Abstract
Purpose
Although, at present, the selection of sperm prior to ICSI is based on motility and morphology, undetectable anomalies, and more importantly damaged DNA are overlooked. In this regard, novel sperm selection procedures have gained much interest. For instance, sperm has been selected by Magnetic-Activated Cell Sorting (MACS) based on early apoptotic marker, the externalization of phosphatidylserine (EPS). Review of the literature has revealed that the efficiency of this technique has been mainly evaluated post Density Gradient Centrifugation (DGC). Therefore, there is a need to prove the efficiency of this technique independent of DGC. In addition, considering the fact that DGC induces EPS due to capacitation and acrosome reaction, therefore, the role of MACS before DGC(MACS-DGC) and MACS after DGC (DGC-MACS) should be assessed.
Methods
Semen samples from fifteen infertile men were divided into three separate fractions: control, DGC, and MACS. To carry out DGC-MACS, DGC samples were further divided into two fractions and MACS was carried on the second fractions. Similarly to carry out MACS-DGC, the MACS samples were further divided into two fractions and DGC was carried on the second fractions. Percentages of sperm with normal morphology, DNA fragmentation, protamine deficiency, EPS and caspase-3 activity were determined in each fraction.
Results
DGC is more efficient than MACS in separating intact sperm only in terms of normal morphology, DNA and chromatin integrity but not for active caspase. However, a combination of these procedures was more efficient than a single procedure to separate intact sperm for the aforementioned parameters. Comparison of the combined procedures showed only higher efficiency to separate active caspase in the MACS-DGC group.
Conclusion
Based on these results, we propose MACS-DGC rather than DGC-MACS to be implemented in clinical settings.
Keywords: Sperm selection, MACS, DNA damage, Protamine deficiency, Active caspase
Introduction
There has been a stable increase in the application of Assisted Reproductive Techniques (ART) for treatment of infertility in the last two decades; however, the success rates of these techniques remain suboptimal [1]. The recognition and selection of the spermatozoa to support development from fertilization to a healthy live birth could improve the ART success rates. Currently, selection of sperm during ICSI is mainly based on motility and morphology. However, in spite of correlation between sperm motility and morphology with the aforementioned sperm functional markers, invisible anomalies such as externalization of plasma membrane phosphatidylserine (EPS), disruption of mitochondrial membrane potential (MMP), caspase-3 activity and more importantly damaged chromatin are relatively ignored [2–4] Therefore, a variety of novel sperm preparation techniques have recently been implemented based on sperm functional characteristics in order to ensure that the selected spermatozoa have intact chromatin [5–7].
Magnetic-Activated Cell Sorting (MACS) is an excellent tool for selecting the desired cells or sperms out of a heterogeneous cell population based on membrane surface markers [8] Externalization of phosphatidylserine (EPS) to the outer membrane of sperm is considered as an early sign of apoptosis. Annexin V is a protein that binds specifically to EPS and enables the identification of cells with altered membrane integrity [9]. Based on this assumption, sperm presenting EPS has spermolemma with impaired function, which has been associated with infertility [10]. Impaired membrane integrity, along with other sperm anomalies are considered as one of the main cause subfertility or infertility. To improve clinical pregnancy rate, separation of Annexin V free sperms by MACS has been proposed. Review of the related literature revealed that Annexin V free sperms have been successfully separated by MACS and the separated sperms have better morphology with reduced EPS [11]. However, the majority of studies have implemented MACS in combination with a sperm density gradient centrifugation (DGC) procedure in the form of DGC prior to MACS (DGC-MACS) [8, 10, 12] or DGC following MACS (MACS-DGC) [13]. Although EPS is a sign of early apoptosis [14], it can also occur following capacitation, and the sperms undergoing DGC that contains albumin, can undergo capacitation as well [12, 15, 16]. These capacitated sperm can be then removed following MACS. Therefore, capacitated sperms with normal function can be eliminated from the insemination sample. To our knowledge, no study has so far defined whether DGC-MACS or MACS-DGC is more suitable for sperm selection. This study has been designed to compare the quality of selected sperm following these two procedures by examining chromatin integrity through assessing the protamine content and DNA fragmentation status, both of which have important influence on fertilization and further development. In addition, the apoptotic status of sperms was determined by assessing the activity of external phosphatidylserine and effector caspase-3.
Materials and methods
Sperm preparation
This study was approved by the Ethics Committee of the Institutional Review Board of Isfahan Fertility and Infertility Center (Isfahan, Iran) and Royan Institute (Tehran, Iran).
Experimental design
Fifteen semen samples were randomly collected from the men attending the Andrology Unit of Isfahan Fertility and Infertility Center, after signing a written informed consent. The semen samples were collected by masturbation into sterile containers after 3–4 days of sexual abstinence. Sperm concentration, morphology and motility tests were carried out by light microscopy according to the protocols of the World Health Organization. In this study, 15 patients were grouped into oligoasthenoteratozoospermic (n = 3), oligoasthenozoospermic (n = 2), asthenozoospermic (n = 3) and normozoospermic (n = 7) categories, according to WHO criteria 2010 [17].
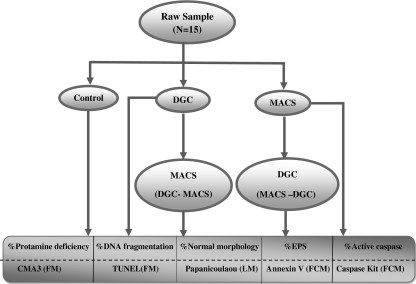
Initially, each sample was washed with Ham’s buffer (N6635; sigma; USA) and divided into three separate fractions and used for control, DGC and MACS. To carry out DGC-MACS, DGC samples were further divided into two fractions and MACS was carried on the second fractions. Similarly to carry out MACS-DGC, the MACS samples were further divided into two fractions and DGC was carried on the second fractions. Therefore, for each sample, five fractions were prepared and the percentages of sperm with normal morphology, DNA fragmentation, protamine deficiency, EPS and caspase-3 activity were determined (Fig. 1).
Fig. 1.
Flow diagram of overall experiment design. FCM Flow Cytometry; LM Light Microscopy; FM Fluorescence Microscopy; EPS Externalization of Phosphatidylserine; CMA3 Chromomycin A3; TUNEL TdT-mediated dUTP Nick-End Labeling
DNA fragmentation detection by fluorescence microscopy (TUNEL)
The DeadEnd™ Fluorometric TUNEL System (DFTS) is a classic TUNEL assay designed for the specific detection and quantification of apoptotic cells within a cell population (Apoptosis Detection System Fluorescein; Promega, Mannheim, Germany, G3250). DFTS measures nuclear DNA fragmentation, an important biochemical hallmark of apoptosis in many cell types. The system is non-radioactive and provides simple, accurate and rapid detection of apoptotic cells in-situ at the single-cell level or in cell suspensions. The DFTS measures the fragmented DNA of apoptotic cells by catalytically incorporating fluorescein-12-dUTP at 3′-OH DNA ends using the enzyme Terminal Deoxynucleotidyl Transferase (TdT), which forms a polymeric tail using the principle of the TUNEL (TdT-mediated dUTP Nick-End Labeling) assay. The fluorescein-12-dUTP-labeled DNA can then be visualized directly by fluorescence microscopy or quantified by flow cytometry.
For evaluation of nuclear DNA fragmentation, sperm suspensions were centrifuged for five minutes at 300 g. The supernatant was discarded and the remaining pellet was washed in phosphate-buffered saline (PBS, pH 7.4). A droplet of this sperm suspension was smeared onto slides, air-dried and fixed by immersion in freshly prepared 4% methanol-free formaldehyde in PBS for 25 minutes at 4°C. The slides were permeabelized with Triton X-100 for five minutes, rinsed and subsequently equilibrated with equilibration buffer for five to ten minutes at room temperature. Nucleotide mix and rTdT were prepared for test and control slides. DNA strand breaks labeled with fluorescein-12-dUTP were kept for 60 minutes at 37°C in a humidified chamber protected from light. Reactions were stopped by immersing slides in 2X SSC for 15 minutes at room temperature. To remove unincorporated fluorescein-12-dUTP, slides were washed three times for five minutes in PBS and stained with freshly diluted propidium iodide solution (1 μg/ml in PBS) for 15 minutes at room temperature in the dark. The slides were washed three times for five minutes in PBS. Samples were analyzed immediately using a fluorescence microscope. On each slide, 200 sperm cells were evaluated; sperm with DNA damage were considered TUNEL positive and recorded.
Protamine deficiency detection by fluorescence microscopy
Chromomycin A3 is an antibiotic that exhibits anti-bacterial, anti-fungal and antitumor activities. It reversibly binds to guanine-cytosine (G-C) base pairs in the minor groove of DNA, thereby inhibiting RNA synthesis by effecting on topoisomerase II activity. This agent is used as a fluorescent chromosome dye and is useful for the detection of protamine deficiency in sperm chromatins (Sigma; C2659. St. Louis, MO, USA). Initially, washed samples of each procedure were fixed in Carnoy’s solution (methanol:glacial acetic acid, 3:1) at 4°C for five minutes. Smears were prepared and each slide was treated for 20 minutes with 100 μl of CMA3 solution [0.25 mg/ml in McIlvaine buffer (7 ml citric acid (0.1 M) 32.9 ml Na2HPO4.7H2O (0.2 M), pH 7.0, containing 10 mM MgCl2)]. The slides were then rinsed in buffer and mounted with buffered glycerol (1:1). Microscopic analysis of the slides was performed on an Olympus fluorescent microscope (BX51, Tokyo, Japan) with the appropriate filters (460–470 nm). CMA3 positive or protamine deficient sperm were defined as having a light yellow stain, whereas CMA3 negative sperm or sperm with a normal amount of protamine were defined as having a dark yellow stain. On each slide, 200 sperm cells were evaluated [18].
Active caspase detection by flow cytometry
Caspase Detection Kits use a novel approach to detect active caspases (Catalog No. APT105; Chemicon.com; USA & Canada). The methodology is based on Fluorochrome Inhibitors of Caspases (FLICA). The inhibitors are cell-permeable and noncytotoxic. Once inside the cell, the inhibitor binds covalently to the active caspase. This kit uses a carboxyfluorescein-labeled fluoromethyl ketone peptide inhibitor of caspase-3 (FAM-DEVD-FMK), which produces a green fluorescence. When added to a population of cells, the FAM-DEVD-FMK probe enters each cell and covalently binds to a reactive cysteine residue that resides on the large subunit of the active caspase heterodimer, thereby inhibiting further enzymatic activity. The bound labeled reagent is retained within the cell, while any unbound reagent will diffuse out of the cell and is washed away. The green fluorescent signal is a direct measure of the amount of active caspase-3 or caspase-7 present in the cell at the time the reagent was added. Cells containing the bound labeled reagent were analyzed by flow cytometry. For evaluation of active caspase, one million washed sperm from each procedure were diluted in PBS buffer. Next, 10 μl of freshly prepared 30x FILICA reagent was added to the cells and mixed. The samples were incubated for one hour at 37°C under 5% CO2 in dark conditions. Then, samples were washed twice with 2 ml of 1X wash buffer. After centrifugation, 2 μl of propidium iodide (PI) was added to each tube. For each procedure, one tube was considered as a control. Then, flow cytometric analysis was carried out.
Detection of externalized phosphatidylserine by flow cytometry
Assessment of externalized phosphatidylserine provides a rapid and reliable method for the detection of apoptosis and necrosis by flow cytometry. During the early stages of apoptosis, phosphatidylserine (PS) becomes exposed on the outer leaflet of the cell membrane. For evaluation of externalized phosphatidylserine, one million washed sperm from each procedure were diluted in calcium buffer. Next, 5 μl of FITC-labeled annexin V was added and the samples were incubated in the dark for 20 minutes at 4°C. Then, samples were washed twice with calcium buffer. After centrifugation, 5 μl of propidium iodide (PI) was added to each tube. Flow cytometric evaluation was conducted within five minutes [19].
Flow cytometry
FITC-annexin V and FITC-FLICA were used for detection of apoptosis and active caspase markers, respectively, as a green fluorescence, which was separately measured using a FACS Calibur flow cytometer (Becton Dikinson) with a 488-nm argon laser. Briefly, 10000 events were acquired for each sample. Artifacts and aggregates were excluded from the analysis by a gate on the sperm population in forward and side scatter (FSC/SSC). Green and red fluorescence (PI) were detected in FL1 with a 530/30 nm band-pass filter and in FL2 with a 585/42 nm band-pass filter, respectively. Instrument setting was performed using secondary control versus sample tests with green and red fluorescence, independently. The data obtained were analyzed with WinMDI 2.9 software”.
Statistical analysis
Results are expressed as means ± SEM. Levene’s test for equality of variances and Shapiro-Wilk were carried out to assess normal distribution. For statistical analysis, the repeated measures ANOVA were used. P values of <0.05 were considered significant.
Results
The descriptive results of semen parameters from 15 infertile men were analyzed. Sperm concentration ranges were from four to 130 million per ml, with a mean of 40.56 ± 37.67. The percentage of mean normal sperm morphology ranged from 1% to 20% (mean, 8.40 ± 5.13). Furthermore, the percentages of motility ranged from 30% to 60% with a mean of 44.00 ± 9.67.
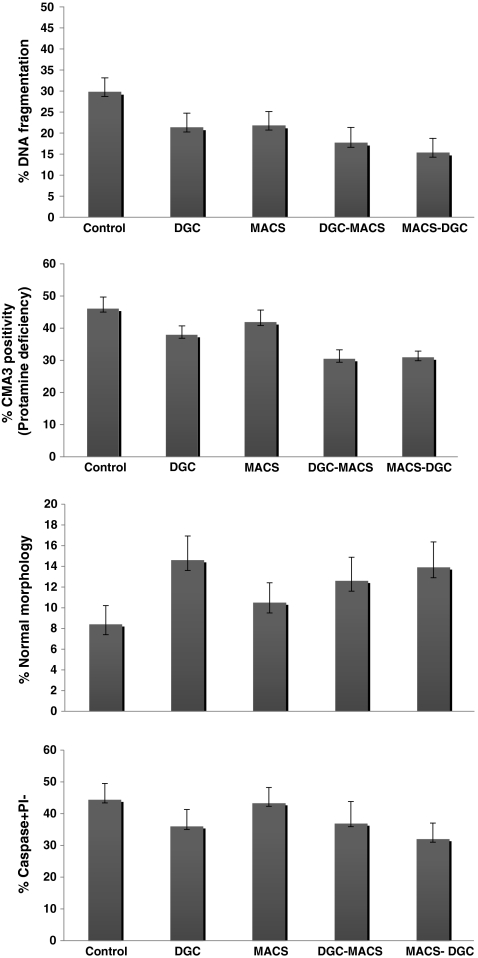
The mean percentages of normal morphology assessed by Papanicoulaou staining in the control, DGC, MACS, DGC-MACS and MACS-DGC procedures were 8.40 ± 1.81, 14.60 ± 2.33, 10.50 ± 1.91, 12.60 ± 2.28 and 13.90 ± 2.46, respectively. Comparison of the mean values of normal morphology showed a significant increase in the percentage of normal morphology following the DGC, DGC-MACS and MACS-DGC procedures when compared with the control group (p < 0.05). The percentage of normal morphology in the DGC group showed significant increase compared with the MACS. In addition, we observed a significant decrease in the percentage of normal morphology in DGC-MACS compared with the DGC (Fig. 2-I). A significant increase was observed in the percentage of normal morphology in MACS-DGC compared to MACS.
Fig. 2.
Comparison of the mean percentages of normal morphology (I), protamine deficiency (II), DNA damage (III) and caspase-3 activity (IV) in the control, DGC, MACS, DGC-MACS and MACS-DGC procedures. Common letters show significant difference at p < 0.05
The mean percentages of protamine deficiency assessed by CMA3 staining in the control, DGC, MACS, DGC-MACS and MACS-DGC procedures were 46.00 ± 3.67, 37.87 ± 2.83, 41.82 ± 3.82, 30.40 ± 2.87and 30.87 ± 2.01, respectively. Comparison of mean percentages of protamine deficiency showed a significant decrease in the percentage of protamine deficiency following the DGC, MACS, DGC-MACS and MACS-DGC procedures when compared with the control group (p < 0.05). The percentage of protamine deficiency in the DGC-MACS and MACS-DGC procedures showed significant decrease compared with the DGC and MACS procedures, respectively (Fig. 2-II).
The mean percentages of DNA damage assessed by TUNEL staining in the control, DGC, MACS, DGC- MACS and MACS-DGC procedures were 29.72 ± 3.41, 21.27 ± 3.47, 21.72 ± 3.41, 17.63 ± 3.72and 15.27 ± 3.49, respectively. Comparison of the mean percentages of DNA damage showed significant decrease in the percentage of DNA damage following the MACS, DGC, DGC-MACS and MACS-DGC procedures when compared with the control group (p < 0.05). The percentage of DNA damage in the DGC-MACS and MACS-DGC procedures showed significant decrease compared with the DGC and MACS procedures, respectively (Fig. 2-III).
The mean percentages of caspase-3 activity in viable sperm in the control, DGC, MACS, DGC-MACS and MACS-DGC procedures were 44.39 ± 5.12, 36.02 ± 5.28, 43.31 ± 4.92, 36.90 ± 6.91and 32.01 ± 5.02, respectively. The mean percentage of caspase-3 activity significantly decreased in the DGC and MACS-DGC procedures when compared with the control group (p < 0.05). The percentage of caspase-3 activity in MACS-DGC procedures showed significant reduction compared with MACS (Fig. 2-IV).
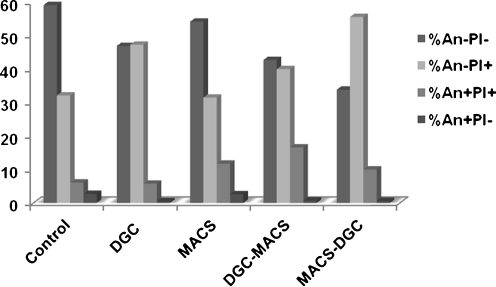
The mean values of the percentage of annexin negative in viable sperm (An-PI-: annexin negative and PI negative) in the control, DGC, MACS, DGC-MACS and MACS-DGC groups were 59.5 ± 3.18, 46.93 ± 3.72, 54.19 ± 3.77, 42.72 ± 5.34, and 33.84 ± 5.68, respectively (Fig. 3). The percentage of annexin negative in viable sperm in the MACS procedure was higher than in the DGC procedure. This difference was close to significant (p = 0.058). In addition, this parameter was significantly reduced in the MACS-DGC procedure when compared with the MACS procedure (p < 0.05). The percentage of annexin negative in viable sperm was also compared between the DGC-MACS and MACS-DGC procedures. The percentages of this parameter increased significantly in the DGC-MACS procedure when compared with the MACS-DGC procedure (p < 0.05).
Fig. 3.
Comparison of mean percentages of annexin in the control, DGC, MACS, DGC-MACS and MACS-DGC procedures
The mean values of the percentage of annexin positive in viable sperm (An + PI-: annexin positive and PI negative) in the control, DGC, MACS, DGC-MACS and MACS-DGC groups were 2.66 ± 0.46, 0.45 ± 0.10, 2.58 ± 0.50, 0.68 ± 0.18, and 0.56 ± 0.11, respectively (Fig. 3). The percentage of annexin positive in viable sperm in MACS was higher than in the DGC procedures (p < 0.05). Additionally, this parameter decreased significantly in the MACS-DGC procedure when compared with the MACS procedure (p < 0.05).
The mean values of the percentage of annexin positive in dead sperm (An + PI+: annexin positive; PI positive) in the control, DGC, MACS, DGC-MACS and MACS-DGC groups were 6.05 ± 1.1, 5.73 ± 2.62, 11.72 ± 2.33, 16.58 ± 3.46, and 9.96 ± 2.17, respectively (Fig. 3). This parameter increased significantly in the DGC-MACS procedure when compared with the DGC procedure (p < 0.05).
The mean values of the percentage of annexin negative in dead sperm (An-PI+: annexin negative; PI positive) in the control, DGC, MACS, DGC-MACS and MACS-DGC groups were 32.12 ± 3.5, 47.29 ± 5.15, 31.50 ± 3.81, 40.00 ± 4.13, and 55.62 ± 6.65, respectively (Fig. 3). For this parameter, a significant difference was observed between the groups (p < 0.05).
Discussion
Management of male infertility mainly depends on our understanding of cellular and molecular aspects of spermatogenesis. Therefore, in addition to standardized semen analysis, evaluation of prognostic markers for assessment of the fertilization potential of an ejaculate is required. In recent years, much attention has been given to the role of apoptosis in reproduction. It is known that apoptosis of germinal cells plays a critical role in normal spermatogenesis. Therefore, altered apoptotic process has been found to be closely associated with sperm anomalies and male infertility [20, 21].
Apoptosis is a cascade of events including the interruption of membrane phospholipid asymmetry, condensation and destruction of the chromatin, compaction of cytoplasmic organelles, reduced mitochondrial transmembrane potential, mitochondrial release of cytochrome c, production of reactive oxygen species, expansion of the endoplasmic reticulum, and a diminishing in cell volume [22]. Among these events, translocation of PS, as an early event of the execution phase of apoptosis, is considered one of the signals for specific recognition and removal of apoptotic cells by phagocytosis [14]. Indeed, increased rates of externalized phosphatidylserine are associated with decreased semen parameters such as sperm motility, morphology or concentration in ejaculated semen [3]. Therefore, the occurrence of apoptotic sperm is reported to be higher in semen samples from infertile men compared with fertile men [23].
Despite that EPS is considered as an early sign of apoptosis in ejaculated sperm, others have shown that EPS is part of the physiological process of capacitation and acrosome reaction taking place upon separation of sperm from the seminal fluid in the presence of natural and chemical mediators of capacitation and acrosome reaction, such as serum, progesterone, ionophore and follicular fluid [24–27].
Recently, different studies reported or proposed the use of MACS together with DGC to prepare human spermatozoa for assisted reproductive techniques. Nevertheless, it is not clear whether annexin positive sperm removed from the insemination sample by MACS post-DGC are residues of an abortive apoptotic process begun before ejaculation, or normal sperm presenting EPS as a normal part of the physiological phenomenon of capacitation and acrosome reaction. Therefore, carrying out MACS before or after DGC might make a difference in the sperm population selected for assisted reproduction techniques.
In this study we aimed to evaluate the effect of the DGC, MACS, MACS-DGC and DGC-MACS procedures on the quality of prepared sperm. The whole process was carried out in absence of serum to mask the effect of PS externalization through capacitation and acrosome reaction, in order to evaluate the efficiency of MACS on its own or in combination with DGC, which has not been evaluated previously. The results reveal that the processes of MACS or DGC reduce the percentage of DNA fragmentation, as an index for the final stage of apoptosis, and no significant difference was observed between the efficiency of these two techniques with regard to this parameter. The addition of MACS to DGC or addition of DGC to MACS, further significantly reduced the percentage of DNA-fragmented sperm in the prepared sample. These two combined procedures did not show any advantage over each other for reducing the percentage of DNA-fragmented sperm.
Assessment of protamine deficiency by CMA3 staining as a sign of sperm immaturity revealed a similar trend to the TUNEL assay results; that is, the percentage protamine deficient sperm was decreased post-MACS or DGC and a combination of these two procedures further potentiated the beneficial effect of these two techniques. To our knowledge this is the first report assessing the efficiency of MACS technique to separate sperm with intact chromatin both in terms of chromatin and DNA integrity. A previous report has shown that chromatin and DNA integrity have important effects on sperm head decondensation and male pronucleus formation. These results are in agreement with the report of Grunewald et al. (2009) which showed a higher rate of sperm head decondensation in annexin negative compared to annexin positive sperm separated by MACS post-DGC, using a hamster oocyte assay [28]. It is of interest that they also did not observe a difference between the MACS negative population and DGC separated sperm.
Assessment of caspase results also showed that, unlike MACS, DGC is more efficient in recovering sperms with respect to caspase activity compared to the control. The percentage of caspase positive sperms was also reduced in both DGC-MACS and MACS-DGC. Furthermore, only the percentage of caspase positive sperms in MACS-DGC was reduced significantly compared to the control. This appears to be due to the DGC procedure and not MACS, because the percentage of caspase positive sperms was not reduced significantly in the MACS as compared to the control.
These results are in disagreement with other literature, which showed that MACS significantly reduces the percentage of caspase positive sperm post-DGC compared to DGC only [11, 23, 28]. However, in these reports, unlike our study, no comparison has been done the using washed semen sample. The possible reason for these differences could be due to the difference in the method of DGC performance (which may vary between different laboratories), the presence of serum in these reports and finally, sample differences between these studies. Regarding the latter point, Paasch et al. (2003) have shown that the percentage of active caspase is higher in infertile individuals as compared to fertile individuals. [23]. In addition, the former group’s active caspase was observed mainly in the post acrosomal region while that of the latter group was in the cytoplasmic residues [23].
With regard to sperm morphology, DGC has also shown to be more efficient than MACS in recovering sperms with normal morphology. In the combination methods, both procedures could recover significantly higher percentage of sperms with normal morphology. Similarly, this effect can be attributed to DGC too. The trend observed here with sperm morphology is consistent with the pervious repots in this filed [13].
In this study we also assessed the annexin in washed samples as the control and in the four groups. The results reveal that in the control group, high percentages of spermatozoa are annexin negative/PI negative after one round of washing. However, upon DGC the percentage of this type of spermatozoa decreased and the percentage of annexin negative /PI positive increased, suggesting that the DGC procedure increases the permeability to PI. Therefore, this increase appears to be independent of apoptosis because we did not observe any change in the percentage of annexin positive/PI negative population, and even a reduction in the caspase 3 activity was observed in the MACS-DGC procedure. Similarly, Martin et al also reported increase in PI permeability independent of apoptosis, and attributed this effect to the process of capacitation and acrosome reaction [26].
In contrast to DGC, no substantial difference was seen following MACS in the pattern of annexin staining. Therefore, the change in the pattern of annexin staining between the control and DGC groups may be attributed to the process of density gradient centrifugation, which is dependent on duration and centrifugation force. Comparison of annexin pattern in the DGC and DGC-MACS procedures also showed no significant difference in the pattern of annexin staining, suggesting that the observed difference to the control is mainly due to the process of centrifugation. Comparison of MACS with MACS-DGC also revealed an increase in the percentage of annexin negative/PI positive, which is due again to the DGC procedure after MACS.
The overall results of this study show that both MACS and DGC are suitable procedures for sperm processing for ICSI. Although no much difference was observed between the combinations of these two procedures (MACS-DGC vs DGC-MACS) with regard to DNA integrity, chromatin maturity and sperm normal morphology, however, the combined procedures separated higher quality sperms than the single procedures. Despite the latter observation, we preferably propose MACS-DGC to be performed rather than DGC-MACS, not only due to significantly lower caspase positive sperms observed in this procedure but also according to below reports stated in the literature: if MACS is performed initially, 1) it separates through the apoptotic sperms rather than the annexin positive ones induced by the initiation of capacitation and acrosome reaction, once sperm being separated from seminal solution during DGC. 2) Although insignificant, the percentage of sperms normal morphology and active caspase was higher and lower in MACS-DGC compared to DGC-MACS, respectively. 3) Sperm population exposed to the MACS column is more susceptible to the mechanical and magnetic forces within the column, which may result in tail defects [11]. Such defects can be removed by applying DGC. 4) Remnant of micro beads remaining after MACS can be separated by using DGC, which makes the procedure safer for clinical purposes. Therefore, in spite of recent clinical trends in the literature, which are mainly implementing DGC-MACS procedure, based on the above discussion, we strongly suggest MACS-DGC rather than DGC-MACS procedure.
Acknowledgements
The authors express their gratitude to the Royan Institute for its financial support, as well as to the staff of Isfahan Fertility and Infertility Center.
Footnotes
Capsule
The results of this study show that for sperm preparation or sperm selection, magnateic-activated cell sorting (MACS) should be carried out before Density gradient centrifugation (DGC) and not after.
References
- 1.Paasch U, Grunewald S, Glander HJ. Sperm selection in assisted reproductive techniques. Soc Reprod Fertil Suppl. 2007;65:515–25. [PubMed] [Google Scholar]
- 2.Benchaib M, Lornage J, Mazoyer C, Lejeune H, Salle B, Franc, et al. Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil Steril. 2007;87:93–100. doi: 10.1016/j.fertnstert.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 3.Zhang HB, Lu SM, Ma CY, Wang L, Li X, Chen ZJ. Early apoptotic changes in human spermatozoa and their relationships with conventional semen parameters and sperm DNA fragmentation. Asian J Androl. 2008;10:227–235. doi: 10.1111/j.1745-7262.2008.00295.x. [DOI] [PubMed] [Google Scholar]
- 4.Bakos HW, Thompson JG, Feil D, Lane M. Sperm DNA damage is associated with assisted reproductive technology pregnancy. Int J Androl. 2008;31:518–526. doi: 10.1111/j.1365-2605.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- 5.Nasr-Esfahani MH, Razavi S, Vahdati AA, Fathi F, Tavalaee M. Evaluation of sperm selection procedure based on hyaluronic acid binding ability on ICSI outcome. J Assist Reprod Genet. 2008;25:197–203. doi: 10.1007/s10815-008-9223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kheirollahi-Kouhestani M, Razavi S, Tavalaee M, Deemeh MR, Mardani M, Moshtaghian J, et al. Selection of sperm based on combined density gradient and Zeta method may improve ICSI outcome. Hum Reprod. 2009;24:2409–16. doi: 10.1093/humrep/dep088. [DOI] [PubMed] [Google Scholar]
- 7.Razavi SH, Nasr-Esfahani MH, Deemeh MR, Shayesteh M, Tavalaee M. Evaluation of zeta and HA-binding methods for selection of spermatozoa with normal morphology, protamine content and DNA integrity. Andrologia. 2010;42:13–9. doi: 10.1111/j.1439-0272.2009.00948.x. [DOI] [PubMed] [Google Scholar]
- 8.Said TM, Agarwal A, Grunewald S, Rasch M, Glander HJ, Paasch U. Evaluation of sperm recovery following annexin V magnetic-activated cell sorting separation. Reprod BioMed Online. 2006;13:336–339. doi: 10.1016/S1472-6483(10)61437-X. [DOI] [PubMed] [Google Scholar]
- 9.Glander HJ, Schaller J. Binding of annexin V to plasma membranes of human spermatozoa: a rapid assay for detection of membrane changes after cryostorage. Mol Hum Reprod. 1999;5:109–15. doi: 10.1093/molehr/5.2.109. [DOI] [PubMed] [Google Scholar]
- 10.Said T, Agarwal A, Grunewald S, Rasch M, Baumann T, Kriegel C, et al. Selection of nonapoptotic spermatozoa as a new tool for enhancing assisted reproduction outcomes: an in-vitro model. Biol Reprod. 2006;74:530–7. doi: 10.1095/biolreprod.105.046607. [DOI] [PubMed] [Google Scholar]
- 11.Aziz N, Said T, Paasch U, Agarwal A. The relationship between human sperm apoptosis, morphology and the sperm deformity index. Hum Reprod. 2007;22:1413–9. doi: 10.1093/humrep/dem016. [DOI] [PubMed] [Google Scholar]
- 12.Grunewald S, Baumann T, Paasch U, Glander HJ. Capacitation and acrosome reaction in nonapoptotic human spermatozoa. Ann N Y Acad Sci. 2006;1090:138–46. doi: 10.1196/annals.1378.015. [DOI] [PubMed] [Google Scholar]
- 13.Dirican EK, Ozgün OD, Akarsu S, Akin KO, Ercan O, Uğurlu M, et al. Outcome of magnetic activated cell sorting of non-apoptotic spermatozoa before density gradient centrifugation for assisted reproduction. J Assist Reprod Genet. 2008;25:375–81. doi: 10.1007/s10815-008-9250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, Schie RC, LaFace DM, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–56. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gadella BM, Harrison RA. Capacitation induces cyclic adenosine 3′,5′-monophosphate-dependent, but apoptosis-unrelated, exposure of aminophospholipids at the apical head plasma membrane of boar sperm cells. Biol Repro. 2002;67:340–350. doi: 10.1095/biolreprod67.1.340. [DOI] [PubMed] [Google Scholar]
- 16.Salicioni AM, Platt MD, Wertheimer EV, Arcelay E, Allaire A, Sosnik J, et al. Signalling pathways involved in sperm capacitation. Soc Reprod Fertil Suppl. 2007;65:245–259. [PubMed] [Google Scholar]
- 17.Examination and processing human semen. 5. New York: Cambridge University Press; 2010. [Google Scholar]
- 18.Tavalaee M, Razavi S, Nasr-Esfahani MH. Influence of sperm chromatin anomalies on assisted reproductive technology outcome. Fertil Steril. 2009;91:1119–26. doi: 10.1016/j.fertnstert.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 19.Hoogendijk CF, Kruger TF, Bouic PJ, Henkel RR. A novel approach for the selection of human sperm using annexin V-binding and flow cytometry. Fertil Steril. 2009;91:1285–92. doi: 10.1016/j.fertnstert.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 20.Bartke A. Apoptosis of male germ cells, a generalized or a cell type-specific phenomenon? Endocrinol. 1995;136:3–4. doi: 10.1210/en.136.1.3. [DOI] [PubMed] [Google Scholar]
- 21.Sakkas D, Mariethoz E, St John JC. Abnormal sperm parameters in humans are indicative of an abortive apoptotic mechanism linked to the Fas-mediated pathway. Exp Cell Res. 1999;251:350–5. doi: 10.1006/excr.1999.4586. [DOI] [PubMed] [Google Scholar]
- 22.Arends MJ, Wyllie AH. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–54. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- 23.Paasch U, Grunewald S, Fitzl G, Glander HJ. Deterioration of plasma membrane is associated with activated caspases in human spermatozoa. J Androl. 2003;24:246–52. doi: 10.1002/j.1939-4640.2003.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 24.Vries KJ, Wiedmer T, Sims PJ, Gadella BM. Caspase-independent exposure of aminophospholipids and tyrosine phosphorylation in bicarbonate responsive human sperm cells. Biol Reprod. 2003;68:2122–2134. doi: 10.1095/biolreprod.102.012500. [DOI] [PubMed] [Google Scholar]
- 25.Muratori M, Porazzi I, Luconi M, Marchiani S, Forti G, Baldi E. AnnexinV binding and merocyanine staining fail to detect human sperm capacitation. J Androl. 2004;25:797–810. doi: 10.1002/j.1939-4640.2004.tb02858.x. [DOI] [PubMed] [Google Scholar]
- 26.Martin G, Sabido O, Durand P, Levy R. Phosphatidylserine externalization in human sperm induced by calcium ionophore A23187: relationship with apoptosis, membrane scrambling and the acrosome reaction. Hum Reprod. 2005;20:3459–3468. doi: 10.1093/humrep/dei245. [DOI] [PubMed] [Google Scholar]
- 27.Barroso G, Taylor S, Morshedi M, Manzur F, Gavino F, Oehninger S. Mitochondrial membrane potential integrity and plasma membrane translocation of phosphatidylserine as early apoptotic markers: a comparison of two different sperm populations. Fertil Steril. 2006;85:149–154. doi: 10.1016/j.fertnstert.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 28.Grunewald S, Reinhardt M, Blumenauer V, Said TM, Agarwal A, Abu Hmeidan F, et al. Increased sperm chromatin decondensation in selected nonapoptotic spermatozoa of patients with male infertility. Fertil Steril. 2009;92:572–7. doi: 10.1016/j.fertnstert.2008.07.1705. [DOI] [PubMed] [Google Scholar]