Abstract
Identifying addicts with higher risk of relapse would provide the opportunity to implement individualized interventions and increase cessation success rates. Unfortunately, the ability to predict the long-term success of drug-cessation treatments continues to elude researchers. We tested whether brain responses to emotional and cigarette-related pictures were predictive of the ability to abstain from smoking. Smokers interested in quitting (n=180) participated in a smoking cessation clinical trial. Before the initiation of any treatment we recorded event-related potentials (ERPs) evoked by emotional (both pleasant and unpleasant), neutral, and cigarette-related images. Cluster analysis was used to assign smokers to two groups based on the amplitude of the late positive potential (LPP) to the experimental stimuli. While both groups showed enhanced responses to cigarette-related cues, one group (n=81) also showed blunted brain responses to intrinsically pleasant stimuli. Smokers in the latter group were significantly less likely to be abstinent at 10, 12, and 24 weeks after their quit date. In conclusion, using event-related potentials, a direct measure of brain activity, we found that smokers with blunted brain responses to intrinsically pleasant stimuli had lower rates of long-term smoking abstinence. This response offers a new biomarker for identifying smokers at higher risk of relapse and for testing the efficacy of new interventions aimed at normalizing brain reward systems’ responses to intrinsically pleasant stimuli.
Keywords: Emotions, ERPs, LPP, nicotine addiction, reward sensitivity, smoking cessation
Introduction
Smoking accounts for 12% of global adult mortality. If current smoking patterns continue, tobacco use will result in about 10 million deaths per year by 2020 (Mackay, Erickson, & Shafey, 2006). A crucial step to changing this trend is to increase the long-term success of smoking cessation interventions. Less that 6% of those who make a cessation attempt are abstinent 1 year later (US Department of Health and Human Services, 2010). Current theoretical models attribute the high relapse rates to drug-induced changes in brain circuits underlying emotion and cognition: Repeated drug use increases motivational relevance of the drug and its associated cues and reduces the motivational relevance of natural rewards (Goldstein & Volkow, 2002; Koob & Volkow, 2010; Volkow et al., 2010). Thus, smokers trying to quit face an environment in which cigarettes and cigarette-related cues are salient and attractive, whereas everyday pleasurable stimuli may have reduced motivational relevance.
Although several empirical studies have supported the idea that smokers process cigarette-related cues as motivationally relevant stimuli (Cinciripini et al., 2006; David et al., 2005; Due, Huettel, Hall, & Rubin, 2002; Littel & Franken, 2007) and others showed that brain responses to these cues predict short-term abstinence (Janes et al., 2010), very few studies have investigated whether smokers actually exhibit reduced sensitivity to natural rewards (i.e., intrinsically pleasant stimuli). It is important to study smokers’ responses to natural rewards because decreased sensitivity to these stimuli has been observed in cocaine addicts (Garavan et al., 2000) and has been shown to predict relapse in alcoholics (Heinz et al., 2007). Thus, brain responses to natural rewards may also provide clinically relevant information in the context of smoking-cessation interventions.
Recently, we used event-related potentials (ERPs), voltage fluctuations in the electroencephalogram that are time locked to external events, to assess smokers’ neural responses in the presence of intrinsically emotional, neutral, and cigarette-related images (Versace et al., 2011). Consistent with the idea that nicotine addiction increases the motivational relevance of drug-related cues, we found that the amplitude of the late positive potential (LPP), the ERP signature of emotional processing (Lang & Bradley, 2009), increased in the presence of cigarette-related images. However, contrary to the idea that nicotine addiction leads to reduced sensitivity to natural rewards, we observed no attenuation of the LPP in response to intrinsically pleasant images. One possible explanation is that by averaging the ERP responses to emotional stimuli across subjects, individual differences in reward sensitivity were obscured. To uncover individual differences in reward sensitivity associated with successful smoking cessation, in the present study we used cluster analysis to group smokers on the basis of their ERP responses to emotional and cigarette-related images.
Materials and Methods
Participants
Participants were recruited via local (Houston metropolitan area) radio and newspaper advertisements requesting volunteers who wanted to quit smoking and were willing to participate in a clinical trial of smoking-cessation medications. The smoking cessation treatment lasted 10 weeks and included behavioral counseling and one of three pharmacological treatments (placebo, bupropion or varenicline). Double blind procedures were followed for the treatment’s pharmacological component. To participate, smokers had to be aged 18-65 years, smoke 5 or more cigarettes per day, have a baseline expired carbon monoxide (CO) level greater than 6 ppm, be fluent in English, have a working telephone, not be currently taking psychotropic medication, not have a current psychiatric disorder including substance abuse (except for smoking), not be involved in any smoking cessation activities, not have contraindications for bupropion or varenicline, and not have any uncontrolled medical illness. The presence of psychiatric disorders was assessed using the MINI International Neuropsychiatric Interview (Lecrubier et al., 1997; Sheehan et al., 1998). A total of 208 eligible participants completed a baseline laboratory session, which was the source of our data. This session was conducted before any treatment randomization or intervention. Because of poor recording quality (24 participants) or technical errors (4 participants), laboratory data from 28 participants were discarded, yielding a total of 180 participants in this study. All participants provided written informed consent before being subjected to any study procedure, and the research was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board.
Material and design
Three equivalent picture sets were created by selecting pictures from the International Affective Picture System (Lang, Bradley, & Cuthbert, 2005) and from cigarette-related picture collections previously used in our (Carter et al., 2006) and other (Gilbert & Rabinovich, 1999) laboratories. Each set included 4 picture categories (unpleasant [UNP], neutral [NEU], pleasant [PLE], and cigarette-related [CIG]) with 24 pictures each (96 total pictures per set). Each category included the same number of pictures depicting scenes in which human beings were present (n=16) or absent (n=8). For pleasant and unpleasant categories, pictures depicting scenes in which human beings were present were further divided into two contents: High emotional arousal (n=8) and low emotional arousal (n=8). This resulted in 10 different semantic contents: Mutilations (MUT; unpleasant high arousal), sad scenes (SAD; e.g., grief, disease; unpleasant low arousal), unpleasant objects (UNPo; e.g., pollution, accidents; unpleasant low arousal), erotic couples (ERO; pleasant high arousal), romantic couples (ROM; pleasant low arousal), pleasant objects (PLEo; e.g., food, landscapes; pleasant low arousal), neutral people (NEUp), neutral objects (NEUo; e.g., household objects), people smoking (CIGp), and cigarette-related objects (CIGo; e.g., ashtrays, cigarettes)1. Pictures were presented in pseudo-random sequences with no more than two pictures of the same category presented consecutively. Each picture was presented for 4 s and was followed by a random intertrial interval of 3-5 s, during which the screen had a black background with a white fixation cross. The entire picture-viewing session lasted approximately 30 min. To improve the signal to noise ratio with the intent of examining ERPs to each semantic content, each picture was presented twice during the session. The stimuli were presented the second time in a different pseudo-random order after the completion of the initial presentation cycle. The session was divided into 8 equivalent blocks lasting 3.2 min each and separated by a 30-s interval, during which the subject was instructed to relax. During the picture presentation, 1/4 of the pictures in each category were startle probed by presenting a burst of 100 dB(A) white noise for 50 ms between 2.5 and 3.5 s after picture onset. Since the LPP peaks between 400 and 700 ms after picture onset, the presentation of the probes did not affect the results reported here. Stimuli were presented with a Pentium 4 computer using Psychology Tools’ E-prime software (v1.4; Pittsburgh, PA) on a plasma screen placed approximately 1.5 m from the participant’s eyes. The pictures subtended a horizontal viewing angle of approximately 24°.
Procedure
Participants were instructed to smoke normally before the laboratory session so as to be in a non-deprived state. Upon arrival at the laboratory, participants provided an expired carbon monoxide (CO) sample and completed the Fagerström Test for Nicotine Dependence (FTND) (Heatherton, Kozlowski, Frecker, & Fagerström, 1991), the Wisconsin Inventory of Smoking Dependence Motives (Piper et al., 2004), the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977), the Depression Proneness Inventory (DPI) (Alloy, Abramson, Metalsky, & Harlages, 1990; Strong, Brown, Kahler, Lloyd-Richardson, & Niaura, 2004), the BIS/BAS scales (Carver & White, 1994) and the Fawcett-Clark Pleasure Scale (FCPS) (Fawcett, Clark, Scheftner, & Gibbons, 1983). After completing the questionnaires, participants were escorted to the laboratory and the recording electrodes were applied. The picture presentation began after a 15-min adaptation period and delivery of six habituation startle probes. Participants were instructed to comfortably sit on a reclining chair, look at the pictures presented on the screen, and ignore the loud noises sometimes delivered through the earphones.
During the picture presentation, the electroencephalogram (EEG) was recorded using a 129-channel Geodesic Sensor Net, amplified with an AC-coupled high input impedance (200 MΩ) amplifier (Geodesic EEG System 200; Electrical Geodesics Inc., Eugene, OR), and referenced to the Cz electrode site. The sampling rate was 250 Hz, and data were filtered online by using 0.1 Hz high-pass and 100 Hz low-pass filters. Scalp impedance of each sensor was kept below 50 KΩ, as suggested by the manufacturer.
ERP data reduction
After data collection, a 30-Hz low-pass filter was applied off-line. Data were visually inspected, and channels contaminated by artifacts for more than 50% of the recording were interpolated with use of spherical splines. On average, approximately 2% of the channels met this criterion and were interpolated. Eye blinks were then corrected with a spatial filtering method as implemented in BESA (v5.1.8.10; MEGIS Software GmbH, Grafelfing, Germany). After eye blink correction, the EEG data were transformed to the average reference and segmented into 900-ms segments starting 100 ms before onset of the picture. Baseline was defined as the 100-ms interval preceding the picture. Using the segmented data, artifacts affecting sensors within specific trials were identified. Artifacts were defined as EEG amplitude above 100 or below − 100 μV, absolute voltage difference between any two data points within the segment larger than 100 μV, voltage difference between two contiguous data points above 25 μV, or variation of less than 0.5 μV for more than 100 ms. A segment was excluded from the subsequent averages if more than 10% of the sensors within the segment were contaminated by artifacts. Overall, fewer than 5% of the segments were excluded. At the end of this process, the average ERPs were calculated at each scalp site for each category (i.e., pleasant, unpleasant, neutral, and cigarette-related).
Visual inspection of grand-averaged ERPs confirmed results from previous studies (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; Keil et al., 2002; Schupp et al., 2000; Weinberg & Hajcak, 2010): Presentation of motivationally relevant pictures (including cigarette-related ones) increased the amplitude of the LPP over central and parietal sensors. The largest difference between neutral and motivationally relevant pictures was observed at about 600 ms after picture onset. Voltages from 10 sensors covering the area with the largest LPP differences between neutral and motivationally relevant pictures were averaged (Fig. 1), and the mean LPP amplitude between 400 and 700 ms after picture onset was calculated for each category (UNP, NEU, PLE, CIG) for each participant. The same procedures described above were also used to obtain LPP amplitude associated with the various semantic contents (i.e., MUT, SAD, UNPo, NEUp, NEUo, ERO, ROM, PLEo, CIGp, CIGo).
Figure 1.
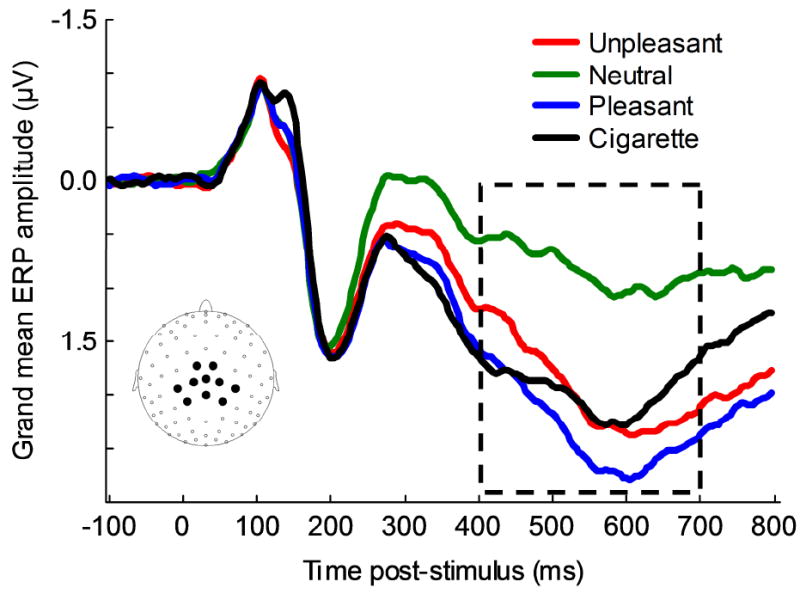
Event-related potentials to unpleasant, neutral, pleasant, and cigarette-related pictures. The waveforms represent grand-averages from 10 electrodes (see inset for electrode location). The box indicates the time window used to calculate the LPP amplitude for each picture category.
Data analyses
The four category LPP values (UNP, NEU, PLE, CIG) from each participant were entered into a cluster analysis (k-means method) to assign smokers to two groups based on their brain responses to the experimental visual stimuli. Cluster analysis is a group of multivariate techniques whose purpose is to classify objects (i.e., participants) based on the characteristics that they possess (Hair & Black, 2000). The k-means method allows the formation of an a priori number of clusters, k. We hypothesized the presence of k = 2 clusters of smokers on the basis of their sensitivity to intrinsically pleasant stimuli. The program starts with k random clusters, and then moves objects between those clusters with the goal of 1) minimizing variability within clusters and 2) maximizing variability between clusters. To prevent the classification algorithm from being biased by individual differences in absolute voltage amplitude, the four LPP values of each participant were z-transformed before cluster analysis. Cluster centers were computed by first sorting distances between all participants and then choosing participants at constant intervals as initial cluster centers. The algorithm performed 90 iterations. The nature of the differences between clusters was examined by means of a two-way ANOVA using “cluster” as a between-subjects factor (cluster 1 vs. cluster 2) and “valence” as a within-subjects factor (unpleasant, neutral, pleasant, cigarette). The cluster analysis and ANOVA were computed using Statistica (v7.1; Statsoft Inc., Tulsa, OK).
The primary abstinence outcomes were self-reported abstinence at 24 hours, 4 weeks, 10 weeks, 12 weeks, and 24 weeks after the quit day. For the first measurement (i.e., 24 hours), self-reported 24 hour abstinence was used, while for all the other measurements, 7-day point prevalence (no smoking, not even a puff in the last 7 days) was used (Hughes et al., 2003). Abstinence at all time points was biochemically verified (expired carbon monoxide level ≤ 10 ppm). We used PROC LOGISTIC procedures in SAS (SAS Institute Inc, Cary, NC, version 9.1) to perform logistic regression analyses that regressed abstinence outcome on cluster membership. Additional logistic regression analyses were conducted to examine the effect of cluster membership on smoking abstinence while controlling for the effect of treatment (medication), which was initiated subsequent to the laboratory session.
Results
Participant Demographics and Smoking History
Table 1 provides the demographic characteristics of the sample used for data analyses.
Table 1.
Baseline Demographic and Smoking Characteristics
| Variable | Cluster 1 (n=99) % (N) | Cluster 2 (n=81) % (N) | Total (N=180) % (N) |
|---|---|---|---|
| Gender | |||
| Female | 31.3 (31) | 39.5 (32) | 35.0 (63) |
| Race/Ethnicity | |||
| African-American, Non-Hispanic | 23.2 (23) | 32.1 (26) | 27.2 (49) |
| White, Including Hispanic | 70.7 (70) | 65.4 (53) | 68.3 (123) |
| Other, Non-Hispanic | 6.1 (6) | 2.5 (2) | 4.4 (8) |
|
| |||
| Mean (SD) | Mean (SD) | Mean (SD) | |
|
| |||
| Age (years) | 45.35 (10.7) | 44.86 (10.3) | 45.13 (10.5) |
| Years smoking | 24.49 (12.1) | 25.03 (11.1) | 24.73 (11.6) |
| Current smoking rate (cigs/day) | 19.20 (8.4) | 18.99 (8.2) | 19.10 (8.3) |
| Expired CO (ppm) | 22.81 (11.6) | 26.22 (13.3) | 24.34 (12.4) |
| FTND score | 4.51 (2.2) | 4.63 (2.1) | 4.57 (2.1) |
| WISDM (Total Score) | 4.14 (1.0) | 4.03 (1.0) | 4.09 (1.0) |
| CES-D (Total Score) | 7.90 (6.4) | 7.42 (7.6) | 7.68 (7.0) |
| Depression Proneness Inventory | 2.50 (1.0) | 2.60 (1.1) | 2.54 (1.0) |
| Fawcett-Clark Pleasure Scale | 3.72 (0.4) | 3.83 (0.4) | 3.77 (0.4) |
|
| |||
| BAS | 27.28 (5.6) | 27.43 (5.1) | 27.35 (5.4) |
| BIS | 14.43 (3.7) | 14.24 (3.3) | 14.34 (3.5) |
Note. All frequencies are calculated within group (column). CO = carbon monoxide; FTND = Fagerstrom Test for Nicotine Dependence; WISDM = Wisconsin Inventory of Smoking Dependence Motives; CES-D= The Center for Epidemiologic Studies’ Depression Scale; BAS = Behavioral Approach System; BIS = Behavioral Inhibition System.
ERPs unmask individual differences in sensitivity to intrinsically pleasant stimuli
The cluster analysis assigned 99 participants to cluster 1 and 81 participants to cluster 2. The mean LPPs to cigarette, pleasant, neutral, and unpleasant pictures as a function of cluster are presented in Figure 2a. The nature of the significant cluster by valence interaction [F(3,534) = 43.49, p < 0.00001] was investigated using post hoc comparisons with Bonferroni corrections. While both neutral and unpleasant pictures prompted similar brain responses in the two groups, pleasant pictures prompted a significantly smaller LPP in cluster 2 than in cluster 1 (p < 0.0001); cigarette-related pictures had the opposite effect, evoking a somewhat larger LPP in cluster 2 than in cluster 1 (p < 0.10). Follow-up analyses using the subcategories defined by semantic content of the pictures determined that the reduced brain reactivity in the presence of intrinsically pleasant stimuli was evident for both low-arousing and high-arousing pleasant stimuli (Figure 2b). Post hoc comparisons with Bonferroni correction showed that the differences between clusters were significant (ps < 0.05) for erotica, romance, and pleasant objects, approached significance (ps < 0.10) for people smoking and cigarette-related objects, and were not significant for all subcategories of unpleasant and neutral pictures [ps > .40]. Taken together these results indicate that while all smokers showed enhanced brain reactions in the presence of cigarette-related stimuli, 45% of them were also characterized by a generalized hypoactivation of the brain reward systems in the presence of intrinsically pleasant stimuli.
Figure 2.
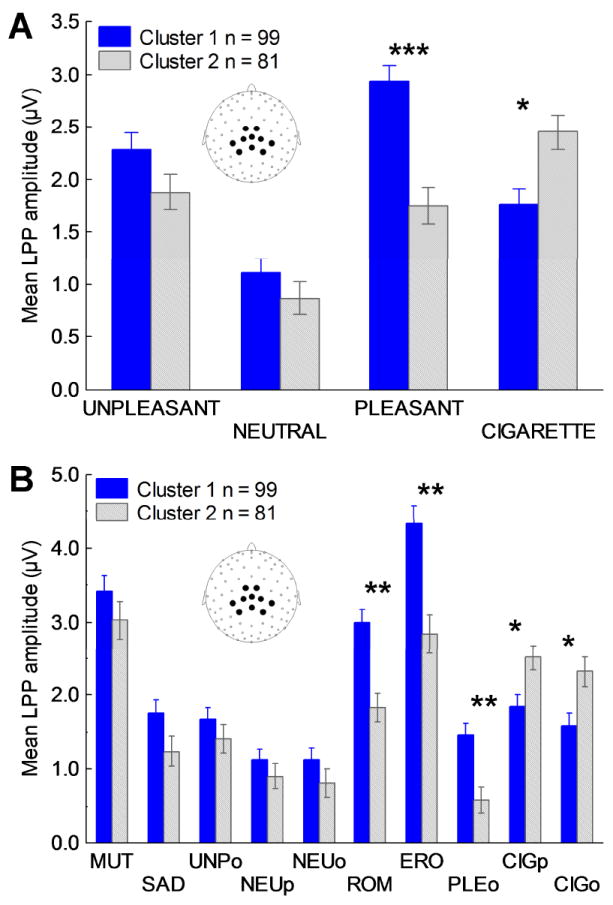
A) Mean late positive potentials (LPPs) from centro-parietal sensors (see inset for electrode locations) evoked before smoking-cessation treatment by unpleasant, neutral, pleasant, and cigarette-related stimuli in smokers assigned to clusters 1 and 2. B) Mean LPPs from centro-parietal sensors evoked by the different semantic contents in smokers assigned to clusters 1 and 2. Unpleasant contents: mutilations (MUT; high emotional arousal), sad (SAD; low emotional arousal; e.g., grief, disease), and objects (UNPo; e.g., pollution, accidents). Pleasant contents: erotic couples (ERO; high emotional arousal), romantic couples (ROM; low emotional arousal), and objects (PLEo; e.g., food, landscapes). Neutral contents: people (NEUp) and objects (NEUo; e.g., household objects). Cigarette-related contents: people smoking (CIGp) and cigarette-related objects (CIGo; e.g., ashtrays, cigarettes). Note: *** = p < 0.00001, ** = p < 0.05, * = p < 0.10. All p levels are corrected for multiple comparisons.
Questionnaire assessment
The presence of differences between the two clusters was further investigated by comparing results obtained from self-report measures of nicotine dependence (cigarette smoked per day, FTND, WISDM), depressed mood (CES-D), and trait affective disposition (BIS/BAS, DPI, FCPS). None of the differences observed between the two groups approached significance (Table 1). The lack of differences when self report questionnaires and demographic variables are considered indicates that ERPs to emotional stimuli might capture an endophenotype marker closer to the underlying biology of smoking addiction than the self-report questionnaires.
Brain sensitivity to intrinsically pleasant stimuli predicts smoking abstinence
To determine whether sensitivity to intrinsically pleasant stimuli influences the ability to successfully quit smoking, we performed separate logistic regressions to regress abstinence status at each post-quit time point (24 hours, 4 weeks, 10 weeks, 12 weeks and 24 weeks) on cluster membership. As shown in Figure 3, at 24 hours (odds ratio [OR] = .67, 95% confidence interval [CI]: .35–1.26) and at 4 weeks (OR =.62, 95% CI: .34-1.11) post-quit, the two groups did not differ significantly in abstinence. However, smokers showing reduced sensitivity to intrinsically pleasant stimuli (cluster 2) were significantly less likely to abstain from smoking at 10 weeks (OR = .52, 95% CI: .28–.94), 12 weeks (OR = .50, 95% CI: .27 – .91), and 24 weeks (OR = .46, 95% CI: .24 – .88) after the quit day. The results remain unchanged after we controlled for the effect of smoking-cessation medication on abstinence.
Figure 3.
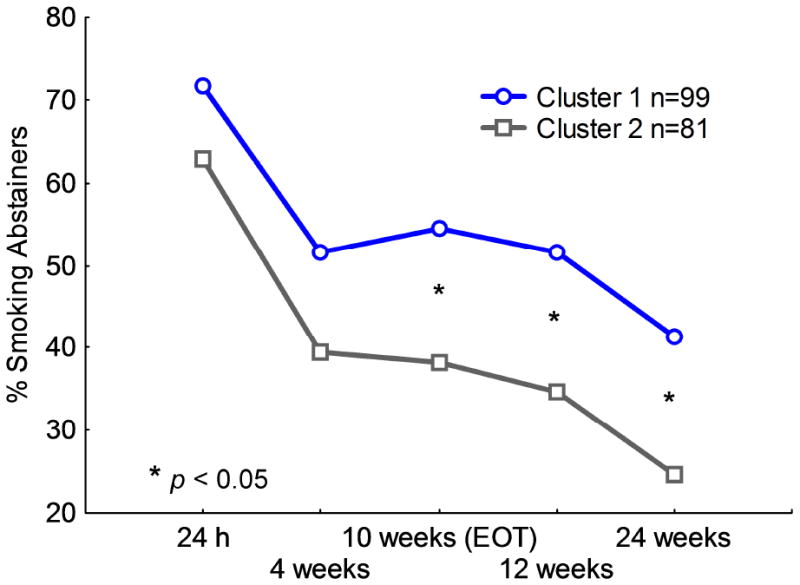
Abstinence rates in smokers with normal sensitivity to intrinsically pleasant stimuli (cluster 1) and with reduced sensitivity to intrinsically pleasant stimuli (cluster 2) during the smoking-cessation trial. EOT = End of treatment.
Discussion
Using ERPs, a direct measure of brain activity, we showed that smokers with blunted brain responses to intrinsically pleasant stimuli prior to quitting have significantly lower long-term abstinence rates. These data are consistent with theoretical models claiming that repeated drug use leads to the attribution of excessive motivational value to the drug of abuse and its associated cues, and to a concomitant reduction of the motivational value attributed to non-drug-related rewards (i.e., intrinsically pleasant stimuli). The predictive relationship between the ability of intrinsically pleasant stimuli to engage brain emotional systems and smoking-cessation outcome has important theoretical and clinical implications.
Chronic drug use is thought to result in the attribution of excessive motivational value to drug cues at the expense of naturally rewarding stimuli (Goldstein & Volkow, 2002; Koob & Volkow, 2010; Volkow et al., 2010). For smokers, several studies have shown significantly larger responses for cigarette cues than neutral cues in brain areas that are involved in the processing of emotionally arousing stimuli (David et al., 2005; Due et al., 2002; Janes et al., 2010; Versace et al., 2010; Versace et al., 2011). Consistent with these findings, cigarette-related pictures prompted more cortical positivity than neutral pictures in both groups of smokers in our study. Yet, unlike findings in previous studies, our results reveal the presence of a subgroup of smokers (45% of our sample) characterized by blunted brain responses in the presence of intrinsically pleasant stimuli. The blunted brain responses found in the presence of both low-arousing (romance and objects) and high-arousing (erotica) pleasant stimuli may indicate a generalized hypoactivation of the brain reward systems. In addition to our current findings in smokers, blunted brain responses to natural rewards have been observed in functional magnetic resonance imaging (fMRI) and ERP studies of cocaine addicts (Dunning et al., 2011; Garavan et al., 2000) and alcoholics (Heinz et al., 2007). Furthermore, cocaine addicts (Goldstein et al., 2010), heroin addicts (de Arcos et al., 2008; Lubman et al., 2009), and alcoholics (de Arcos, Verdejo-Garcia, Peralta-Ramirez, Sanchez-Barrera, & Perez-Garcia, 2005) all report lower subjective ratings of pleasantness than non-addicts when exposed to rewarding cues. Thus, as predicted by theoretical models (Goldstein & Volkow, 2002; Koob & Volkow, 2010; Volkow et al., 2010), devaluation of natural rewards appears to be a consequence of substance addiction in general. It is also possible that reduced reward sensitivity is a trait pre-existing smoking initiation that might increase sensitivity to the rewarding properties of nicotine. In line with this hypothesis, we observed higher LPPs to cigarette-related cues in smokers characterized by blunted brain responses to pleasant stimuli. Rubinstein and co-workers (Rubinstein, Luks, Dryden, Rait, & Simpson, 2011) also showed that decreased brain responses to pleasurable food pictures are already present in adolescent light smokers. The findings reported by Rubinstein et al. (2011) are in line with the hypothesis that decreased sensitivity to natural rewards might be a trait preceding addiction.
The subgroup of smokers with blunted brain responses to natural rewards were less likely to be abstinent at follow-up sessions ranging from 10 – 24 weeks after the beginning of a smoking-cessation attempt. This is consistent with a previous report from a study of alcoholics, where blunted fMRI responses to pleasant pictures in brain reward systems predicted relapse (Heinz et al., 2007). Another fMRI study found that female smokers with the largest responses to cigarette-related cues were most likely to relapse (Janes et al., 2010). That result is consistent with the trend toward larger LPP to cigarette cues in the group that had the higher relapse rate in our study (cluster 2). It is important to note though that Janes and co-workers did not include in their study intrinsically pleasant stimuli, the category where we observed the largest differences between groups. Given the correlation existing between electrocortical and hemodynamic measures of emotional reactivity (Sabatinelli, Lang, Keil, & Bradley, 2007), future fMRI studies including both emotional and drug-related stimuli will identify the specific brain regions with reduced reactivity to intrinsically pleasant stimuli.
By analyzing responses to specific semantic contents within each emotional valence, we showed that the ERP differences between groups span across a range of pleasant stimuli and are independent of emotional arousal. This finding supports the hypothesis that reward sensitivity, rather than overall levels of emotional reactivity, is responsible for differences between groups. However, it should be noted that although the largest LPP differences between groups were observed when pleasant stimuli were presented, the clustering algorithm took into account brain reactions to all picture categories when assigning individuals to clusters. In fact, when we tried to predict smoking cessation outcomes only on the basis of the LPP to pleasant pictures, the results were less accurate than those obtained using cluster analysis to determine group assignment.
Our findings suggest that a pattern characterized by both blunted brain reactivity to pleasant stimuli and enhanced reactivity to cigarette-related stimuli provides a more accurate metric for identifying individuals who might be at higher risk of relapse. Interestingly, previous studies that used ERPs to evaluate smokers’ brain responses to emotional stimuli (Gilbert et al., 2004; Gilbert et al., 2007) have not found significant effects involving reactivity to pleasant stimuli; rather, they suggest that nicotine deprivation biases smokers’ attention towards unpleasant stimuli. In fact, current theories of nicotine addiction emphasize the role of situational and state variables such as negative affect, withdrawal symptoms, stress, and cravings as the primary forces underlying relapse (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Kassel, Stroud, & Paronis, 2003; Piasecki, Fiore, McCarthy, & Baker, 2002; Shiffman et al., 2007). Our data were collected when participants were still smoking at their regular rate; thus, our findings do not challenge the relevance of abstinence-related negative affect in causing relapse. Rather, it appears that reduced sensitivity to natural rewards might be another significant factor that prevents smokers from achieving a smoke-free life.
It is also important to note that none of our self-report measures of smoking behavior, smoking reinforcement, nicotine addiction, depressed mood, and trait affective disposition differentiated between the two groups in the present study. This result demonstrates the potential clinical utility of the LPP as diagnostic biomarker. In future trials, decreased LPPs to intrinsically pleasant stimuli relative to cigarette-related and other emotional stimuli, could be used to identify smokers who are at the highest risk of relapse. Those individuals could be recommended for interventions specifically designed to address their hyposensitivity to natural rewards.
In this study we used ERPs rather than fMRI as a physiological predictor of relapse. ERP techniques have several advantages over fMRI for use in clinical settings. The risks associated with ERP recordings are negligible, and the recording setup is well tolerated by patients. Also, the acquisition of ERP data is relatively inexpensive, allowing data collection from large samples. These characteristics make ERPs a widely used tool in the diagnosis of certain neurological and sensory disorders. ERPs have also been proposed as biomarkers that could be used to improve the development of treatments for schizophrenia and other psychiatric disorders (Kenemans & Kahkonen, 2011; Luck et al., 2010). For example, Foti et al. (Foti, Olvet, Klein, & Hajcak, 2010) showed that symptoms of depression are associated with reduced LPP to fearful and angry faces. We are currently collecting ERP data in unmedicated depressed smokers attempting to quit to extend the results obtained by Foti et al. (Foti et al., 2010). We expect that evaluating brain responses to emotional stimuli using ERPs in smokers may one day be routinely used to refine diagnoses and choose the most appropriate treatment to maximize the probability of long-term smoking abstinence.
Overall, these data demonstrate that a large percentage of smokers are characterized by blunted brain responses to intrinsically pleasant stimuli. We think that this endophenotype might reflect the reduced ability of their brain reward systems to extract pleasure from non-drug-related activities, a trait that jeopardizes smokers’ goal of prolonged smoking abstinence. Future studies will clarify whether the reward system’s reduced sensitivity to intrinsically pleasant stimuli that characterizes these smokers is, as hypothesized, a consequence of chronic nicotine use or is a pre-existing condition that preceded smoking initiation and may have facilitated nicotine addiction in the first place. A recent ERP study by Dunning and co-workers (Dunning et al., 2011) supports the idea that drug use interferes with the motivational systems. In line with our findings, Dunning et al. (2011) showed that, unlike non-addicted controls and abstinent users, current cocaine users have reduced LPP to pleasant cues in a 400-1000 ms time window. They also reported that cocaine users did not show emotional modulation of the LPP in a later time window (1000-2000 ms). The authors attributed the latter result to a generalized impairment of the emotional system during cocaine use. Because of the relatively high cutoff of the low pass filter used to collect our data, we could not test emotional modulation in a later LPP time window. Future studies will evaluate the ability of smokers in sustaining motivated attention to emotional stimuli.
Another aspect that we did not evaluate in this study is the effect that stimulus repetition might have on ERPs. Previous work showed that the emotional modulation of the LPP is a robust phenomenon that is present even when pictures are repeated 90 times (Codispoti, Ferrari, & Bradley, 2006; Codispoti, Ferrari, & Bradley, 2007). In light of these results, it is unlikely that repeating each stimulus twice, as we did in this experiment, significantly reduced the LPP emotional modulation. However, it is possible that individuals with reduced sensitivity to pleasant stimuli might show rapid habituation to pleasant stimuli. Future experiments will test this hypothesis and will contribute to further clarifying the effects that nicotine use has on the reward system.
A final consideration is related to the presence of the rare startle probes during picture presentation. Although the startle probes were delivered almost 2 s after the LPP time window, their presence might have differentially affected picture processing. When we compared results from studies where startles probes were either present (Bradley, Codispoti, Cuthbert, & Lang, 2001; Schupp et al., 2004) or absent (Cuthbert et al., 2000; Lang, Greenwald, Bradley, & Hamm, 1993) during picture viewing, we concluded that the presence of startle probes does not affect the pattern of reactivity to emotional pictures It seems unlikely that the presence of startle probes may specifically alter the reactivity to cigarette-related pictures.
In conclusion, our data show that smokers with blunted brain responses to intrinsically pleasant stimuli have more difficulties in achieving long-term smoking abstinence. Because of their sensitivity to brain responses to both natural and drug-related rewards, ERPs might be an ideal biomarker to identify smokers who will benefit the most from interventions specifically aimed at restoring the saliency of natural rewards (Volkow & Li, 2005).
Acknowledgments
This work was supported by the National Institute on Drug Abuse through grant 1R01DA017073-01 to Paul M. Cinciripini, by a faculty fellowship from The University of Texas MD Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment to Francesco Versace, and by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
Dr. Cinciripini has served on the scientific advisory board of Pfizer Pharmaceuticals, has received grant support from Pfizer, and has conducted educational talks sponsored by Pfizer on smoking cessation for physicians.
Footnotes
Authors contribution All authors were responsible for the study concept and design. FV, JDR, and VLB contributed to the data collection. FV and JAM carried out the data reduction. FV and CYL performed the statistical analyses. FV and JME drafted the manuscript. All authors revised the manuscript and intellectually contributed to it, critically reviewed its content, and approved the final version for publication.
The other authors declare no conflict of interest.
Average IAPS normative ratings for valence and arousal were: MUT: 1.79, 6.36; SAD: 2.91, 4.82; UNPo: 3.01, 5.45; ERO: 6.63, 6.29; ROM: 7.40, 4.90; PLEo: 6.92, 4.67; NEUp: 5.30, 3.55; NEUo: 4.94, 2.76. To ensure the equivalence of the three sets as far as valence and arousal were concerned, two ANOVAs were conducted using the IAPS normative ratings of valence and arousal of each picture as dependent variable. Both analyses used picture set (A, B, C) and semantic category (MUT, SAD, UNPo, ERO, ROM, PLEo, NEUp, NEUo) as categorical factors. In both analyses the interaction picture set by semantic category was far from significance (Fs(16, 179) < 0.5, p > 0.96). By including stimuli belonging to the same categories in each picture set, we achieved the goal of probing appetitive and defensive motivational systems with both low and high arousing stimuli while controlling for semantic content. IAPS pictures used in this study were: Set A: MUT: 3000, 3030, 3068, 3101, 3130, 3261, 3300, 9433; SAD: 2095, 2455, 2490, 2700, 3280, 9190, 9410, 9520; UNPo: 6000, 6200, 6230, 9090, 9320, 9373, 9621, 9911; ERO: 4611, 4650, 4660, 4666, 4676, 4696, 4800, 4810; ROM: 4610, 4624, 4626, 4643; PLEo: 5250, 5626, 5660, 5764, 7340, 7350, 7470, 7485; NEUp: 2102, 2215, 2220, 2235, 2305, 2312, 2372, 2396, 2435, 2441, 2495, 2515, 2570, 2594, 9070; NEUo: 7000, 7006, 7038, 7040, 7052, 7053, 7110, 7130; Set B: MUT: 3053, 3060, 3069, 3100, 3102, 3140, 3170, 9420; SAD: 2205, 2399, 2520, 2800, 2810, 9265, 9429, 9926; UNPo: 6020, 6190, 6410, 9110, 9290, 9301, 9600, 9901; ERO: 4647, 4653, 4658, 4677, 4680, 4687, 4693, 4694; ROM: 2208, 4623, 4645, 4700; PLEo: 5628, 5661, 5780, 5781, 7270, 7430, 7450, 7482; NEUp: 2190, 2191, 2200, 2214, 2221, 2358, 2500, 2513, 2575, 2579, 2598, 2600, 2830, 7550; NEUo: 7002, 7004, 7009, 7020, 7034, 7055, 7056, 7140; Set C: MUT: 3051, 3080, 3110, 3120, 3168, 3225, 3350, 9253; SAD: 2141, 2276, 2491, 2590, 2703, 2900, 9421, 9530; UNPo: 6210, 6260, 9010, 9300, 9390, 9560, 9620, 9912; ERO: 4649, 4659, 4669, 4689, 4690, 4691, 4695, 4698; ROM: 4599, 4625, 4640, 4641; PLEo: 5629, 5631, 5700, 5711, 7330, 7410, 7460, 7480; NEUp: 2104, 2210, 2230, 2383, 2393, 2397, 2485, 2493, 2499, 2510, 2512, 2593, 2595, 2597, 2630, 2850; NEUo: 7010, 7030, 7041, 7054, 7059, 7080, 7090.
Reference List
- Alloy L, Abramson LY, Metalsky G, Harlages S. The Depression Proneness Inventory: A Stress reponsiveness measure of vulnerability to depression. 1990 Unpublished manuscript. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Carter BL, Robinson JD, Lam CY, Wetter DW, Day SX, Tsan JY, Cinciripini PM. A psychometric evaluation of cigarette stimuli used in a cue reactivity study. Nicotine & Tobacco Research. 2006;8:361–369. doi: 10.1080/14622200600670215. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Cinciripini PM, Robinson JD, Carter BL, Lam CY, Wu X, De Moor CA, Baile WS, Wetter DW. The effects of smoking deprivation and nicotine administration on emotional reactivity. Nicotine & Tobacco Research. 2006;8:379–392. doi: 10.1080/14622200600670272. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Bradley MM. Repetitive picture processing: autonomic and cortical correlates. Brain Research. 2006;1068:213–220. doi: 10.1016/j.brainres.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Bradley MM. Repetition and event-related potentials: Distinguishing early and late processes in affective picture perception. Journal of Cognitive Neuroscience. 2007;19:577–586. doi: 10.1162/jocn.2007.19.4.577. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- David SP, Munafo MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, Walton RT. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;58:488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Arcos FA, Verdejo-Garcia A, Ceverino A, Montanez-Pareja M, Lopez-Juarez E, Sanchez-Barrera M, Lopez-Jimenez A, Perez-Garcia M. Dysregulation of emotional response in current and abstinent heroin users: negative heightening and positive blunting. Psychopharmacology. 2008;198:159–166. doi: 10.1007/s00213-008-1110-2. [DOI] [PubMed] [Google Scholar]
- de Arcos FA, Verdejo-Garcia A, Peralta-Ramirez MI, Sanchez-Barrera M, Perez-Garcia M. Experience of emotions in substance abusers exposed to images containing neutral, positive, and negative affective stimuli. Drug and Alcohol Dependence. 2005;78:159–167. doi: 10.1016/j.drugalcdep.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Dunning JP, Parvaz MA, Hajcak G, Maloney T, ia-Klein N, Woicik PA, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Motivated attention to cocaine and emotional cues in abstinent and current cocaine users - an ERP study. Eur J Neurosci. 2011;33:1716–1723. doi: 10.1111/j.1460-9568.2011.07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J, Clark DC, Scheftner WA, Gibbons RD. Assessing anhedonia in psychiatric patients. Archives of General Psychiatry. 1983;40:79–84. doi: 10.1001/archpsyc.1983.01790010081010. [DOI] [PubMed] [Google Scholar]
- Foti D, Olvet DM, Klein DN, Hajcak G. Reduced electrocortical response to threatening faces in major depressive disorder. Depress Anxiety. 2010;27:813–820. doi: 10.1002/da.20712. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Rabinovich NE. The international smoking image series (with neutral counterparts), v 1.2. Carbondale, IL: Department of Psychology, Southern Illinois University; 1999. [Google Scholar]
- Gilbert DG, Sugai C, Zuo Y, Eau Claire N, McClernon FJ, Rabinovich NE, Markus T, Asgaard G, Radtke R. Effects of nicotine on brain responses to emotional pictures. Nicotine & Tobacco Research. 2004;6:985–996. doi: 10.1080/14622200412331324947. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Sugai C, Zuo Y, Rabinovich NE, McClernon FJ, Froeliger B. Brain indices of nicotine’s effects on attentional bias to smoking and emotional pictures and to task-relevant targets. Nicotine & Tobacco Research. 2007;9:351–363. doi: 10.1080/14622200701188810. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Woicik PA, Moeller SJ, Telang F, Jayne M, Wong C, Wang GJ, Fowler JS, Volkow ND. Liking and wanting of drug and non-drug rewards in active cocaine users: the STRAP-R questionnaire. J Psychopharmacol. 2010;24:257–266. doi: 10.1177/0269881108096982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair FJ, Black WC. Cluster Analysis. In: Grimm LG, Yarnold PR, editors. Reading and understanding more multivariate statistics. Washington, DC: American Psychological Association; 2000. pp. 147–205. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Wrase J, Kahnt T, Beck A, Bromand Z, Grusser SM, Kienast T, Smolka MN, Flor H, Mann K. Brain activation elicited by affectively positive stimuli is associated with a lower risk of relapse in detoxified alcoholic subjects. Alcoholism-Clinical and Experimental Research. 2007;31:1138–1147. doi: 10.1111/j.1530-0277.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine & Tobacco Research. 2003;5:13–25. [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, deB FB, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biological Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang PJ. Large-scale neural correlates of affective picture processing. Psychophysiology. 2002;39:641–649. doi: 10.1017.S0048577202394162. [DOI] [PubMed] [Google Scholar]
- Kenemans JL, Kahkonen S. How human electrophysiology informs psychopharmacology: from bottom-up driven processing to top-down control. Neuropsychopharmacology. 2011;36:26–51. doi: 10.1038/npp.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM. Emotion and the motivational brain. Biological Psychology. 2009;84:437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report no A-6. Gainesville, FL: University of Florida; 2005. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Sheehan KH, Janavs J, Dunbar GC. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. European Psychiatry. 1997;12:224–231. [Google Scholar]
- Littel M, Franken IH. The effects of prolonged abstinence on the processing of smoking cues: an ERP study among smokers, ex-smokers and never-smokers. J Psychopharmacol. 2007;21:873–882. doi: 10.1177/0269881107078494. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Kettle JW, Scaffidi A, Mackenzie T, Simmons JG, Allen NB. Responsiveness to drug cues and natural rewards in opiate addiction: associations with later heroin use. Archives of General Psychiatry. 2009;66:205–212. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Mathalon DH, O’Donnell BF, Hamalainen MS, Spencer KM, Javitt DC, Uhlhaas PJ. A Roadmap for the Development and Validation of Event-Related Potential Biomarkers in Schizophrenia Research. Biological Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay CJ, Erickson M, Shafey O. The Tobacco Atlas. 2. Washington, D.C.: American Cancer Society; 2006. [Google Scholar]
- Piasecki TM, Fiore MC, McCarthy DE, Baker TB. Have we lost our way? The need for dynamic formulations of smoking relapse proneness. Addiction. 2002;97:1093–1108. doi: 10.1046/j.1360-0443.2002.00216.x. [DOI] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: The Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) Journal of Consulting and Clinical Psychology. 2004;72:139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rubinstein ML, Luks TL, Dryden WY, Rait MA, Simpson GV. Adolescent Smokers Show Decreased Brain Responses to Pleasurable Food Images Compared With Nonsmokers. Nicotine & Tobacco Research. 2011 doi: 10.1093/ntr/ntr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Keil A, Bradley MM. Emotional perception: correlation of functional MRI and event-related potentials. Cereb Cortex. 2007;17:1085–1091. doi: 10.1093/cercor/bhl017. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Hillman CH, Hamm AO, Lang PJ. Brain processes in emotional perception: Motivated attention. Cognition and Emotion. 2004;18:593–611. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Shiffman S, Balabanis MH, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, Hickcox M, Paton SM. Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug and Alcohol Dependence. 2007;91:159–168. doi: 10.1016/j.drugalcdep.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong DR, Brown RA, Kahler CW, Lloyd-Richardson EE, Niaura R. Depression proneness in treatment-seeking smokers: A taxometric analysis. Personality and Individual Differences. 2004;36:1155–1170. [Google Scholar]
- US Department of Health and Human Services. How tobacco smoke causes disease: The biology and behavioral basis for smoking-attributable disease: A report of the surgeon general. Atlanta, GA: U.S Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [Google Scholar]
- Versace F, Minnix JA, Robinson JD, Lam CY, Brown VL, Cinciripini PM. Brain reactivity to emotional, neutral and cigarette-related stimuli in smokers. Addiction Biology. 2011;16:296–307. doi: 10.1111/j.1369-1600.2010.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Robinson JD, Lam CY, Minnix JA, Brown VL, Carter BL, Wetter DW, Cinciripini PM. Cigarette cues capture smokers’ attention: Evidence from event-related potentials. Psychophysiology. 2010;47:435–441. doi: 10.1111/j.1469-8986.2009.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Li TK. The neuroscience of addiction. Nat Neurosci. 2005;8:1429–1430. doi: 10.1038/nn1105-1429. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Beyond good and evil: the time-course of neural activity elicited by specific picture content. Emotion. 2010;10:767–782. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]


