Abstract
Objective
To evaluate and compare standard sperm parameters and sperm chromatin integrity by sperm chromatin dispersion test (SCD) in ejaculates from men whose partners have a history of recurrent pregnancy loss and from control group of fertile men.
Methods
Thirty couples with unexplained recurrent abortion (case group) and 30 fertile couples (control group) referring to Shiraz infertility center were included. Sperm parameters were assessed in semen samples from two groups and then staining with SCD procedure. The results were analyzed by performing ANOVA and Tukey,s tests.
Results
In control group, nucleoids with big (65.93 ± 2.35), small (12.4 ± 0.60) and without halo (11.6 ± 0.50) showed significant difference with case group (41.40 ± 1.43), (21.16 ± 1.11) and (23.26 ± 1.10) respectively. In the RPL group spermatozoa with high percentage of abnormal parameters (morphology and motility) was observed (p ≤ 0.05).
Conclusion
This study strengthens the current literature associating sperm quality with recurrent pregnancy loss, and emphasizes the important of evaluating male factor by tests such as SCD in addition to conventional sperm parameters.
Keywords: DNA, Sperm, Recurrent pregnancy loss, Sperm chromatin dispersion test
Introduction
Recurrent pregnancy loss is defined as the miscarriage of two or more consecutive pregnancies in the first or early second trimester of gestation [1]. The various etiologies like chromosomal, anatomic, hormonal, immunological have been studied extensively in females with this problem but sperm characteristics have not been examined in detail till now [2]. Some evidence suggests that abnormal integrity of sperm DNA may affect embryo development and possibly increase miscarriage [3]. However, these data are still very preliminary, and it is not known how often sperm defects contribute to recurrent miscarriage.
In 1999, Kobayashi and colleagues [4] demonstrated in in vitro fertilization cycles that low percentages of normal sperm morphology were associated not only with lower successful fertilization rates and pregnancy rates per cycle, but also with a greater risk for miscarriages even if embryo transfer was successful. Carrell and colleagues [5] found higher rates of sperm DNA fragmentation in couples with recurrent early pregnancy loss following spontaneous conception. Similarly, Borini and colleagues [6] found higher early pregnancy loss rates in couples undergoing assisted reproduction techniques, both by in vitro fertilization (IVF) and by conception with intracytoplasmic sperm injection (ICSI) when high sperm DNA fragmentation and abnormal morphology were present. A recent review by Puscheck and Jevendran [7] suggests that the male contributes to recurrent pregnancy loss due to genetic factors, semen factors, or due to other factors such as age and sperm morphology may reflect these underlying deleterious conditions. In addition, DNA fragmentation and high DNA stainability have also been correlated with both abnormal sperm morphology and recurrent pregnancy loss. In a very recent study showed that [2] abnormalities of sperm DNA structure, high DNA fragmentation and high DNA stainability (HDS), were not correlated with IVF or ICSI fertilization rates, good embryo rates or pregnancy rates, but did appear to be correlated higher post implantation spontaneous abortion rates.
With increase in number of infertile couples opting for assisted reproduction techniques (ART), the decline in fertility potential and growing concern about role of male factor in RPL, highlight the need for diagnostic techniques which could asses paternal germ cell DNA damage.
Recently, a new method, the sperm chromatin dispersion test (SCD) was introduced for evaluating sperm DNA fragmentation [8]. The SCD test is based on the principle that sperm with fragmented DNA fail to produce the characteristic halo of dispersed DNA loop that is observed in sperm with non fragmented DNA following acid denaturation and removal of nuclear proteins. SCD test results have been shown to be highly correlated with sperm chromatin structure assay (SCSA) that a very powerful technique mainly on human sperm samples [9]. In addition, this method is simple, less expensive and can be performed in a short period of time.
The main objective of this study was to prospectively evaluate the predictive value of the SCD test to correlate DNA dispersion with sperm parameters in recurrent pregnancy loss.
Materials & methods
Patient selection
In this prospective study, 30 couples with recurrent pregnancy loss and 30 fertile couples were enrolled at Shiraz—Human Infertility Centre between December 2010 and Jan 2011. All the participants were divided into two groups: case group (n = 30) consisted of the men whose partners had ≥ 3unexplained recurrent spontaneous abortion at least than 20 weeks of gestation. Control group (n = 30) consisted of healthy men (with no known medical conditions) whose partners with no history of pregnancy loss.
Semen sample
The semen samples of the two groups were collected by masturbation after 2–5 days of sexual abstinence. After complete liquefaction of the sample, semen analysis was performed according to World Health Organization guidelines [10] and sperm morphology was analyzed following the Kruger strict criteria [11]. Sperm count was performed in a Neuberger counting chamber. After immobilizing the cells with distilled water, morphology was evaluated by the Diffquick staining technique. Motility was expressed as a percentage of rapid and/or progressive spermatozoa.
SCD test
Aliquots of 0.2 mL of fresh sample semen diluted in medium to obtain sperm concentrations that ranged were between 5 and 10 million/mL. The suspensions were mixed with 1% low-melting-point aqueous agarose (to obtain a 0.7% final agarose concentration) at 37 º. Aliquots of 50 μL of the mixture were pipetted onto Coverslips were carefully covered, and slides were immediately immersed horizontally in a tray with freshly prepared acid denaturation solution (0.08 N HCL) for 7 min. a glass slide precoated with 0.65% standard agarose dried at 80 º C, covered with a coverslip (24 by 60 mm), and left to solidify at 4°C for 4 min. The agarose matrix allows for work with unfixed sperm on a slide in a suspension like environment. At 22°C in the dark to generate restricted single- stranded DNA (ss DNA) motifs from DNA breaks. The denaturation was then stopped, and proteins were removed by a transfer of the slides to a tray with neutralizing and lysing solution (0.4 M Tris, 0.8 M DTT, 1% SDS, 2 M NaCl, 0.05 M Triplex) for 25 min at room temperature. Removal of nuclear proteins results in nucleoids with a central core and a peripheral halo of dispersed DNA loop. Slides were thoroughly washed twice in water for 5 min, dehydrated in sequential 70%, 90% and 100% ethanol baths (2 min each), and air dried. At the end cells were stained with Wright and PBS (1:1) for 10 min. After air dried, the degree of DNA dispersion was assessed by bright field microscopy. A minimum of 200 spermatozoa were evaluated by 2 different observers [8].
Statistical analysis
The results were analyzed by performing ANOVA and Tukey,s tests, with p < 0.05 considered as statistically significant. The mean and standard deviation (SD) was also calculated for each value.
Results
The comparison of semen analysis of RPL and the control groups is shown in Table 1. On comparing the routine semen analysis in males of RPL group with the control group, semen volume, count were within normal range in both groups. Sperm motility (56.31 ± 2.30) and morphology (51.56 ± 1.40) were found to be significantly lower in the RPL group versus control group (64.26 ± 2.82) & (26.73 ± 1.88) respectively (p ≤ 0.05).
Table 1.
Seminal parameters of 30 couples with spontaneous recurrent abortion (Case group) compared to 30 fertile couples (Control group)
| Semen parameters | Control group(n = 30) | Case group(n = 30) |
|---|---|---|
| Ejaculation volume (ml) | 3.01 ± 0.23 | 2.58 ± 0.18 |
| Concentration (×106/ml) | 62.65 ± 7.21 | 64.08 ± 6.51 |
| Progressive motility (a + b%) | 64.26 ± 2.82 | 56.31 ± 2.30 * |
| Morphologic alterations (%) | 26.73 ± 1.88 | 51.56 ± 1.40 * |
* Significant difference with control group. (P < 0.05)
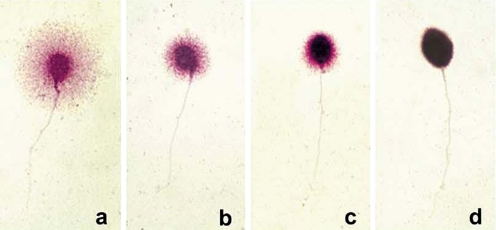
Determination of sperm DNA fragmentation was tested by SCD test using conventional bright-field microscopy. Four SCD patterns were established (Fig. 1): [1] sperm cells with large halos: those whose halo width is similar or higher than the minor diameter of the core; [2] sperm cells with medium size halos: their halo size is between those with high and with small halo; [3] sperm cell with small size halo: the halo width is similar or smaller than one third of the minor diameter of the core; [4] sperm cell without a halo.
Fig. 1.
Nucleoides from human sperm cells obtained with the SCD procedure: Nucleoides with big halo of DNA dispersion (a), medium sized (b), small (c) and without halo and degenerated
Table 2 showed a substantial difference in the percentage of positive SCD stained spermatozoa. In control group, nucleoids with big (65.93 ± 2.35), small (12.4 ± 0.60) and without halo (11.6 ± 0.50) showed significant difference with case group (41.40 ± 1.43), (21.16 ± 1.11) and (23.26 ± 1.10) respectively.
Table 2.
Sperm chromatin dispersion (SCD) data (mean ± standard error of mean) from semen samples spontaneous recurrent abortion (case group) and fertile subjects (control group)
| Semen sample | % Big halo | % Medium halo | % Small halo | % Without halo |
|---|---|---|---|---|
| Control group (n = 30) | 65.93 ± 2.35 | 7.9 ± 0.47 | 12.4 ± 0.60 | 11.6 ± 0.50 |
| Case group (n = 30) | 41.40 ± 1.43 * | 8.50 ± 0.63 | 21.16 ± 1.11* | 23.26 ± 1.10 * |
* Significant difference with control group. (P < 0.05)
Discussion
The relation between standard semen parameters and recurrent pregnancy loss has been a controversial subject [12]. To evaluate the role of male factors in RPL we examined the semen parameters and sperm DNA integrity thorough SCD test. This study strengthens the current literature associating sperm quality with recurrent pregnancy loss, and emphasizes the important of evaluating male factor by tests such as SCD in addition to conventional sperm parameters. According to Kruger’s explain our semen analysis data showed that abnormal sperm morphology has been associated with increased miscarriage rates [13]. Hill et al. was successful in identifying an association between increases in abnormal sperm morphology in unexplained recurrent miscarriage in comparison with the general population [14]. In addition, it was shown that in couples with RPL, abnormalities of sperm parameters can reflect DNA structure that may subsequently increase the risk of early miscarriage even after successful conception, spontaneous and assisted [15–17]. On the other hand, DNA integrity analysis is a relatively independent measure of semen quality that yields diagnostic and prognostic information complementary to, but distinct and more significant than standard sperm parameters.
Different studies on infertile patients and in patients with recurrent pregnancy loss have shown varying results concerning the extent of sperm DNA damage.
Gopalkkrishnan et al. investigated the sperm quality in greater detail, particularly looking at the sperm nuclei and chromatin condensation. This group did found an association with poor sperm quality and repeated early pregnancy loss as measured by an increase in sperm nuclear vacuoles or abnormal chromatin condensation [18]. In 2008, Saxena and colleagues demonstrated that sperm functional tests were significantly lower in the RPL group [2]. Recently, a new procedure for the determination of the DNA fragmentation in human sperm cells, called the sperm chromatin dispersion (SCD) test [8]. To date, there have been few reports demonstrating the usefulness of SCD test in recurrent pregnancy loss. In present study, higher percentage of sperm cells with high degree of nuclear dispersion was found in the RPL patients. The SCD test is performed by conventional bright- field microscopy, and it has been shown recently that the SCD test results are highly correlated with those from the SCSA [9], thus confirming the validity of SCD.
It was shown that the SCD test could be a good and cost-effective alternative to the SCSA. The discrimination of different degree of DNA fragmentation is an interesting ability of the SCD test [9]. Also this test allows for the detection of an extreme degree of DNA damage that possibly affects nuclear structure and that could not be detected using the other DNA damage techniques [19].
In conclusion, sperm DNA damage assessment may be valuable among routine tests for infertility investigations. The possibility for DNA assessment using conventional bright- field microscopy should be universally applicable and the SCD test could allow for the routine screening of sperm DNA fragmentation in the basic andrology laboratories. The SCD test is a simple, cost effective, rapid, reliable and accurate procedure for routinely sperm DNA fragmentation in the clinical andrology laboratory.
Footnotes
Capsule
Sperm quality is correlated with RPL and SCD test could be a good and cost-effective method for detection of extreme degree of DNA damage.
References
- 1.American Society for Reproductive Medicine (ASRM). Recurrent pregnancy loss. Patients Fact Sheet. Created February. 2005. Available at: http://www.asrm.org/patients/FactSheet/fac.html.Accessed January 9, 2008
- 2.Saxena D, Misro MM, Roy S, Chopra K, Sinha D, Nandan D, Trivedi SS. Possible role of male factors in recurrent pregnancy loss. Indian J Physio Pharmacol. 2008;52(3):274–282. [PubMed] [Google Scholar]
- 3.Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update. 2003;9:331–45. doi: 10.1093/humupd/dmg027. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi T, Miyazaki T, Natori M, Nozava S. Protective role of superoxide dismutase in human sperm motility: superoxide dismutase activity and lipid peroxide in human semina; p;asma and spermatozoa. Human Reprod. 1991;6(7):983–6. doi: 10.1093/oxfordjournals.humrep.a137474. [DOI] [PubMed] [Google Scholar]
- 5.Carrel DT, Liu L, Peterson CM, Jones KP, Hatasaka HH, Erikson L, Campbell B. Sperm DNA fragmentation is increased in couples with unexplained recurrent pregnancy loss. Arch Androl. 2003;49:49–55. doi: 10.1080/01485010290099390. [DOI] [PubMed] [Google Scholar]
- 6.Borini A, Tarozzi N, Bizzaro D, Bonu MA, Fava L, Flamigni C, Coticchio G. Sperm DAN fragmentation: paternal effect on early post-implantation embryo development in ART. Hum Reprod. 2006;21(11):2876–81. doi: 10.1093/humrep/del251. [DOI] [PubMed] [Google Scholar]
- 7.Puscheck EE, Jevendran RS. The impact of male factor on recurrent pregnancy loss. Curr Opin Obstet Gynecol. 2007;19:222–28. doi: 10.1097/GCO.0b013e32813e3ff0. [DOI] [PubMed] [Google Scholar]
- 8.Fernandes JL, Muriel L, Rivero MT, Goyanes V, Vasquez R, Alvarez JG. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003;24:59–66. [PubMed] [Google Scholar]
- 9.Fernandes JL, Muriel L, Goyanes V, Segrelles E, Gosalves J, Enciso M, Iafromboise M, Jonge C. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil Steril. 2005;84:833–842. doi: 10.1016/j.fertnstert.2004.11.089. [DOI] [PubMed] [Google Scholar]
- 10.Laboratory manual for the examination of human semen and sperm cervical mucus interaction. 4. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 11.Kruger TF, Menkveld R, Stander FS, Lombard CJ, der Merwe JP Wan, van Zyl JA. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril. 1986;46. [DOI] [PubMed]
- 12.Zini A, Sigman M. Are tests of DNA damage clinically useful? Pros and Cons J of Androl. 2009;30:220–229. doi: 10.2164/jandrol.108.006908. [DOI] [PubMed] [Google Scholar]
- 13.Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988;49:112–117. doi: 10.1016/s0015-0282(16)59660-5. [DOI] [PubMed] [Google Scholar]
- 14.Hill JA, Abbott AF, Politch JA. Sperm morphology and recurrent abortion. Fertil Steril. 1994;9:1180–1183. [PubMed] [Google Scholar]
- 15.Kenneth F, Trifatter JR. Abnormal sperm morphology and recurrent pregnancy loss? Fertil Steril 2007
- 16.Guzick DS, Overstreet GW, Factor-Litvak P, Brazil CK, Nakajima ST, et al. National Cooperative Reproductive Medicine Network. Sperm morphology, motility and concentration in infertile and fertile men. N Ingel J Med. 2001;345:1388–1393. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 17.Dada R. Recurrent pregnancy loss: Male factor. In: Deka D, Malhotra N, editors. An introduction to genetics and fetal medicine. New Delhi: Jaypee Publications; 2009. pp. 31–7. [Google Scholar]
- 18.Gopalkkrishnan K, Padwal V, Meherji PK. Poor quality of sperm as it affects repeated early pregnancy loss. Arch Androl. 2000;45:111–117. doi: 10.1080/014850100418800. [DOI] [PubMed] [Google Scholar]
- 19.Chen C, Lee S, Chen D, Chien H, Chen I, Chu Y. Apoptosis and kinematics of ejaculated spermatozoa in patients with varicocele. J Androl. 2004;25:348–53. doi: 10.1002/j.1939-4640.2004.tb02799.x. [DOI] [PubMed] [Google Scholar]