152. doi: 10.1056/NEJM199210153271607. [DOI] [PubMed] [Google Scholar]
- 2.Lemann J, Jr, Pleuss JA, Worcester EM, Hornick L, Schrab D, Hoffmann RG. Urinary oxalate excretion increases with body size and decreases with increasing dietary calcium intake among healthy adults. Kidney Int. 1996;49:200–208. doi: 10.1038/ki.1996.27. [DOI] [PubMed] [Google Scholar]
- 3.Asplin J, Mandel N, Coe F. Evidence for calcium phosphate supersaturation in the loop of Henle. Am J Physiol. 1996;270:F604–F613. doi: 10.1152/ajprenal.1996.270.4.F604. [DOI] [PubMed] [Google Scholar]
- 4.Kok DJ, Khan SR. Calcium oxalate nephrolithiasis, a free or fixed particle disease. Kidney Int. 1994;46:847–854. doi: 10.1038/ki.1994.341. [DOI] [PubMed] [Google Scholar]
- 5.Pak CY, Holt K. Nucleation and growth of brushite and calcium oxalate in urine of stone-formers. Metabolism. 1976;25:665–673. doi: 10.1016/0026-0495(76)90064-0. [DOI] [PubMed] [Google Scholar]
- 6.Ryall RL, Harnett RM, Marshall VR. The effect of urine, pyrophosphate, citrate, magnesium and glycosaminoglycans on the growth and aggregation of calcium oxalate crystals in vitro. Clin Chim Acta. 1981;112:349–356. doi: 10.1016/0009-8981(81)90458-7. [DOI] [PubMed] [Google Scholar]
- 7.Worcester EM, Beshensky AM. Osteopontin inhibits nucleation of calcium oxalate crystals. Ann NY Acad Sci. 1995;760:375–377. doi: 10.1111/j.1749-6632.1995.tb44661.x. [DOI] [PubMed] [Google Scholar]
- 8.Evan AP, Coe FL, Lingeman JE, Shao Y, Sommer AJ, Bledsoe SB, Anderson JC, Worcester EM. Mechanism of formation of human calcium oxalate renal stones on Randall’s plaque. Anat Rec (Hoboken ) 2007;290:1315–1323. doi: 10.1002/ar.20580. [DOI] [PubMed] [Google Scholar]
- 9.Asplin JR, Parks JH, Coe FL. Dependence of upper limit of metastability on supersaturation in nephrolithiasis. Kidney Int. 1997;52:1602–1608. doi: 10.1038/ki.1997.491. [DOI] [PubMed] [Google Scholar]
- 10.Khan SR. Calcium oxalate crystal interaction with renal tubular epithelium, mechanism of crystal adhesion and its impact on stone development [editorial]. [Review] Urol Res. 1995;23:71–79. doi: 10.1007/BF00307936. [DOI] [PubMed] [Google Scholar]
- 11.Olszta MJ, Odom DJ, Douglas EP, Gower LB. A new paradigm for biomineral formation: mineralization via an amorphous liquid-phase precursor. Connect Tissue Res. 2003;44 (Suppl 1):326–334. [PubMed] [Google Scholar]
- 12.Lieske JC, Hammes MS, Toback FG. Role of calcium oxalate monohydrate crystal interactions with renal epithelial cells int he pathogenesis of nephrolithiasis: a review. Scanning Microsc. 1998;10:519–534. [PubMed] [Google Scholar]
- 13.Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao Y, Sommer AJ, Paterson RF, Kuo RL, Grynpas M. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003;111:607–616. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandersius S, Rez P. Morphology of crystals in calcium oxalate monohydrate kidney stones. Urol Res. 2007;35:287–293. doi: 10.1007/s00240-007-0115-3. [DOI] [PubMed] [Google Scholar]
- 15.Gower LB, Amos FF, Khan SR. Mineralogical signatures of stone formation mechanisms. Urol Res. 2010;38:281–292. doi: 10.1007/s00240-010-0288-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorian HH, Rez P, Drach GW. Evidence for aggregation in oxalate stone formation: atomic force and low voltage scanning electron microscopy. J Urol. 1996;156:1833–1837. doi: 10.1016/s0022-5347(01)65547-2. [DOI] [PubMed] [Google Scholar]
- 17.Grases F, Costa-Bauza A, Conte A. Studies on structure of calcium oxalate monohydrate renal papillary calculi. Mechanism of formation. Scanning Microsc. 1993;7:1067–1074. [PubMed] [Google Scholar]
- 18.Khan SR, Hackett RL. Role of organic matrix in urinary stone formation: an ultrastructural study of crystal matrix interface of calcium oxalate monohydrate stones. J Urol. 1993;150:239–245. doi: 10.1016/s0022-5347(17)35454-x. [DOI] [PubMed] [Google Scholar]
- 19.Asplin JR, Mandel NS, Coe FL. Evidence of calcium phosphate supersaturation in the loop of Henle. Am J Physiol. 1996;270:F604–F613. doi: 10.1152/ajprenal.1996.270.4.F604. [DOI] [PubMed] [Google Scholar]
- 20.Robertson WG, Peacock M, Nordin BEC. Calcium crystalluria in recurrent renal stone formers. Lancet. 1969:21–24. doi: 10.1016/s0140-6736(69)92598-7. [DOI] [PubMed] [Google Scholar]
- 21.de Bruijn WC, Boeve ER, van Run PRWA, van Miert PPMC, de Water R, Romijn JC, Verkoelen CF, Cao LC, Van’t N, Schroder FH. Etiology of calcium oxalate nephrolithiasis in rats. II. The role of the papilla in stone formation. Scanning Microsc. 1995;9:115–125. [PubMed] [Google Scholar]
- 22.de Bruijn WC, Boeve ER, van Run PR, van Miert PP, de Water R, Romijn JC, Verkoelen CF, Cao LC, Schroder FH. Etiology of calcium oxalate nephrolithiasis in rats. I. Can this be a model for human stone formation? Scanning Microsc. 1995;9:103–114. [PubMed] [Google Scholar]
- 23.Koul HK, Menon M, Chaturvedi LS, Koul S, Sekhon A, Bhandari A, Huang M. COM crystals activate the p38 mitogen-activated protein kinase signal transduction pathway in renal epithelial cells. J Biol Chem. 2002;277:36845–36852. doi: 10.1074/jbc.M200832200. [DOI] [PubMed] [Google Scholar]
- 24.Mandel N. Crystal-membrane interaction in kidney stone disease. J Am Soc Nephrol. 1994;5:S37–S45. doi: 10.1681/ASN.V55s37. [DOI] [PubMed] [Google Scholar]
- 25.Riese RJ, Riese JW, Kleinman JG, Wiessner JH, Mandel GS, Mandel NS. Specificity in calcium oxalate adherence to papillary epithelial cells in cultures. Am J Physiol. 1988;255:F1025–F1032. doi: 10.1152/ajprenal.1988.255.5.F1025. [DOI] [PubMed] [Google Scholar]
- 26.Riese RJ. Adherence of kidney stone microcrystals to renal papillary collecting tubule cells in primary culture. 0. 1989. [Google Scholar]
- 27.Riese RJ, Kleinman JG, Wiessner JH, Mandel GS, Mandel NS. Uric acid crystal binding to renal inner medullary collecting duct cells in primary culture. J Am Soc Nephrol. 1990;1:187–192. doi: 10.1681/ASN.V12187. [DOI] [PubMed] [Google Scholar]
- 28.Riese RJ, Mandel NS, Wiessner JH, Mandel GS, Becker CG, Kleinman JG. Cell polarity and calcium oxalate crystal adherence to cultured collecting duct cells. Am J Physiol (Renal Fluid Electrolyte Physiol ) 1992;262(31):F117–F184. doi: 10.1152/ajprenal.1992.262.2.F177. [DOI] [PubMed] [Google Scholar]
- 29.Bigelow MW, Wiessner JH, Kleinman JG, Mandel NS. Surface exposure of phosphatidylserine increases calcium oxalate crystal attachment to IMCD cells. Am J Physiol (Renal Fluid Electrolyte Physiol ) 1997;272:F55–F62. doi: 10.1152/ajprenal.1997.272.1.F55. [DOI] [PubMed] [Google Scholar]
- 30.Asselman M, Verhulst A, De Broe ME, Verkoelen CF. Calcium Oxalate Crystal Adherence to Hyaluronan-, Osteopontin-, and CD44-Expressing Injured/Regenerating Tubular Epithelial Cells in Rat Kidneys. J Am Soc Nephrol. 2003;14:3155–3166. doi: 10.1097/01.asn.0000099380.18995.f7. [DOI] [PubMed] [Google Scholar]
- 31.Sorokina EA, Wesson JA, Kleinman JG. An acidic peptide sequence of nucleolin-related protein can mediate the attachment of calcium oxalate to renal tubule cells. 2004:2057–2065. doi: 10.1097/01.ASN.0000133024.83256.C8. [DOI] [PubMed] [Google Scholar]
- 32.Kumar V, Farell G, Deganello S, Lieske JC. Annexin II is present on renal epithelial cells and binds calcium oxalate monohydrate crystals. J Amer Soc Neph. 2003;14:289–297. doi: 10.1097/01.asn.0000046030.24938.0a. [DOI] [PubMed] [Google Scholar]
- 33.Sheng X, Ward MD, Wesson JA. Crystal surface adhesion explains the pathological activity of calcium oxalate hydrates in kidney stone formation. J Am Soc Nephrol. 2005;16:1904–1908. doi: 10.1681/ASN.2005040400. [DOI] [PubMed] [Google Scholar]
- 34.Grover PK, Marshall VR, Ryall RL. Dissolved urate salts out calcium oxalate in undiluted human urine in vitro: implications for calcium oxalate stone genesis. Chem Biol. 2003;10:271–278. doi: 10.1016/s1074-5521(03)00057-7. [DOI] [PubMed] [Google Scholar]
- 35.Pak CY, Sakhaee K, Peterson RD, Poindexter JR, Frawley WH. Biochemical profile of idiopathic uric acid nephrolithiasis. Kidney Int. 2001;60:757–761. doi: 10.1046/j.1523-1755.2001.060002757.x. [DOI] [PubMed] [Google Scholar]
- 36.Grover P, Ryall R, Marshall V. Calcium oxalate crystallization in urine: Role of urate and glycosaminoglycans. Kidney Int. 1992;41:149–154. doi: 10.1038/ki.1992.20. [DOI] [PubMed] [Google Scholar]
- 37.Farell G, Huang E, Kim SY, Horstkorte R, Lieske JC. Modulation of proliferating renal epithelial cell affinity for calcium oxalate monohydrate crystals. J Am Soc Nephrol. 2004;15:3052–3062. doi: 10.1097/01.ASN.0000144205.49134.64. [DOI] [PubMed] [Google Scholar]
- 38.Moe OW, Abate N, Sakhaee K. Pathophysiology of uric acid nephrolithiasis. Endocrinol Metab Clin North Am. 2002;31:895–914. doi: 10.1016/s0889-8529(02)00032-4. [DOI] [PubMed] [Google Scholar]
- 39.Ebrahimpour A, Perez L, Nancollas GH. Induced crystal growth of calcium oxalate monohydrate at hydroxyapatite surfaces. The influence of human serum albumin, citrate, and magnesium. Langmuir. 1991;7:577–583. [Google Scholar]
- 40.Dent CE, SENIOR B. Studies on the treatment of cystinuria. Br J Urol. 1955;27:317–332. doi: 10.1111/j.1464-410x.1955.tb03486.x. [DOI] [PubMed] [Google Scholar]
- 41.Kok DJ, Papapoulos SE, Blomen LJMJ, Bijvoet OLM. Modulation of calcium oxalate monohydrate crystallization kinetics in vitro. Kidney Int. 1988;34:346–350. doi: 10.1038/ki.1988.187. [DOI] [PubMed] [Google Scholar]
- 42.Lieske JC, Leonard R, Toback FG. Adhesion of calcium oxalate monohydrate crystals to renal epithelial cells is inhibitied by specific anions. Am J Physiol (Renal Fluid Electrolyte Physiol ) 1995;268(37):F604–F612. doi: 10.1152/ajprenal.1995.268.4.F604. [DOI] [PubMed] [Google Scholar]
- 43.Fleisch H. Inhibitors and promoters of stone formation. Kidney Int. 1978;13:361–371. doi: 10.1038/ki.1978.54. [DOI] [PubMed] [Google Scholar]
- 44.Grases F, Conte A. Urolithiasis, inhibitors and promoters. Urol Res. 1992;20:86–88. doi: 10.1007/BF00294344. [DOI] [PubMed] [Google Scholar]
- 45.Ryall RL, Harnett RM, Marshall VR. The effect of urine, pyrophosphate, citrate, magnesium and glycosaminoglycans on the growth and aggregation of calcium oxalate crystals in vitro. Clin Chim Acta. 1981;112:349–356. doi: 10.1016/0009-8981(81)90458-7. [DOI] [PubMed] [Google Scholar]
- 46.Grases F, Isern B, Sanchis P, Perello J, Torres JJ, Costa-Bauza A. Phytate acts as an inhibitor in formation of renal calculi. Front Biosci. 2007;12:2580–2587. doi: 10.2741/2256. [DOI] [PubMed] [Google Scholar]
- 47.Lieske JC, Farell G, Deganello S. The effect of ions at the surface of calcium oxalate monohydrate crystals on cell-crystal interactions. Urol Res. 2004;32:117–123. doi: 10.1007/s00240-003-0391-5. [DOI] [PubMed] [Google Scholar]
- 48.FARAGALLA FF, GERSHOFF SN. INTERELATIONS AMONG MAGNESIUM, VITAMIN B6, SULFUR AND PHOSPHORUS IN THE FORMATION OF KIDNEY STONES IN THE RAT. J Nutr. 1963;81:60–66. doi: 10.1093/jn/81.1.60. [DOI] [PubMed] [Google Scholar]
- 49.Massey L. Magnesium therapy for nephrolithiasis. Magnes Res. 2005;18:123–126. [PubMed] [Google Scholar]
- 50.Worcester EM, Blumenthal SS, Beshensky AM, Lewand DL. The calcium oxalate crystal growth inhibitor protein produced by mouse kidney cortical cells in culture is osteopontin. J Bone Miner Res. 1992;7:1029–1036. doi: 10.1002/jbmr.5650070905. [DOI] [PubMed] [Google Scholar]
- 51.Worcester EM, Kleinman JG, Beshensky AM. Osteopontin production by cultured kidney cells. Ann NY Acad Sci. 1995;760:266–278. doi: 10.1111/j.1749-6632.1995.tb44637.x. [DOI] [PubMed] [Google Scholar]
- 52.Hoyer JR, Asplin JR, Otvos L., Jr Phosphorylated osteopontin peptides suppress crystallization by inhibiting the growth of calcium oxalate crystals. Kidney Int. 2001;60:77–82. doi: 10.1046/j.1523-1755.2001.00772.x. [DOI] [PubMed] [Google Scholar]
- 53.Yamate T, Kohri K, Umekawa T, Amasaki N, Isikawa Y, Kurita T. The effect of osteopontin on the adhesion of calcium oxalate crystals to Madin-Darby canine kidney cells. Eur Urol. 1996;30:388–393. doi: 10.1159/000474201. [DOI] [PubMed] [Google Scholar]
- 54.Yamate T, Kohri K, Umekawa T, Iguchi M, Kurita T. Osteopontin antisense oligonucleotide inhibits adhesion of calcium oxalate crystals in Madin-Darby canine kidney cell. J Urol. 1998;160:1506–1512. [PubMed] [Google Scholar]
- 55.Wesson JA, Ganne V, Beshensky AM, Kleinman JG. Regulation by macromolecules of calcium oxalate crystal aggregation in stone formers. Urol Res. 2005;33:206–212. doi: 10.1007/s00240-004-0455-1. [DOI] [PubMed] [Google Scholar]
- 56.van Rooijen JJ, Voskamp AF, Kamerling JP, Vliegenthart JF. Glycosylation sites and site-specific glycosylation in human Tamm-Horsfall glycoprotein. Glycobiology. 1999;9:21–30. doi: 10.1093/glycob/9.1.21. [DOI] [PubMed] [Google Scholar]
- 57.Hess B, Nakagawa Y, Parks JH, Coe FL. Molecular abnormality of Tamm-Horsfall glycoprotein in calcium oxalate nephrolithiasis. Am J Physiol. 1991;260:F569–F578. doi: 10.1152/ajprenal.1991.260.4.F569. [DOI] [PubMed] [Google Scholar]
- 58.Viswanathan PRJDKAMWMDKJGWJD. Calcium oxalate monohydrate aggregation induced by aggregation of desialylated Tamm-Horsfall protein. Urol Res. doi: 10.1007/s00240-010-0353-7. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar V, Farell G, Lieske JC. Whole urinary proteins coat calcium oxalate monohydrate crystals to greatly decrease their adhesion to renal cells. J Urol. 2003;170:221–225. doi: 10.1097/01.ju.0000059540.36463.9f. [DOI] [PubMed] [Google Scholar]
- 60.Konya E, Amasaki N, Umekawa T, Iguchi M, Kurita T. Influence of urinary sialic acid on calcium oxalate crystal formation. Urol Int. 2002;68:281–285. doi: 10.1159/000058451. [DOI] [PubMed] [Google Scholar]
- 61.Webber D, Radcliffe CM, Royle L, Tobiasen G, Merry AH, Rodgers AL, Sturrock ED, Wormald MR, Harvey DJ, Dwek RA, Rudd PM. Sialylation of urinary prothrombin fragment 1 is implicated as a contributory factor in the risk of calcium oxalate kidney stone formation. FEBS J. 2006;273:3024–3037. doi: 10.1111/j.1742-4658.2006.05314.x. [DOI] [PubMed] [Google Scholar]
- 62.Pragasam V, Kalaiselvi P, Subashini B, Sumitra K, Varalakshmi P. Structural and functional modification of THP on nitration: comparison with stone formers THP. Nephron Physiol. 2005;99:28–34. doi: 10.1159/000081800. [DOI] [PubMed] [Google Scholar]
- 63.Lien YH, Lai LW. Liposome-mediated gene transfer into the tubules. [Review] [22 refs] Exp Nephrol. 1997;5:132–136. [PubMed] [Google Scholar]
- 64.Worcester EM, Sebastian JL, Hiatt JG, Beshensky AM, Sadowski JA. The effect of warfarin on urine calcium oxalate crystal growth inhibition and urinary excretion of calcium and nephrocalcin. Calcif Tissue Int. 1993;53:242–248. doi: 10.1007/BF01320909. [DOI] [PubMed] [Google Scholar]
- 65.Atmani F, Lacour P, Jungers P, Drueke T, Daudon M. Reduced inhibitory activity of uronic-acid-rich protein in urine of stone formers. Urol Res. 1994;22:257–260. doi: 10.1007/BF00541903. [DOI] [PubMed] [Google Scholar]
- 66.Tang Y, Grover PK, Moritz RL, Simpson RJ, Ryall RL. Is nephrocalcin related to the urinary derivative (bikunin) of inter-alpha-trypsin inhibitor? Br J Urol. 1995;76:425–430. doi: 10.1111/j.1464-410x.1995.tb07738.x. [DOI] [PubMed] [Google Scholar]
- 67.Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: A 5-year randomized prospective study. J Urol. 1996;155:839–843. [PubMed] [Google Scholar]
- 68.Harvey JA, Hill KD, Pak CY. Similarity of urinary risk factors among stone-forming patients in five regions of the United States. J Lithotr Stone Dis. 1990;2:124–132. [PubMed] [Google Scholar]
- 69.Worcester EM. Stones from bowel disease. Endocrinol Metab Clin North Am. 2002;31:979–999. doi: 10.1016/s0889-8529(02)00035-x. [DOI] [PubMed] [Google Scholar]
- 70.Worcester EM, Coe FL. New insights into the pathogenesis of idiopathic hypercalciuria. Semin Nephrol. 2008;28:120–132. doi: 10.1016/j.semnephrol.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li X-Q, Tembe V, Horwitz GM, Bushinsky DA, Favus MJ. Increased intestinal vitamin D receptor in genetic hypercalciuric rats. A cause of intestinal calcium hyperabsorption. J Clin Invest. 1993;91:661–667. doi: 10.1172/JCI116246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zerwekh JE, Hughes MR, Reed BY, Breslau NA, Heller HJ, Lemke M, Nasonkin I, Pak CYC. Evidence for normal vitamin D receptor messenger ribonucleic acid and genotype in adsorptive hypercalciuria. J Clin Endocrinol Metab. 1995;80:2960–2965. doi: 10.1210/jcem.80.10.7559881. [DOI] [PubMed] [Google Scholar]
- 73.Reed BY, Gitomer WL, Heller HJ, Hsu MC, Lemke M, Padalino P, Pak CY. Identification and characterization of a gene with base substitutions associated with the absorptive hypercalciuria phenotype and low spinal bone density. J Clin Endocrinol Metab. 2002;87:1476–1485. doi: 10.1210/jcem.87.4.8300. [DOI] [PubMed] [Google Scholar]
- 74.Borghi L, Schianchi T, Meschi T, Guerra A, Allegri F, Maggiore U, Novarini A. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346:77–84. doi: 10.1056/NEJMoa010369. [DOI] [PubMed] [Google Scholar]
- 75.Robertson WG, Hughes H. Importance of mild hyperoxaluria in the pathogenesis of urolithiasis--new evidence from studies in the Arabian peninsula. Scanning Microsc. 1993;7:391–401. [PubMed] [Google Scholar]
- 76.Huang HS, Ma MC, Chen CF, Chen J. Lipid peroxidation and its correlations with urinary levels of oxalate, citric acid, and osteopontin in patients with renal calcium oxalate stones. Urology. 2003;62:1123–1128. doi: 10.1016/s0090-4295(03)00764-7. [DOI] [PubMed] [Google Scholar]
- 77.Sumitra K, Pragasam V, Sakthivel R, Kalaiselvi P, Varalakshmi P. Beneficial effect of vitamin E supplementation on the biochemical and kinetic properties of Tamm-Horsfall glycoprotein in hypertensive and hyperoxaluric patients. Nephrol Dial Transplant. 2005;20:1407–1415. doi: 10.1093/ndt/gfh794. [DOI] [PubMed] [Google Scholar]
- 78.Tungsanga K, Sriboonlue P, Futrakul P, Yachantha C, Tosukhowong P. Renal tubular cell damage and oxidative stress in renal stone patients and the effect of potassium citrate treatment. Urol Res. 2005;33:65–69. doi: 10.1007/s00240-004-0444-4. [DOI] [PubMed] [Google Scholar]
- 79.Voss S, Hesse A, Zimmermann DJ, Sauerbruch T, von Unruh GE. Intestinal oxalate absorption is higher in idiopathic calcium oxalate stone formers than in healthy controls: measurements with the [(13)C2]oxalate absorption test. J Urol. 2006;175:1711–1715. doi: 10.1016/S0022-5347(05)01001-3. [DOI] [PubMed] [Google Scholar]
- 80.Nguyen QV, Kalin A, Drouve U, Casez JP, Jaeger P. Sensitivity to meat protein intake and hyperoxaluria in idiopathic calcium stone formers. Kidney Int. 2001;59:2273–2281. doi: 10.1046/j.1523-1755.2001.00744.x. [DOI] [PubMed] [Google Scholar]
- 81.Holmes RP, Assimos DG. The impact of dietary oxalate on kidney stone formation. Urol Res. 2004;32:311–316. doi: 10.1007/s00240-004-0437-3. [DOI] [PubMed] [Google Scholar]
- 82.McDonald GB, Earnest DL, Admirand WH. Hyperoxaluria correlates with fat malabsorption in patients with sprue. Gut. 1977;18:561–566. doi: 10.1136/gut.18.7.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sinha MK, Collazo-Clavell ML, Rule A, Milliner DS, Nelson W, Sarr MJ, Kuman R, Lieske JC. Hyperoxaluria and nephrolithiasis after Roux-en-Y gastric bypass for obesity. Kidney Int. 2007 doi: 10.1038/sj.ki.5002194. [DOI] [PubMed] [Google Scholar]
- 84.Taylor EN, Curhan GC. Determinants of 24-hour urinary oxalate excretion. Clin J Am Soc Nephrol. 2008;3:1453–1460. doi: 10.2215/CJN.01410308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet. 2006;38:474–478. doi: 10.1038/ng1762. [DOI] [PubMed] [Google Scholar]
- 86.Levi M, Breusegem S. Renal phosphate-transporter regulatory proteins and nephrolithiasis. N Engl J Med. 2008;359:1171–1173. doi: 10.1056/NEJMe0805943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ha YS, Tchey DU, Kang HW, Kim YJ, Yun SJ, Lee SC, Kim WJ. Phosphaturia as a promising predictor of recurrent stone formation in patients with urolithiasis. Korean J Urol. 2010;51:54–59. doi: 10.4111/kju.2010.51.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karim Z, Gerard B, Bakouh N, Alili R, Leroy C, Beck L, Silve C, Planelles G, Urena-Torres P, Grandchamp B, Friedlander G, Prie D. NHERF1 mutations and responsiveness of renal parathyroid hormone. N Engl J Med. 2008;359:1128–1135. doi: 10.1056/NEJMoa0802836. [DOI] [PubMed] [Google Scholar]
- 89.Coe FL. Treated and untreated recurrent calcium nephrolithiasis in patients with idiopathic hypercalciuria, hyperuricosuria, or no metabolic disorder. Ann Intern Med. 1977;87:404–410. doi: 10.7326/0003-4819-87-4-404. [DOI] [PubMed] [Google Scholar]
- 90.Coe FL, Parks JH, Moore ES. Familial idiopathic hypercalciuria. N Engl J Med. 1979;300:337–340. doi: 10.1056/NEJM197902153000703. [DOI] [PubMed] [Google Scholar]
- 91.Sakhaee K, Nigam S, Snell P, Hsu MC, Pak CY. Assessment of the pathogenetic role of physical exercise in renal stone formation. J Clin Endocrinol Metab. 1987;65:974–979. doi: 10.1210/jcem-65-5-974. [DOI] [PubMed] [Google Scholar]
- 92.Borghi L, Meschi T, Amato F, Novarini A, Romanelli A, Cigala F. Hot occupation and nephrolithiasis. J Urol. 1993;150:1757–1760. doi: 10.1016/s0022-5347(17)35887-1. [DOI] [PubMed] [Google Scholar]
- 93.Pak CY, Poindexter JR, Peterson RD, Heller HJ. Biochemical and physicochemical presentations of patients with brushite stones. J Urol. 2004;171:1046–1049. doi: 10.1097/01.ju.0000104860.65987.4a. [DOI] [PubMed] [Google Scholar]
- 94.Hildebrandt F, Jungers P, Grunfeld J. In: Nephronophthisis, Medullary Cystic and Medullary Sponge Kidney Disease. Schrier RW, editor. 2001. [Google Scholar]
- 95.Sayer JA, Pearce SH. Diagnosis and clinical biochemistry of inherited tubulopathies. Ann Clin Biochem. 2001;38:459–470. doi: 10.1177/000456320103800503. [DOI] [PubMed] [Google Scholar]
- 96.Ismail EA, Abul SS, Sabry MA. Nephrocalcinosis and urolithiasis in carbonic anhydrase II deficiency syndrome. Eur J Pediatr. 1997;156:957–962. doi: 10.1007/s004310050751. [DOI] [PubMed] [Google Scholar]
- 97.Griffith DP, Musher DM, Itin C. Urease. The primary cause of infection-induced urinary stones. Invest Urol. 1976;13:346–350. [PubMed] [Google Scholar]
- 98.Usui Y, Matsuzaki S, Matsushita K, Shima M. Urinary citrate in kidney stone disease. Tokai J Exp Clin Med. 2003;28:65–70. [PubMed] [Google Scholar]
- 99.Domrongkitchaiporn S, Stitchantrakul W, Kochakarn W. Causes of hypocitraturia in recurrent calcium stone formers: focusing on urinary potassium excretion. Am J Kidney Dis. 2006;48:546–554. doi: 10.1053/j.ajkd.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 100.Levy FL, Adams-Huet B, Pak CY. Ambulatory evaluation of nephrolithiasis: an update of a 1980 protocol. Am J Med. 1995;98:50–59. doi: 10.1016/S0002-9343(99)80080-1. [DOI] [PubMed] [Google Scholar]
- 101.Amanzadeh J, Gitomer WL, Zerwekh JE, Preisig PA, Moe OW, Pak CY, Levi M. Effect of high protein diet on stone-forming propensity and bone loss in rats. Kidney Int. 2003;64:2142–2149. doi: 10.1046/j.1523-1755.2003.00309.x. [DOI] [PubMed] [Google Scholar]
- 102.Aruga S, Wehrli S, Kaissling B, Moe OW, Preisig PA, Pajor AM, Alpern RJ. Chronic metabolic acidosis increases NaDC-1 mRNA and protein abundance in rat kidney. Kidney Int. 2000;58:206–215. doi: 10.1046/j.1523-1755.2000.00155.x. [DOI] [PubMed] [Google Scholar]
ADDITIONAL READING
- Kok DJ. Clinical implications of physicochemistry of stone formation. Endocrinol Metab Clin North Am. 2002;31:855–867. doi: 10.1016/s0889-8529(02)00037-3. [DOI] [PubMed] [Google Scholar]