Abstract
Objective
To compare cytogenetic data of first-trimester missed abortions in intracytoplasmic sperm injection (ICSI) for non-male factor-mediated and spontaneous pregnancies.
Methods
Using karyotype analysis, we conducted a retrospective cohort trial of missed abortions following ICSI for non-male factor and spontaneous pregnancies. Patients experienced missed abortions during the first 12 weeks of pregnancy. Dilation and curettage procedure was performed followed by cytogenetic evaluations. Two patient groups were created: ICSI (n = 71) and spontaneous pregnancies (n = 81). At least 20 GTG-banded metaphases were analyzed in each case for cytogenetic analyses. Statistical analyses were performed using NCSS 2007 Statistical Program software. The significance level and confidence interval for all analyses were set to p < 0.05 and a 95% confidence interval, respectively.
Results
A total of 49.3% (75/152) of the miscarriages were cytogenetically abnormal among the patients. We detected cytogenetically abnormalities in 47.9% (34/71) of the ICSI group and 50.6% (41/81) of the control group, which were not statistically significant differences (p=NS). The sex chromosome abnormalities were similar between the ICSI and control groups (p=NS). The most prevalent abnormalities that were observed in the ICSI and control groups with first-trimester pregnancy loss were trisomy (n = 42; 27.6%), Turner syndrome (45, X0, n = 13; 8.6%), triploidy (n = 13; 8.6%), 48 chromosomes (n = 5; 3.3%), and mixed chromosomal abnormalities (n = 3; 1.2%). In addition, the karyotypes were similar between the ICSI and control groups (p=NS). We observed increases in fetal aneuploidy rates with increased maternal age (<30 years = 23.9% vs. 31–34 years = 37.0% vs. 35–39 years = 82.9% vs. >39 years = 90.9%). However, the observed increases in fetal aneuploidy rates were not statistically significant (p=NS).
Conclusion
The aneuploidy rates and sex chromosome anomalies following ICSI for non-male factor were similar to those following natural conception.
Keywords: Karyotype, Aneuploidy, ICSI, Missed abortion, Spontaneous pregnancy
Introduction
First-trimester miscarriage is the most common complication of human reproduction with an incidence ranging between 50% and 70% of all conceptions [1]. Genetic defects, especially chromosomal abnormalities, are the most common causes of spontaneous miscarriage during the first trimester. Chromosomal abnormalities occur in approximately 60% of such cases [2–4]. The majority of first-trimester miscarriages exhibit aneuploidies. Multiple cytogenetic studies have demonstrated aneuploidy rates ranging from 50%–80% in various populations [5]. Autosomal trisomies are the most frequent karyotypic abnormalities. However, polyploidies, sex chromosome monosomies, and structural rearrangements account for a substantial number of miscarriages. Several studies have demonstrated increased risk of miscarriage and aneuploidy rates with increased maternal age [5, 6].
The American and European registries of assisted reproductive technology (ART) reported the initiation of 352769 IVF cycles in 2002 [7]. In approximately one-half of these cycles, the women were ≥35 years of age [7, 8], reflecting the demographic trend toward a delay in starting a family [9]. Most ART studies have indicated that the offspring are healthy, normally developed children with cognitive and behavioral outcomes that are similar to their naturally conceived peers [5, 10]. It is unclear whether pregnancies that are conceived through ART are at an increased risk of pregnancy loss compared with naturally conceived pregnancies after accounting for maternal age and ART procedures [3, 4]. Due to the increased frequency of multiple births as well as human intervention and manipulation, a new emphasis has been placed on the developmental follow-up of children who are born after IVF and IVF-related procedures [11]. Human manipulations of oocytes and spermatozoa, and fertility drug use as well as embryo culture and transfer may affect the growing fetus. Although most studies involving ART offspring to date have demonstrated no additional risks for developmental problems, potential difficulties for children who are born after ART include the following: (a) genetic disorders, (b) congenital anomalies, (c) preterm delivery, (d) developmental disabilities, and (e) mental health difficulties [12].
Most chromosomally abnormal embryos do not develop to term [13]. ICSI is associated with an increased incidence of aneuploidy and de novo sex chromosome aberrations [14]. The additional risk of chromosomal abnormalities for children who are conceived through ICSI is approximately 1% above the baseline risk, which is small but not negligible. Therefore, prenatal diagnosis is a reasonable consideration [14, 15]. ICSI bypasses natural selection mechanisms and could potentially lead to higher aneuploidy rates in the first trimester [16]. ICSI procedure-dependent risks are as follows: (a) physical or biochemical disturbance of ooplasm or the meiotic spindle; (b) injection of biochemical contaminants; and (c) injection of sperm-associated exogenous DNA. ICSI procedure-independent risks are as follows: (a) injection of sperm carrying a chromosomal anomaly; (b) transmission of a genetic defect, which may be related to the underlying male factor infertility; (c) male gamete structural defects; (d) anomalies of sperm-activating factors; (e) potential for incorporating sperm mitochondrial DNA; and (f) female gamete anomalies [15].
ICSI has enabled the successful treatment of many conditions that are associated with infertility. ICSI has brought hope to millions of infertile couples and success to many. However, the introduction of ICSI has raised concerns about the health and well being of conceived children due to its invasive nature and the type of conditions that ICSI is used to treat [17–19]. The literature on pregnancy outcomes after intracytoplasmic sperm injection (ICSI) is limited and inconclusive regarding the risk of miscarriage and aneuploidy.
In this study, we compared chromosomal abnormalities in missed abortions following spontaneous and ICSI for non-male factor-mediated pregnancies.
Material and methods
The current study was a retrospective cohort trial and included two groups of patients who had first-trimester missed abortions during ICSI-mediated (n = 71) or spontaneous (n = 81) pregnancies, which were used as controls. All of the patients signed an informed consent form according to the Helsinki declaration. Patient demographic characteristics and karyotype analyses were determined. Semen analyses were not performed in the control group. Therefore, sperm parameters were excluded in this study. A dilatation and curettage (D&C) was offered to all women after the diagnosis of missed abortion was established. D&Cs were performed immediately after the diagnosis of missed abortion (1–26 h). The gestational ages were calculated based on the date of embryo transfer in the ICSI group or the date of the last menstrual period and ultrasonography in the control group. D&Cs were performed in the operating room under general anesthesia.
In the ICSI group, patients underwent stimulation using various protocols, including GnRH-agonist (Lucrine®; Abbott, Cedex, France) down-regulation and stimulation with exogenous gonadotropins or gonadotropins (Gonal-F®; Serono or Puregon®; Organon, Oss, the Netherlands) followed by GnRH antagonist treatment. After meeting the ultrasonographic criteria for follicular maturity, a single dose of human chorionic gonadotropin (Ovitrelle, 250 mcg; Serono) was administered. Transvaginal follicular aspiration was performed approximately 35 h after hCG administration. Embryos were transferred 3–5 days after the follicular aspiration.
Samples were obtained by D&C after spontaneous abortion and collected in sterile containers containing 10 ml of RPMI-1640 with L-glutamine (Invitrogen, Carlsbad, CA, USA). The trained embryologists dissected and selected the placental chorionic villi, which were cultured in BIO-AMP-2 complete medium (Biological Industries Ltd., Haemek, Israel) at 37°C in a 5% CO2 incubator. All procedures, including cell harvesting, slide preparation, and staining, were conducted following standard protocols [20]. At least 20 GTG-banded metaphases were analyzed in each case.
The statistical analysis was performed using NCSS 2007 Statistical Program software. Group comparisons of normally distributed variables were assessed using the Student’s t-test and Fisher’s exact test. Categorical variables were compared using the chi-squared test. The significance level for all analyses was set at p < 0.05 with a 95% confidence interval (CI).
Results
We obtained 152 specimens and isolated their chromosomal constituents using conventional cytogenetic methods. Of these abortuses, 71 and 81 abortuses were derived from ICSI-mediated and spontaneous pregnancies respectively.
The mean ages of the female and male partners were 32.68 ± 4.58 years and 34.59 ± 5.31 years, respectively. These results indicate that mean ages of female and male partners are not significantly different (p=NS). The mean weeks of gestation in the ICSI and control groups were 8.93 ± 1.37 weeks and 9.25 ± 1.12 weeks, respectively (Table 1). The miscarriage history was higher in the control group than the ICSI group (p < 0,001). Eight patients were grand multipar with more than three miscarriages and four patients had histories of five miscarriages in the control group. Because of this reason, miscarriages rates in the control group were significantly higher than that in the ICSI group. In the control group, no patients had infertility problems in their past history. We identified the following indications for ART: tubal factors (n = 21, 30%), anovulation and polycystic ovary syndrome (n = 32, 45%), and unexplained infertility (n = 18, 25%). The male factor infertility was excluded from this study due to the high rate of karyotype anomalies. Eight patients (11%) in the ICSI group had poor response.
Table 1.
Demographics of patients
| ICSI | Control | p | |
|---|---|---|---|
| Women age (years) | 32.68 ± 4.58 | 32.69 ± 4.19 | 0.983 |
| Men age (years) | 34.59 ± 5.31 | 35.78 ± 5.17 | 0.166 |
| Gestational week | 8.93 ± 1.37 | 9.25 ± 1.12 | 0.118 |
| Gravida | 0.3 ± 0.71 | 2.81 ± 1.13 | <0.001 |
| Smoking | 11 (15.5%) | 19 (23.4%) | 0.867 |
| Miscarriage history | 0.4 ± 0.47 | 1.3 ± 0.81 | <0.001 |
| BMI | 26.81 ± 3.11 | 28.02 ± 4.17 | 0.833 |
ICSI intracytoplasmic sperm injection, BMI body mass index
Overall, 49.3% of the miscarriages (75/152) were cytogenetically abnormal among the patients [47.9% (34/71) in the ICSI group and 50.6% (41/81) in the control group]. No statistically significant differences were detected (p=NS). Among the miscarriages with normal cytogenetic results, 20.4% (31/152) were normal male karyotypes and 30.3% (46/152) were normal female karyotypes. Sex chromosome abnormalities (n = 16, 21,3%) were not different between the groups (ICSI; n = 7/34, 21% and control; n = 9/41, 22%). The most prevalent abnormalities that were observed in first-trimester pregnancy losses were trisomy (n = 42; 27.6%) and Turner syndrome (n = 13; 8.6%), triploidy (n = 13; 8.6%), 48 chromosomes (n = 5; 3.3%), and mixed chromosomal abnormalities (n = 3; 1.2%; Table 2).
Table 2.
Karyotype analysis results in groups
| ICSI | Control | Total | ||
|---|---|---|---|---|
| Karyotype | Normal | 37 (52.1%) | 40 (49.4%) | 77 (50.7%) |
| Abnormal | 34 (47.9%) | 41 (50.6%) | 75 (49.3%) | |
| Sex chromosome (normal/abnormal karyotype) | Male | 31 (43.7%) | 26 (32.1%) | 57 (37.5%) |
| Female | 40 (56.3%) | 55 (67.9%) | 95 (62.5%) | |
| Distribution of karyotype | Normal | 37 (52.1%) | 40 (49.4%) | 77 (50.7%) |
| Trisomy | 19 (26.8%) | 23 (28.4%) | 42 (27.6%) | |
| Turner Syndrome | 5 (7.0%) | 8 (9.9%) | 13 (8.6%) | |
| 48 chromosomes | 4 (5.6%) | 1 (1.2%) | 5 (3.3%) | |
| Triploidy | 5 (7.0%) | 8 (9.9%) | 13 (8.6%) | |
| Mix | 1 (1.4%) | 1 (1.2%) | 2 (1.3%) | |
| Sex chromosome (Normal) | 46 XX | 20 (28.2%) | 26 (32.1%) | 46 (30.3%) |
| 46 XY | 17 (23.9%) | 14 (17.3%) | 31 (20.4%) | |
The karyotype revealed that the rate of trisomy was not different between the ICSI and control groups (RR, 0.96; CI, 0.65–1.41). In addition, Turner syndrome, triploidy, 48 chromosomes, and mixed chromosomal abnormalities were similar in the ICSI and control groups. These results reveal no significant differences between the stimulation protocols and karyotype (p=NS).
Trisomy 21 [9.9% (7/71)] was the most frequent anomaly in the ICSI group followed by trisomy 15 [5.6% (4/71)], trisomy 18 [4.2% (3/71)], trisomy 13 [4.2% (3/71)], and trisomies 8 and 10 [1.4% (1/71) for each]. In the control group, trisomy 21 [7.4% (6/81)] was the most common followed by trisomies 15 and 13 [4.9% (4/81) for each], trisomy 7 [3.7% (3/81)], trisomy 16 [2.5% (2/81)], and trisomies 2, 18, 22, and 47 [1.2% (1/81) for each; Table 3].
Table 3.
Distribution of trisomies
| Total | ICSI | Control | |
|---|---|---|---|
| Trisomy 2 | 1 (0.7%) | 0 (0%) | 1 (1.2%) |
| Trisomy 7 | 3 (2%) | 0 (0%) | 3 (3.7%) |
| Trisomy 8 | 1 (0.7%) | 1 (1.4%) | 0 (0%) |
| Trisomy 10 | 1 (0.7%) | 1 (1.4%) | 0 (0%) |
| Trisomy 13 | 7 (4.6%) | 3 (4.2%) | 4 (4.9%) |
| Trisomy 15 | 8 (5.3%) | 4 (5.6%) | 4 (4.9%) |
| Trisomy 16 | 2 (1.3%) | 0 (0%) | 2 (2.5%) |
| Trisomy 18 | 4 (2.6%) | 3 (4.2%) | 1 (1.2%) |
| Trisomy 21 | 13 (8.6%) | 7 (9.9%) | 6 (7.4%) |
| Trisomy 22 | 1 (0.7%) | 0 (0%) | 1 (1.2%) |
| Trisomy 47 | 1 (0.7%) | 0 (0%) | 1 (1.2%) |
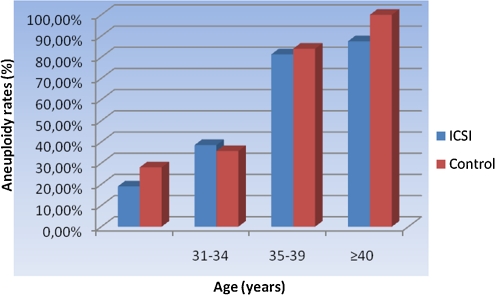
Patients were further subdivided by maternal age into the following groups: <30 years; 31–34 years; 35–39 years; and >39 years (Table 4). Cytogenetic results were further subdivided into normal female, normal male, Turner syndrome, autosomal trisomy, 48 chromosomes, and other aneuploidies. An increase in the fetal aneuploidy rate was noted with increased maternal age (<30 years = 23.9%; 31–34 years = 37.0%; 35–39 years = 82.9%; >39 years = 90.9%; Fig. 1). However, this increase was not statistically significant (p=NS).
Table 4.
Distribution of cytogenetic results according to age
| Age | ICSI | Control | Total | ||||
|---|---|---|---|---|---|---|---|
| <30 | Euploid | 17 | (81%) | 18 | (72%) | 35 | (76.1%) |
| Trisomy | 0 | (0%) | 5 | (20%) | 5 | (10.9%) | |
| Turner Syndrome | 2 | (9.5%) | 1 | (4%) | 3 | (6.5%) | |
| 48 chromosomes | 2 | (9.5%) | 0 | (0%) | 2 | (4.3%) | |
| Triploidy | 0 | (0%) | 1 | (4%) | 1 | (2.2%) | |
| 31–34 | Euploid | 16 | (61.5%) | 18 | (64.3%) | 34 | (63%) |
| Trisomy | 5 | (19.2%) | 5 | (17.9%) | 10 | (18.5%) | |
| Turner Syndrome | 3 | (11.5%) | 4 | (14.3%) | 7 | (13%) | |
| Triploidy | 2 | (7.7%) | 1 | (3.6%) | 3 | (5.6%) | |
| 35–39 | Euploid | 3 | (18.8%) | 4 | (16%) | 7 | (17.1%) |
| Trisomy | 9 | (56.3%) | 12 | (48%) | 21 | (51.2%) | |
| Turner Syndrome | 0 | (0%) | 2 | (8%) | 2 | (4.9%) | |
| 48 chromosomes | 1 | (6.3%) | 1 | (4%) | 2 | (4.9%) | |
| Triploidy | 2 | (12.5%) | 5 | (20%) | 7 | (17.1%) | |
| Mix | 1 | (6.3%) | 1 | (4%) | 2 | (4.9%) | |
| ≥40 | Euploid | 1 | (12.5%) | 0 | (0%) | 1 | (9.1%) |
| Trisomy | 5 | (62.5%) | 1 | (33.3%) | 6 | (54.5%) | |
| Turner Syndrome | 0 | (0%) | 1 | (33.3%) | 1 | (9.1%) | |
| 48 chromosomes | 1 | (12.5%) | 0 | (0%) | 1 | (9.1%) | |
| Triploidy | 1 | (12.5%) | 1 | (33.3%) | 2 | (18.2%) | |
Fig. 1.
Aneuploidy rate according to ages
Discussion
First-trimester miscarriage is the most common complication of human reproduction with an incidence that ranges between 50% and 70% of all conceptions [1]. Aneuploidy is found in the majority of first-trimester miscarriages. Multiple cytogenetic studies have demonstrated aneuploidy rates that range from 50 to 80% in various populations [5]. Autosomal trisomies are the most frequent karyotypic abnormalities. However, polyploidies, sex chromosome monosomies, and structural rearrangements account for a substantial number of miscarriages [5, 6].
ICSI bypasses mechanisms of natural selection and may increase first-trimester aneuploidy rates [16]. Previous studies have not demonstrated a correlation between aneuploidy and ICSI-mediated pregnancies [14, 21]. However, more sex chromosome anomalies were shown among pregnancies resulting from ICSI in our study, which confirms findings of previous studies [14, 21–24]. We did not find an increased risk for chromosomal abnormalities or aneuploidy in ICSI-mediated compared with spontaneous pregnancies. In contrast, the rates of sex chromosomal abnormalities were not different in ICSI-mediated and spontaneous pregnancies in this study. We did not observe any correlation between sex chromosomal anomalies in both groups. However, recent studies have indicated that sex chromosomal anomalies are higher in ICSI than in control groups. Differences between these studies and our study may be attributed to the relatively small sample size in our study compared to that in recent studies.
We have shown that autosomal trisomy is the most common abnormal karyotype in the ICSI-mediated and spontaneous pregnancy groups. Trisomies 21, 15, 18, and 13 were the most frequent trisomies. Kushnir and Frattarelli [14] and Kim et al. [21] have reported that autosomal trisomy is the most common abnormal cytogenetic result in ICSI-mediated and spontaneous pregnancies in first-trimester miscarriages [14, 21]. Kushnir and Frattarelli [14] has shown that 52.62% (201/382) of miscarriages are cytogenetically abnormal [48.44% (62/128) in controls, 54.29% (76/140) after ICSI, and 55.26% (63/114) after conventional IVF] with no statistically significant differences [14]. Kim et al. [21] has shown that 50.1% of the miscarriages are cytogenetically abnormal among all patients undergoing IVF [21]. In the current study, 49.3% of the miscarriages (75/152) were cytogenetically abnormal [47.9% (34/71)] in the ICSI group and 50.6% (41/81) in the control group.
A relationship between maternal age and first-trimester pregnancy loss has been indicated in previous studies. Aneuploidy rates dramatically increase with maternal age in ICSI-mediated and spontaneous pregnancies [14, 16, 21, 25]. However, we did not determine that the aneuploidy rates increased with maternal age. Because the age distribution and the number of patients based on age was varied, no statistical correlations were detected between aneuploidy and maternal age.
ICSI is an effective modality for the treatment of couples with infertility. In summary, our data indicated that the aneuploidy rates and sex chromosome anomalies following ICSI for non-male factor were similar to the aneuploidy rates and sex chromosome anomalies following natural conception. As a retrospective cohort study, we have shown that there is no relationship between aneuploidy, sex chromosomal abnormalities, and ICSI for non-male factor. This study supports the safety of ICSI as an effective treatment for infertility. Although no statistical differences were detected, the study groups were small and heterogenic with a number of potential biases. Therefore, further investigation of ICSI and karyotype analysis is required using large, randomized, clinical trials.
Footnotes
Statement for authors submitting original research: The responsible authors at the institute where the work has been carried out have approved the enclosed manuscript “Comparison of Chromosomal Abnormality Rates in ICSI for Non-Male Factor and Spontaneous Conception”. Authors certify that none of the materials in this manuscript has been published previously in any form and that none of this material is currently under consideration for publication elsewhere. All authors have participated sufficiently in the intellectual content, the analysis of data and the writing the manuscript to take public responsibility for it.
Capsule
The chromosomal anomalies and aneuploidy rates following ICSI for non-male factor were similar to those following natural conception.
Contributor Information
Banu Bingol, Phone: +90-212-2666646, FAX: +90-212-2884437, Email: banubingol1975@yahoo.com.
Faruk Abike, Phone: +90-533-6385168, Email: farukabike@gmail.com.
References
- 1.Simpson JL. Causes of fetal wastage. Clin Obstet Gynecol. 2007;50:10–30. doi: 10.1097/GRF.0b013e31802f11f6. [DOI] [PubMed] [Google Scholar]
- 2.Wang JX, Norman RJ, Wilcox AJ. Incidence of spontaneous abortion among pregnancies produced by assisted reproductive technology. Hum Reprod. 2004;19:272–277. doi: 10.1093/humrep/deh078. [DOI] [PubMed] [Google Scholar]
- 3.Schieve LA, Tatham L, Peterson HB, Toner J, Jeng G. Spontaneous abortion among pregnancies conceived using assisted reproductive technology in the United States. Obstet Gynecol. 2003;101:959–967. doi: 10.1016/S0029-7844(03)00121-2. [DOI] [PubMed] [Google Scholar]
- 4.Farr SL, Schieve LA, Jamieson DJ. Pregnancy loss among pregnancies conceived through assisted reproductive technology, United States, 1999–2002. Am J Epidemiol. 2007;165:1380–1388. doi: 10.1093/aje/kwm035. [DOI] [PubMed] [Google Scholar]
- 5.Simpson JL, Bombard AT. Chromosomal abnormalities in spontaneous abortion: frequency, pathology and genetic counseling. In: Edmonds KBMJ, editor. Spontaneous abortion. London: Blackwell; 1987. pp. 51–76. [Google Scholar]
- 6.Angell RR. Aneuploidy in older women. Higher rates of aneuploidy in oocytes from older women. Hum Reprod. 1994;9:1199–2000. doi: 10.1093/oxfordjournals.humrep.a138675. [DOI] [PubMed] [Google Scholar]
- 7.Wright VC, Schieve LA, Reynolds MA, Jeng G. Assisted reproductive technology surveillance—United States, 2002. MMWR Surveill Summ. 2005;54:1–24. [PubMed] [Google Scholar]
- 8.Andersen AN, Gianaroli L, Felberbaum R, Mouzon J, Nygren KG. Assisted reproductive technology in Europe, 2002: results generated from European registers by ESHRE. Hum Reprod. 2006;21:1680–1697. doi: 10.1093/humrep/del075. [DOI] [PubMed] [Google Scholar]
- 9.Baird DT, Collins J, Egozcue J, et al. Fertility and ageing. Hum Reprod Update. 2005;11:261–276. doi: 10.1093/humupd/dmi006. [DOI] [PubMed] [Google Scholar]
- 10.Woldringh GH, Besselink DE, Tillema AH, Hendriks JC, Kremer JA. Karyotyping, congenital anomalies and follow-up of children after intracytoplasmic sperm injection with non-ejaculated sperm: a systematic review. Hum Reprod Update. 2010;16:12–19. doi: 10.1093/humupd/dmp030. [DOI] [PubMed] [Google Scholar]
- 11.Squires J, Kaplan P. Developmental outcomes of children born after assisted reproductive technologies. Infants Young Children. 2007;20(1):2–10. doi: 10.1097/00001163-200701000-00002. [DOI] [Google Scholar]
- 12.Wright V, Chang J, Jeng G, Macaluso M. Assisted reproductive technology surveillance—United States, 2003. MMWR Surveill Summ. 2006;55(SS04):1–22. [PubMed] [Google Scholar]
- 13.Wilton L. Preimplantation genetic diagnosis for aneuploidy screening in early human embryos: a review. Prenat Diagn. 2002;22:512–518. doi: 10.1002/pd.388. [DOI] [PubMed] [Google Scholar]
- 14.Kushnir VA, Frattarelli JL. Aneuploidy in abortuses following IVF and ICSI. J Assist Reprod Genet. 2009;26:93–97. doi: 10.1007/s10815-009-9292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonduelle M, Assche E, Joris H, Keymolen K, Devroey P, Steirteghem A, Liebaers I. Prenatal testing in ICSI pregnancies: incidence of chromosomal anomalies in 1586 karyotypes and relation to sperm parameters. Hum Reprod. 2002;17:2600–2614. doi: 10.1093/humrep/17.10.2600. [DOI] [PubMed] [Google Scholar]
- 16.Munne S, Alikani M, Tomkin G, Grifo J, Cohen J. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertil Steril. 1995;64:382–391. [PubMed] [Google Scholar]
- 17.Patrizio P. Intracytoplasmic sperm injection (ICSI): potential genetic concerns. Hum Reprod. 1995;10:2520–2523. doi: 10.1093/oxfordjournals.humrep.a135734. [DOI] [PubMed] [Google Scholar]
- 18.Kretser DM. The potential of intracytoplasmic sperm injection (ICSI) to transmit genetic defects causing male infertility. Reprod Fertil Dev. 1995;7:137–142. doi: 10.1071/RD9950137. [DOI] [PubMed] [Google Scholar]
- 19.Seamark RF, Robinson JK. Potential health problems stemming from assisted reproduction programmes. Hum Reprod. 1995;10:1321–1322. doi: 10.1093/humrep/10.6.1321. [DOI] [PubMed] [Google Scholar]
- 20.Simoni G, Brambati B, Danesino C, Rossella F, Terzoli GL, Ferrari M, Fraccaro M. Efficient direct chromosome analyses and enzyme determinations from chorionic villi samples in the first trimester of pregnancy. Hum Genet. 1983;63:349–357. doi: 10.1007/BF00274761. [DOI] [PubMed] [Google Scholar]
- 21.Kim JW, Lee WS, Yoon TK, Seok HH, Cho JH, Kim YS, Lyu SW, Shim SH. Chromosomal abnormalities in spontaneous abortion after assisted reproductive treatment. BMC Med Genet. 2010;11:153. doi: 10.1186/1471-2350-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonduelle M, Camus M, Vos A, Staessen C, Tournaye H, Assche E, Verheyen G, Devroey P, Liebaers I, Steirteghem A. Seven years of intracytoplasmic sperm injection and follow-up of 1987 subsequent children. Hum Reprod. 1999;14(Suppl 1):243–264. doi: 10.1093/humrep/14.suppl_1.243. [DOI] [PubMed] [Google Scholar]
- 23.Herve C, Moutel G. Sex chromosome abnormalities after intracytoplasmic sperm injection. Lancet. 1995;346:1096–1097. [PubMed] [Google Scholar]
- 24.Meschede D, Horst J. Sex chromosomal anomalies in pregnancies conceived through intracytoplasmic sperm injection: a case for genetic counselling. Hum Reprod. 1997;12:1125–1127. doi: 10.1093/humrep/12.6.1125. [DOI] [PubMed] [Google Scholar]
- 25.Benadiva CA, Kligman I, Munne S. Aneuploidy 16 in human embryos increases significantly with maternal age. Fertil Steril. 1996;66:248–255. [PubMed] [Google Scholar]