Abstract
Background
The rapidly increasing number of available plant genomes opens up almost unlimited prospects for biology in general and molecular phylogenetics in particular. A recent study took advantage of this data and identified a set of nuclear genes that occur in single copy in multiple sequenced angiosperms. The present study is the first to apply genomic sequence of one of these low copy genes, agt1, as a phylogenetic marker for species-level phylogenetics. Its utility is compared to the performance of several coding and non-coding chloroplast loci that have been suggested as most applicable for this taxonomic level. As a model group, we chose Tildenia, a subgenus of Peperomia (Piperaceae), one of the largest plant genera. Relationships are particularly difficult to resolve within these species rich groups due to low levels of polymorphisms and fast or recent radiation. Therefore, Tildenia is a perfect test case for applying new phylogenetic tools.
Results
We show that the nuclear marker agt1, and in particular the agt1 introns, provide a significantly increased phylogenetic signal compared to chloroplast markers commonly used for low level phylogenetics. 25% of aligned characters from agt1 intron sequence are parsimony informative. In comparison, the introns and spacer of several common chloroplast markers (trnK intron, trnK-psbA spacer, ndhF-rpl32 spacer, rpl32-trnL spacer, psbA-trnH spacer) provide less than 10% parsimony informative characters. The agt1 dataset provides a deeper resolution than the chloroplast markers in Tildenia.
Conclusions
Single (or very low) copy nuclear genes are of immense value in plant phylogenetics. Compared to other nuclear genes that are members of gene families of all sizes, lab effort, such as cloning, can be kept to a minimum. They also provide regions with different phylogenetic content deriving from coding and non-coding parts of different length. Thus, they can be applied to a wide range of taxonomic levels from family down to population level. As more plant genomes are sequenced, we will obtain increasingly precise information about which genes return to single copy most rapidly following gene duplication and may be most useful across a wide range of plant groups.
Background
Molecular phylogenetics has made remarkable progress in recent decades toward the reconstruction of a largely resolved 'tree of life' [1]. In flowering plants, the relationships are now clear from the deepest branches through to the family level, with only a few exceptions [2-4]. However, developing strong phylogenetic hypotheses for more closely related plants at the interface of species level and population level has been, and still is, quite challenging. At this level, gene exchange and recombination is still possible, and low sequence divergence often limits phylogenetic resolution. As a result, multiple independent phylogenetic markers with sufficient sequence divergence are necessary, and analytical approaches from population genetics may in some cases be more appropriate than traditional phylogenetic methods [5,6].
The most commonly used marker regions in plant phylogenetics are coding and non-coding sequences from the chloroplast genome and ribosomal gene regions located in the nucleus. Recently, whole plastid genomes (plastomes) have been used to address deep branching questions [e.g. [7-11]] in plants, and a rapid increase in plastome scale data is underway. Mitochondrial genes are not as widely used because of typically very slow sequence evolution and some complexities such as extreme rate variation in some lineages and occurrences of horizontal gene transfer [12,13], gene conversion and processed paralogy [e.g. [14]]. Nevertheless, these genes do play a substantial role for phylogenies of for instance parasitic plants [e.g. [13,15]] where plastid genomes can be highly modified [16,17]. However, some of the most extensive efforts to date to resolve the "deep branches" in flowering plant phylogenetics have involved large taxon samples with sequence data obtained from up to 17 genes representing all three genomic compartments [18].
"The Tortoise and the Hare" series [19-21] addressed the utility of nuclear and chloroplast loci for low level phylogenetics. These authors noticed an increased resolution for recent branching events with the nuclear gene Adh, but the chloroplast markers required much less laboratory effort. Subsequently, a number of promising chloroplast regions have been used to address questions at low taxonomic levels [21]. Plastomes provide numerous genes, introns, and intergenic regions; amplification and direct sequencing of PCR product is easy as a single cell harbors 1000 or more plastids with multiple (essentially) identical plastid genomes. Plastomes provide an almost unmatched source of orthologous sequence that is not complicated by gene family duplication, and multiple genes can be concatenated to provide a very long sample of orthologous sequence. However, plastome markers are tightly linked on a single (typically) non-recombining molecule that usually reflects only the maternal lineage in angiosperms, limiting the generality of plastid DNA as the sole source of evolutionary markers at and below species level. In addition, plastomes are limited in their variability (i.e. substitution rates, parsimony informative sites) and therefore their utility for low taxonomic level studies may be restricted [19,22].
The intergenic spacer (ITS) regions of nrDNA often provide higher variability, but their orthology usually remains an assumption not tested prior to their use in phylogenetic analysis. Hundreds to thousands of copies of highly conserved nrDNA gene regions located in the nucleus make those markers easy to amplify, but their evolution by tandem duplication events results in a gene array more accurately described as a gene family or large collection of paralogs [23,24]. The paralogous nature of nrDNA can complicate the reconstruction of phylogenetic relationships, as it is impossible to determine orthologous copies from such a large copy number. As a consequence, divergent paralogs may be inadvertently sampled from different organisms in a study; when included in phylogenetic reconstruction, artifacts can appear [e.g. [25]].
Nuclear markers (excluding nrDNA marker) are essential for a broad range of evolutionary investigation, including systematics and character evolution [e.g. [26]], hybridization [e.g. [27]], polyploidization [e.g. [28]], biogeography [29], origins of domestication [e.g. [30]], and speciation [e.g. [31]]. Occurring independently all over the nuclear genome in a virtually inexhaustible repertoire in terms of both number and variability, bi-parentally inherited nuclear loci are promising on different levels, especially when compared to organellar markers.
The first attempts to establish nuclear markers other than ribosomal genes yielded a substantial number of low copy nuclear genes (LCNG) [32]. LCNG, such as the ADH-genes [33], pistillata [34], GPAT [35], PRK [36] or LEAFY [37] were applied in numerous studies. These genes are known to occur either in single copy in one or more focal species in the study group, or as members of small gene families [38] and may not occur in any specific plant lineage. Complete sampling of gene families often involves intense experimental efforts to clone and sequence all members of a family. Thus, for practical reasons, single loci are mostly preferred.
Many different approaches have been used to identify useful nuclear single copy loci in plants, with results varying widely in terms of both the general idea and computational effort. In recent years, the advent of next generation sequencing technologies (NGS), bioinformatic progress, and publicly available sequence data have greatly facilitated the identification of such loci [e.g. [39]]. These rich sources of sequence information have been utilized by many research groups, who identified new nuclear markers for plant, animal and fungal phylogenetics to reveal relationships that could not be resolved with organelle or nuclear ribosomal DNA markers [22,40-47]. All of them focus on genes that occur in the nucleus in single copy in a sample of organisms with sequenced genomes. The nucleus provides a vast repertoire of unlinked markers. However, since there are thousands of genes in the nucleus, marker selection is challenging and the majority of nuclear genes occur in small to large gene families. The present study is based on the approach published in Duarte et al. [22], where a global classification of plant protein coding sequences (Tribes) was used to identify a collection of 959 genes that are represented by exactly one copy in each of four sequenced angiosperm genomes (Arabidopsis, Populus, Vitis, and Oryza). "Tribes" are collections of related genes in a specified set of genomes produced by the gene clustering program MCL-Tribe [39,48]. While many PlantTribes approximate gene families [39], the global classification also identifies genes that are members of highly distinctive clusters that lack closely related paralogs, including clusters with only a single gene in each taxon. The set of genes identified in the analysis was called APVO SSCG (Arabidopsis, Populus, Vitis, Oryza shared single copy genes). The agt1 gene, applied in the present study, is part of that set and the abbreviation nSCG (nuclear single copy gene) that is used here refers to this approach. We use the term nSCG operationally, referring to a gene that has been identified as single copy in global gene classification of a specified set of genomes, here APVO. This does not necessarily mean that the gene will be single copy in any given lineage, particularly in very recent polyploids [22,49], where the entire gene set has been recently duplicated, and the process of duplicate gene loss is underway. However, as larger numbers of genomes are interrogated, genes that continue to be found in single copy in all but the most recent polyploids are more likely to be single copy in a given uncharacterized lineage.
Hughes et al. [50] proposed the exploration of nuclear loci other than nrDNA for phylogenetic reconstruction, which would require the abandonment of 'universal thinking'. The enhanced variability of nuclear loci compared to other markers provides great potential, but limits the likelihood of identifying universal amplification primers that will function across a wide taxonomic range. In fact, it might be difficult to identify universal gene loci because polyploidy is prevalent and frequent in plants. Duplicated genes are often lost by both random and selective processes in a short time, but that also means there is a chance to encounter multiple gene copies in a specific lineage [22].
Peperomia (Piperaceae) ranks among the ten largest angiosperm genera, with approximately 1,650 species [51]. The phylogenetics and classification of such species-rich clades has long been very challenging. In addition, morphological characters have been shown to be subject to parallel evolution and extreme reduction, resulting in a paucity of synapomorphies [52,53]. Moreover, speciation within Peperomia has likely happened comparatively recently [51], and as a consequence, reconstructed phylogenies often lack resolution at the species and population level [54]. Recent backbone phylogenies were based on over 4000 molecular characters to overcome the lack of variability [52,53]. Therefore, Peperomia is an ideal candidate for testing the performance of a nSCG region and comparing outcomes with variable chloroplast markers such as those suggested by Shaw et al. [20,21]. Within this study, we focus on closely related species belonging to Peperomia subgenus Tildenia where currently 59 species are recognized [55,56].
Results
The agt1 gene studied here is an ortholog of At2G13360 in Arabidopsis thaliana, where it is known to catalyze the Alanine:Glyoxylate Aminotransferase reaction located in peroxisomes [57]. It was identified to be shared between four angiosperm genomes (Arabidopsis, Populus, Vitis, and Oryza) as a nSCG [22]. We have chosen this region as an example to test the utility of a nSCG for low-level phylogenies in comparison to eight widely studied chloroplast markers [20,21]. A single copy of this gene was identified in large EST sets from multiple basal angiosperms [22], making it a suitable candidate for our basal angiosperm test group Tildenia. The agt1 region was amplified, sequenced, and aligned for 62 accessions covering 33 out of the 59 species of Peperomia subgenus Tildenia resulting in a dataset of 2088 characters (Additional File 1: Taxa used in the present study, Additional File 2: Sequence statistics for the coding and non-coding regions for all markers in this study calculated with SeqState). A dataset with an identical sampling was generated for the trnK intron, the matK gene and the trnK-psbA spacer resulting in an aligned dataset of 3049 characters (sequences obtained in part from [51]. To allow further exploration of the utility of the agt1, we added additional chloroplast markers for a sub-sample of 26 representative accessions. These markers comprised the psbA-trnH spacer plus short parts of flanking genes (353 bp), as well as the ndhF-rpl32-trnL gene region, consisting of a partial sequence of the ndhF gene (204 bp), the ndhF-rpl32 spacer (620 bp), the rpl32 gene (158 bp) and partial sequence of the rpl32-trnL spacer (1048 bp) (Additional File 2: Sequence statistics for the coding and non-coding regions for all markers in this study calculated with SeqState). The ndhF-rpl32 spacer, rpl32 gene and rpl32-trnL spacer were co-amplified and yielded an aligned dataset of 2030 bp. All markers are among the most variable loci for phylogenetic studies according to Shaw et al. [20,21].
An important feature of agt1 in Peperomia is that PCR amplicons typically were resolved as a single sharp target sized band on agarose gels and, for most samples, could be sequenced directly without cloning. Only a few (roughly 10%) accessions required a cloning step. In those cases, a single band was detected in the agarose gel, but sequencing revealed short (1 to 10 bp) indels.
Structure and characterization of agt1
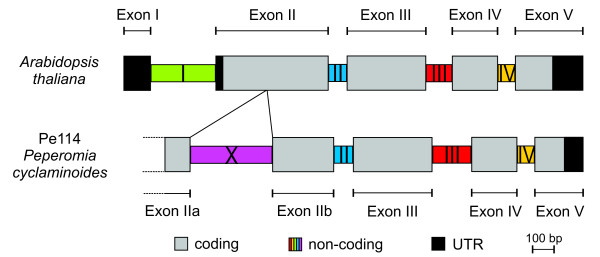
In Peperomia, the agt1 gene comprises five coding regions (exons) separated by four intronic sections with a total length of 1542 to 1827 bp. In comparison to agt1 in Arabidopsis thaliana, the Peperomia gene contains an additional intron, which is located in the second exon and has a length of 211-390 bp (Figure 1). This intron is also present in the genomic sequences of Populus trichocarpa, Medicago truncatula, Solanum lycopersicon, Oryza sativa, and Carica papaya, as well as in all Peperomia species generated in the present study. Hence, it can be inferred that it was lost during the diversification of Brassicales. We named this additional non-coding section that splits exon II into two parts, intron X, and the two resulting exons that derive from exon II were named exon IIa and exon IIb (Figure 1). In the Peperomia accessions we studied, the agt1 exons are highly conserved in length and lack any indels. In contrast, intron X was the most variable in length among the respective four introns, showing a length range of 552-739 bp.
Figure 1.
The Alanine:Glyoxylate-Aminotransferase (agt1) gene model in Arabidopsis thaliana and in Peperomia cyclaminoides. The gene model for the agt1 gene in P. cyclaminoides Pe114 was derived from both genomic and cDNA sequences. Compared with the gene model in Arabidopsis thaliana, Peperomia cyclaminoides has an additional intron (intron X) that is 380 bp long and located in exon II. This intron can be found among all sequenced Peperomia accessions and ranges in length from 211-390 bp. We also checked its presence in a number of sequenced plant genomes available in Phytozome (v.7.0); the intron is absent only from Arabidopsis and grasses. Since it can be found in papaya, we suggest two independent losses of this intron: within the diversification of both Poaceae (or possibly a larger monocot group) and Brassicales.
Variability of agt1 versus the chloroplast regions
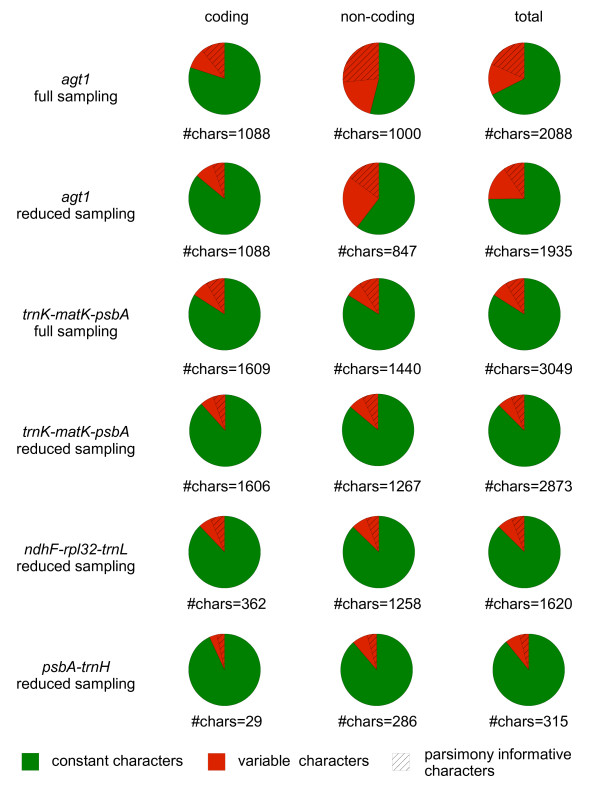
To characterize the markers in more detail, several variability parameters were estimated for both coding and non-coding regions and for the full as well as the reduced sampling datasets (Figure 2, Additional File 2: Sequence statistics for the coding and non-coding regions for all markers in this study calculated with SeqState). In the agt1 full sampling dataset, introns and exons are roughly equally represented (introns 1000 bp, exons 1088 bp, see Additional File 2: Sequence statistics for the coding and non-coding regions for all markers in this study calculated with SeqState). Coding parts possess 20% variable characters (VC) and 11% parsimony informative characters (PIC), whereas non-coding parts contain 46% VC and 27% PIC. Thus, the content of PIC varies largely among different sections of the gene and in non-coding parts it is twice as high as in coding parts. In total, one fifth of the characters in the agt1 gene are parsimony informative.
Figure 2.
Characteristics of utilized markers. Portions of constant (green), variable (red) and parsimony informative characters (dashed) for coding and non-coding parts of all markers used in this study. Chloroplast markers suggested by Shaw et al. [19,20] for species level were combined into the longer regions that we co-amplified with single primer pairs. Full sampling comprises 62 accessions. Reduced sampling is a dataset that contains a sub-sampling of 26 selected accessions from the full sampling. The percentage of variability of the chloroplast markers is very similar between regions and among coding and non-coding portions at the taxonomic level in this study. In contrast, the nuclear gene has highly variable introns, which yields a total variability that is two to three times greater than any of the chloroplast markers.
The trnK-matK-psbA region does not vary significantly among coding and non-coding regions on this taxonomic level (Additional File 2: Sequence statistics for the coding and non-coding regions for all markers in this study calculated with SeqState). Compared to the agt1 with the identical sampling this region shows less than half of the ratio of VC and PIC (Additional File 2: Sequence statistics for the coding and non-coding regions for all markers in this study calculated with SeqState). Thus, the agt1 is much more effective in yielding PIC per sequenced bp than the widely applied trnK-matK-psbA region. Furthermore, the nuclear gene segments provide different amounts of variability, which make them useful for many taxonomic levels.
A similar pattern is visible in the reduced sampling of all utilized markers (Additional File 2: Sequence statistics for the coding and non-coding regions for all markers in this study calculated with SeqState). In total, the percentages of both variable sites (25%) and PIC (15%) of agt1 are much higher than those of the chloroplast markers. However, it has to be noted that the VC percentage of the reduced sampling is 7% lower than that of the full sampling and that there are only half of the PIC in the reduced dataset. In contrast, the trnK-matK-psbA does not possess large differences between reduced and full sampling datasets. The decrease of VC in the reduced sampling suggests that the potential of agt1 is not exhausted and deeper sampling would likely increase available variability and thus phylogenetic resolution.
The variabilities of the different gene regions are graphically displayed in Figure 2. It is remarkable that the chloroplast markers are very similar in their overall fractions of variable sites (Figure 2). Thus, the number of PIC is directly proportional to the number of sequenced bp and thus on this phylogenetic level marker selection does not seem to be crucial in terms of phylogenetic content. Furthermore, non-coding regions of all chloroplast markers are just as informative as non-coding regions on this phylogenetic level. To look for potential saturation of the markers, the retention index (RI) was calculated with and without indel-coded length mutations. Generally, for the chloroplast markers the RI does not differ between the datasets that include indels and those without indels (Table 1). The RI values of both trnK-matK-psbA datasets (full sampling including indels: RI = 0.873, reduced sampling including indels: RI = 0.828) as well as ndhF-rps32-trnL dataset (reduced sampling including indels RI = 0.836) indicate low homoplasy. The homoplasy of agt1 full sampling (including indels, RI = 0.802) is little homoplastic as well. Only the RI values of psbA-trnH (reduced sampling including indels RI = 0.697) and agt1 reduced sampling datasets (including indels: RI = 0.658) datasets are a little lower, which suggests that they are more homoplastic and likely closer to saturation. Considering the RI, both trnK-matK-psbA and ndhF-rpl32-trnL can be regarded as little homoplastic. The RI of the different agt1 datasets differs and is lower in the reduced sampling. Consequently, a smaller sampling is more homoplastic than a denser sampling with the same breadth. This fact is congruent with the phylogenies of the agt1 gene, resulting in less resolution and support in the reduced dataset. This leads to the conclusion that a high variability as detected for the agt1 gene requires a dense sampling to keep the risk for artifacts low.
Table 1.
Statistics based on a Maximum Parsimony Ratchet analysis showing the retention index as a measure of homoplasy.
| total characters (with indels) | Trees found (with indels) | Steps (with indels) | RI (with indels) | ||
|---|---|---|---|---|---|
| agt1 full sampling | coding | 1088 (1088) | 1253 (N/A) | 405 (N/A) | 0.827 (N/A) |
| non-coding | 1009 (1192) | 1721 (1539) | 978 (1205) | 0.799 (0.794) | |
| total | 2097 (2280) | 232 (362) | 1395 (1622) | 0.802 (0.798) | |
| agt1 reduced sampling | coding | 1088 (1088) | 206 (N/A) | 260 (N/A) | 0.742 (N/A) |
| non-coding | 856 (971) | 323 (55) | 613 (746) | 0.636 (0.628) | |
| total | 1944 (2059) | 50 (64) | 876 (1010) | 0.667 (0.658) | |
| matK-trnK-psbA full sampling | coding | 1609 (1621) | 158 (172) | 448 (461) | 0.882 (0.883) |
| non-coding | 1475 (1554) | 39 (85) | 422 (518) | 0.879 (0.877) | |
| total | 3085 (3174) | 1007 (1356) | 881 (990) | 0.874 (0.873) | |
| matK-trnK-psbA reduced sampling | coding | 1606 (1614) | 18 (15) | 303 (312) | 0.840 (0.383) |
| non-coding | 1310 (1361) | 9 (28) | 287 (343) | 0.837 (0.831) | |
| total | 2916 (2974) | 12 (21) | 587 (651) | 0.832 (0.828) | |
| ndhF-rpl32-trnL reduced sampling | coding | 362 (367) | 2 (2) | 58 (63) | 0.962 (0.964) |
| non-coding | 1668 (1783) | 10 (8) | 246 (382) | 0.822 (0.823) | |
| total | 2030 (2150) | 1 (65) | 305 (448) | 0.847 (0.836) | |
| psbA-trnH reduced sampling | coding | 29 (29) | 1 (N/A) | 2 (N/A) | 1.000 (N/A) |
| non-coding | 324 (370) | 810 (28) | 46 (100) | 0.727 (0.714) | |
| total | 353 (399) | 1044 (38) | 49 (103) | 0.696 (0.700) |
To summarize, a quarter of the total agt1 dataset is parsimony informative, while having a low homoplasy level at the same time. All calculations were based on nucleotide substitutions only (indels not included), as RIs of all data sets revealed this to be less homoplastic than the data sets containing indels (Table 1).
Phylogenetic output
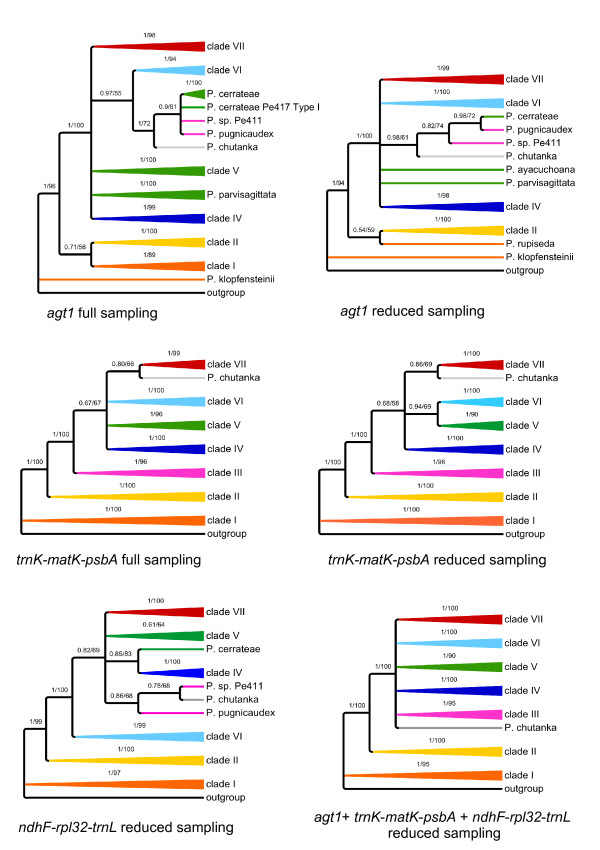
To obtain insights into agt1's phylogenetic performance in terms of topology, resolution and support, we performed identical phylogenetic analyses with identical sampling and a reduced sub-sampling for all markers (Figure 3). For those analyses, Maximum Likelihood (ML) and Bayesian Inference (BI) were used. For simplicity, the clades have been named with numbers based on the study of Symmank et al. [51].
Figure 3.
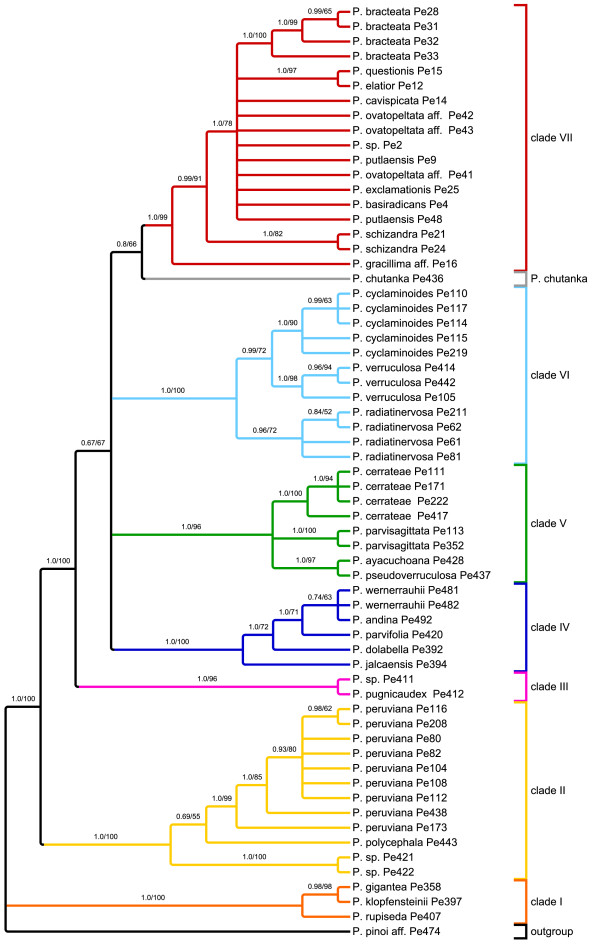
Schematic summary of topologies. Summarized topologies are based on Bayesian inference. Numbers above branches are posterior probabilities (PPs) from Bayesian inference (BI) (left) and ML bootstrap values (right). Agt1 and trnK-matK-psbA are shown for both full and reduced sampling, and ndhF-rpl32-trnH for reduced sampling. Clades were named based on the topology obtained from the trnK-matK-psbA region. Full sampling reflects a 63 accession dataset, while reduced sampling comprises 26 accessions. The chloroplast regions do not provide congruent signal. From all these trees, the nuclear gene provides the best resolution with the full sampling. In this tree, an additional clade is formed, consisting of P. chutanka, P. pugnicaudex and P. cerrateae. Clade VI is sister to this new clade. These (statistically supported) relationships cannot be observed in any of the trees based on chloroplast markers.
By looking at the trees derived from different markers, here summarized at the clade level (Figure 3), it is obvious that agt1 and the chloroplast gene trees result in different topologies. Although the majority of clades are recovered as monophyletic among all gene trees, a few clades are unresolved in the agt1 tree (Figure 3).
All phylogenetic trees obtained from the different markers show species from clade I and II as the first diverging branches followed by a polytomy formed by the remaining clades. The nuclear trees all show four clades out of seven being monophyletic and statistically supported (clade II, IV, VI and VII) (Figure 3). Clade I is polyphyletic in the agt1 tree, but monophyletic with any of the chloroplast markers. A new clade is formed in the agt1 phylogeny, consisting of P. cerrateae, P. sp. Pe411, P. pugnicaudex, and P. chutanka. Clade VI is sister to this clade, which is supported with 0.97 Posterior Probability (PP) in the full sampling. Thus, clades III and V are not monophyletic in the nuclear-based tree or in the tree resulting from ndhF-rps32-trnL. In the trnK-matK-psbA tree, P. cerrateae was part of clade V, P. sp. Pe411 and P. pugnicaudex formed clade III and the position of P. chutanka could not be resolved. In summary, agt1 yields a deeper resolution compared to any individual or combined chloroplast dataset by forming a new clade that has clade VI as a sister. Sampling density does not seem to influence the phylogenies obtained from the chloroplast markers as much as it affects the nuclear trees. Denser sampling seems to improve resolution with agt1. Statistical evaluation of phylogenetic signals between datasets was done using SH tests [58]. These tests reveal a significant conflict between the chloroplast markers and the nuclear agt1 gene, expressed by the rejection of all respective topology/dataset combinations (Additional File 3: Results from topology tests). The only exception to this pattern is the combination of the ndhF-rpl32-trnL topology and the agt1 dataset (p = 0.070). The trnH-psbA spacer was not used for this test due to insufficient phylogenetic resolution. In accordance with the result of Shaw et al. [20], this finding states the inappropriateness of this region for phylogenetic studies on a low taxonomic level.
To determine the source of conflict between the nuclear and chloroplast markers we tested 10 alternative hypotheses by changing the topologies of the reduced agt1 and trnK-matK-psbA datasets. In most cases a single clade was replaced according to its phylogenetic position in the opposed topology. Additionally, clades that appear polyphyletic in one topology were constrained to be monophyletic and placed at different positions. All alternative hypotheses resulted in significant rejection by the tested datasets. The alternative topologies and the SH-values of the accordant combinations are summarized in Additional File 3: Results from topology tests. The results indicate a general incongruence between the phylogenetic signal derived from the nuclear and chloroplast genome. The rejection of the alternative hypothesis clearly shows that the conflict is not caused by a single subset of species or clade, but is reflecting multiple inconsistencies between the nuclear versus the chloroplast gene regions at lower nodes of the tree. Such a pattern might be expected if an early hybridization and chloroplast capture event had occurred early in the evolution of the subgenus. Such an event can lead to incongruence between the histories of nuclear and plastid marker regions.
The combination of agt1, trnK-matK-psbA, ndhF-rpl32-trnL, and trnH-psbA data set (7243 total characters) yields a topology possessing all the monophyletic clades identified by trnK-matK-psbA with maximal support. In addition, P. chutanka is resolved in the combined analysis as sister to clade III (0.99 PP). A phylogeny of trnH-psbA alone did not yield any resolution (results not shown).
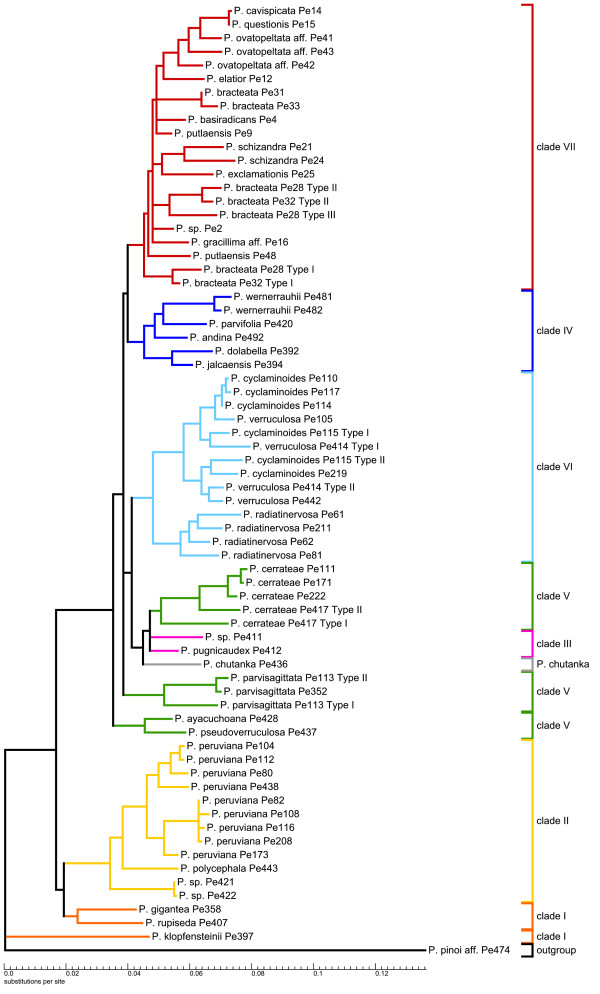
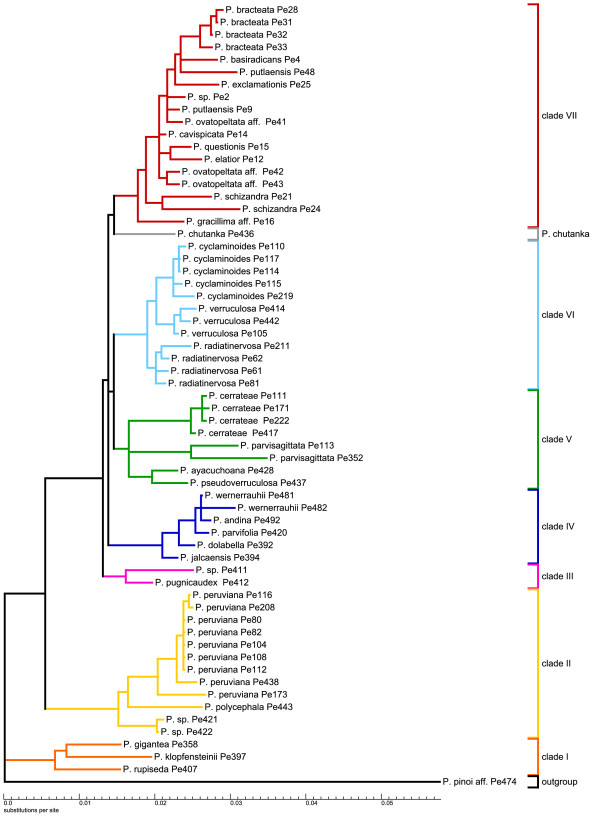
The resolution of the two full sampling phylogenies (Figure 4, Figure 5) was measured using the normalized consensus fork index (CFI), which divides the number of nodes found in a strict consensus tree by the number of possible nodes [59]. To increase the information value of this index with respect to the resolution capacity of the respective molecular markers, we consider the nodes that appear in the trees inferred from BI and ML, where nodes below 50% were collapsed (Figure 4 and 5). According to this, the agt1 yields a considerably higher total resolution (CFI = 0.74) than the trnK-matK-psbA region (CFI = 0.58). Comparison of the phylograms show that the substitution rate in the agt1 data set is about twice as great as in the trnK-matK-psbA dataset (BI, Figure 6 and 7).
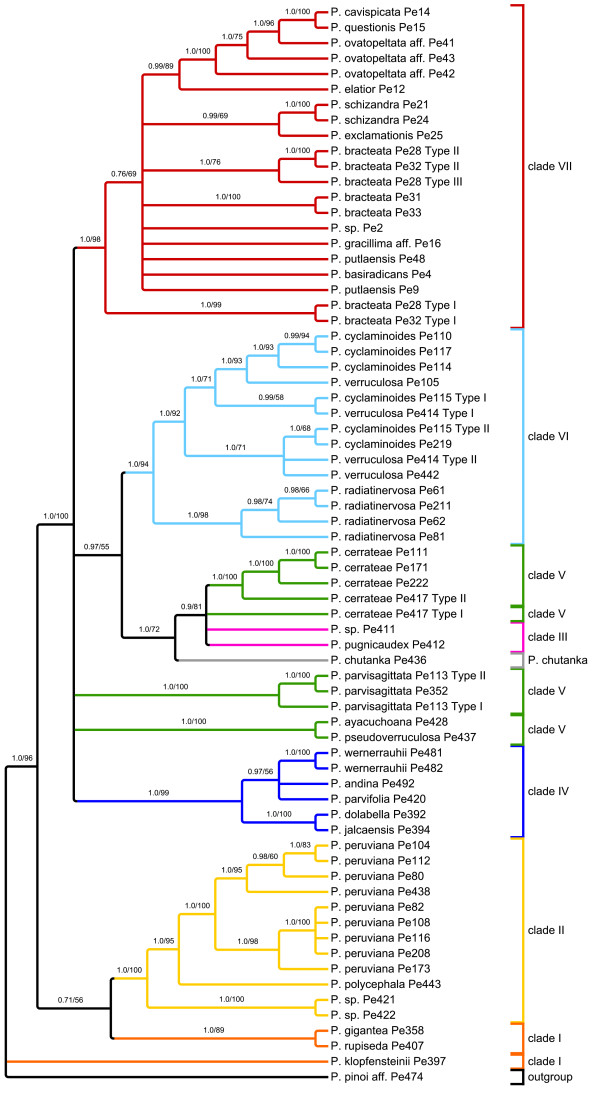
Figure 4.
Phylogenetic hypothesis for Peperomia subgenus Tildenia based on ML and BI analysis of agt1. This topology is derived from ML; nodes supported at < 50% in at least one of the two analyses were collapsed. Numbers above branches are PPs from BI (left) and ML bootstrap values (right).
Figure 5.
Phylogenetic hypothesis for Peperomia subgenus Tildenia based on ML and BI analysis of trnK-matK-psbA. This topology is derived from ML; nodes supported at < 50% in at least one of the two analyses were collapsed. Numbers above branches are PPs from BI (left) and ML bootstrap values (right).
Figure 6.
Phylogram of agt1 based on Bayesian inference. Phylogram obtained from the Bayesian inference of the agt1 data set with relative substitution rates using the GTR+G+I model and posterior probabilities plotted above the branches. This phylogenetic tree is summarized in Figure 3. Clades are colored according to Figure 3. In accessions that are represented in the tree by different 'types', multiple copies were detected that are not monophyletic. A closer look to the resolution within the clades shows highly supported relationships down to the population level.
Figure 7.
Phylogram of the trnK-matK-psbA region based on Bayesian inference. Phylogram from Bayesian inference of the trnK-matK-psbA data set with relative substitution rates using the GTR+G model and posterior probabilities plotted above the branches. This phylogenetic tree is summarized in Figure 3. Clades are colored according to Figure 3.
The phylograms also suggest that a sudden and fast radiation occurred that gave rise to clades III-VII. This radiation has been linked directly to the formation of new habitats during the uplift of the inner Andean valleys in Peru and Bolivia [51]. Generally, the agt1 resolution is much higher within the clades and on population level, which is very obvious for P. peruviana, P. cerrateae, and P. radiadinervosa as well as within clade IV and VI (Figure 4). Furthermore, the agt1 phylogeny suggests that P. cyclaminoides and P. verruculosa are not monophyletic and P. bracteata is polyphyletic on the agt1 tree. Clade VII shows better resolution of the deeper branches with the chloroplast dataset (Figure 5), whereas the nuclear gene resolves relationships better in shallower parts of the tree. In the trnK-matK-psbA phylogenetic tree, P. gracillima aff. is the first branch, followed by P. schizandra as sister to the rest of clade VII. The nuclear gene, however, reveals a well-resolved sub-clade comprising P. elatior as the first branch, followed by P. ovatopeltata aff., P. questionis and P. cavispicata. Furthermore, in the nuclear phylogeny, P. exclamationis is resolved as sister to P. schizandra.
Single Copy vs. Multicopy status of agt1
As described above, approximately 90% of the PCR amplicons could be directly sequenced from PCR product; only 10% required cloning to obtain distinct sequences. Multiple copies were detected in at least one accession from every clade except clade I and III. Putative pseudogenes, e.g. ones with frameshift mutations, were removed from the analysis. A resulting phylogeny, which contained all detected copies excluding putative pseudogenes, and which did not comprise sequence data downstream from intron III, revealed that the copies of eight accessions are monophyletic, and thus do not influence the final analysis (Additional File 4: Phylogram of agt1 based on BI of entire dataset including cloned sequences). Therefore, a cloned copy representing the particular accession could have been randomly chosen for the main phylogeny. However, agt1 copies sequenced from four of the accessions are not monophyletic. These copies cluster together with copies from other accessions of the respective species, indicating duplication during radiation of the respective species (P. cerrateae Pe417, P. parvisagittata Pe113, P. schizandra Pe24 and P. wernerrauhii Pe481) (Additional File 4: Phylogram of agt1 based on BI analysis of the entire dataset including cloned sequences). The only accessions for which the situation seems to be more complex are P. bracteata Pe28 and Pe32, as well as P. cyclaminoides Pe115 and P. verruculosa Pe414. Both cases show a similar pattern: copies of a single accession are not monophyletic, but are sister to their orthologous copy from other accessions. Thus, cloned sequences of such an accession are likely to be paralogous.
Discussion
Rapidly radiating genera and single copy nuclear genes
Rapidly radiating taxa and species-rich groups have been, and remain, challenging for high resolution phylogenetic analyses with widely used chloroplast markers [e.g. [60-62]].
Independent of the applied marker, most of these groups tend to share a well-resolved base with several long branches followed by several clades branching from a polytomy with rather short branches. This could indicate that some lineages within species-rich groups radiated over a comparatively short time span, possibly as a result of the availability of new habitat or some other opportunity. In the case of Tildenia, this branching pattern can be seen in the phylograms (Figure 6 and 7) and has been linked to geological events during the uplift of the Andes as well as the climatic oscillation during the period of diversification in the mid-Miocene between 15-10 MYA [51]. As we had expected, the agt1 gene resolves short and deep branches much better in Tildenia, since substitution rates for the non-coding parts are much greater than for coding or for non-coding chloroplast markers. Shaw et al. [21] concluded that non-coding chloroplast regions are the most useful for resolving relationships of closely related species, but we found non-coding parts of all chloroplast markers employed in the present study to be very similar in terms of variability compared to the coding sections of the chloroplast genome at this very low taxonomic level. In contrast, non-coding parts of nuclear genes show a three-fold increased variability compared to non-coding chloroplast markers.
Another reason why species rich genera are not as well studied as other groups is certainly because they are much more difficult to sample thoroughly and require both extensive field and herbarium work. This is the case for Peperomia where highly reduced flower morphology makes correct species identification a significant challenge [53]. The study by Wanke et al. [52] was the first to apply molecular markers to Peperomia. Currently, Tildenia is the best-studied subgenus of Peperomia, since there were many new species from the Andes and Mexico described recently [[55,56] respectively] and an extensive phylogeny was combined with a molecular dating approach to detect biogeographical migration patterns [51]. Our current study is primarily focused on the phylogenetic performance of agt1 compared to chloroplast markers, and thus, we applied a broad taxon sampling rather than several nSCG markers with a less dense sampling. For this reason, only limited conclusions can be drawn concerning the origin of the multiple copies as well as incongruent topologies of nuclear versus chloroplast trees. However, obtaining different signals from the nuclear gene in comparison to various chloroplast regions highlights how important it is to apply independent markers. In fact, many cases of incongruence between chloroplast and nuclear marker based topologies can be found in the literature [e.g. [63]]. However, the potential sources of novel clades in the nuclear gene tree seem to be very complex. There are completely different relationships formed on an interspecific level, deriving from many different clades in the trnK-matK-psbA tree. Peperomia cerrateae, P. parvisagittata, P. ayacuchoana and P. pseudoverruculosa formed a clade in the chloroplast-based tree. In the nuclear-based tree however, the species themselves are still monophyletic, but the relationships among species are poorly resolved. The simplest explanation for this pattern is that the trnK-matK-psbA tree lacks resolution at this level. This is because in the ndhF-rpl32-trnL tree a similar tendency is visible, in which P. cerrateae, P. parvisagittata, P. ayacuchoana and P. pseudoverruculosa are not a monophyletic clade and that P. chutanka might be related to clade III with P. pugnicaudex.
In the nuclear-based phylogenetic tree some cases are found where duplicated, paraphyletic copies of agt1 occur in a particular accession. An example for this is clade VI, which is formed of P. radiatinervosa, P. cyclaminoides and P. verruculosa. Since the four copies from P. radiatinervosa form a monophyletic clade, the duplication event could, for example, be due to alloploidy between the two other taxa. Another scenario that would explain this pattern is a duplication event that occurred after the split from P. radiatinervosa, but prior to the diversification of P. cyclaminoides and P. verruculosa. Under the latter scenario, duplicated copies in P. radiatinervosa and the other species evolved independently and are still retained in the respective accessions.
A second case of multiple agt1 copies in a species involves P. bracteata. Agt1 copies from this species form three different clades within clade VII: in two clades the different copies of Pe28 and Pe32 cluster together, whereas Pe31 and Pe33 lack duplicates. Incomplete lineage sorting could be an explanation for this pattern, since it is not shared with other species in this clade.
Our results confirm the conclusion that it is essential to use several unlinked markers for phylogenetic reconstruction to improve phylogenetic resolution, and to identify and limit gene tree artifacts, which can be caused by hybridization, polyploidy, introgression or lineage sorting [e.g. [50]]. However, to refine our understanding of the reasons for the observed conflicts between agt1 and chloroplast regions, additional nuclear loci would be required. The suite of AVPO SSCG loci identified by Duarte et al. [22] provide numerous candidates for further analysis.
To be or not to be single copy
The starting point for the present study was a Tildenia phylogeny based on several chloroplast markers. In order to obtain a better resolution in Tildenia, in particular below the species level, we investigated the phylogenetic performance of the non-coding parts of one of the APVO SSCG [22]. Among the large collection of 965 SSCGs identified in Arabidopsis, Populus, Vitis, and Oryza, several were selected for further analysis of public EST datasets, and a trial analysis of Brassicaceae was performed using direct sequencing and phylogenetic analysis of a dozen of the genes using RT-PCR (cDNA) amplicons [22]. In both analyses, results were generally consistent with a single gene being detected in the expressed gene sets across diverse angiosperms, though duplicates were indicated in some species, particularly in recent polyploid taxa [49]. Thus, we reasoned that genes from among this collection were good candidates for further study in Peperomia, a basal angiosperm without prior nuclear gene sequence data. By amplifying and sequencing through intronic as well as exonic regions using primers grounded in conserved exons, a large collection of highly variable sites was obtained that were especially valuable for phylogenetic at and below the species level.
An advantage of the approach developed here is that it should be possible to take any angiosperm group of interest and quickly identify whether SSCG are likely to work well for species level phylogenetics. The only major requirement is a pair of unambiguous amplification primers that will work for the particular group of interest. In some cases, primers from Duarte et al. [22] or this study may serve as amplification or sequencing primers. However, additional steps can be taken to enhance the chances of success in any given study. Publicly available EST data can easily be queried to extract sequences from closely related taxa, perform alignments with genome and coding regions of the sequenced species obtained from the PlantTribes database [39], and design primers that have an increased likelihood of success. Given the large number of 965 APVO SSCG [22], successful primer pairs should be identified for at least a handful of genes for any angiosperm group. In our study, after one initial round of primer design for Tildenia and the first sequencing run, agt1 was one of six genes that immediately yielded good results. This gene did prove to be low copy (rather than single copy) in some Tildenia accessions, which is not unexpected given that all angiosperms have a history of ancient polyploidy and both polyploidy and gene duplication is a frequent process in plants [64-66].
The rapidly increasing number of sequenced plant genomes allows the identification of genes that are present in single copy across increasingly many species. The recently updated PlantTribes database 2.0 [67] provides a publicly available tool that can be used for identifying such genes. If the database is queried (at moderate stringency, 3.0) for Tribes containing a single gene in each of the seven angiosperm genomes (Arabidopsis, Populus, Vitis, Oryza, Sorghum, Medicago and Carica), this number decreases to 223 genes and if three non-angiosperm plant genomes are added (Selaginella, Physcomitrella, and Chlamydomonas), 36 genes pass the criteria. We see that as new genomes are added to this approach, there is a decrease in the number of shared single copy genes. However, this set of genes is much more refined and thus may be more likely to be single copy throughout plants in general. In the case of agt1, beside the APVO species, it occurs in a single copy in Sorghum bicolor and Carica papaya, but there are six copies in the Medicago truncatula (Version MT3.5) genome. In the three non-angiosperm plant genomes, two annotated copies can be identified in Selaginella moellendorfii, three in Chlamydomonas reinhardtii, and five in Physcomitrella patens.
The approach we develop here differs from another recent method to identify orthologous nuclear markers within a particular plant family or other relatively specific lineage [e.g. [44]]. In a group that already has multiple genome-scale datasets, such as the large angiosperm families Asteraceae, Solanaceae, Poaceae, and Fabaceae, genomes and EST datasets can be leveraged to identify genes that exist in single copy across the range of sampled taxa. In this case, larger numbers of shared single copy genes may be obtained within a family, but the marker set will include many genes that are multicopy in other lineages.
Even if a number of 'nearly universal' nSCGs are eventually identified in angiosperms, universal amplification and sequencing primers that can be used in an arbitrarily chosen lineage are perhaps less likely to exist than for chloroplast genes due to the relatively rapid underlying substitution rates for nuclear genes. Nucleoribosomal markers, such as the intergenic transcribed spacer region (ITS), can often be amplified and sequenced with widely conserved primers because it is flanked by highly conserved nucleoribosomal gene sequence [68] and do provide an independent estimate of phylogenetic history in comparison to chloroplast DNA. However, because ITS regions form a large multicopy "family" where copies are (often incompletely) homogenized through concerted evolutionary processes, assessment of orthology in nuclear ITS sequences is very complex, even when multiple copies are cloned and sequenced [23,24]. We found that nSCG amplification and primer design requires a little more lab effort compared to chloroplast markers, but on the other hand, unlinked loci from different genomic compartments are essential in order to reconstruct and fully interpret phylogenetic relationships [69]. It is comparably easy to generate a data set of several chloroplast markers, but those results need to be complemented and compared to unlinked nuclear markers.
Conclusions
Considering topology, variability and homoplasy, we can conclude that agt1 is highly valuable for phylogenetics at a low taxonomic level such as species level and below. Beyond that, agt1 was useful for identifying further evolutionary biological processes such as recent gene duplication. When combined with additional nuclear genes, numerous concurrent duplications would be indicative of recent polyploidization in a species or even in particular populations. In combination with uniparentally inherited loci, biparentally inherited nSCGs can also provide evidence for hybridization and potentially for organelle capture. The nSCG applied in the present study provides regions with different quantitative levels of variability deriving from coding and non-coding parts of different length. While the percentage of PIC of the coding sections does not vary significantly among the chloroplast and nuclear markers, the non-coding sections of the agt1 in Peperomia is three times higher than the most rapidly evolving chloroplast regions.
Methods
Sampling Strategy
Based on Symmank et al. [51], Mathieu et al. [55] and Samain et al. [56], we sampled a phylogenetically representative subset (29 out of 59 species) of Peperomia subgenus Tildenia (Additional File 1: Taxa used in the present study). As outgroup taxon, Peperomia pinoi aff. Pe474, closely related to this subgenus, was chosen [52,53]. For a comprehensive approach to compare the agt1 gene with the trnK intron, matK gene and the trnK-psbA spacer region, 70 accessions including the outgroup species were sampled. In addition, the agt1 gene was compared to two additional chloroplast gene clusters containing both introns and spacers (ndhF-rpl32-trnL and psbA-trnH). A restricted set of 26 accessions including outgroup was utilized for this.
Marker selection
The starting point for the present study were 959 genes identified by Duarte et al. [22], that are shared in single copy in the annotated genomes of Arabidopsis thaliana, Populus trichocarpa, Vitis vinifera and Oryza sativa. These authors used a high throughput comparative proteomic approach to identify genes that occur in single copy in all of the four genomes. This comprehensive approach suggests candidate markers for any angiosperm of interest, because the genes captured this way are present in phylogenetically diverse angiosperms and thus potentially suitable useful as common phylogenetic markers in varied taxa.
Starting from 13 potential nSCGs compared by Duarte et al. [22], ESTs (Expressed Sequence Tags) obtained from the MAGIC database from the Ancestral Angiosperm Genome Project [70] were screened for homologues in other representatives of basal angiosperm lineages. The sequences of these 13 loci were extracted from all available plant genomes in order to check the variation of total length as well as the length and number of introns. Our decision to use the agt1 gene was made based on the number of introns and the positive impact that introns would have on the expected variability for that gene. Comparison of EST data and further characterization was done using PlantTribes [39,67] and TIGR Plant Transcript Assemblies [71,72]. Initial primer sets were designed for this particular region using both Sanger and 454 (Roche) EST data from other basal angiosperm lineages (Amborella trichopoda, Nuphar advena, Liriodendron tulipifera, Persea americana, Aristolochia fimbriata) to subsequently amplify and sequence the gene region in a RT-PCR approach for Piper nigrum and Peperomia prostrata (4474-390F: ACCAGGGAGGAACCATCTCTTTG; 4474-1530R: TTYTTCARMCCCCATGCTTC). These sequences were used to build primers that are more specific for Piperaceae to amplify and sequence Peperomia accessions. A single primer was designed in a highly conserved 5' region of the gene (Pe-4474-1800F: TTCTTTGAYTGGAATGACTACTTGA) which was applied in a 3'-RACE (3' RACE System for Rapid Amplification of cDNA Ends, Invitrogen Corporation) for Peperomia cyclaminoides Pe114. Based on the resulting sequence, primers at the outermost end of the gene were generated and then used for a broad set of Peperomia accessions (Additional File 5: Primers used for amplification and sequencing of both the nuclear and chloroplast markers applied in this study).
DNA isolation, RNA isolation, cloning, amplification, RT-PCR and sequencing
DNA was isolated from silica dried material using the CTAB method. Most of the sequences of the trnK-matK-psbA gene region are obtained from Symmank et al. [51]. Amplification of additional trnK-matK-psbA sequences as well as other chloroplast sequences followed the procedure described in Symmank et al. [51]. Primers used for amplification and sequencing of the chloroplast markers are listed in Additional File 5: Primers used for amplification and sequencing of both the nuclear and chloroplast markers applied in this study. For the nuclear marker, both 25 μl and 50 μl reactions were run containing between 0.5 and 4 μl DNA template (100 to 200 ng) for a 50 μl reaction. A 'master mix' for a 50 μl reaction contained 8 μl dNTP (Roth, 1.25 mM each), 5 μl red Taq-buffer for high yields (PeqLab), 1.5 μl MgCl2 (25 mM), 1 μl of each primer (50 pmol/μl) and 0.5 μl of Taq DNA polymerase (PeqLab). Water was added to obtain a total reaction volume of 50 μl. For amplification of the agt1 we modified a standard PCR-program for low concentration of target DNA. The program started with an initial denaturation step at 94°C for 2 min, followed by 45 cycles of denaturation at 96°C for 45 sec, annealing at 51°C for 30 sec and elongation at 72°C for 90 sec, and a final elongation 72°C for 7 min. Annealing temperature was adjusted to the melting point of the primers. PCR was run on a T3 Thermocycler (Biometra). After gel electrophoresis through a 1.2% agarose gel, the PCR products were purified using a gel extraction kit (Macherey&Nagel). Some of the PCR products required an additional cloning step. In those cases the T/A Cloning Kit (Genaxxon BioScience) was applied following the manufacturer's protocol. A ligation reaction was set up with 2-4 μl PCR-product, 1 μl of each ligase buffer, ligase and vector and filled up with water to a total reaction volume of 10 μl. Three to nine clones were arbitrarily chosen and directly amplified via colony PCR using M13 primers under the following conditions: initial denaturation at 95°C for 2 min, denaturation at 95°C for 1 min, annealing at 55°C for 1 min, elongation at 72°C for 1.5 min and a final elongation at 72°C for 10 min. This PCR was run with 36 cycles and the products were directly sequenced after purification (Macherey&Nagel).
RNA was isolated using peqGold Plant RNA Kit (PeqLab) following the manufacturer's instructions. A 3' RACE kit (Invitrogen Life Science) was used following the manufacturer's protocol. For RT-PCR the Access RT-PCR System (Promega) was used following the manufacturer's instructions.
Direct sequencing was conducted either using a Beckman Coulter CEQ DTCS Quick Start Kit (Beckman Coulter) with the CEQ 8000 sequencer or using Macrogen's sequencing service (Macrogen Inc., Korea). Sequences were edited and aligned manually using PhyDE [73]. Regions of uncertain homology (e.g. long monobase repeats) were excluded from all subsequent analyses.
Due to the length of the agt1 gene, DNA was not amplified in a single reaction but in three parts with substantial overlap (50 to 150 bp) to ensure efficient amplification. The ESTs and cDNA sequences of basal angiosperms mentioned above, which were available to generate initial amplification primers, did not cover the entire region. The 3'-ends of the ESTs usually reached exon IV, making a RACE approach necessary to include full-length gene information including intron IV and exon V. The middle section comprising only the short intron II (between exon IIb and exon III) yielded sequences without any length mutations, whereas either the first or the third part or both sections required a cloning step for several accessions and were treated individually. Very few PCR amplicons (approximately 10%) revealed differences in length among amplified products. The three sections of those accessions had to be assembled together to gain a full sequence. The assemblage yielded up to three different 'types' of copies and the fragments were sorted phylogenetically. This method allowed us to avoid artifacts due to incorrect sequence combinations.
However, most of the detected multicopy accessions were monophyletic in the phylogenetic tree and thus, a copy could be chosen at random for the further analysis. For the few accessions that appeared polyphyletic in our analyses, a copy of each 'type' was left in the dataset and was distinguished by 'type I' and 'type II'. All major clades of subgenus Tildenia contained at least one accession that required cloning steps; thus multiple copies are not unique to a particular lineage.
Phylogenetic analyses
Several mostly small regions of uncertain sequence homology (hotspots) had to be excluded from the different data matrices (Additional File 6: Hotspots excluded, due to ambiguous homology assessments). All alignments are available from TreeBASE.
Indel matrices were calculated using the "simple indel coding" approach (SIC) [74]. This indel matrix was generated automatically by the indel coding tool of SeqState [75]. Substitution models for Bayesian inference (BI) were determined using jModelTest [76]. For the agt1 dataset the general time reversible model of nucleotide substitution and site-specific rate categories following a gamma distribution (GTR+I+Γ) was assigned as the best fitting model considering the Akaike information criterion (AIC). For the three chloroplast datasets, GTR+Γ was the best fitting model. Bayesian MCMC inferences were performed with MrBayes v3.1 [77] using the substitution models mentioned above.
The BI was applied with four Markov chains running simultaneously for 4 million generations, saving trees every 100 generations. The burn-in was individually set for each analysis between 5% and 20% after determining stationarity of each run with Tracer v1.5 [78]. At least ten runs were assembled to generate the consensus trees and posterior probabilities for each individual analysis. Maximum Likelihood as implemented in RAxML Version 7.2.7.a [79] using the rapid bootstrap algorithm was used in order to increase the number of bootstrap replicates to 1,000.
The degree of homoplasy of each dataset both with and without indels was assessed on a Maximum Parsimony (MP) tree that was obtained using a parsimony ratchet approach. Command files for MP analyses were created using PRAP [80] and executed in PAUP*4b10 [81]. Topologies were obtained with the heuristic search strategy and 10 random addition cycles of 200 iterations each with a 25% upweighting of the characters in the iterations. For compiling and drawing all trees TreeGraph2 [82] was employed.
Sequence statistics for specified regions of each marker were obtained utilizing SeqState [75]. The Shimodaira-Hasegawa (SH) test [58] was performed in PAUP*4b10 [81] with full optimization to evaluate the topologies obtained by the different genetic markers against each other. The SH test simultaneously compensates for a posteriori hypotheses of multiple alternative topologies by adjusting the expected difference in log-likelihood values. Topology tests were performed on the agt1 dataset containing one randomly selected single copy to comply with the congruence of sampling. In case of conflict between two markers (p ≤ 0.05) the test was repeated with a manually modified topology to determine the conflict. In the process, one hypothesis (topology) was stepwise adjusted to the phylogenetic results of the conflicting marker to evaluate the effects of single clade position changes.
Authors' contributions
JN: laboratory work for the nSCG dataset, cloning, phylogenetic analyses and drafted the paper. LS: collected samples, contributed the cpDNA datasets, carried out phylogenetic analyses, statistical tests, and helped draft the paper. MSS: collected samples, helped with the sampling design (including identification and nomenclature) and proofread the paper. KFM: supplied funding for the study, edited the draft version, helped with the statistical analysis. CN: supplied funding for the study and lab space for parts of the study, and proofread the paper. CWD: helped draft the manuscript, supplied funding and lab space for parts of the study, contributed in the discussion in various phases, analysis of EST datasets, and proofread the paper. SW: designed and supervised the study, supplied funding for the study, performed RT-PCR, primer design, and sample collection, and helped draft the paper. All authors read and approved the final manuscript.
Supplementary Material
Taxa used in the present study. Excel file listing the species used in this study including voucher information and GenBank accession numbers (provided upon final acceptance of manuscript). For detailed information of origin and collection sites see [49].
Sequence statistics for the coding and non-coding regions for all markers in this study calculated with SeqState. This table provides information about the general characteristics of the coding, and non-coding parts of the study sequences for different sampling densities.
Results from topology tests. Excel file showing Shimodaira-Hasegawa (SH) test results of different topology/dataset combinations. Asterisk indicates a significant rejection (p ≤ 0.05) of the respective combination.
Phylogram of agt1 based on a Bayesian inference of full dataset containing cloned sequences. Phylogram from the Bayesian inference of the agt1 data set with relative substitution rates using the GTR+G+I model and posterior probabilities plotted above the branches. Clades are colored according to Figure 3. The applied data set comprises part I and part II of the nuclear gene including all cloned sequences.
Primers used for amplification and sequencing of both the nuclear and chloroplast markers applied in this study. Excel file listing primer sequences of the respective gene region that were used for both PCR and sequencing.
Hotspots excluded, due to ambiguous homology assessments. Regions of uncertain sequence homology (hotspots) excluded from the different datasets. Positions are given equivalent to files downloadable from TreeBase.
Contributor Information
Julia Naumann, Email: julia.naumann@tu-dresden.de.
Lars Symmank, Email: lars.symmank@tu-dresden.de.
Marie-Stéphanie Samain, Email: mariestephanie.samain@ugent.be.
Kai F Müller, Email: kaimueller@uni-muenster.de.
Christoph Neinhuis, Email: christoph.neinhuis@tu-dresden.de.
Claude W dePamphilis, Email: cwd3@psu.edu.
Stefan Wanke, Email: stefan.wanke@tu-dresden.de.
Acknowledgements
This work was financially supported by the German Research Foundation (DFG; NE681/5-1, NE681/5-2, NE681/10-1) to CN, SW; the Research Foundation Flanders (FWO-Vlaanderen; FWO G.0172.07; FWO travel grants to MSS); a postdoctoral fellowship from the DAAD to SW; the German Academic Exchange Service (PPP Colombia) to SW, the German Academic Exchange Service (PPP USA) to KFM, CWD, SW; "Gesellschaft der Freunde und Förderer der TU Dresden" ("Friends of the TU Dresden"), and the Friends of the Botanical Garden Ghent. We are grateful to the Botanical Gardens of Ghent University and University of Technology Dresden for general support. We thank the authorities of Bolivia (permit number MDRAyMA-VBRFMA-DGBAP-UAVPS N° 046/08), Mexico (permit numbers SGPA/DGGFS/712/1397/07 and SGPA/DGGFS/712/2486/09) and Peru (permit number 009-2009-AG-DGFFS-DGEFFS) for permission to collect material. We are grateful to the following persons for assistance with field work and our Peperomia research in general: Joaquina Albán, Pieter Asselman, Carlos Bambarén, Brian Bates, Stephan Beck, Nelson Cieza, Hilda Flores, Paul Goetghebeur, Esteban Martínez, Robert Maijer, Guido Mathieu, Victor Morales, Helga Ochotorena and Guillermo Pino.
References
- Maddison DR, Schulz KS, Maddison WP. The Tree of Life Web Project. Zootaxa. 2007;1668:19–40. [Google Scholar]
- Angiosperm Phylogeny Website. http://www.mobot.org/mobot/research/apweb/
- APG III. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc. 2009;161:105–121. [Google Scholar]
- Burleigh JG, Bansal MS, Eulenstein O, Hartmann S, Wehe A, Vision TJ. Genome-scale phylogenetics: Inferring the plant tree of life from 18,896 gene trees. Syst Biol. 2011;60:117–125. doi: 10.1093/sysbio/syq072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson DH, Bryant D. Application of Phylogenetic Networks in Evolutionary Studies. Mol Biol Evol. 2006;23(2):254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Fu BY, Allaby RG. Phylogenetic network of Linum species as revealed by non-coding chloroplast DNA sequences. Genet Resour Crop Evo. 2010;57:667–677. doi: 10.1007/s10722-009-9502-7. [DOI] [Google Scholar]
- Parks M, Cronn R, Liston A. Increasing phylogenetic resolution at low taxonomic levels using massively parallel sequencing of chloroplast genomes. BMC Biol. 2009;7:84. doi: 10.1186/1741-7007-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RK, Cai Z, Raubeson LA, Daniell H, dePamphilis CW, Leebens-Mack J, Müller KF, Guisinger-Bellian M, Haberle RC, Hansen AK, Chumley TW, Lee S, Peery R, McNeal JR, Kuehl JV, Boore JL. Analysis of 81 genes from 64 plastid genomes resolves relationships in angiosperms and identifies genome-scale evolutionary patterns. PNAS. 2007;104:19369–19374. doi: 10.1073/pnas.0709121104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MJ, Bell CD, Soltis PS, Soltis DE. Using plastid genome-scale data to resolve enigmatic relationships among basal angiosperms. PNAS. 2007;104(49):19363–19368. doi: 10.1073/pnas.0708072104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HC, Moore MJ, Soltis PS, Bell CD, Brockington SF, Alexandre R, Davis CC, Latvis M, Manchester SR, Soltis DE. Rosid radiation and the rapid rise of angiosperm-dominated forests. PNAS. 2009;10:3853–3858. doi: 10.1073/pnas.0813376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MJ, Soltis PS, Bell CD, Burleigh JG, Soltis DE. Phylogenetic analysis of 83 plastid genes further resolves the early diversification of eudicots. PNAS. 2010;107:4623–4628. doi: 10.1073/pnas.0907801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AO, Palmer JD. Horizontal gene transfer in plants. J Exp Bot. 2007;58(1):1–9. doi: 10.1093/jxb/erl148. [DOI] [PubMed] [Google Scholar]
- Barkman TJ, McNeal JR, Lim S-H, Coat G, Croom HB, Young ND, dePamphilis CW. Mitochondrial DNA suggests at least 11 origins of parasitism in angiosperms and reveals genomic chimerism in parasitic plants. BMC Evol Biol. 2007;7:248. doi: 10.1186/1471-2148-7-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe LM, dePamphilis CW. Effects of RNA editing and gene processing on phylogenetic reconstruction. Mol Biol Evol. 1996;13:1159–1166. doi: 10.1093/oxfordjournals.molbev.a025680. [DOI] [PubMed] [Google Scholar]
- Nickrent DL, Blarer A, Qiu Y-L, Soltis DE, Soltis PS, Zanis M. Molecular data place Hydnoraceae with Aristolochiaceae. Am J Bot. 2002;89:1809–1817. doi: 10.3732/ajb.89.11.1809. [DOI] [PubMed] [Google Scholar]
- dePamphilis CW, Palmer JD. In: Physiology, biochemistry, and genetics of nongreen plastids. Boyer CD, Shannon JC, Hardison RC, editor. Rockville: The American Society of Plant Physiologists; 1989. Evolution and Function of Plastid DNA: A Review With Special Reference to Nonphotosynthetic Plants; pp. 182–202. [Google Scholar]
- Wicke S, Schneeweiss GM, Mueller KF, dePamphilis CW, Quandt D. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Mol Biol. 2011. DOI 10.1007/s11103-011-9762-4. [DOI] [PMC free article] [PubMed]
- Soltis DE, Smith SA, Cellinese N, Wurdack KJ, Tank DC, Brockington SF, Refulio-Rodriguez NF, Walker JB, Moore MJ, Carlsward BS, Bell CD, Latvis M, Crawley S, Black C, Diouf D, Xi Z, Rushworth CA, Gitzendanner MA, Sytsma KJ, Qiu YL, Hilu KW, Davis CC, Sanderson MJ, Beaman RS, Olmstead RG, Judd WS, Donoghue MJ, Soltis PS. Angiosperm phylogeny: 17 genes, 640 taxa. Am J Bot. 2011;98:704–730. doi: 10.3732/ajb.1000404. [DOI] [PubMed] [Google Scholar]
- Small RL, Ryburn JA, Cronn RC, Seelanan T, Wendel JF. The tortoise and the hare: choosing between non-coding plastome and nuclear Adh sequences for phylogeny reconstruction in a recently diverged plant group. Am J Bot. 1998;85:301–1315. [PubMed] [Google Scholar]
- Shaw J, Lickey EB, Beck JT, Farmer SB, Liu W, Miller J, Siripun KC, Winder CT, Schilling EE, Small RL. The tortoise and the hare II: relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. Am J Bot. 2005;92:142–166. doi: 10.3732/ajb.92.1.142. [DOI] [PubMed] [Google Scholar]
- Shaw J, Lickey EB, Schilling EE, Small RL. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare III. Am J Bot. 2007;94:275–288. doi: 10.3732/ajb.94.3.275. [DOI] [PubMed] [Google Scholar]
- Duarte JM, Wall KP, Edger PP, Landherr LL, Ma H, Pires JC, Leebens-Mack J, dePamphilis CW. Identification of shared single copy nuclear genes in Arabidopsis, Populus, Vitis and Oryza and their phylogenetic utility across various taxonomic levels. BMC Evol Biol. 2010;10:61. doi: 10.1186/1471-2148-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez I, Wendel JF. Ribosomal ITS sequences and plant phylogenetic inference. Mol Phylogenet Evol. 2003;29:417–434. doi: 10.1016/S1055-7903(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Bailey CD, Carr T, Harris S, Hughes C. Characterization of angiosperm nrDNA polymorphim, paralogy, and pseudogenes. Mol Phylogenet Evol. 2003;29:435–455. doi: 10.1016/j.ympev.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Koonin EV. Orthologs, Paralogs, and Evolutionary Genomics. Annu Rev Genet. 2005;39:309–38. doi: 10.1146/annurev.genet.39.073003.114725. [DOI] [PubMed] [Google Scholar]
- Schultheis LM, Baldwin BG. Molecular phylogenetics of Fouquieriaceae: evidence from nuclear rDNA ITS studies. Am J Bot. 1999;86:578–589. doi: 10.2307/2656819. [DOI] [PubMed] [Google Scholar]
- Sang T, Crawford DJ, Stuessy TF. Chloroplast DNA phytogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae) Am J Bot. 1997;84:1120–1136. doi: 10.2307/2446155. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL, Rauscher JT, Brown AHD. Diploid and polyploid reticulate evolution throughout the history of the perennial soybeans (Glycine subgenus Glycine) New Phytol. 2004;161:121–132. [Google Scholar]
- Lavin M, Schrire BP, Lewis G, Pennington RT, Delgado-Salinas A, Thulin M, Hughes CE, Matos AB, Wojciechowski MF. Metacommunity process rather than continental tectonic history better explains geographically structured phylogenies in legumes. Philos Trans R Soc B. 2004;359:1509–1522. doi: 10.1098/rstb.2004.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt TC, Tanksley SD. Comparative sequencing in the genus Lycopersicon. Implications for the evolution of fruit size in the domestication of cultivated tomatoes. Genetics. 2002;162:365–379. doi: 10.1093/genetics/162.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraclough T, Nee S. Phylogenetics and speciation. Trends Ecol Evol. 2001;16:391–399. doi: 10.1016/S0169-5347(01)02161-9. [DOI] [PubMed] [Google Scholar]
- Strand AE, Leebens-Mack J, Milligan BG. Nuclear DNA-based markers for plant evolutionary biology. Mol Ecol. 1997;6:113–118. doi: 10.1046/j.1365-294X.1997.00153.x. [DOI] [PubMed] [Google Scholar]
- Sang T, Zhang D. Reconstructing hybrid speciation using sequences of Low Copy Nuclear Genes: hybrid origins of five Paeonia species based on Adh gene phylogenies. Syst Bot. 1999;24:148–163. doi: 10.2307/2419546. [DOI] [Google Scholar]
- Bailey CD, Doyle JJ. Potential Phylogenetic Utility of the Low-Copy Nuclear Gene pistillata in Dicotyledonous Plants: Comparison to nrDNA ITS and trnL Intron in Sphaerocardamum and Other Brassicaceae. Mol Phylogenet Evol. 1999;1:20–30. doi: 10.1006/mpev.1999.0627. [DOI] [PubMed] [Google Scholar]
- Tank D, Sang T. Phylogenetic utility of the glycerol-3-phosphate acyltransferase gene: Evolution and implications in Paeonia (Paeoniaceae) Mol Phylogenet Evol. 2001;19:421–429. doi: 10.1006/mpev.2001.0931. [DOI] [PubMed] [Google Scholar]
- Thomas MM, Garwood NC, Baker WJ, Henderson SA, Russell SJ, Hodel DR, Bateman RM. Molecular phylogeny of the palm genus Chamaedorea, based on the low-copy nuclear genes PRK and RPB2. Mol Phylogenet Evol. 2006;38:398–415. doi: 10.1016/j.ympev.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Kim S, Sultan SE, Donoghue MJ. Allopolyploid speciation in Persicaria (Polygonaceae): Insights from a low-copy nuclear region. PNAS. 2008;105:12370–12375. doi: 10.1073/pnas.0805141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang T. Utility of low-copy nuclear gene sequences in plant phylogenetics. Crit Rev Biochem Mol. 2002;37:121–147. doi: 10.1080/10409230290771474. [DOI] [PubMed] [Google Scholar]
- Wall PK, Leebens-Mack J, Müller KF, Dawn Field, Altman NS, dePamphilis CW. PlantTribes: a gene and gene family resource for comparative genomics in plants. Nucleic Acids Res. 2008;36:D970–D976. doi: 10.1093/nar/gkm972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton TM, Van der Hoeven R, Eannetta NT, Tanksley SD. Identification, analysis, and utilization of conserved ortholog set markers for comparative genomics in higher plants. Plant Cell. 2002;14:1457–1467. doi: 10.1105/tpc.010479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Mueller LA, Crouzillat D, Petiard V, Tanksley SD. Combining bioinformatics and phylogenetics to identify large sets of single-copy orthologous genes (COSII) for comparative, evolutionary and systematic studies: A test case in the Euasterid plant clade. Genetics. 2006;174:1407–1420. doi: 10.1534/genetics.106.062455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittall JB, Medina-Marino A, Zimmer EA, Hodges SA. Generating single copy nuclear gene data for a recent adaptive radiation. Mol Phylogenet Evol. 2006;39:124–134. doi: 10.1016/j.ympev.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Chapman BA, Bowers JE, Feltus FA, Paterson AH. Buffering of crucial functions by paleologous duplicated genes may contribute cyclicality to angiosperm genome duplication. Proc Natl Acad Sci USA. 2006;103(8):2730–2735. doi: 10.1073/pnas.0507782103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez I, Costa A, Feliner GN. Selecting Single-Copy Nuclear Genes for Plant Phylogenetics: A Preliminary Analysis for the Senecioneae (Asteraceae) J Mol Evol. 2008;66:276–291. doi: 10.1007/s00239-008-9083-7. [DOI] [PubMed] [Google Scholar]
- Wahlberg N, West Wheat C. Genomic Outposts Serve the Phylogenomic Pioneers: Designing Novel Nuclear Markers for Genomic DNA Extractions of Lepidoptera. Syst Biol. 2008;57(2):231–242. doi: 10.1080/10635150802033006. [DOI] [PubMed] [Google Scholar]
- Wiegmann B, Trautwein M, Kim J, Cassel B, Bertone M, Winterton S, Yeates D. Single-copy nuclear genes resolve the phylogeny of the holometabolous insects. BMC Biol. 2009;7:34. doi: 10.1186/1741-7007-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguileta G, Marthey S, Chiapello H, Lebrun MH, Rodolphe F, Fournier E, Gendrault-Jacquemard A, Giraud T. Assessing the performance of single-copy genes for recovering robust phylogenies. Syst Biol. 2008;57:613–627. doi: 10.1080/10635150802306527. [DOI] [PubMed] [Google Scholar]
- Hutcheon K, Ditt RF, Beilstein M, Comai L, Schroeder J, Goldstein E, Shewmaker CK, Nguyen T, De Rocher J, Kiser J. Polyploid genome of Camelina sativa revealed by isolation of fatty acid synthesis genes. BMC Plant Biol. 2010;10:233. doi: 10.1186/1471-2229-10-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright AJ, Van Dongen S, Ouzounis CA. An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res. 2002;30(7):1575–1584. doi: 10.1093/nar/30.7.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CE, Eastwood RJ, Donovan Bailey C. Review. From famine to feast? Selecting nuclear DNA sequence loci for plant species-level phylogeny reconstruction. Phil Trans R Soc Lond B. 2006;361:211–225. doi: 10.1098/rstb.2005.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symmank L, Samain MS, Smith JF, Pino G, Stoll A, Goetghebeur P, Neinhuis C, Wanke S. From the Andean cradle in Peru to the Trans-Volcanic Mexican Belt: the extraordinary journey of the geophytic Peperomia subgenus Tildenia (Piperaceae) J Biogeography. doi:10.1111/j.1365-2699.2011.02586.x.
- Wanke S, Samain MS, Vanderschaeve L, Mathieu G, Goetghebeur P, Neinhuis C. Phylogeny of the genus Peperomia (Piperaceae) inferred from the trnK/matK region (cpDNA) Plant Biol. 2006;8:93–102. doi: 10.1055/s-2005-873060. [DOI] [PubMed] [Google Scholar]
- Samain MS, Vanderschaeve L, Chaerle P, Goetghebeur P, Neinhuis C, Wanke S. Is morphology telling the truth about the evolution of the species rich genus Peperomia (Piperaceae)? Plant Sys Evol. 2009;278:1–21. doi: 10.1007/s00606-008-0113-0. [DOI] [Google Scholar]
- Bradley U. PhD thesis. University of Dublin, Trinity College; 2002. Biogeography and speciation of the genus Peperomia Ruiz and Pavon in Eastern Polynesia. [Google Scholar]
- Mathieu G, Symmank L, Callejas R, Wanke S, Neinhuis C, Goetghebeur P, Samain MS. New geophytic Peperomia (Piperaceae) species from Mexico, Belize and Costa Rica. Rev Mex Biodiv. 2011;82:357–382. [Google Scholar]
- Samain MS, Mathieu G, Pino G, Symmank L, Cieza N, Neinhuis C, Goetghebeur P, Wanke S. The geophytic Peperomia subgenus Tildenia (Piperaceae) in the Andes with the description of new species in a phylogenetic framework. Plant Ecol Evol. 2011;144(2):1–29. [Google Scholar]
- Liepman AH, Olsen LJ. Alanine aminotransferase homologs catalyze the glutamate:glyoxylate aminotransferase reaction in peroxisomes of Arabidopsis. Plant Physiol. 2003;131:215–227. doi: 10.1104/pp.011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimodaira H Hasegawa M Multiple comparisons of log-likelihoods with applications to phylogenetic inference Mol Biol Evol 199916114–1116.10331256 [Google Scholar]
- Colless DH. Congruence between morphometric and allozyme data for Menidia species: a reappraisal. Syst Zool. 1980;29:288–299. doi: 10.2307/2412663. [DOI] [Google Scholar]
- Berry PE, Hipp AL, Wurdack KJ, Van Ee B, Riina R. Molecular phylogenetics of the giant genus Croton and tribe Crotoneae (Euphorbiaceae sensu stricto) using ITS and trnL-trnF DNA sequence data. Am J Bot. 2005;92:1520–1534. doi: 10.3732/ajb.92.9.1520. [DOI] [PubMed] [Google Scholar]
- Richardson JE, Chatrou LW, Mols JB, Erkens RHJ, Pirie MD. Historical biogeography of two cosmopolitan families of flowering plants: Annonaceae and Rhamnaceae. Phil Trans R Soc Lond B. 2004;359:1495–1508. doi: 10.1098/rstb.2004.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kårehed J, Groeninckx I, Dessein S, Motley TJ, Bremer B. The phylogenetic utility of chloroplast and nuclear DNA markers and the phylogeny of the Rubiaceae tribe Spermacoceae. Mol Phylogenet Evol. 2008;49:843–866. doi: 10.1016/j.ympev.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Mort ME, Soltis DE, Soltis PS, Francisco-Ortega J, Santos-Guerra A. Phylogenetics and Evolution of the Macaronesian Clade of Crassulaceae Inferred from Nuclear and Chloroplast Sequence Data. Syst Bot. 2002;27(2):271–288. [Google Scholar]
- Cui L, Wall PK, Leebens-Mack JH, Lindsay BG, Soltis DE, Doyle JJ, Soltis PS, Carlson JE, Arumuganathan K, Barakat A, Albert VA, Ma H, dePamphilis CW. Widespread genome duplications throughout the history of flowering plants. Genome Res. 2006;16:738–749. doi: 10.1101/gr.4825606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali A, Landherr L, Ralph PE, Tomsho LP, Hu Y, Liang H, Soltis PS, Soltis DE, Clifton SW, Schlarbaum SE, Schuster SC, Ma H, Leebens-Mack J, dePamphilis CW. Ancestral polyploidy in seed plants and angiosperms. Nature. 2011. doi:10.1038/nature09916 Letter. [DOI] [PubMed]
- Van de Peer Y. A mystery unveiled. Genome Biol. 2011;12:113. doi: 10.1186/gb-2011-12-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PlantTribes. http://fgp.bio.psu.edu/tribedb/10_genomes/index.pl
- Wicke S, Costa A, Muñoz J, Quandt D. Restless 5S: The re-arrangement(s) and evolution of the nuclear ribosomal DNA in land plants. Mol Phylogenet Evol. 2011;61:321–332. doi: 10.1016/j.ympev.2011.06.023. [DOI] [PubMed] [Google Scholar]
- Nichols R. Gene trees and species trees are not the same. Trends Ecol Evol. 2001;16:358–64. doi: 10.1016/S0169-5347(01)02203-0. [DOI] [PubMed] [Google Scholar]
- Ancestral Angiosperm Genome Project. http://ancangio.uga.edu/
- TIGR Plant Transcript Assemblies. http://plantta.jcvi.org
- Childs KL, Hamilton JP, Zhu W, Ly E, Cheung F, Wu H, Rabinowicz PD, Town CD, Buell CR, Chan AP. The TIGR Plant Transcript Assemblies database. Nucleic Acids Res. 2007;35:D846–51. doi: 10.1093/nar/gkl785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J, Müller KF, Neinhuis C, Quandt D. PhyDE - Phylogenetic Data Editor. 2006. http://www.phyde.de
- Simmons MP, Ochoterena H. Gaps as Characters in Sequence-Based Phylogenetic Analyses. Syst Biol. 2000;49(2):369–381. [PubMed] [Google Scholar]
- Müller KF. SeqState: Primer design and sequence statistics for phylogenetic DNA datasets. Appl Bioinformatics. 2005;4:65–69. doi: 10.2165/00822942-200504010-00008. [DOI] [PubMed] [Google Scholar]
- Posada D. jModelTest: Phylogenetic Model Averaging. Mol Biol Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes. 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Rambaut A, Drummond A. Tracer. 2009. http://tree.bio.ed.ac.uk/software/tracer version 1.5.
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Müller KF. PRAP - computation of Bremer support for large data sets. Mol Phylogenet Evol. 2004;31:780–782. doi: 10.1016/j.ympev.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP* Phylogenetic Analysis using Parsimony (*and other Methods) Sunderland, MA: Sinauer Associates; 2003. Version 4. [Google Scholar]
- Stöver BC, Müller KF. TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinformatics. 2010;11:7. doi: 10.1186/1471-2105-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Taxa used in the present study. Excel file listing the species used in this study including voucher information and GenBank accession numbers (provided upon final acceptance of manuscript). For detailed information of origin and collection sites see [49].
Sequence statistics for the coding and non-coding regions for all markers in this study calculated with SeqState. This table provides information about the general characteristics of the coding, and non-coding parts of the study sequences for different sampling densities.
Results from topology tests. Excel file showing Shimodaira-Hasegawa (SH) test results of different topology/dataset combinations. Asterisk indicates a significant rejection (p ≤ 0.05) of the respective combination.
Phylogram of agt1 based on a Bayesian inference of full dataset containing cloned sequences. Phylogram from the Bayesian inference of the agt1 data set with relative substitution rates using the GTR+G+I model and posterior probabilities plotted above the branches. Clades are colored according to Figure 3. The applied data set comprises part I and part II of the nuclear gene including all cloned sequences.
Primers used for amplification and sequencing of both the nuclear and chloroplast markers applied in this study. Excel file listing primer sequences of the respective gene region that were used for both PCR and sequencing.
Hotspots excluded, due to ambiguous homology assessments. Regions of uncertain sequence homology (hotspots) excluded from the different datasets. Positions are given equivalent to files downloadable from TreeBase.