Abstract
Purpose
Men are exposed to various doses of ionizing radiation due to living in regions with high natural background radiation, accidentally, occupationally or for cancer treatment. To study genomic instability of AZFc region to gamma radiation, blood samples from normal, oligozoospermia, and azoospermia individuals were irradiated by a Co-60 source.
Methods
Irradiated cells were kept for 48 h in order to repair initial DNA damages. Real time PCR was performed for three markers (SY 1206, SY 1197, SY 579) for testing copy number variation before and after irradiation. Copy number variations were compared by calculation of cycle threshold comparative method.
Results
Copy number variations of studied markers in AZFc region (microdeletion and duplication) in all samples after exposure to radiation increased with a dose dependent fashion. The frequency of instability was significantly higher in samples from infertile men in comparison with fertile ones (p < 0.001).
Conclusion
No significant difference was seen between the two infertile groups (p > 0.05). This observation might be a possible explanation for induction of azoospermia and oligozoospermia after radiotherapy. Increased frequency of induced microdeletion and duplication in infertile men compared with normal might be attributed to the deficiency in repair systems and the genetic factors involved in incomplete spermatogenesis of infertile men.
Keywords: AZFc instability, Gamma radiation, Infertile men, Leukocytes, Real time PCR
Introduction
Ionizing radiation is a known carcinogen and inducer of chromosomal abnormalities. There are some studies conducted on temporary, permanent infertility and also recurrent abortion which are caused after exposure to gamma radiation. More specifically in this area there is a variety of studies showing very high sensitivity of germinal epithelium to radiation-induced damage, with changes to spermatogenesis process following as little as 0.2 Gy [1]. On the other hand studies in animal models showed doses as low as 0.05 Gy radiation was able to cause the perturbations in the steady-state spermatogenesis of mouse, which was not restored even up to 70 days post irradiation [2].
Other studies showed problems of infertility, as oligoszoopermia and azoospermia, following cancer therapy in childhood and adolescence [3–5]. Moreover, there has been little information about the radiation effects on a chromosome or specific genes located on a chromosome, until recently when some papers are published on the effects of natural background radiation and Cs-137 gamma rays on Y chromosome [6–10]. Actually Y chromosome is a convenient candidate in this regard for its clonal inheritance and being haploid. About 10–15% of Azoospermia and severe-oligozoospermia patients have microdeletion in AZF region of Y chromosome [11]. A great part of microdeletions happen in AZFc region, which includes 80% of them.
It is worth noting that 15–20% of azoospermia and 7–10% of oligozoospermia patients have microdeletions in the AZFc region. There are many genes in this region (namely BPY2, CDY1, CSPG4LY, TTTY3, TTTY4, TTTY17, GOLGAL2Y, and DAZ) from which DAZ gene is the most susceptible candidate for deletion in oligozoospermia and azoospermia males [12].
Regarding the sensitivity of infertile men to exposure of mutagenic agents, Hughes and colleagues studied the damaging effect of roentgenograms and hydrogen peroxide in fertile and infertile men [13]. They showed, in agreement with other studies, that asthenozoospermic men were more prone to these damaging agents compared with normozoospermic infertile men, who in turn presented an increased level of cytogenetic damage compared with fertile men [13, 14]. Papachristou and colleagues [15] have shown increased genomic and chromosomal instability (in terms of chromosome aberrations and sister chromatid exchange, SCE) of infertile men exposed to mytomicin C (MMC) and caffeine.
The effect of radiation on the process of spermatogenesis is well documented [16–18]. Its effects lead to impaired spermatogenesis, fertilization failure, spontaneous abortion and possibly childhood cancer. Radiation as a mutagenic and clastogenic agent causes genomic instability in different ways, like polymorphism as single nucleotide polymorphism (SNP), deletion, duplication and inversion. In a series of investigations sensitivity of genes on Y chromosome of individuals exposed to high natural background radiation and accidental Cs-137 gamma rays have been reported; among which are copy number variation and SNP in SRY gene [7], DYZ1 micro-satellite sequence [6], microdeletion and increasing copy number of AZF region [8–10]. AZFc microdeletion is one of the important factors causing impaired spermatogenesis and susceptibility of this region in exposure to natural background radiation has been shown previously. Therefore, in this study we investigated possible induction of copy number variation in AZFc region following exposure on blood samples from normal, oligozoospermia, and azoospermia males to gamma radiation, by the use of real time PCR. Several techniques have been used to detect copy number variation in AZFc region such as short fragment variant (SFV), fiber-FISH or Southern blotting. These methods have shown limitations for example problems in interpreting results obtained by SFV [19–21], time consuming FISH method [20] or difficult to set up of southern blot and quantities of genetic material needing in this method [21].
Real time PCR is shown to be a valid and appropriate method for copy number variation studies [22]. Real time PCR is easy to adjust, highly reproducible, and can be used on large number of samples as well as having several others advantages compared to conventional methods.
Material and methods
Donors
Blood donors were divided into three groups according to the World Health Organization criteria [23]. Mean sperm count for normal and oligozoospermia males was 50 × 106 ± 3.1 (47–51× 106) and 15 × 106 ± 2.4 (11–17 × 106) respectively. Men with no spermatozoa in their semen were considered as azoospermia. Mean ages of these individuals were 35.1 ± 3.7, 34.4 ± 4.1, and 34.9 ± 3.9 respectively; and each group consists of 10 donors. Normal samples were obtained from men referring to the infertility clinic because of infertility problems of their spouse. They themselves had no indications of infertility problem. Also all patients were new cases and samples were obtained from them at their first visit. The study was approved by the Ethical Committee of the Faculty of Medical Sciences of Tarbiat Modares University (Tehran, Iran). Patients gave their informed written consent too. All donors completed a written questionnaire to obtain information related to their life style, such as dietary habits, medical history and exposure to chemical and physical agents. Therefore to limit confounding factors and be sure of the effects seen on samples is due to ionizing radiation, all samples had been screened to exclude previous radiation exposure, smoker, varicocele, genital tract infections, hepatitis, and HIV.
Irradiation of blood samples and DNA extraction
Whole blood samples, in ethylene diamine tetraacetic acid (EDTA) as anticoagulant, were irradiated to 2 and 4 Gy gamma-rays generated from a Co-60 source (Theratron II, 780C, AECL, Kanata, Canada) with a dose rate of 1.23 Gy/min. After irradiation RPMI-1640 supplemented with fetal calf serum (FCS, 15%, Gibco BRL) was add to blood cells and left in a CO2 incubator (5% CO2 in air) for 48 h to repair initial DNA damage. Samples were washed with 1% phosphate buffer saline (PBS) three times, and then DNA was extracted from peripheral blood using DNA extraction Kit (Diatom DNA Prep-100, Genefanavaran, Tehran, Iran). The concentration of isolated DNA was measured using Nano Drop 2000c (Thermo, USA) spectrophotometer at 280 nm, and finally DNA samples with A260/A280 ratios of more than 1.5 were selected for quantitative analysis.
Real-time PCR and comparative threshold cycle method
Beta actin gene was used as an internal control for analysis of the copy number variation in this experiment. Three markers in AZFc region SY1197, SY1206, SY 579 (DAZ genes) (Fig. 1) and beta actin gene were amplified with appropriate primers (Table 1). All primers were designed using gene runner software. SYBR Green I real time PCR assay was carried out in final reaction volumes of 20 μl with 10 μl of SYBR Green I master mix (Takara, Shiga, Japan), 200 nM of forward and reverse primers and 10 ng of genomic DNA. Thermal cycling was performed on the ABI-7500 (Applied Biosystem, USA) sequence detection system using the following cycling condition: 30 s at 95°C as first denaturation step, followed by 40 cycles at 95°C for 5 s and 60°C for 34 s. Each complete amplification stage was followed by dissociation stage; at 95°C for 15 s, 60°C for 1 min and 95°C for 15 s.
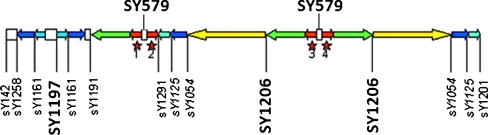
Fig. 1.
AZFc region is located in repeated DNA blocks (amplicons), different markers present in this region. The figure is showing STS markers selected for study in this region. The selected STS markers include SY 1206 and SY 1197 markers used as hot spots to radiation and SY579 marker as a possible unstable marker in DAZ gene
Table 1.
The oligonucleotid primers used in real time PCR assay
| STS markers and internal control gene | Primer | Sequence | Amplification size (bp) |
|---|---|---|---|
| SY 1197 | F | TCATTTGTGTCCTTCTCTTGG CTTAAAGTTGCCTGGTTATCTC | 224 |
| SY 1197 | R | ||
| SY 1206 | F | AACTCTTCCTTACCCTTGGTC CTAAAGTCAACCTGAGCCTTC | 240 |
| SY 1206 | R | ||
| SY579 | F | GACAACACCACCGTACTCCAG GGAAGGAAGTGGAGGGAAAC | 130 |
| SY579 | R | ||
| Beta actin | F | GCTTTTAGGTTTGACCCATCC TCACTTCTTTCACTGAACCG | 250 |
| Beta actin | R |
Optimization of real-time PCR assay
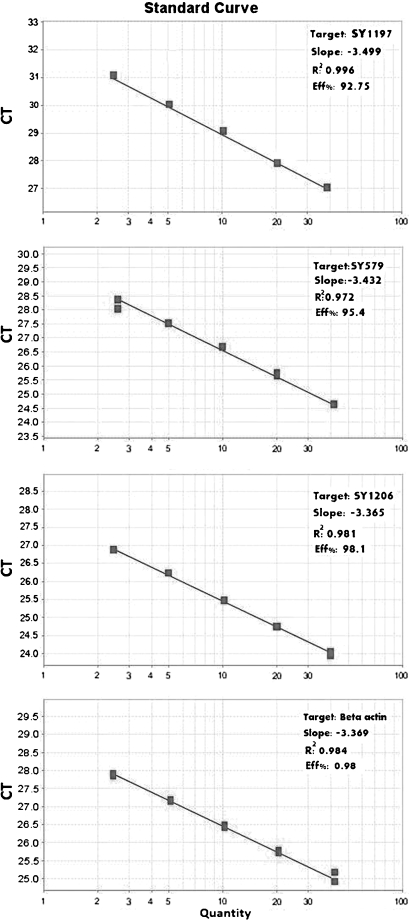
The efficiency of each marker was determined by using two fold series of normal genomic DNA dilutions. Dissociation curve analysis was performed for each amplification reaction to observe the presence of any possible primer dimmer or non-specifically amplified product formation. Validation of experiments was performed to determine the PCR efficiencies of target and reference gene. The slope of the fit lines were within the acceptable range of −3.6 < slope <−3.1 (Fig. 2). The PCR efficiencies of the targets and reference gene were approximately equal, indicating validity of the comparative threshold cycle method for copy number variation assay. Results of melt curve analysis showed that no detectable non-specific products was present in the reaction; and for each amplicon, gel electrophoresis analysis of PCR products revealed a single band with expected length as well.
Fig. 2.
Standard curves showing PCR efficiencies of target and reference genes as well as the slope of the fit lines within the acceptable range of −3.6 < slope <−3.1
Each assay was repeated at least two times before drawing any conclusion. Calculation of the gene copy number variation was carried out using comparative threshold cycle as described by Livak and Schmittgen (2001) [24]. Briefly, the mean threshold cycle mCT (mean cycle threshold) was obtained from duplicate amplification during the exponential phase of amplification. Then mCT value of reference gene (Beta actin) was subtracted from mCT value of target markers (SY1197, SY1206, SY 579) to obtain ΔCT. Subsequently, a spreadsheet was designed so that ΔCT and ΔΔCT values of each sample could be calculated from corresponding CT values; where 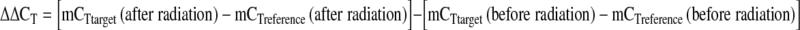 . Finally this ΔΔCT was converted to ratio using the ratio formula
. Finally this ΔΔCT was converted to ratio using the ratio formula 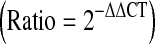 .
.
Statistical analysis
To determine the significant difference between studied groups, statistical analysis including mean, standard deviation (SD), correlation coefficients (R2) using SPSS (version 17) soft ware (SPSS Inc., Chicago, IL, USA), one way analysis of variance (ANOVA) as well as unpaired t-test analysis were carried out. P-value of <0.05 was considered as statistically significant.
Results
Copy number variation analysis of STS markers
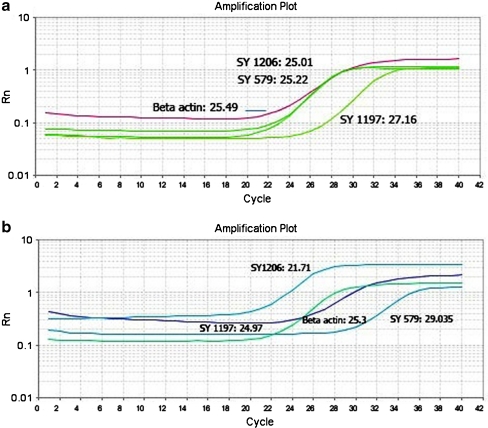
To validate copy number of STS markers on the basis of samples before irradiation, two normal samples were used as calibrator and the gene dosage ratios of markers before radiation were determined relative to the mean ΔCT value of these samples (Table 2). All samples in three studied groups (n = 30) showed the ratio of about 1 for three markers of (SY1197, SY1206, SY579), indicating that all samples had no microdeletion and/or amplification before exposure to radiation. This result suggests the cut off value of 1 for discrimination copy number variation of markers before and after exposure to radiation. Results of ΔΔCT method showed ratio of 1 (no change), <1 (microdeletion), >1 (amplification) in normal, oligozoospermia and azoospermia samples (Table 3). An example of amplification plot and CT value of Beta actin, SY 1197, SY 1206, and SY 579 markers in (A6) sample are shown in Fig. 3a (before irradiation) and Fig. 3b (after exposure to radiation).
Table 2.
Validation of copy number for STS markers on the basis of samples before gamma-irradiation in 3 study groups (normal, oligozoospermia and azoospermia)
| STS markers | Studied groups | Ratio (2−ΔΔCT) |
|---|---|---|
| SY1197 | normal | 1.06 ± 0.1 |
| oligosperm | 1.17 ± 0.14 | |
| azoosperm | 1.05 ± 0.12 | |
| SY1206 | normal | 1.12 ± 0.12 |
| oligosperm | 1.09 ± 0.1 | |
| azoosperm | 1.02 ± 0.1 | |
| SY579 | normal | 1.1 ± 0.1 |
| oligosperm | 1.12 ± 0.11 | |
| azoosperm | 1.1 ± 0.08 |
Table 3.
Results of ΔΔCT calculations to detect STS markers copy number variation after exposure to different doses of radiation. *denotes for amplification and °denotes for microdeletion
| Normal | STS markers | 2−ΔΔCT 2Gy | 2−ΔΔCT 4Gy | Oligo | STS markers | 2−ΔΔCT 2Gy | 2−ΔΔCT 4Gy | Azoo | STS markers | 2−ΔΔCT 2Gy | 2−ΔΔCT 4Gy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | SY1197 | 1.02 | 1.1 | O1 | SY1197 | 1.17 | 3.7* | A1 | SY1197 | 0.99 | 7.1* |
| SY1206 | 1.1 | 1.18 | SY1206 | 1.15 | °0.06 | SY1206 | 1.1 | 12.59* | |||
| SY579 | 1.14 | 1.13 | SY579 | °0.35 | 0. 13° | SY579 | 0.995 | 3.55* | |||
| N2 | SY1197 | 1.12 | 1.25 | O2 | SY1197 | 0.22° | 1.23 | A2 | SY1197 | 1.3 | 0.18° |
| SY1206 | 1.06 | 1.18 | SY1206 | 1.2 | 4.02* | SY1206 | 0.031° | 0.17° | |||
| SY579 | 1.12 | 1.17 | SY579 | 1.12 | 1.15 | SY579 | 1.06 | 1.16 | |||
| N3 | SY1197 | 0.99 | 1.12 | O3 | SY1197 | 1.14 | 1.2 | A3 | SY1197 | 1.12 | 1.2 |
| SY1206 | 1.15 | 1.13 | SY1206 | 1.12 | 1.2 | SY1206 | 2.8* | 0.019° | |||
| SY579 | 1.2 | 4.02* | SY579 | 8.1* | 9.71* | SY579 | 0.17° | 0.02° | |||
| N4 | SY1197 | 1 | 1.01 | O4 | SY1197 | 5.8* | 6.4* | A4 | SY1197 | 1.1 | 1.04 |
| SY1206 | 1.18 | 4.62* | SY1206 | 1 | 1.33 | SY1206 | 1.12 | 1.16 | |||
| SY579 | 1.11 | 1.3 | SY579 | 1.17 | 1.07 | SY579 | 0.997 | 3.56* | |||
| N5 | SY1197 | 1.01 | 1.1 | O5 | SY1197 | 1.13 | 13.4* | A5 | SY1197 | 0.983 | 0.38° |
| SY1206 | 1.2 | 1 | SY1206 | 5.02* | 2.89* | SY1206 | 1.2 | 3.1* | |||
| SY579 | 1.16 | 1.18 | SY579 | 1.15 | 7.72* | SY579 | 1.1 | 1.21 | |||
| N6 | SY1197 | 1.11 | 4.56* | O6 | SY1197 | 1.2 | 13.45* | A6 | SY1197 | 1.13 | 4* |
| SY1206 | 0.99 | 5.7* | SY1206 | 3.71* | 0.091° | SY1206 | 1.17 | 8.63* | |||
| SY579 | 1.13 | 1.17 | SY579 | 1.2 | 7.72* | SY579 | 1.1 | 0.06° | |||
| N7 | SY1197 | 1.25 | 0.078° | O7 | SY1197 | 1.2 | 4.09* | A7 | SY1197 | 2.1* | 8* |
| SY1206 | 2.85* | 2.44* | SY1206 | 1.1 | 1.25 | SY1206 | 1.1 | 1.16 | |||
| SY579 | 1.17 | 1.23 | SY579 | 1.01 | 1.31 | SY579 | 1.12 | 1.09 | |||
| N8 | SY1197 | 1.19 | 1.11 | O8 | SY1197 | 2.15* | 0.03° | A8 | SY1197 | 4.4* | 8.5* |
| SY1206 | 1.13 | 0.04° | SY1206 | 1.33 | 1.17 | SY1206 | 1.11 | 1.25 | |||
| SY579 | 1.02 | 1.1 | SY579 | 1.07 | 2.741* | SY579 | 0.991 | 1.18 | |||
| N9 | SY1197 | 1.15 | 1.055 | O9 | SY1197 | 1.3 | 0.17° | A9 | SY1197 | 1.08 | 3.96* |
| SY1206 | 1.14 | 1.08 | SY1206 | 2.89* | 2.86* | SY1206 | 3.1* | 1.25 | |||
| SY579 | 0.997 | 1.15 | SY579 | 1.05 | 1.24 | SY579 | 1.2 | 3.5* | |||
| N10 | SY1197 | 1.12 | 3.36* | O10 | SY1197 | 0.992 | 1.09 | A10 | SY1197 | 2.1* | 1.03 |
| SY1206 | 1.13 | 1.16 | SY1206 | 1.14 | 1.23 | SY1206 | 1.31 | 1.07 | |||
| SY579 | 5.22* | 2.71* | SY579 | 1.01 | 1.27 | SY579 | 1.2 | 3.151* |
Fig. 3.
Amplification plot and CT value of SY 1197, 1206 and 579 markers before radiation (a) and after exposure to 4 Gy radiation (b) in a typical sample (A6). This sample had amplification in SY 1197, 1206 markers and microdeletion in SY579 marker
Frequency of microdeletion and amplification in studied groups
After irradiation of blood samples obtained from three groups for study, the frequency of microdeletion and amplification increased as a dose dependent manner (Table 4, Fig. 3). As seen, the frequency of both microdeletion and amplification increases when radiation dose increases from 2 Gy to 4 Gy for all study groups. The frequency of amplification was found higher than microdeletion for all studied STS markers. Normal samples showed no microdeletion after 2 Gy irradiation in three markers but, with the increase of radiation dose to 4 Gy the frequency of microdeletion increased to 6.6%. Amplification frequencies in these samples after 2 Gy and 4 Gy were 6.6% and 23.3% respectively. On the other hand, in oligozoospermia samples microdeletion and amplification frequencies after 2 Gy were 6.6% and 20%, but these changes increased following the dose of 4 Gy to 16.6% and 40% respectively.
Table 4.
Total frequency of AZFc microdeletion and amplification in three studied groups after 4 Gy or 2 Gy (values indicated in brackets) gamma radiation
| Groups | Number of samples with STS marker deletion | Frequency of microdeletion in AZFc region | Number of samples with STS marker amplification | Frequency of amplification in AZFc region | Frequency of microdeletion and amplification in AZFc region |
|---|---|---|---|---|---|
| Normal | SY1197: 1 (0) | SY1197: 2 (0) | |||
| SY1206: 1 (0) | 2/30 = 6.6% | SY1206: 3 (1) | 7/30 = 23.3% | 9/30 = 30% | |
| SY579: 0 (0) | (0/30 = 0%) | SY579: 2 (1) | (2/30 = 6.6%) | (2/30 = 6.6%) | |
| Oligosperm | SY1197: 2 (1) | SY1197: 5 (2) | |||
| SY1206: 2 (0) | 5/30 = 16.6% | SY1206: 3 (3) | 12/30 = 40% | 17/30 = 56.6%ab | |
| SY579: 1 (1) | (2/30 = 6.6%) | SY579: 4 (1) | (6/30 = 20%) | (8/30 = 26.6%)ab | |
| Azoosperm | SY1197: 2 (0) | SY1197: 5 (3) | |||
| SY1206: 2 (1) | 6/30 = 20% | SY1206: 3 (2) | 12/30 = 40% | 18/30 = 60%ab | |
| SY579: 2 (1) | (2/30 = 6.6%) | SY579: 4 (0) | (5/30 = 16.6%) | (7/30 = 23.3%)ab |
aSignificantly different from normal group (p < 0.01 for 4 Gy and p < 0.05 for 2 Gy)
bNot statistically significant between oligozoospermia and azoospermia groups (p > 0.05)
In azoospermia patients the frequency of microdeletion in three markers after 2 Gy was 6.6% but increased to 20% after 4 Gy; and also amplification frequencies after 2 and 4 Gy were 16.6% and 40%.
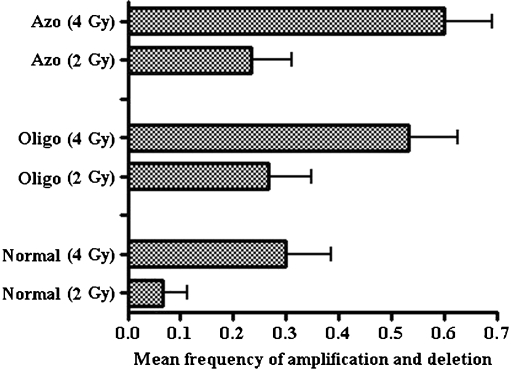
Totally, the frequencies of instability in AZFc region (microdeletion and amplification) for three markers (i.e. SY1197, SY1206, SY579) in normal, oligozoospermia and azoospermia samples were 6.6%, 26.6%, and 23.6% after 2 Gy and 30%, 56%, and 60% after 4 Gy respectively (Fig. 4). The mean frequency of total copy number variation (microdeletion and amplification) between normal and infertile patients for both 2 and 4 Gy gamma irradiation (P < 0.05 and p < 0.01) was statistically significant (see Fig. 4), but this difference was not statistically significant between oligozoospermia and azoospermia individuals (Table 4).
Fig. 4.
Mean total frequency of microdeletion and amplification observed in three study groups (normal, oligozoospermia and azoospermia) following different doses (2 and 4 Gy) of radiation. Error bars show standard deviation of mean values
Discussion
Exposure of mammalian germ cells to ionizing radiation can cause impaired spermatogenesis; and in human populations such effects would cause male infertility [25]. The genetic mechanism of infertility in these individuals is yet unknown; although the effect of ionizing radiation on reproduction system of men is attributed to mitotic and apoptotic death of spermatogonia and spermatocyte cells [26]. On the other hand most of the studies in this regard have been done on experimental models and few studies have examined infertility relative to low level ionizing irradiation in in vivo system. This is partly because infertility is difficult to measure and definitions vary widely and partly because exposure to artificial sources of ionizing irradiation is rare and investigations have been limited to specific groups. Therefore in this study we have investigated the effects of gamma radiation on genomic instability of AZFc region on Y chromosome in in vitro system because of importance of this region in male infertility. Some studies have investigated instability of AZFc region of Y chromosome in exposure to high natural background radiation and gamma rays generated from Cs-137 source [8, 10]. These studies show instability of this region as microdeletion in some proximal and distal markers, and copy number polymorphism of DAZ gene as amplification.
Generally speaking, many patients may be exposed to diagnostic or therapeutic radiation in childhood or early adulthood, and also abdominal and pelvic irradiation plays an important role in modern treatment modalities of locally advanced cancers such as prostate, testicular or rectal. As a result different degrees of infertility have been reported in these patients depending on radiotherapy doses, abdominal and pelvic radiation, etc. Radiotherapy patients receive 2 Gy at each treatment fraction; however, mean cumulative radiation exposure to the testicles is shown to be about 3.56 Gy [27, 28] therefore we chose both 2 and 4 Gy doses to study genomic instability of Y chromosome.
In previous study proximal and distal markers of AZFc region has introduced as hot spot in exposure to background radiation, so, because of amplification of DAZ gene (in center of AZFc region) following exposure to background radiation, we used (SY1197) and (SY1206) markers as proximal and distal markers of AZFc region and SY 579 marker in DAZ gene as well [8].
Our results showed instability of this region as microdeletion and amplification in proximal (SY1197) and distal (SY1206) markers of AZFc region and SY579 marker in DAZ gene (Table 3). Therefore the whole AZFc region is affected by radiation because of the presence of these markers in proximal, center, and distal region of AZFc. These observations are indicative of non random intrachromosomal variations. Therefore is not in line with the suggestion made by Snjay et al. (2007) for random intrachromosomal breakage as a probable mechanism for radiation induced microdeletion [8]. Statistical significant difference in the frequency of instability in lymphocytes of infertile individuals compared to normal males was seen after both 2 and 4 Gy gamma irradiation (Fig. 4 and Table 4). This observation is in agreement with the results reported regarding susceptibility of infertile men compared to normal individuals to mutagenic agents [13–15, 29].
Among three markers, after 4 Gy gamma exposures, SY1197 was more vulnerable than others; because the frequency of instability in this marker, in 3 studied groups, was 56.6% but for SY1206 and SY579 was 46.6% and 43.3% respectively. In the report by Snjay et al., [8] AZFc instability and copy number variation of DAZ gene after exposure to natural background radiation was shown in the blood DNA samples, but they noticed no change in the germ line samples. Therefore, they suggested that germ cells are either putatively protected by some innate mechanism or the affected germ cells do not survive [8]. Another study showed mutations in loci linked to Y chromosome (AZF region) in the offspring born to individuals accidentally exposed to cesium-137, suggesting that doses higher than background radiation can affect germ cells [9, 10]. The instability induced in AZFc region in blood samples (Fig. 4 and Table 4) with doses of 2 and 4 Gy, might also affect genome instability in sperms and germ cells with similar radiation doses. Increased apoptosis as well as mitotic death due to chromosomal aberrations is likely responsible for progressive regression of spermatogenic potential, especially in irradiated persons [26]. Recently Yamada et al. [30] have shown that increased apoptosis of germ cells is responsible for progressive decline of spermatogenic potential in patients with AZFc deletions. Therefore, instability of genes in the AZFc region might imply a possible mechanism leading to azoospermia and oligozoospermia after radiotherapy [30].
The susceptibility of AZFc region to radiation exposure might be due to repeated DNA blocks (amplicons) and highly recombinogenic locus. Deletions and amplification represent one of the most functionally relevant structural rearrangements in AZFc, under normal conditions, which are essentially the product of homologous recombination and microdeletion in this region; which is one of the causes of male infertility.
Recombination is triggered by the generation of a DNA double strand break (DSB) within an amplicon. The occurrence of such lesions are particularly frequent in the male germ line, owing to the fact that spermatogenesis requires multiple cell divisions in an oxidative environment with depleted DNA repair enzymes [31]. The DSB generated by intrinsic mechanisms or exogenously, are known as principal DNA lesions causing adverse biological effects in cells exposed to ionizing radiations. The biological consequences of exposure to ionizing radiation include gene mutation, chromosome aberrations, cellular transformation and cell death [16]. These effects are attributed to the DNA-damaging effects of ionizing radiation which results in irreversible changes during DNA replication or during the enzymatic repair processes of the DNA damage. Initially, a 4-Gy dose of ionizing radiation produces approximately 120–160 double-strands DNA breaks (DSB), 1000–2000 single-strand DNA breaks, and a similar number of base damaging events in the cell [32]. Some of these occur very soon after radiation exposure; if left unrepaired may result in alterations affecting the maintenance of chromosome stability.
It is proposed that DSBs or SSBs induced in the DNA, by endogenous processes or exogenous agents (such as ionizing radiation), can in principle be repaired either by non-homologous end joining (NHEJ) or homology directed repair (HDR) [33]. Studies have also shown that elevated rate of DNA nicks and double strand breaks in sperm of infertile men could lead to infertility and 50% of miscarriages; this means that this individuals have a background genetic instability that can be caused by their inability to repair DNA damage and are susceptible to mutagenic and clastogenic agents [34–37]. However, even if DNA damage is repaired in repair proficient cells, signaling of a single DSB triggers the cells to make a genomic rearrangement at the crossover points of a looped chromatin domain, possibly a transcription factory [38, 39].
Intrachromosomal recombination events can also occur between elements that are located apart on the chromosome, leading to large deletions or duplications. Both mitotic and meiotic recombination events can be stimulated by agents that produce DSBs. Different pathways involved in the repair of radiation induced DSBs and small sequence deletions or additions are introduced during the repair process [40].
In this study we illustrated that susceptibility and instability of AZFc region in lymphocytes of infertile men exposed to radiation is more than in normal males. One of the reasons for higher instability of AZFc region of infertile male might be deficiency in repair system so that genes that encode proteins and tightly regulate the recombination of genetic material and the repair of DNA have mutations, and this leads to infertility characterized by arrest in meiosis I at a meiotic checkpoint [41, 42].
Acknowledgements
This work was supported by Research Department of the Faculty of Medical Sciences of Tarbiat Modares University, Tehran, Iran. The authors wish to express their sincere thanks to all patients and healthy individuals for their blood donation and kind contribution and Ms Zahra Tizmaghz for irradiation of samples.
Conflict of Interest statement The authors declare that there are no conflicts of interest.
Footnotes
Capsule
Lymphocytes from infertile individuals showed higher frequency of genome instability in AZFc region on Y chromosome after gamma irradiation compared to normal controls.
References
- 1.Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Canc Inst Monogr. 2005;34:12–17. doi: 10.1093/jncimonographs/lgi003. [DOI] [PubMed] [Google Scholar]
- 2.Gagetia GC, Krishnamurthy H. Effect of low doses of gamma-radiation on the steady-state spermatogenesis of mouse: a flow-cytometric study. Mutat Res. 1995;332:97–107. doi: 10.1016/0027-5107(95)00158-8. [DOI] [PubMed] [Google Scholar]
- 3.Ash P. The influence of radiation on fertility in man. Br J Radiol. 1980;53:271–278. doi: 10.1259/0007-1285-53-628-271. [DOI] [PubMed] [Google Scholar]
- 4.Meirow D, Schenker JG. Cancer and male infertility. Hum Reprod. 1995;10:2017–2022. doi: 10.1093/oxfordjournals.humrep.a136228. [DOI] [PubMed] [Google Scholar]
- 5.Kuczyk M, Machtens S, Bokemeyer C, Schultheiss D, Jonas U. Sexual function and fertility after treatment of testicular cancer. Curr Opin Urol. 2000;10:473–477. doi: 10.1097/00042307-200009000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Deepali P, Snjay P, Jyoti S, Sebastian PC, Sher A. Genomic instability of the DYZ1 repeat in patient with Y chromosome anomalies and males exposed to natural background radiation. DNA Res. 2006;13:103–109. doi: 10.1093/dnares/dsl002. [DOI] [PubMed] [Google Scholar]
- 7.Snjay P, Jyoti S, Sebastian PC, Ahamd J, Sher A. Tandem duplication and copy number polymorphism of the SRY gene in patients with sex chromosome anomalies and males exposed to natural background radiation. Mol Hum Reprod. 2006;12:113–121. doi: 10.1093/molehr/gal012. [DOI] [PubMed] [Google Scholar]
- 8.Snjay P, Jyoti S, Sebastian PC, Sher A. AZFc somatic microdeletions and copy number polymorphism of the DAZ genes in human males exposed to natural background radiation. Hum Genet. 2007;121:337–346. doi: 10.1007/s00439-006-0318-7. [DOI] [PubMed] [Google Scholar]
- 9.Arruda JT, Silva DM, Silva CC, Moura KKVO, da-Cruz AD. Homologous recombination between HERVs causes duplications in the AZFa region of men accidentally exposed to cesium-137 in Goiânia. Genet Mol Res. 2008;7:1063–1069. doi: 10.4238/vol7-4gmr492. [DOI] [PubMed] [Google Scholar]
- 10.Arruda JT. Occurrence of mutations in loci linked to Y chromosome in the offspring born to individuals exposed to ionizing radiation. Genet Mol Res. 2009;8:938. doi: 10.4238/vol8-3ta021. [DOI] [Google Scholar]
- 11.Reijo R, Algappan RK, Patrizio P, Page DC. Severe oligospermia resulting from deletion of azoospermia factor gene on Y chromosome. Lancet. 1996;347:1290–1293. doi: 10.1016/S0140-6736(96)90938-1. [DOI] [PubMed] [Google Scholar]
- 12.McElreavey K, Krausz C, Bishop CE. The human Y chromosome and male infertility. Results Probl Cell Differ. 2000;28:2211–2232. doi: 10.1007/978-3-540-48461-5_9. [DOI] [PubMed] [Google Scholar]
- 13.Hughes CM, Lewis SE, McKelvey-Martin VJ, Thompson W. A comparison of baseline and induced DNA damage in human spermatozoa from fertile and infertile men, using a modified comet assay. Mol Hum Reprod. 1996;2:613–619. doi: 10.1093/molehr/2.8.613. [DOI] [PubMed] [Google Scholar]
- 14.Pasqualotto FF, Sharma RK, Kobayashi H, Nelson DR, Thomas AJ, Jr-Agarwal A. Oxidative stress in normospermic men undergoing infertility evaluation. J Androl. 2001;22:316–322. [PubMed] [Google Scholar]
- 15.Papachristou F, Simopoulou M, Touloupidis S. DNA damage and chromosomal aberrations in various types of male factor infertility. Fertil Steril. 2008;9:1774–1781. doi: 10.1016/j.fertnstert.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Hall EJ. Radiobiology for the radiologist. 5. New York: Lippincott Williams and Wilkins; 2000. [Google Scholar]
- 17.Mozdarani H, Salimi M. Numerical chromosome abnormalities in 8-cell embryos generated from gamma-irradiated male mice in the absence and presence of vitamin E. Int J Radiat Biol. 2006;82:817–822. doi: 10.1080/09553000600973343. [DOI] [PubMed] [Google Scholar]
- 18.Mozdarani H, Nazari E. Cytogenetic damage in preimplantation mouse embryos generated after paternal and parental gamma-irradiation and the influence of vitamin C. Reproduction. 2009;137:35–43. doi: 10.1530/REP-08-0073. [DOI] [PubMed] [Google Scholar]
- 19.Repping S, de-Vries JW, Van-Daalen SK, Korver CM, Leschot NJ, Van-Der-Veen F. The use of sperm HALO-FISH to determine DAZ gene copy number. Mol Hum Reprod. 2003;9:183–188. doi: 10.1093/molehr/gag032. [DOI] [PubMed] [Google Scholar]
- 20.De-Vries JW, Hoffer MJV, Repping S, Hoovers JMN, Leschot NJ, Van-Der-Veen F. Reduced copy number of DAZ genes in subfertile and infertile men. Fertil Steril. 2002;77:68–75. doi: 10.1016/S0015-0282(01)02935-1. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes S, Huellen K, Goncalves J, Dukal H, Zeisler J, Rajpert-De-Meyts E, Skakkebaek NE, Habermann B, Krause W, Sousa M, Barros A, Vogt PH. High frequency of DAZ1/DAZ2 gene deletions in patients with severe oligozoospermia. Mol Hum Reprod. 2002;8:286–298. doi: 10.1093/molehr/8.3.286. [DOI] [PubMed] [Google Scholar]
- 22.Rozé V, Bresson J, Luc-Fellmann F. Quantitative PCR technique for the identification of microrearrangements of the AZFc region. J Assist Reprod Genet. 2007;24:241–248. doi: 10.1007/s10815-006-9055-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO manual for the standardized investigation and diagnosis of the infertile couple. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Neel JV, Lewis SE. The comparative radiation genetics of humans and mice. Annu Rev Genet. 1990;24:327–362. doi: 10.1146/annurev.ge.24.120190.001551. [DOI] [PubMed] [Google Scholar]
- 26.Meistrich ML. Male gonadal toxicity. Pediatr Blood Canc. 2009;53:261–266. doi: 10.1002/pbc.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colpi GM, Contalbi GF, Nerva F, Sagone P, Piediferro G. Testicular function following chemo–radiotherapy. Eur J Obstet Gynecol Reprod Biol. 2004;113S:S2–S6. doi: 10.1016/j.ejogrb.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Hermann RM, Henkel K, Christiansen H, Vorwerk H, Hille A. Testicular dose and hormonal changes after radiotherapy of rectal cancer. Radiother Oncol. 2005;75:83–88. doi: 10.1016/j.radonc.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 29.Papachristou F, Lialiaris T, Touloupidis S, Kalaitzis C, Simopoulos C, Sofikitis N. Evidence of increased chromosomal instability in infertile males after exposure to mitomycin C and caffeine. Asian J Androl. 2006;8:199–204. doi: 10.1111/j.1745-7262.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- 30.Yamada K, Fujita K, Quan J, Sekine M, Kashima K, Yahata T, Tanaka KJ. Increased apoptosis of germ cells in patients with AZFc deletions. J Assist Reprod Genet. 2010;27:293–297. doi: 10.1007/s10815-010-9400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crow JF. The origins, patterns and implications of human spontaneous mutation. Nat Rev Genet. 2000;1:40–47. doi: 10.1038/35049558. [DOI] [PubMed] [Google Scholar]
- 32.Wyman C, Kanaar R. DNA double-strand break repair: all’s well that ends well. Annu Rev Genet. 2006;40:363–383. doi: 10.1146/annurev.genet.40.110405.090451. [DOI] [PubMed] [Google Scholar]
- 33.Gasior SL, Wakeman TP, Xu B, Deininger PL. The human LINE-1 retrotransposon creates DNA double-strand breaks. J Mol Biol. 2006;357:1383–1393. doi: 10.1016/j.jmb.2006.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahowald GK, Baron JM, Sleckman BP. Collateral damage from antigen receptor gene diversification. Cell. 2008;135:1009–1012. doi: 10.1016/j.cell.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 35.Nili HA, Mozdarani H, Aleyasin A. Correlation of sperm DNA damage with protamine deficiency in Iranian subfertile men. Reprod BioMedicine Online. 2009;184:479–485. doi: 10.1016/S1472-6483(10)60123-X. [DOI] [PubMed] [Google Scholar]
- 36.Falk M, Lukasova E, Kozubek S. Higher-order chromatin structure in DSB induction repair and misrepair. Mutat Res. 2010;704:88–100. doi: 10.1016/j.mrrev.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Moskovtsev SI, Mullen JB, Lecker I, Jarvi K, White J, Roberts M. Frequency and severity of sperm DNA damage in patient with confirmed cases of male infertility of different etiology. Reprod BioMedicine Online. 2010;20:759–763. doi: 10.1016/j.rbmo.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Bryant PE. The signal model a possible explanation for the conversion of DNA double strand breaks in to chromatid breaks. Int J Radiat Biol. 1998;73:243–251. doi: 10.1080/095530098142338. [DOI] [PubMed] [Google Scholar]
- 39.Bryant PE, Mozdarani H. Mechanisms underlying the conversion of DNA double strand breaks in to chromatid breaks. Int J Low Radiat. 2004;1:223–230. doi: 10.1504/IJLR.2004.003874. [DOI] [Google Scholar]
- 40.Sankaranarayanan K, Wassom JS. Ionizing radiation and genetic risks XIV. Potential research directions in the post-genome era based on knowledge of repair of radiation-induced DNA double-strand breaks in mammalian somatic cells and the origin of deletions associated with human genomic disorders. Mutat Res. 2005;578:333–370. doi: 10.1016/j.mrfmmm.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 41.Baker S, Plug A, Prolla T, Bronner C, Harris A, Yao X, Christie D, Monell C, Arnheim N, Bradley A, Ashley T, Liskay R. Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat Genet. 1996;13:336–342. doi: 10.1038/ng0796-336. [DOI] [PubMed] [Google Scholar]
- 42.Mark S, Renee A, Reijo P. Male infertility, genetic analysis of the DAZ on Y chromosome and genetic analysis of DNA repair. Mol Cell Endocrinol. 2001;184:41–49. doi: 10.1016/S0303-7207(01)00646-3. [DOI] [PubMed] [Google Scholar]