Summary
Purpose
To evaluate the proportions of abnormal and normal embryos detected by preimplantation genetic diagnosis (PGD) of infertile couples of whom one was a Robertsonian translocation (RT) carrier, and to provide practical information, including details of reproductive outcomes, to aid in genetic counseling of such couples.
Methods
We retrospectively analyzed all PGD cycles conducted to deal with RT at our center between January 2000 and December 2009. Subject demographic and clinical data were compared with the results of PGD.
Results
Employing PGD, we conducted a total of 66 cycles on 34 couples of whom one was an RT carrier, including 24 female and 10 male carriers. Of the 514 blastomeres tested, 161 (31.3%) were normal or balanced. Of the 57 cycles that included embryo transfer, 17 (29.8%) attained positivity for human chorionic gonadotropin (hCG). A total of 17 embryos were implanted and 16 babies, including two sets of twins, were born. The takehome baby rate was 41.2% per couple and the loss rate 6.6%. Receiver operating characteristic curve analysis showed that the proportion of alternate embryos associated with a sensitivity of 70.6% for prediction of clinical pregnancy following PGD was 0.31. Sex of the carrier and type of translocation were not significantly associated with pregnancy outcomes.
Conclusion
Couples with RT may benefit from PGD; pregnancy success rate is improved and embryo loss reduced. We found that about 30% of embryos were of normal or balanced chromosomal constitution and that the percentage of normal or balanced embryos was predictive of PGD outcome.
Keywords: Preimplantation genetic diagnosis, Robertsonian translocation, Recurrent pregnancy loss, Translocation carrier
Introduction
Robertsonian translocation (RT) is one of the most common balanced structural translocations in humans. RT is a result of the centromeric fusion of two acrocentric chromosomes (i.e., chromosomes 13, 14, 15, 21, and/or 22). A healthy RT carrier is usually phenotypically normal, but has an increased risk of developing unbalanced gametes. RT carriers frequently report infertility problems, including recurrent pregnancy loss, oligozoospermia, and azoospermia. Importantly, unbalanced embryos that are trisomic for chromosomes 13 and 21 can survive throughout the course of pregnancy, resulting in the birth of children with Patau syndrome and Down syndrome, respectively. The incidence of RT is 1.2 per 1,000 in the general population, 1.8% in couples with recurrent pregnancy loss, and 2–3% in infertile men [1–3].
The most frequent type of RT translocation occurs between chromosomes 13 and 14. Translocations between chromosomes 14 and 21 are less common, and other possible combinations are infrequent. Theoretically, alternate segregation during meiosis leads to six types of gametes; two are normal or balanced and four are unbalanced. Previous studies have shown that chromosome segregation in male and female RT carriers predominantly results in the production of normal or balanced gametes (alternate segregation). Moreover, the frequency of normal/balanced gametes is much higher in male than in female carriers [4, 5]. RT impacts obstetric outcomes after fertilization, as aneuploidy has very little effect on fertilization capacity.
Preimplantation genetic diagnosis (PGD) is a prenatal diagnostic procedure that has been used successfully to identify RT [6] throughout the world. PGD in RT carriers is an alternative to prenatal diagnosis and the termination of abnormal fetuses. Moreover, this approach results in a significant reduction in spontaneous abortions and a significant increase in successful pregnancies. Previous studies of the chromosomal patterns of embryos in RT carriers subjected to PGD have shown mixed results. Some have suggested that RT prompts abnormal chromosome segregation, resulting in high levels of mosaicism and chaotic embryos [7–9], whereas others have reported that RT does not cause chromosomal malsegregation during cleavage and that PGD is a useful alternative to prenatal diagnosis [10].
Genetic counseling for RT carriers includes detailing the risk of eventful reproductive outcomes and appropriate evidence-based treatment strategies. PGD may facilitate pregnancy in RT carriers, but due to the extensive effort and high cost associated with the procedure, its use should be restricted. PGD has only been used to assess embryos in a few RT carriers. The present study details 10 years of experience with PGD in RT carriers. Specifically, the number of superior embryos revealed with PGD in RT carriers was assessed. Moreover, the factors that predicted pregnancy and the number of retrieved oocytes and embryos that were required to achieve successful outcomes were analyzed. This study provides practical information regarding the use of PGD as a treatment option for RT carriers.
Methods
Patients
This retrospective analysis included all RT carriers who underwent PGD cycles at our center between January 2000 and December 2009. Sixty-six cycles of PGD were performed in 34 couples with an RT, including 24 female and 10 male carriers. Each couple’s ages, reproductive histories, and reasons for PGD were assessed. During the same time period, 410 PGD cycles were performed on 214 couples at the infertility center at CHA General Hospital.
Counseling and informed consent
All couples were evaluated and counseled by infertility specialists and medical geneticists. The procedure and limitations of PGD were explained to the couples. The risk of misdiagnosis attributable to embryonic mosaicism and the 1–2% technical error rate of the fluorescent in situ hybridization (FISH) procedure used in PGD were also detailed. Informed consent was provided by each couple prior to enrollment. The study was approved by the Institutional Review Board at CHA University Hospital.
In vitro fertilization and preimplantation genetic diagnosis
All PGD couples underwent in vitro fertilization (IVF) using a gonadotropin-releasing hormone (GnRH) agonist or antagonist protocol for pituitary suppression, and were stimulated with recombinant follicular-stimulating hormone (FSH). Ovarian activity was monitored by regular ultrasound (US) scans and measurements of serum estrogen concentration. Recombinant human chorionic gonadotropin (hCG) was administered when the leading follicles exceeded 18 mm in diameter. Thirty-five hours later, oocytes were retrieved via transvaginal puncture under US guidance. Retrieved oocytes were fertilized by intracytoplasmic sperm injection (ICSI) and fertilized embryos were cultured in Quinn's Advantage Cleavage Medium (Sage In-Vitro Fertilization, Inc., Trumbull, CT, USA) at 37°C under 5% (v/v) CO2 in air for 3 days. Fertilization and embryo development were assessed daily and embryos that reached the 6–8-cell stage on day 3 were biopsied. A single blastomere was taken from each embryo, after which the embryo was washed, transferred to Quinn's Advantage Blastocyst Medium (Sage In-Vitro Fertilization, Inc.) and cultured as described above.
Diagnostic efficiency of fluorescent in situ hybridization probes used for preimplantation genetic diagnosis
The FISH probes used included LSI 13, RB1, LSI14 (IGH), CEP15, LSI21 (D21S259-342), and TelVysion 22qtel, all of which were purchased from Vysis Inc. (Des Plaines, IL, USA). The specificity and sensitivity of the probes had previously been tested using patient lymphocyte cultures. The probe signal patterns of 50 metaphase and 50 interphase nuclei were scored as previously described [11]. All probes had specificities of 100% and efficiencies of 84–95%.
Fluorescent in situ hybridization
Slides of biopsied blastomeres were prepared as described in Coonen et al. [12], and probe mixtures were prepared according to the manufacturer’s protocol. FISH reactions were performed overnight using the HYBriteTM denaturation/hybridization system (Vysis Inc). FISH signals were independently scored and interpreted by two technologists.
Embryo transfer and pregnancy evaluation
Embryos with normal and balanced FISH signals were transferred into the uterine cavity on the fourth day after oocyte retrieval. Serum β-hCG concentration was measured 12 days after transfer. Clinical pregnancy was defined as the presence of a fetal heartbeat on vaginal US at 4–6 weeks after embryo transfer. Miscarriage was defined as the spontaneous termination of a clinical pregnancy before the fetus was viable. Twins were regarded as a single live birth. After the confirmation of clinical pregnancy, the patients were followed throughout the pregnancy and delivery.
Statistical analysis
Data were analyzed using X2 tests and receiver operating characteristic (ROC) curve construction. All statistical analyses were performed using SPSS software (ver. 17; SPSS Inc., Chicago, IL, USA). Statistical significance was defined as p < 0.05.
Results
During the 10-year time period, 66 cycles of PGD were performed in 34 couples with an RT, including 24 female and 10 male carriers. The most common type of RT was der(13;14)(q10;q10), present in 67.6% of carriers. The mean age of female carriers was 32.7 years (range: 26–39 years). All female carriers had experienced two or more pregnancy losses (range: 2–5; mean: 2.8). Eight of the male carriers were identified via an infertility workup following a diagnosis of male factor infertility. Two other males knew that they were RT carriers due to a family history, and both requested genetic counseling before trying to conceive. All male carriers had oligozoospermia, with counts of 0.2–20 × 106 spermatozoa/ml. The karyotype of each male carrier’s partner was normal, and none of the couples had previously conceived a live-born child. The characteristics and clinical outcomes of the PGD cycles conducted in RT carriers are summarized in Tables 1 and 2.
Table 1.
Patient characteristics and PGD outcomes of couples of whom one was a Robertsonian translocation carrier
| Female | Male | Total | |
|---|---|---|---|
| Number of couples | 24 | 10 | 34 |
| Number of cycles | 42 | 24 | 66 |
| Mean age of the carrier (years) | 32.04 ± 3.47 | 36.12 ± 4.97 | |
| Mean age of the partner (years) | 35.61 ± 5.09 | 34.48 ± 3.59 | |
| Mean number of previous miscarriages | 2.87 ± 0.86 | 0.3 ± 0.67 | |
| Type of translocation | |||
| 45,XX,der(13;14)(q10:q10) | 16 | 7 | 23 |
| 45,XX,der(14;21)(q10:p10) | 2 | 2 | 4 |
| 45,XX,der(13;21)(q10:q10) | 3 | 0 | 3 |
| 45,XX,der(21;22)(q10:q10) | 3 | 0 | 3 |
| 45,XY,der(15;22)(q10:p10) | 0 | 1 | 1 |
| Retrieved oocytes | 478 (11.38 ± 5.72*) | 492 (20.5 ± 8.74*) | 970 (14.69 ± 8.20) |
| 2PN embryos | 321 (7.64 ± 4.10*) | 298 (12.41 ± 7.58*) | 619 (9.37 ± 6.02) |
| Fertilization rate (%) | 67.9 ± 15.3* | 58.0 ± 18.6* | 64.3 ± 17.2 |
| Biopsied embryos | 275 (6.54 ± 3.43*) | 239 (9.95 ± 4.80*) | 514 (7.78 ± 4.28) |
| Normal or balanced embryos | 85 (2.02 ± 1.64*) | 76 (3.16 ± 1.63*) | 161(2.43 ± 1.71) |
| Proportion of normal or balanced embryos (%) | 27.7 ± 16.6 | 32.1 ± 15.8 | 29.3 ± 16.4 |
| Number of embryos transferred | 84 (2.00 ± 1.60*) | 71 (2.95 ± 1.45*) | 155 (2.34 ± 1.61) |
| Cancellation rate (%) | 16.7 (7/42) | 8.3 (2/24) | 13.6 (9/66) |
| Pregnancy rate per ET (%) | 31.4 (11/35) | 27.3 (6/22) | 29.8 (17/57) |
| Clinical pregnancy rate per ET (%) | 25.7 (9/35) | 27.3 (6/22) | 26.3 (15/57) |
| Live birth rate per couple (%) | 37.5 (9/24) | 50 (5/10) | 41.2 (14/34) |
| Miscarriage rate (%) | 0 (0/9) | 16.6 (1/6) | 6.6 (1/15) |
Data are expressed as means±SDs or numbers
*P < 0.05
Table 2.
Results of 66 PGD cycles of 34 couples of whom one was a Robertsonian translocation carrier
| Patient | Karyotype | Cycle | Oocytes | FISH result | ET | Pregnancy (hCG/Clinical/LBa) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Retrieved | Fertilized | Biopsied | Normal/Balanced | Abnormal | Inconclusive | |||||
| Female | ||||||||||
| 1 | 45,XX,der(13;14)(q10:q10) | 1 | 9 | 7 | 7 | 2 | 4 | 1 | 2 | No |
| 2 | 45,XX,der(13;14)(q10:q10) | 1 | 11 | 8 | 5 | 2 | 3 | 0 | 2 | No |
| 2 | 9 | 7 | 7 | 2 | 5 | 0 | 2 | No | ||
| 3 | 11 | 5 | 2 | 0 | 2 | 0 | None | |||
| 3 | 45,XX,der(13;14)(q10:q10) | 1 | 8 | 6 | 6 | 3 | 2 | 1 | 3 | Yes (+/1/1) |
| 4 | 45,XX,der(13;14)(q10:q10) | 1 | 20 | 15 | 12 | 4 | 5 | 3 | 4 | Yes (+/1/1) |
| 5 | 45,XX,der(13;21)(q10:q10) | 1 | 8 | 6 | 6 | 3 | 2 | 1 | 3 | Yes (+/1/1) |
| 6 | 45,XX,der(13;14)(q10:q10) | 1 | 12 | 9 | 5 | 1 | 4 | 0 | 1 | No |
| 7 | 45,XX,der(13;14)(q10:q10) | 1 | 15 | 11 | 11 | 2 | 7 | 2 | 2 | Yes (+/0/0) |
| 2 | 11 | 8 | 8 | 2 | 6 | 0 | 2 | No | ||
| 3 | 26 | 22 | 10 | 4 | 6 | 0 | 4 | No | ||
| 8 | 45,XX,der(21;22)(q10:q10) | 1 | 8 | 5 | 5 | 2 | 2 | 1 | 2 | No |
| 9 | 45,XX,der(13;14)(q10:q10) | 1 | 4 | 4 | 4 | 1 | 3 | 0 | 1 | No |
| 10 | 45,XX,der(13;14)(q10:q10) | 1 | 12 | 7 | 7 | 1 | 5 | 1 | 1 | No |
| 2 | 14 | 8 | 5 | 1 | 4 | 0 | 1 | No | ||
| 11 | 45,XX,der(13;14)(q10:q10) | 1 | 12 | 8 | 6 | 2 | 3 | 1 | 2 | No |
| 12 | 45,XX,der(13;21)(q10:q10) | 1 | 10 | 9 | 9 | 4 | 5 | 0 | 4 | No |
| 13 | 45,XX,der(21;22)(q10:q10) | 1 | 18 | 10 | 10 | 4 | 6 | 0 | 4 | No |
| 14 | 45,XX,der(21;22)(q10:q10) | 1 | 4 | 2 | 2 | 1 | 1 | 0 | 1 | No |
| 2 | 6 | 3 | 4 | 0 | 3 | 1 | None | |||
| 3 | 5 | 4 | 2 | 1 | 1 | 0 | 1 | Yes (+/1/1) | ||
| 15 | 45,XX,der(13;14)(q10:q10) | 1 | 15 | 8 | 8 | 3 | 5 | 0 | 3 | Yes (+/1/1) |
| 16 | 45,XX,der(13;14)(q10:q10) | 1 | 16 | 11 | 9 | 3 | 6 | 0 | 3 | No |
| 17 | 45,XX,der(13;14)(q10:q10) | 1 | 16 | 11 | 5 | 0 | 5 | 0 | None | |
| 2 | 19 | 12 | 12 | 6 | 6 | 0 | 6 | No | ||
| 3 | 25 | 15 | 15 | 6 | 6 | 3 | 6 | Yes (+/1/1) | ||
| 18 | 45,XX,der(13;14)(q10:q10) | 1 | 25 | 15 | 15 | 6 | 9 | 0 | 6 | Yes (+/2/2) |
| 19 | 45,XX,der(13;14)(q10:q10) | 1 | 12 | 10 | 10 | 2 | 8 | 0 | 2 | No |
| 2 | 10 | 6 | 6 | 2 | 4 | 0 | 2 | No | ||
| 3 | 9 | 7 | 6 | 1 | 5 | 0 | 1 | No | ||
| 4 | 6 | 6 | 3 | 0 | 3 | 0 | None | |||
| 5 | 11 | 6 | 6 | 2 | 3 | 1 | 2 | No | ||
| 20 | 45,XX,der(14;21)(q10:p10) | 1 | 9 | 7 | 6 | 1 | 5 | 0 | 1 | No |
| 2 | 13 | 10 | 1 | 1 | 9 | 0 | 1 | No | ||
| 3 | 9 | 4 | 4 | 2 | 1 | 1 | 2 | No | ||
| 21 | 45,XX,der(14;21)(q10:p10) | 1 | 6 | 4 | 4 | 1 | 2 | 1 | 1 | Yes(+/1/1) |
| 22 | 45,XX,der(13;14)(q10:q10) | 1 | 9 | 6 | 6 | 2 | 4 | 0 | 2 | Yes(+/1/1) |
| 23 | 45,XX,der(13;14)(q10:q10) | 1 | 13 | 8 | 8 | 4 | 4 | 0 | 4 | Yes(+/0/0) |
| 2 | 9 | 5 | 3 | 0 | 2 | 1 | None | |||
| 3 | 9 | 4 | 4 | 1 | 3 | 0 | 1 | No | ||
| 24 | 45,XX,der(13;14)(q10:q10) | 1 | 1 | 1 | 1 | 0 | 1 | 0 | None | |
| 2 | 3 | 1 | 1 | 0 | 1 | 0 | None | |||
| Male | ||||||||||
| 1 | 45,XY,der(13;14)(q10:q10) | 1 | 39 | 28 | 14 | 5 | 5 | 4 | 5 | No |
| 2 | 20 | 16 | 15 | 3 | 12 | 0 | 3 | No | ||
| 2 | 45,XY,der(13;14)(q10:q10) | 1 | 19 | 10 | 9 | 4 | 5 | 0 | 4 | No |
| 2 | 18 | 15 | 15 | 5 | 9 | 1 | 5 | Yes(+/2/2) | ||
| 3 | 45,XY,der(13;14)(q10:q10) | 1 | 12 | 4 | 4 | 2 | 2 | 0 | 2 | No |
| 2 | 9 | 3 | 3 | 0 | 3 | 0 | None | |||
| 4 | 45,XY,der(13;14)(q10:q10) | 1 | 19 | 9 | 9 | 2 | 7 | 0 | 2 | No |
| 2 | 15 | 13 | 13 | 3 | 8 | 2 | 3 | Yes(+/1/1) | ||
| 5 | 45,XY,der(13;14)(q10:q10) | 1 | 16 | 6 | 6 | 3 | 3 | 0 | 3 | No |
| 2 | 11 | 6 | 6 | 3 | 3 | 0 | 3 | No | ||
| 3 | 23 | 10 | 8 | 2 | 4 | 2 | 2 | Yes(+/1/0) | ||
| 6 | 45,XY,der(14;21)(q10:p10) | 1 | 18 | 5 | 2 | 0 | 2 | 0 | None | |
| 2 | 16 | 10 | 9 | 5 | 4 | 0 | 5 | No | ||
| 3 | 20 | 14 | 12 | 3 | 7 | 2 | 3 | No | ||
| 4 | 17 | 10 | 10 | 5 | 2 | 3 | 4 | No | ||
| 5 | 23 | 19 | 19 | 5 | 14 | 0 | 4 | No | ||
| 7 | 45,XY,der(14;21)(q10:p10) | 1 | 10 | 5 | 5 | 1 | 4 | 0 | 1 | No |
| 2 | 12 | 5 | 5 | 2 | 3 | 0 | 2 | Yes(+/1/1) | ||
| 8 | 45,XY,der(15;22)(q10:p10) | 1 | 17 | 11 | 9 | 5 | 4 | 0 | 5 | Yes(+/1/1) |
| 9 | 45,XY,der(13;14)(q10:q10) | 1 | 26 | 15 | 11 | 11 | 4 | 7 | 4 | No |
| 2 | 37 | 28 | 17 | 2 | 15 | 0 | 2 | No | ||
| 3 | 29 | 8 | 7 | 2 | 5 | 0 | 2 | No | ||
| 10 | 45,XY,der(13;14)(q10:q10) | 1 | 25 | 20 | 13 | 5 | 7 | 1 | 4 | No |
| 2 | 41 | 28 | 18 | 5 | 7 | 6 | 3 | Yes(+/1/1) | ||
aLB live birth
The mean number of oocytes aspirated from the 34 couples, including couples with either a male or female RT carrier, was 14.7 per cycle. The fertilization rate was 64.3%, and 53.0% of fertilized embryos reached a developmental stage that permitted successful biopsy. Of the 514 blastomeres tested, 161 (31.3%) were normal or balanced, 313 (60.9%) were abnormal, and 40 (7.8%) yielded inconclusive results. The number of retrieved oocytes and the fertilization rate differed significantly between couples containing male and female carriers, but no difference was evident in pregnancy outcome or the proportion of normal or balanced embryos.
Embryo transfer was performed in 57/66 (86.4%) PGD cycles, with a mean of 2.0 embryos transferred per cycle. No normal or balanced embryo was available for transfer during the other nine cycles. Of the 57 cycles of embryo transfer, 17 (29.8%) were associated with positive serum hCG, including two associated with a chemical pregnancy. One embryo spontaneously aborted at 9 weeks of gestation following the confirmation of a heartbeat at 7 weeks; chromosomal analysis of the aborted fetus revealed a normal karyotype of 46,XX. Eight couples with ongoing pregnancies requested confirmatory prenatal diagnoses by amniocentesis to exclude possible misdiagnosis; of these fetuses, six were RT carriers and two were normal. Fourteen cycles resulted in live births; the delivery rate was 21.2% (14/66) per cycle featuring oocyte retrieval and 24.6% (14/57) per cycle featuring embryo transfer. A total of 17 embryos were implanted, and 16 babies, including two sets of twins, were born. The live birth delivery rate per couple was 41.2% and the miscarriage rate was 6.6% (1/15).
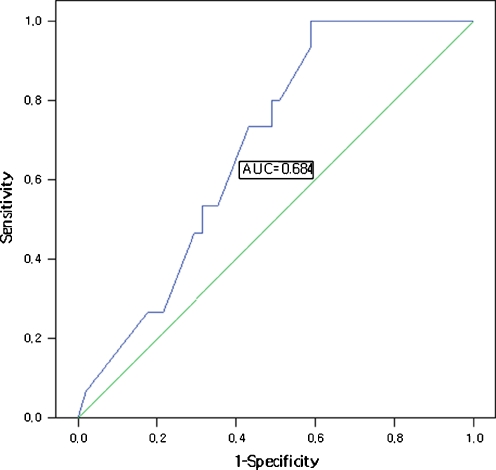
The proportion of normal or balanced embryos significantly affected outcomes. The area under the ROC curve was 0.68 (Fig. 1). A cutoff value of 31% for normal or balanced embryos resulted in high sensitivity (70.6%) and moderate specificity (57.1%).
Fig. 1.
Receiver-operating characteristic curve analysis of the relationship between the proportion of normal/balanced embryos and clinical pregnancy. A cutoff of 31% was the best predictor of clinical pregnancy
Discussion
Patients with RT, although phenotypically normal, commonly experience infertility and repeated miscarriages because approximately two-thirds of their gametes feature disomy or nullisomy and result in abnormal embryos [13, 14]. All couples in the present study sought help at our center because of recurrent abortion and male factor infertility. These couples had been trying to produce a child for a mean of 3.2 years prior to enrollment in the PGD program; all previous attempts had ended in abortion or termination as a result of fetal anomalies. The pregnancy rate was 29.8% per embryo transfer in these couples, with an overall delivery rate of 24.6% per embryo transfer after PGD and 41.2% per couple. These results are consistent with previously reported pooled PGD results [15]. The pregnancy rate with PGD was significantly lower than the mean pregnancy rate obtained with conventional IVF (38.9%/embryo transfer) at our center during the same period. The lower pregnancy rate with the use of PGD may have been due to the smaller number of embryos suitable for transfer or to the effects of the biopsy. The high proportion of chromosomal abnormalities found in embryos of RT carriers may also have reduced the pregnancy rate [8, 16]. In addition, factors other than chromosomal abnormalities that result in recurrent abortions may have influenced our results, as many couples undergoing PGD suffer from immunological disorders and/or luteal phase defects [17].
With PGD, the rate of pregnancy loss in patients who had lost 69 previous pregnancies was only 6.6%. The mean duration of success (from the start of the cycle to a positive serum hCG) was 3.3 months and the mean number of cycles was 1.6. Thus, PGD significantly reduced the miscarriage rate and significantly increased the successful pregnancy rate compared with the couples’ histories prior to PGD. Although the results of PGD following the confirmation of balanced translocation have been previously reported, RT carriers were not included. Table 3 shows the overall pregnancy and loss rates after PGD for RT carriers in several studies, including the present work and several case reports of natural reproductive outcomes in RT carriers. Compared with the natural reproductive history of such carriers, PGD increased the pregnancy rate, reduced the time required to obtain a successful pregnancy, and significantly reduced the spontaneous abortion rate. In contrast, the natural reproductive outcomes of translocation carriers after chromosomal analysis showed a high probability (45–80%) of the birth of a healthy child. Moreover, the mean time required to achieve a successful natural pregnancy was 5.3 years, a significantly greater proportion of carrier than non-carrier couples experienced one or more miscarriages after chromosomal analysis, and an increased incidence of termination due to chromosomal abnormalities was evident [18–20]. Although a successful natural pregnancy is probable in couples with a balanced translocation, repeated miscarriages and terminations of abnormal fetuses are associated with psychological trauma and a significant physical burden. Therefore, PGD may be a better option for RT carriers, allowing couples to take home a healthy child in a shorter period of time without the trauma associated with miscarriages and terminations.
Table 3.
Pregnancy outcomes in Robertsonian translocation carriers with or without PGD treatment
| Reference | Patient number (cycles) | Mean number of prior miscarriages | Miscarriage rate | Proportion of normal or balanced embryos | Clinical pregnancy rate per transfer | Cumulative live birth rate | Mean time frame for success |
|---|---|---|---|---|---|---|---|
| With PGD | |||||||
| Scriven et al. [10] | 4 (7) | 1.3 | 0% (83.3%a) | 62% | 42.8% | 75% | 1.8 cycles |
| Kuliev and Verlinsky [13] | 17(19) | NA | 25% (81%a) | 33.8% | 33% | NA | NA |
| Huang [30] | 37 (41) | NA | 22.2% (100%a) | 33.5% | 27.3% | NA | NA |
| Gianaroli et al. [31] | 15 (23) | 2.0 | 25% | 23% | 61.5% | 40% | 1.5 cycles |
| Lim et al. [32] | 6 (11) | NA | 0% (93.3%a) | 22.3% | 10% | 16.7% | 1.4 cycles |
| Fischer et al. [33] | NA (52) | 3.8 | 6% (88.5%a) | 27% | 38% | NA | 1.4 cycles |
| Current study | 34 (66) | 2.9 | 6.6% (100%a) | 29.3% | 26.3% | 41.2% | 1.6 cycles |
| Without PGD | |||||||
| Sugiura-Osawara [19] | 11 | > 2b | 36.4%c | – | – | 63.6% | NA |
| Goddjin [34] | 3 | 2.9 | 26% | – | – | 72% | 6 years |
| Stephenson and Sierra [17] | 12 | 3.8 | 31%b | – | – | 67% | 4.2 years |
| Franssen [20] | 278 | > 2b | 49%c | – | – | 80% | 5.8 years |
NA data not available
amiscarriage rate before PGD
bGreater than 2
cMiscarriage rate after diagnosis of Robertsonian translocation
Various factors can affect PGD outcomes, including the sex of the carrier, the type of translocation, the type of biopsy used, and the number of normal/balanced embryos. Educating couples about the chance of successful pregnancy prior to PGD can eliminate concerns regarding these associated factors. The principal factor that affects pregnancy outcome is the proportion of normal/balanced embryos, which was confirmed in the present work [5, 21].
Analyses of chromosomal segregation in RT carriers have shown that the proportion of normal/balanced gametes is much higher in male than in female carriers [4, 5]. Moreover, the percentage of abnormal gametes is correlated with the proportion of abnormal embryos in translocation carriers [22, 23]. The frequencies of miscarriage and stillbirth are higher in female than in male der(13;14) carriers [24]. Thus, the sex of the carrier may influence PGD results. We found that the proportion of normal or balanced embryos was higher in couples with a male (32.1%) than in those with a female (27.7%) RT carrier, although this difference was not statistically significant. No between-group difference in clinical pregnancy or live birth rate was found. Assessments of meiotic segregation in the spermatozoa of male RT carriers have indicated that alternate segregation is very common. However, alternate segregation patterns differed in male and female RT carriers, with the latter showing a higher production rate of unbalanced gametes [16, 25–27]. Embryo karyotype did not correlate with the number of alternate embryos analyzed by carrier age, sex, or type of translocation [28]. Thus, although sex differences may influence the proportion of normal/balanced gametes, such differences do not occur at the embryonic stage and therefore do not affect the outcomes of PGD. These findings may be attributable to the high degree of complexity characterizing human gamete production, post-meiotic events, and embryonic development, with differences blurred by the natural selection process that takes place within the embryo. In addition, abnormal gametes may be present in the partner (with a normal karyotype) of a carrier, and embryonic data may thus be biased toward a higher proportion of unbalanced embryos [27]. Although PGD outcomes were not correlated with the sex of the RT carriers in our study, the sample size was insufficient to draw a firm conclusion on this topic and further investigation is warranted.
Pregnancy rates were 26.1% for der(13;14) RT carriers, 18.2% for der(14;21) RT carriers, and 25% for carriers of the remaining minor types of RT. Although the pregnancy rate was somewhat higher for der(13;14) than for der(14;21) RT carriers, this difference was not statistically significant. Moreover, no significant difference was present between these RT types and any other RT type.
No significant difference in the morphological characteristics of normal/balanced and abnormal embryos was evident. Morphological criteria have been found to be unreliable when used to select normal blastocysts from normal or balanced embryos [29]. We experienced embryo transfer failure in nine cycles because no normal embryo was available. Due to the relatively low fertilization and embryonic development rates in RT carriers compared with normal individuals, the proportional association between pregnancy rate and the available number of normal gametes differs, as successful outcomes require a satisfactory number of oocytes and embryos. Thus, to obtain one or more normal or balanced embryos for transfer, at least six oocytes must be retrieved and at least four embryos per cycle must be biopsied.
We encountered two incidences of chemical pregnancy and one miscarriage after confirmation of a positive heartbeat. Cytogenetic analysis of the aborted fetus revealed a normal karyotype (46,XX). No other known factor contributed to recurrent abortion in these three couples. Although the miscarried fetus had a normal karyotype, spontaneous abortion may also be caused by embryonic mosaicism. Indeed, higher levels of mosaicism have been reported in the embryos of RT carriers than in those of normal couples [28].
PGD outcomes were not correlated with the sex of the RT carrier or the type of translocation. However, the proportion of normal/balanced embryos and the number of embryos available for transfer significantly affected PGD outcomes. Generally, >50% of embryos obtained from RT carriers showed abnormal chromosomal constitutions, and only ~31% were normal or balanced. Therefore, to obtain one or more normal or balanced embryos for transfer, at least six oocytes should be retrieved and at least four per cycle should be biopsied.
We found that PGD dramatically reduced the pregnancy loss rate and shortened the time required for a successful live birth. However, the ability of RT carriers to achieve successful natural pregnancies must be carefully considered before PGD is suggested, especially in couples who are young, fertile, and have no history of recurrent miscarriage. PGD may not be appropriate for male RT carriers with normal sperm analyses. However, PGD may be an appropriate option for couples with a history of pathologic pregnancy (malformation or stillbirth), recurrent abortion, one or more abortions associated with chromosomal imbalance, advanced age, or infertility.
Acknowledgments
This study was supported by a grant (no. A084923) from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea.
This work was presented, in part, at the 64th Annual Meeting of the American Society for Reproductive Medicine, San Francisco, CA, on November 8, 2008.
Footnotes
Capsule Couples with RT may have benefit from PGD by improving success rate of pregnancy with reducing loss and ratio of normal or balance embryo has value in predicting PGD outcome.
References
- 1.Fryns J-P, Buggenhout G. Structural chromosome rearrangements in couples with recurrent fetal wastage. Eur J Obstet Gynecol Reprod Bio. 1998;81:171–176. doi: 10.1016/S0301-2115(98)00185-7. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen J, Wohlert M. Chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Hum Genet. 1991;87:81–83. doi: 10.1007/BF01213097. [DOI] [PubMed] [Google Scholar]
- 3.Therman E, Susman B, Denniston C. The nonrandom participation of human acrocentric chromosomes in Robertsonian translocations. Ann Hum Genet. 1989;53:49–65. doi: 10.1111/j.1469-1809.1989.tb01121.x. [DOI] [PubMed] [Google Scholar]
- 4.Guttenbach M, Engel W, Schmid M. Analysis of structural and numerical chromosome abnormalities in sperm of normal men and carriers of constitutional chromosome aberrations. A review. Hum Genet. 1997;100:1–21. doi: 10.1007/s004390050459. [DOI] [PubMed] [Google Scholar]
- 5.Munne S, Sandalinas M, Escudero T, Fung J, Gianaroli L, Cohen J. Outcome of preimplantation genetic diagnosis of translocations. Fertil Steril. 2000;73:1209–1218. doi: 10.1016/S0015-0282(00)00495-7. [DOI] [PubMed] [Google Scholar]
- 6.Munne S, Scott R, Sable D, Cohen J. First pregnancies after preconception diagnosis of translocations of maternal origin. Fertil Steril. 1998;69:675–681. doi: 10.1016/S0015-0282(97)00568-2. [DOI] [PubMed] [Google Scholar]
- 7.Tharapel AT, Tharapel SA, Bannerman RM. Recurrent pregnancy losses and parental chromosome abnormalities: a review. BJOG. 1985;92:899–914. doi: 10.1111/j.1471-0528.1985.tb03069.x. [DOI] [PubMed] [Google Scholar]
- 8.Conn CM, Harper JC, Winston RM, Delhanty JD. Infertile couples with Robertsonian translocations: preimplantation genetic analysis of embryos reveals chaotic cleavage divisions. Hum Genet. 1998;102:117–123. doi: 10.1007/s004390050663. [DOI] [PubMed] [Google Scholar]
- 9.Emiliani S, Gonzalez-Merino E, Bergh M, Abramowicz M, Englert Y. Higher degree of chromosome mosaicism in preimplantation embryos from carriers of robertsonian translocation t(13:14) J Assist Reprod Genet. 2003;20:95–100. doi: 10.1023/A:1021796226031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scriven PN, Flinter FA, Braude PR, Ogilvie CM. Robertsonian translocations–reproductive risks and indications for preimplantation genetic diagnosis. Hum Reprod. 2001;16:2267–2273. doi: 10.1093/humrep/16.11.2267. [DOI] [PubMed] [Google Scholar]
- 11.Munne S, Cohen J. Chromosome abnormalities in human embryos. Hum Reprod Update. 1998;4:842–855. doi: 10.1093/humupd/4.6.842. [DOI] [PubMed] [Google Scholar]
- 12.Coonen E, Dumoulin JC, Ramaekers FC, Hopman AH. Optimal preparation of preimplantation embryo interphase nuclei for analysis by fluorescence in-situ hybridization. Hum Reprod. 1994;9:533–537. doi: 10.1093/oxfordjournals.humrep.a138540. [DOI] [PubMed] [Google Scholar]
- 13.Verlinsky Y, Tur-Kaspa I, Cieslak J, Bernal A, Morris R, Taranissi M, Kaplan B, Kuliev A. Preimplantation testing for chromosomal disorders improves reproductive outcome of poor-prognosis patients. Reprod Biomed Online. 2005;11:219–225. doi: 10.1016/S1472-6483(10)60961-3. [DOI] [PubMed] [Google Scholar]
- 14.Otani T, Roche M, Mizuike M, Colls P, Escudero T, Munne S. Preimplantation genetic diagnosis significantly improves the pregnancy outcome of translocation carriers with a history of recurrent miscarriage and unsuccessful pregnancies. Reprod Biomed Online. 2006;13:869–874. doi: 10.1016/S1472-6483(10)61037-1. [DOI] [PubMed] [Google Scholar]
- 15.Sermon KD, Michiels A, Harton G, Moutou C, Repping S, Scriven PN, SenGupta S, Traeger-Synodinos J, Vesela K, Viville S, Wilton L, Harper JC. ESHRE PGD Consortium data collection VI: cycles from January to December 2003 with pregnancy follow-up to October 2004. Hum Reprod. 2007;22:323–336. doi: 10.1093/humrep/del402. [DOI] [PubMed] [Google Scholar]
- 16.Munne S, Escudero T, Sandalinas M, Sable D, Cohen J. Gamete segregation in female carriers of Robertsonian translocations. Cytogenet Cell Genet. 2000;90:303–308. doi: 10.1159/000056793. [DOI] [PubMed] [Google Scholar]
- 17.Stephenson MD, Sierra S. Reproductive outcomes in recurrent pregnancy loss associated with a parental carrier of a structural chromosome rearrangement. Hum Reprod. 2006;21:1076–1082. doi: 10.1093/humrep/dei417. [DOI] [PubMed] [Google Scholar]
- 18.Carp HJ, Dirnfeld M, Dor J, Grudzinskas JG. ART in recurrent miscarriage: preimplantation genetic diagnosis/screening or surrogacy? Hum Reprod. 2004;19:1502–1505. doi: 10.1093/humrep/deh293. [DOI] [PubMed] [Google Scholar]
- 19.Sugiura-Ogasawara M, Ozaki Y, Sato T, Suzumori N, Suzumori K. Poor prognosis of recurrent aborters with either maternal or paternal reciprocal translocations. Fertil Steril. 2004;81:367–3. doi: 10.1016/j.fertnstert.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Franssen MT, Korevaar JC, Veen F, Leschot NJ, Bossuyt PM, Goddijn M. Reproductive outcome after chromosome analysis in couples with two or more miscarriages: index [corrected]-control study. BMJ. 2006;332:759–763. doi: 10.1136/bmj.38735.459144.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verlinsky Y, Cieslak J, Evsikov S, Galat V, Kuliev A. Nuclear transfer for full karyotyping and preimplantation diagnosis for translocations. Reprod Biomed Online. 2002;5:300–305. doi: 10.1016/S1472-6483(10)61836-6. [DOI] [PubMed] [Google Scholar]
- 22.Escudero T, Abdelhadi I, Sandalinas M, Munne S. Predictive value of sperm fluorescence in situ hybridization analysis on the outcome of preimplantation genetic diagnosis for translocations. Fertil Steril. 2003;79:1528–1534. doi: 10.1016/S0015-0282(03)00252-8. [DOI] [PubMed] [Google Scholar]
- 23.Escudero T, Lee M, Carrel D, Blanco J, Munne S. Analysis of chromosome abnormalities in sperm and embryos from two 45, XY, t(13:14) Prenat Diag. 2000;20:599–602. doi: 10.1002/1097-0223(200007)20:7<599::AID-PD883>3.3.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 24.Engels H, Eggermann T, Caliebe A, Jelska A, Schubert R, Schüler HM, Panasiuk B, Zaremba J, Latos-Bieleńska A, Jakubowski L, Zerres KP, Schwanitz G, Midro AT. Genetic counseling in Robertsonian translocations der(13;14): frequencies of reproductive outcomes and infertility in 101 pedigrees. Am J Med Genet Part A. 2008;146A:2611–2616. doi: 10.1002/ajmg.a.32500. [DOI] [PubMed] [Google Scholar]
- 25.Ogur G, Assche E, Vegetti W, Verheyen G, Tournaye H, Bonduelle M, Steirteghem A, Liebaers I. Chromosomal segregation in spermatozoa of 14 Robertsonian translocation carriers. Mol Hum Reprod. 2006;12:209–215. doi: 10.1093/molehr/gah253. [DOI] [PubMed] [Google Scholar]
- 26.Anahory T, Hamamah S, Andreo B, Hedon B, Claustres M, Sarda P, Pellestor F. Sperm segregation analysis of a (13;22) Robertsonian translocation carrier by FISH: a comparison of locus-specific probe and whole chromosome painting. Hum Reprod. 2005;20:1850–1854. doi: 10.1093/humrep/deh886. [DOI] [PubMed] [Google Scholar]
- 27.Munne S. Analysis of chromosome segregation during preimplantation genetic diagnosis in both male and female translocation heterozygotes. Cytogenet Genome Res. 2005;111:305–309. doi: 10.1159/000086904. [DOI] [PubMed] [Google Scholar]
- 28.Jin H, Ping L, Jie Q, Ying L, Yongjian C. Translocation chromosome karyotypes of the Robertsonian translocation carriers' embryos. Fertil Steril. 2009;93:1061–1065. doi: 10.1016/j.fertnstert.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 29.Evsikov S, Cieslak J, Verlinsky Y. Effect of chromosomal translocations on the development of preimplantation human embryos in vitro. Fertil Steril. 2000;74:672–677. doi: 10.1016/S0015-0282(00)01513-2. [DOI] [PubMed] [Google Scholar]
- 30.Huang J, Lian Y, Qiao J, Chen Y, Ren X, Liu P. Characteristics of embryo development in Robertsonian translocations' preimplantation genetic diagnosis cycles. Prenat Diagn. 2009;29:1167–1170. doi: 10.1002/pd.2376. [DOI] [PubMed] [Google Scholar]
- 31.Gianaroli L, Magli MC, Ferraretti AP, Munne S, Balicchia B, Escudero T, Crippa A Possible interchromosomal effect in embryos generated by gametes from translocation carriers. Hum Reprod. 2002;17:3201–3207. doi: 10.1093/humrep/17.12.3201. [DOI] [PubMed] [Google Scholar]
- 32.Kyu Lim C, Hyun Jun J, Mi Min D, Lee HS, Young Kim J, Koong MK, Kang IS. Efficacy and clinical outcome of preimplantation genetic diagnosis using FISH for couples of reciprocal and Robertsonian translocations: the Korean experience. Prenat Diagn. 2004;24:556–561. doi: 10.1002/pd.923. [DOI] [PubMed] [Google Scholar]
- 33.Fischer J, Colls P, Escudero T, Munne S. Preimplantation genetic diagnosis (PGD) improves pregnancy outcome for translocation carriers with a history of recurrent losses. Fertil Steril. 2009;94:283–289. doi: 10.1016/j.fertnstert.2009.02.060. [DOI] [PubMed] [Google Scholar]
- 34.Goddijn M, Joosten JH, Knegt AC, VanderVeen F, Franssen MT, Bonsel GJ, Leschot NJ. Clinical relevance of diagnosing structural chromosome abnormalities in couples with repeated miscarriage. Hum Reprod. 2004;19:1013–1017. doi: 10.1093/humrep/deh172. [DOI] [PubMed] [Google Scholar]