Abstract
Objective
Present study was aimed to determine whether aqueous neem leaf extract (NLE) induces generation of reactive oxygen species (ROS) and apoptosis through mitochondria-mediated pathway in rat oocytes.
Design
A controlled prospective study.
Setting
Laboratory research setting at Department of Zoology of Banaras Hindu University.
Animal(s)
Forty eight sexually immature female rats that were 20–30 days of age.
Intervention(s)
Sexually immature female rats were fed palatable dose of NLE (10 mg/g dry feed palate) for 10 days and then subjected to superovulation induction protocol. Thereafter, rats were euthanized, ovulated cumulus oocyte complexes were collected from oviduct and oocytes were denuded.
Main outcome measure(s)
Rate of morphological apoptotic changes, measurement of hydrogen peroxide, nitric oxide and cytochrome c concentrations, caspase-9, caspases-3 activities and DNA fragmentation in oocytes.
Results
In vivo NLE treatment induced morphological apoptotic changes were associated with increased hydrogen peroxide, nitric oxide and cytochrome c concentrations, caspase-9, caspase-3 activities and DNA fragmentation in oocyte.
Conclusion
NLE induces generation of ROS that leads to oocytes apoptosis through mitochondria-mediated pathway.
Keywords: Aqueous neem leaf extract, Oocyte, Reactive oxygen species, Cytochrome c, Caspase-9, Caspase-3, DNA fragmentation, Morphological apoptotic features
Neem plant (Azadirachta indica) has been extensively used for the treatment of several diseases in ayurveda, unani and homoeopathic systems of medicine and has become a cynosure of modern medicine. All parts of the neem tree i.e. leaves, flowers, seeds, fruits, roots and bark possess antifertility properties [1]. Due to its antifertility property, neem leaves are frequently used to control fertility in traditional system of medicine worldwide. We hypothesize that the antifertility effect of neem extracts in female could be due to its direct or indirect action at the level of oocytes. This notion is supported by our previous observations that the aqueous neem leaf extract (NLE) induces apoptosis in rat oocytes cultured in vitro [2]. However, the mechanism(s) by which NLE induces oocyte apoptosis remains to be elucidated.
Quercetin is one of the major bioactive flavonoids found in NLE [1]. The quantitative analysis indicates that the aqueous NLE contain 6 to 48 mg% (w/w) quercetin [3]. Quercetin induces generation of reactive oxygen species (ROS) in somatic cells possibly by two pathways. One potential mechanism is that quercetin, after scavenging the peroxyl radical, is converted to radicals in the form of quercetin-O. [4]. Another mechanism is that quercetin inhibits antioxidant systems such as thioredoxin or glutathione [5–7]. Quercetin induces generation of ROS and thereby apoptosis in cancer cells [4, 5, 8–11]. Since, quercetin is one of the major bioactive ingredients of aqueous NLE [3], possibility exist that the generation of ROS and cytochrome c release from mitochondria might be involved during aqueous NLE-induced oocyte apoptosis but there is no evidence to support this hypothesis.
The release of cytochrome c from mitochondria may initiate the apoptotic signals in wide variety of cells including oocytes [12–17]. In the cytosol, cytochrome c binds to apoptotic factor-1 (Apaf-1) leading to the recruitment and activation of procaspase-9 in a large complex termed the apoptosome [18, 19]. As a result, procaspase-9 auto processes and cleaves the effectors procaspase including procaspase-3. The activated procaspase-3 cleaves key structural and regulatory proteins that result in the biochemical and morphological changes associated to apoptosis [20, 21].
Quercetin-induced apoptosis in somatic cells is associated with loss of mitochondrial membrane potential thereby cytochrome c release, increase in procaspase-9 and procaspase-3 activities [21, 22]. Similarly, a possibility exists that aqueous NLE could induce generation of ROS and thereby apoptosis in preovulatory oocytes through mitochondria-mediated pathway. However, there is no evidence to support this possibility. Therefore, present study was designed to test whether in vivo treatment of NLE induces generation of ROS and if so, whether NLE-induced apoptosis is mediated through mitochondrial pathway. For this purpose, in vivo and in vitro studies with NLE and/or quercetin were carried out to analyze morphological apoptotic changes, intracellular hydrogen peroxide (H2O2), nitric oxide (NO) and cytochrome c levels, caspase-9 and caspase-3 activities, and DNA fragmentation in oocytes.
Materials and methods
Chemicals and preparation of culture medium
All chemicals used in the present study were purchased from Sigma Chemical Co. St. Louis, MO, USA unless stated otherwise. The culture medium (M-199; HiMedia, Mumbai, India) was freshly prepared as per company manual protocol. The penicillin (100 IU/ml) and streptomycin (100 μg/ml) were added to the culture medium.
Experimental animal
Sexually immature female rats (20–30 days old; 45 ± 5 g body weight) along with their mother of Charles-Foster strain were separated from existing colony of departmental animal facility and maintained in normal husbandry conditions with food and water ad libitum. All procedures confirmed to the stipulations of the Departmental Animal Ethical Committee of Banaras Hindu University, Varanasi-221005 and followed the guidelines for the care and use of laboratory animals (NIH Publication).
Preparation of aqueous NLE and determination of its palatable dose
Green leaves were procured from neem tree of university campus and aqueous fraction of NLE was collected and then lyophilized following extraction procedure published earlier [2]. The NLE powder was stored at 4°C before use. The rat dry feed palate (Pranav Agro Industries Ltd. Sangli, India) was grinded and NLE powder was mixed to get various doses of NLE (5, 10, 15 and 20 mg/g dry feed palate). The rat dry feed powder with or without various doses of NLE were used to prepare palate and then air-dried. To determine the palatable dose, 15 sexually mature female rats were divided in five groups of three each. Control rats were fed normal dry feed, while remaining four groups were provided dry feed palate containing various doses of NLE (5, 10,15 and 20 mg/g dry feed palate) for 3 days. On the basis of daily consumption, a palatable dose (10 mg/g dry feed) was determined (data not shown) and used for in vivo studies.
Effect of NLE on morphological changes in oocytes in vivo
The sexually immature female rats of 20 days old (body weight, 35 ± 5 g; three rats in each group) along with their mother were used for in vivo studies. Control rats were fed normal dry feed palate, while experimental rats were provided palatable dose (10 mg/g dry feed palate) of NLE and water ad libitum for 10 days. These 30 days old experimental as well as control animals (body weight, 46 ± 4 g) were subjected to superovulation induction protocol for the collection of oocytes as described earlier [2]. The ovulated cumulus oocytes complexes from control and NLE –treated animals were photographed for the analysis of cumulus cells intactness and morphology. The denuded oocytes from control and experimental animals were quickly analyzed for their morphological status. To analyze the susceptibility of these oocytes towards apoptosis, morphologically normal oocytes (15 to 16 oocytes in each group) from control animals as well as from NLE-treated animals were further cultured for 3 h in medium-199 and then analyzed for morphological status using phase-contrast microscope (Nikon, Eclipse; E600, Japan) at 400X magnification. The experiment was repeated three times and representative photographs are shown in the result section.
Effects of various concentrations of NLE and quercetin on morphological apoptotic in oocytes in vitro
For in vitro studies, oocytes were collected from 24 to 25 days old female rats (body weight, 40 ± 5 g) subjected to superovulation induction protocol as described earlier [2]. Quercetin is one of the major bioactive ingredients of aqueous NLE [3], we used quercetin as a positive control for in vitro studies. Groups of 14–15 oocytes were incubated separately in 2 ml of plain medium (control group) and medium containing various concentrations of NLE (1.25, 2.5, 5, 10 and 20 mg/ml) or quercetin (0.375, 0.75, 1.5, 3 and 6 μg/ml). These concentrations of NLE and quercetin have been reported to induce apoptosis in oocytes and cancer cells cultured in vitro [2, 23]. All petridishes were maintained at 37°C for 3 h in a CO2 incubator (Thermo Pharma, Ohio, USA) and then examined for morphological apoptotic changes using a phase-contrast microscope. The experiment was repeated three times to confirm the results.
Effect of NLE on intraoocyte H2O2 and total nitrite concentrations
The intracellular H2O2 concentration in oocyte lysates was analyzed using H2O2 assay kit purchased from Northwest Life Science Specialties, LLC, WA, USA and total nitrite concentration was analyzed using NO assay kit purchased from R&D Systems MN, USA. The oocytes from control and NLE-treated animals (100 oocytes from each group) were transferred to a microcentrifuge tube containing 200 μL of hypotonic lysis buffer (5 mM Tris, 20 mM EDTA, 0.5% TritonX-100, pH 8) for 1 h on ice for lysis. Lysates were centrifuged at 10,000 x g at 4°C for 15 min and clear supernatant was immediately diluted by 5-fold with sample diluent and then used for the quantitative estimation of H2O2 and total nitrite concentrations by colorimetric assay as per company manual protocols. The optical density (OD) was determined using a Microplate Reader (Micro Scan MS5608A, ECIL, Hyderabad, India) set at 560 nm for H2O2 and 540 nm for total nitrite. The samples were run in triplicate and all samples were run in one assay to avoid inter-assay variation. The intra-assay variation for H2O2 and total nitrite concentrations were 3% and 2.5%, respectively.
Effect of NLE on intraoocyte cytochrome c concentration
The cytochrome c concentration in oocyte lysates was analyzed using cytochrome c assay kit purchased from R&D Systems MN, USA. The oocyte lysates were prepared as described above for the measurement of H2O2 and total nitrite concentrations. The cytochrome c concentration in oocyte lysates was analyzed as per company manual protocol. The plate was read at 450 nm using Microplate Reader. All samples were run in triplicate to avoid inter-assay variation and intra-assay variation was 1.02%.
Effects of NLE on caspase-9 and caspase-3 activities
The intracellular caspase-3 and caspase-9 activities in oocyte lysates were analyzed using caspase-3 and caspase-9 colorimetric assay kits purchased from R&D Systems MN, USA. The oocyte lysates were prepared as described above for the measurement of H2O2 and total nitrite concentrations. All reagents, working standards and samples were brought to room temperature and caspase-9 and caspase-3 assays were performed simultaneously as per company manual protocol. All samples were run in triplicate to avoid inter-assay variation and intra-assay variation for caspase-9 and caspase-3 were 1.5% and 1.7%, respectively. The samples OD was analyzed using standards graph and mean ± SE OD values are used to depict caspase-9 and caspase-3 activities.
Effect of NLE on DNA fragmentation in oocytes
The DNA fragmentation was detected using terminal deoxynucleotidyl transferase (TDT) nick-end labeling (TUNEL) kit purchased from R&D Systems (USA). Oocytes from control and 10 mg/ml NLE-treated groups (15–16 oocytes in each group) were separately transferred on clean glass slides and fixed in 3.7% (v/v) buffered formaldehyde for 15 min at 18–20°C. After washing, slides were used for DNA fragmentation analysis as described earlier [2]. Slides were analyzed for TUNEL positive staining under phase contrast microscope at 400 X magnification. The TUNEL analysis was repeated three times and representative photographs are shown in the result section.
Statistical analysis
Data are expressed as mean ± standard error of mean (SE) of triplicate samples. All percentage data were subjected to arcsine square-root transformation before statistical analysis. Data were analyzed by either Student’s t-test or One-way ANOVA using SPSS software, version 17.0 (SPSS, Inc., Chicago, IL, USA). A probability of P < 0.05 was considered significant.
Results
In vivo NLE treatment induces morphological apoptotic features in oocytes
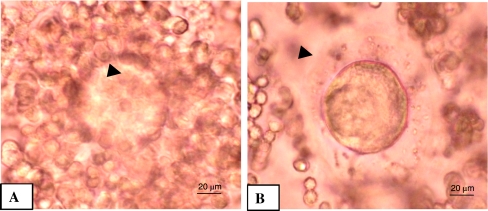
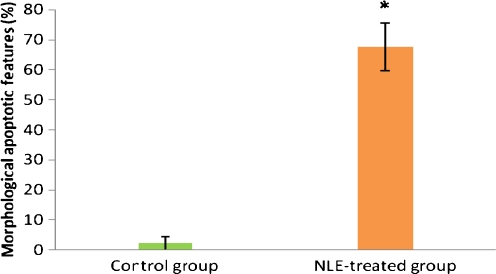
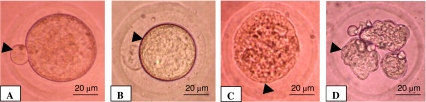
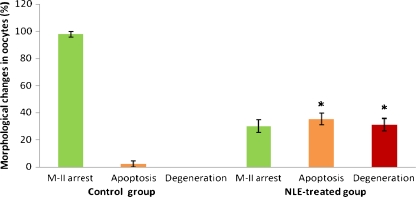
As shown in Fig. 1B, NLE treatment (10 mg/g dry feed palate for 10 days) induced cumulus cells dispersion and reduced number of cumulus cells attached to the oocytes as compare to control oocytes that had compact and large number of cumulus cells encircling the oocyte (1A). Further, NLE treatment significantly induced morphological apoptotic features in majority of oocytes (67.55 ± 7.98%; Fig. 2). Although, shrinkage (27%; Fig. 3B) and cytoplasmic fragmentation (26.4%; Fig. 3D) were most frequent morphological apoptotic features observed after NLE treatment in vivo, cytoplasmic granulation was also seen in few number of oocytes (14.7%; Fig. 3C). On the other hand, majority of control oocytes were normal (97.7% Fig. 3A) and only few oocytes showed morphological apoptotic features (2.3%; Table 1). Culture of remaining morphologically normal oocytes (collected from NLE-treated animals; total 52 oocytes) for 3 h under in vitro culture conditions in plain medium further induced morphological apoptotic features (35.50 ± 4.12%; Fig. 4). The shrinkage (13.5%), cytoplasmic fragmentation (9.6%) and cytoplasmic granulation (11.5%) were the morphological apoptotic features observed prior to degeneration (32.7%). On the other hand, majority of oocytes collected from control animals remained with first polar body with normal morphology (97.6%) and only few oocytes (2.4%) showed morphological apoptotic features even after 3 h of in vitro culture (Table 2).
Fig. 1.
Representative photographs showing effects of NLE on the number of cumulus cells and their intactness with oocytes in vivo. Control cumulus oocytes complex showing greater number and tightly intact cumulus cells with the oocytes (A; arrow). Treatment of 10 mg/gm dry feed palate of NLE reduced number of cumulus cells attached to the oocytes and increased cumulus cell dispersion (B; arrow). Bar = 20 μm
Fig. 2.
Effect of NLE treatment on the induction of morphological apoptotic features in vivo. Animals were fed NLE palatable dose (10 mg/gm dry feed palate) for 10 days and then subjected to superovulation induction protocol. Ovulated oocytes were observed for presence or absence of morphological apoptotic features. Data are mean ± SE of three replicates. “*” Denotes significantly (P < 0.05) higher as compare to control group. Data were analyzed by Student’s t-test
Fig. 3.
Representative photographs showing NLE (10 mg/gm dry feed palate) treatment in vivo on morphologic apoptotic features in oocytes. (A) control oocyte showing first polar body with normal morphology (arrow), (B) NLE-treated oocyte showing shrinkage (arrow), (C) cytoplasmic granulation (arrow), and (D) cytoplasmic fragmentation (arrow). Bar = 20 μm
Table 1.
Effect of NLE (10 mg/g dry feed) oral feeding on induction of morphological apoptotic features in oocytes in vivo
| Morphological changes in oocytes | ||||||
|---|---|---|---|---|---|---|
| Oocytes collected from control animals | ||||||
| Total Oocytes | Normal oocytes | Apoptotic oocytes | ||||
| Shrinkage | Cytoplasmic fragmentation | Cytoplasmic granulation | Total apoptotic oocytes | Degeneration | ||
| 171 | 167 | 1 | 2 | 1 | 4 | 0 |
| (100%) | (97.7%) | (0.6%) | (1.2%) | (0.6%) | (2.3%) | (0%) |
| Oocytes collected from NLE (10 mg/g dry feed) treated animals | ||||||
| Total Oocytes | Normal oocytes | Apoptotic oocytes | ||||
| Shrinkage | Cytoplasmic fragmentation | Cytoplasmic granulation | Total apoptotic oocytes | Degeneration | ||
| 163 | 52 | 44 | 43 | 24 | 111 | 0 |
| (100%) | (31.9%) | (27.0%) | (26.4%) | (14.7%) | (68.1%) | (0%) |
Fig. 4.
Effect of in vivo NLE (10 mg/gm dry feed palate) treatment on the susceptibility of oocytes towards apoptosis in vitro. Oocytes collected from control as well as from NLE-treated animals that had normal morphology were further cultured in plain medium for 3 h and observed for their morphological apoptotic changes. Data are mean ± SE of three replicates. “*” Denotes significantly (P < 0.05) higher as compare to control group. Data were analyzed by Student’s t-test
Table 2.
Effect of NLE (10 mg/g dry feed) oral feeding on the susceptibility of oocytes to undergo apoptosis if cultured in plain medium for 3 h in vitro
| Morphological changes in oocytes | ||||||
|---|---|---|---|---|---|---|
| Remaining normal oocytes collected from control animals | ||||||
| Total Oocytes | Normal oocytes | Apoptotic oocytes | ||||
| Shrinkage | Cytoplasmic fragmentation | Cytoplasmic granulation | Total apoptotic oocytes | Degeneration | ||
| 167 | 163 | 2 | 1 | 1 | 4 | 0 |
| (100%) | (97.6%) | (1.2%) | (0.6%) | (0.6%) | (2.4%) | (0%) |
| Remaining normal oocytes collected from NLE (10 mg/g dry feed) treated animals | ||||||
| Total Oocytes | Normal oocytes | Apoptotic oocytes | ||||
| Shrinkage | Cytoplasmic fragmentation | Cytoplasmic granulation | Total apoptotic oocytes | Degeneration | ||
| 52 | 17 | 7 | 5 | 6 | 18 | 17 |
| (100%) | (32.7%) | (13.5%) | (9.6%) | (11.5%) | (34.6%) | (32.7%) |
NLE and quercetin induce morphological apoptotic features in oocytes in vitro
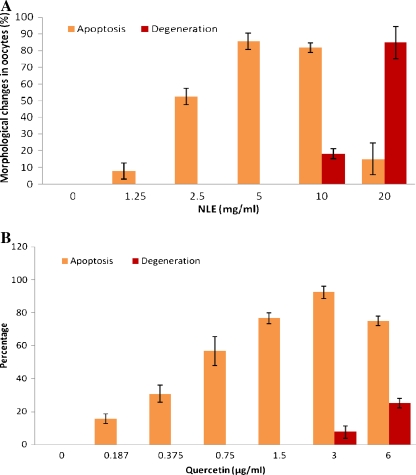
The NLE-induced oocyte apoptosis in vitro was reconfirmed in the present study. As shown in Fig. 5A, NLE-induced morphological apoptotic features in a concentration-dependent manner (One-way ANOVA: F = 40.86, P < 0.05). The initiation of degeneration was observed at 10 mg/ml NLE (18.27 ± 2.85%) and majority of oocytes underwent degeneration if exposed to 20 mg/ml of NLE for 3 h in vitro. Similarly, quercetin induced morphological apoptotic features in a concentration-dependent manner (One-way ANOVA: F = 57.52, P < 0.05; Fig. 5B). Degeneration of was observed if the oocytes were exposed to higher concentrations of quercetin (3 and 6 μg/ml). Indeed, quercetin (one of the major bioactive ingredients of NLE) was more potent in inducing oocyte apoptosis as compare to NLE under in vitro culture conditions.
Fig. 5.
Effects of various concentrations of NLE and quercetin on morphological changes in oocytes cultured in vitro. Oocytes were exposed to various concentrations of NLE (A) or quercetin (B) for 3 h in vitro and then morphological apoptotic changes were analyzed. Data are mean ± SE of three replicates. Data were analyzed by one-way ANOVA
NLE treatment increases H2O2 and total nitrite concentrations in oocytes
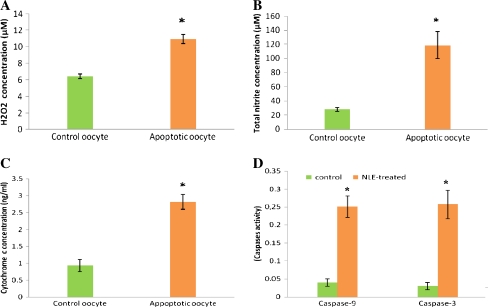
As shown in Fig. 6A, NLE treatment significantly (P < 0.05) increased intracellular H2O2 concentration (10.92 ± 0.54 μM) in oocytes collected from NLE-treated animals as compare to control animals (6.45 ± 0.28 μM). Similarly, a significant (P < 0.05) increase in intracellular total nitrite concentration (118.83 ± 19.48 μM) was noticed in oocyte collected from NLE-treated animals as compare to oocyte (27.74 ± 2.54 μM) collected from control animals (Fig. 6B).
Fig. 6.
In vivo effects of 10 mg/gm dry feed palate of NLE treatment on intraoocyte H2O2 concetration (A), total nitrite concentration (B), cytochrome c concentrations (C), caspase-9 and caspase-3 activities (D). Data are mean ± SE of three replicates. “*” Denotes significantly (P < 0.05) higher as compare to control oocyte (Student’s t-test)
NLE treatment increases cytochrome c concentration in oocytes
As shown in Fig. 6C, NLE treatment significantly (P < 0.05) increased intracellular cytochrome c concentration in oocytes collected from NLE-treated animals that had morphological apoptotic features (2.82 ± 0.18 ng/ml) as compare to oocytes that were collected from control animals and had normal morphology with first polar body (0.93 ± 0.22 ng/ml).
NLE treatment increases caspae-9 and caspase-3 activities in oocytes
As shown in Fig. 6D, NLE treatment significantly (P < 0.05) increased both caspase-9 (0.251 ± 0.03 OD value) and caspase-3 activities (0.258 ± 0.04 OD value) in oocytes that had morphological apoptotic features as compare to their respective control oocytes (caspase-9, 0.04 ± 0.02 OD value; caspase-3, 0.03 ± 0.02 OD value).
NLE treatment induces DNA fragmentation in oocytes
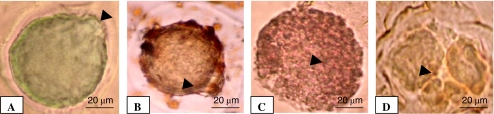
To confirm the occurrence of DNA fragmentation, oocytes were collected from control as well as from NLE-treated animals that had morphological apoptotic features were subjected to TUNEL assay. As shown in Fig. 7, NLE induced morphological apoptotic features such as shrinkage (B), cytoplasmic granulation (C) and cytoplasmic fragmentation (D) and showed TUNEL positive staining as evidenced by dark brown DAB staining. On the other hand, oocytes collected from control animals that had normal morphology with first polar body showed TUNEL negative (methyl green) stain (A).
Fig. 7.
Representative photograph showing the effects of 10 mg/gm dry feed palate of NLE-induced DNA fragmentation in oocytes. (A) control oocyte showing TUNEL negative staining (arrow). (B) NLE-treatment induced DNA fragmentation as evidenced by TUNEL positive staining in oocytes showing shrinkage (arrow), (C) cytoplasmic granulation (arrow) and (D) cytoplasmic fragmentation (arrow). The irregular shape of zona pellucida is due to the proteinase K treatment during TUNEL assay. Bar = 20 μm
Discussion
The aqueous NLE induces apoptosis in rat oocytes cultured in vitro [2]. This result led us to find out whether NLE can induce oocyte apoptosis in vivo. If yes, what is the possible mechanism underlying NLE-induced oocyte apoptosis? Data of present study suggest that NLE (10 mg/g dry palate) treatment for 10 days induced morphological apoptotic features in more than 67% of ovulated oocytes. The remaining 32% oocytes were looking morphologically normal suggesting that the dose (10 mg/g dry feed) and treatment time (10 days) may not be sufficient to induce 100% apoptosis in vivo. However, these oocytes had reduced number and dispersed cumulus cells as compare to control cumulus oocytes complex. Since cumulus cells not only provide nutrients and signaling molecules for survival of the oocytes, they protect oocytes from any adverse changes under vitro conditions. Hence, the reduced number and dispersed cumulus cells may deprive the oocytes from survival factors and induce susceptibility to undergo apoptosis. Culture of these morphologically normal oocytes in plain medium for 3 h in vitro further induced morphological apoptotic features in more than 35% of oocytes. These results suggest that NLE induces both apoptosis in vivo and susceptibility to undergo apoptosis followed by degeneration if cultured in vitro. This possibility is further strengthened by in vitro observations that NLE induced apoptotic features in a concentration-dependent manner, and degeneration was also observed if the oocytes were exposed to higher concentrations of NLE (10 and 20 mg/ml). These data reconfirm our previous finding that NLE induces morphological apoptotic features in rat oocytes cultured in vitro [2]. Further studies are underway to find out the optimal dose and treatment time that induces 100% apoptosis in vivo, which may be helpful to develop a herbal contraceptive.
Quercetin is one of the major bioactive flavonoids of NLE [1]. Hence, we used quercetin as positive control in the present study. Our data suggest that quercetin induced oocyte apoptosis in a concentration-dependent manner in vitro. The higher concentrations (3 and 6 μg/ml) also induced degeneration of oocytes. The quercetin was more potent in inducing apoptotic features as compare to NLE in vitro. These results suggest that the quercetin is one of the major bioactive ingredients associated with NLE-induced oocyte apoptosis in vivo as well as in vitro. Although quercetin induced oocyte apoptosis in vitro has not been reported in any mammalian species till date, concentrations of quercetin used in the present study (1.5 to 6 μg/ml) have been reported to induce apoptosis in various cancer cells cultured in vitro [4, 8–10, 23].
The aqueous NLE contain 6 to 48 mg% (w/w) quercetin [3]. A possibility exist that quercetin might have induced oocyte apoptosis in NLE-treated animals possibly through the generation of ROS as has been reported for various somatic cell types [4, 11, 23, 24]. This possibility is further strengthened by the data of present study that in vivo NLE treatment significantly increased H2O2 and total nitrite concentrations and thereby morphological apoptotic features in oocytes. The increased intracellular levels of ROS have been reported to induce morphological apoptotic features such as shrinkage, membrane blebbing, cytoplasmic granulation and cytoplasmic fragmentation in rat oocytes [25–28].
The increased intracellular level of ROS can modulate mitochondria membrane potential and triggers a release of cytochrome c which initiate apoptotic signals in wide variety of somatic cells [12–17, 23, 29, 30]. Similarly, NLE-induced generation of ROS can modulate mitochondria membrane potential and triggers a release of cytochrome c to initiate apoptotic pathway in oocytes. This notion is further supported by our data that NLE treatment significantly increased cytochrome c concentration in oocyte that had morphological apoptotic features. Although NLE-induced generation of ROS and cytochrome c release in oocyte have not been reported till date, quercetin-induced generation of ROS and cytochrome c release from mitochondria has been reported in various somatic cell types [23, 24, 30]. Taken together, these findings suggest that NLE-induced generation of ROS trigger cytochrome c release from mitochondria that may initiate apoptotic signals in oocytes.
The release of cytochrome c from mitochondria activates upstream caspases in a cell. In the cytosol, cytochrome c binds to apoptotic factor Apaf-1 leading to the recruitment and activation of procaspase-9 [18, 19]. As a result, caspase-9 cleaves procaspase-3 and then caspase-3 cleaves key structural and regulatory proteins that result in the biochemical and morphological changes associated to apoptosis [21]. Similarly, data of the present study suggest that NLE significantly increased both caspase-9 and caspase-3 activities in oocytes collected from NLE-treated animals that had morphological apoptotic features. These data together with previous observations [2] suggest that NLE induces generation of ROS and thereby cytochrome c release from mitochondria that leads to morphological changes associated to oocyte apoptosis.
The DNA fragmentation in 180–200 base-pair DNA ladder is a hallmark feature of apoptosis [31]. The fragmented DNA can be detected in a single cell using in situ technique such as TUNEL assay [15, 32–35]. Data of the present study suggest that NLE induced DNA fragmentation as evidenced by TUNEL positive staining in oocytes that had morphological apoptotic features. These results further confirm our previous finding that NLE induces DNA fragmentation in rat oocytes in vitro [2].
In summary, data of the present study suggest that NLE induced generation of ROS and thereby cytochrome c release from mitochondria of the oocyte. A rise in cytochrome c concentration increased caspase-9 and caspase-3 activities which finally induced DNA fragmentation in oocytes. These results supports our hypothesis that NLE induces generation of ROS and thereby oocyte apoptosis through mitochondria-mediated pathway. However, further studies are required to investigate the involvement of other pathway during NLE-induced oocytes apoptosis.
Acknowledgement
Authors are thankful to Department of Science and Technology, New Delhi, India for financial assistance.
Footnotes
Capsule
Aqueous Neem (Azadirachta indica) leaf extract induces generation of ROS and thereby oocyte apoptosis through mitochondria-mediated pathway
References
- 1.Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anti-Canc Agents. 2005;5:149–56. doi: 10.2174/1568011053174828. [DOI] [PubMed] [Google Scholar]
- 2.Chaube SK, Prasad PV, Khillare B, Shrivastav TG. Extract of Azadirachta indica (Neem) leaf induces apoptosis in rat oocytes cultured in vitro. Fertl Steril. 2006;85:1223–31. doi: 10.1016/j.fertnstert.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 3.Sithisarn P, Gritsanapan W. Variability of antioxidative quercetin content in Siamese neem tree leaves in Thailand by TLC-densitometry. Acta Hort (ISHS) 2008;786:161–9. [Google Scholar]
- 4.Lee YK, Hwang JT, Kwon DY, Surh YJ, Park OJ. Induction of apoptosis by quercetin is mediated through AMPKα1/ask1/p38 pathway. Cancer Lett. 2010;292:228–36. doi: 10.1016/j.canlet.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Jeong JH, An JY, Kwon YT, Rhee JG, Lee YJ. Effects of low dose quercetin: cancer cell-specific inhibition of cell cycle progression. J Cell Biochem. 2009;106:73–82. doi: 10.1002/jcb.21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo PL, Chen CY, Hsu YL. Isoobtusilactone A induces cell cycle arrest and apoptosis through reactive oxygen species/apoptosis signal-regulating kinase 1 signaling pathway in human breast cancer cells. Cancer Res. 2007;67:7406–20. doi: 10.1158/0008-5472.CAN-07-1089. [DOI] [PubMed] [Google Scholar]
- 7.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Update. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Cheng S, Gao N, Zhang Z, Chen G, Budhraja A, Ke Z, Son YO, Wang X, Luo J, Shi X. Quercetin induces tumor-selective apoptosis through downregulation of Mcl-1 and activation of bax. Clin Cancer Res. 2010;16:5679–91. doi: 10.1158/1078-0432.CCR-10-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou CC, Yang JS, Lu HF, Ip SW, Lo C, Wu CC, Lin JP, Tang NY, Chung JG, Chou MJ, Teng YH, Chen DR. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch Pharm Res. 2010;33:1181–91. doi: 10.1007/s12272-010-0808-y. [DOI] [PubMed] [Google Scholar]
- 10.Chien SY, Wu YC, Chung JG, Yang JS, Lu HF, Tsou MF, Wood WG, Kuo SJ, Chen DR. Quercetin-induced apoptosis acts through mitochondrial- and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Hum Exp Toxicol. 2010;28:493–503. doi: 10.1177/0960327109107002. [DOI] [PubMed] [Google Scholar]
- 11.Lee YK, Park SY, Kim YM, Lee WS, Park OJ. AMP kinase/cyclooxygenase-2 pathway regulates proliferation and apoptosis of cancer cells treated with quercetin. Exp Mol Med. 2009;41:201–7. doi: 10.3858/emm.2009.41.3.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun T, Dar S, Vorobiov D, Lindenboim L, Dascal N, Stein R. Expression of Bcl-x(S) in Xenopus oocytes induces BH3-dependent and caspase-dependent cytochrome c release and apoptosis. Mol Cancer Res. 2003;1:186–94. [PubMed] [Google Scholar]
- 13.Johnson CE, Freel CD, Kornbluth S. Features of programmed cell death in intact Xenopus oocytes and early embryos revealed by near-infrared fluorescence and real-time monitoring. Cell Death Differ. 2010;17:170–9. doi: 10.1038/cdd.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–6. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–57. doi: 10.1016/S0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–32. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Li XH, Ma X, Wang ZH, Lu S, Guo YL. Redox-induced apoptosis of human oocytes in resting follicles in vitro. J Soc Gynecol Investig. 2006;13:451–8. doi: 10.1016/j.jsgi.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Acehan D, Jiang X, Morgan DG, Heuser JE, Wang X, Akey CW. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol Cell. 2002;9:423–32. doi: 10.1016/S1097-2765(02)00442-2. [DOI] [PubMed] [Google Scholar]
- 19.Zou H, Li Y, Liu X, Wang X. An APAF-1 cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–56. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 20.Fuentes-Prior P, Salvesen GS. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem J. 2004;384:201–32. doi: 10.1042/BJ20041142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin MC, Allan LA, Lickrish M, Sampson C, Morrice N, Clarke PR. Protein kinase A regulates caspase-9 activation by Apaf-1 downstream of cytochrome c. J Biol Chem. 2005;280:15449–55. doi: 10.1074/jbc.M414325200. [DOI] [PubMed] [Google Scholar]
- 22.Brown GC, Borutaite V. Regulation of apoptosis by the redox state of cytochrome c. Biochem Biophys Acta. 2008;1777:877–1781. doi: 10.1016/j.bbabio.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Priyadarsini RV, Murugan RS, Maitreyi S, Ramalingam K, Karunagaran D, Nagini S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur J Pharmacol. 2010;649:84–91. doi: 10.1016/j.ejphar.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Jung JH, Lee JO, Kim JH, Lee SK, You GY, Park SH, Park JM, Kim EK, Suh PG, An JK, Kim HS. Quercetin suppresses HeLa cell viability via AMPK-induced HSP70 and EGFR down-regulation. J Cell Physiol. 2010;223:408–14. doi: 10.1002/jcp.22049. [DOI] [PubMed] [Google Scholar]
- 25.Chaube SK, Prasad PV, Thakur SC, Shrivastav TG. Hydrogen peroxide modulates meiotic cell cycle and induces morphological features characteristic of apoptosis in rat oocytes in vitro. Apoptosis. 2005;10:863–74. doi: 10.1007/s10495-005-0367-8. [DOI] [PubMed] [Google Scholar]
- 26.Chaube SK, Dubey PK, Mishra SK, Prasad PV, Shrivastav TG. Calcium ionophore-induced egg activation or apoptosis is associated with the generation of intracellular hydrogen peroxide. Free Radic Res. 2008;42:212–20. doi: 10.1080/10715760701868352. [DOI] [PubMed] [Google Scholar]
- 27.Chaube SK, Tripathi A, Khatun S, Mishra SK, Prasad PV, Shrivastav TG. Extracellular calcium protects against verapamil-induced metaphase-II arrest and initiation of apoptosis in aged rat eggs. Cell Biol Intl. 2009;33:337–43. doi: 10.1016/j.cellbi.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Tripathi A, Khatun S, Pandey AN, Mishra SK, Chaube R, Shrivastav TG, Chaube SK. Intracellular levels of hydrogen peroxide and nitric oxide in oocytes at various stages of meiotic cell cycle and apoptosis. Free Radic Res. 2009;43:287–94. doi: 10.1080/10715760802695985. [DOI] [PubMed] [Google Scholar]
- 29.Harish Kumar G, Chandra Mohan KV, Jagannadha Rao A, Nagini S. Nimbolide a limonoid from Azadirachta indica inhibits proliferation and induces apoptosis of human choriocarcinoma (BeWo) cells. Invest New Drugs. 2009;27:246–52. doi: 10.1007/s10637-008-9170-z. [DOI] [PubMed] [Google Scholar]
- 30.Harish Kumar G, Priyadarsini RV, Vinothini G, Vidjaya Letchoumy P, Nagini S. The neem limonoids azadirachtin and nimbolide inhibit cell proliferation and induce apoptosis in an animal model of oral oncogenesis. Invest New Drugs. 2010;28:392–401. doi: 10.1007/s10637-009-9263-3. [DOI] [PubMed] [Google Scholar]
- 31.Jurisicova A, Acton BM. Deadly decisions: the role of genes regulating programmed cell death in human preimplantation embryo development. Reproduction. 2004;128:281–91. doi: 10.1530/rep.1.00241. [DOI] [PubMed] [Google Scholar]
- 32.Ansari B, Coates PJ, Greenstein BD, Hall PA. In situ end-labelling detects DNA strand breaks in apoptosis and other physiological and pathological states. J Pathol. 1993;170:1–8. doi: 10.1002/path.1711700102. [DOI] [PubMed] [Google Scholar]
- 33.Hao Y, Lai L, Mao J, Im GS, Bonk A, Prather RS. Apoptosis in parthenogenetic preimplantation porcine embryos. Biol Reprod. 2004;70:1644–9. doi: 10.1095/biolreprod.103.026005. [DOI] [PubMed] [Google Scholar]
- 34.Henkel R, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, Menkveld R, Gips H, Schill WB, Kruger TF. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertl Steril. 2004;81:965–72. doi: 10.1016/j.fertnstert.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 35.Roth Z, Hansen PJ. Involvement of apoptosis in disruption of developmental competency of bovine oocytes by heat shock during maturation. Biol Reprod. 2004;71:1898–906. doi: 10.1095/biolreprod.104.031690. [DOI] [PubMed] [Google Scholar]