Abstract
Patients suffering from neuropsychiatric disorders often exhibit a loss of regulation of their biological rhythms which leads to altered sleep/wake cycle, body temperature rhythm and hormonal rhythms. Whereas these symptoms have long been considered to result from the pathology of the underlying disease, increasing evidence now indicates that the circadian system may be more directly involved in the etiology of psychiatric disorders. This emerging view originated with the discovery that the genes involved in the generation of biological rhythms are expressed in many brain structures where clocks function – and perhaps malfunction. It is also due to the interesting phenotypes of clock mutant mice. Here we summarize recent reports showing that alteration of circadian clocks within key brain regions associated with neuropsychiatric disorders may be an underlying cause of the development of mental illness. We discuss how these alterations take place at both systems and molecular levels.
Introduction
Neuropsychiatric disorders represent the second largest cause of morbidity and premature mortality worldwide. They include major depressive disorder, anxiety, schizophrenia, bipolar disorder, obsessive-compulsive disorder, alcohol and substance abuse, and attention-deficit hyperactivity disorder. One prevalent symptom often associated with these mental illnesses is a disruption of biological rhythms with deregulation of the sleep/wake cycle, body temperature rhythm and hormonal rhythms. Whereas these biological rhythm dysfunctions have been considered to mainly result from the pathology of the mental illnesses, increasing data now show that the circadian system may be more directly involved in disease etiology. Indeed, genes involved in the generation of biological rhythms (i.e., “clock genes”) are expressed in many brain structures where they fine tune biological and physiological functions to be optimal at the most appropriate times of day. Alteration of clock gene expression within the brain regions associated with neuropsychiatric disorders can lead to development of mental illnesses. We discuss here how these alterations take place at both the molecular and system levels.
1. Clock genes are expressed in many brain structures
1.1 Communication between the central clock and the peripheral clocks
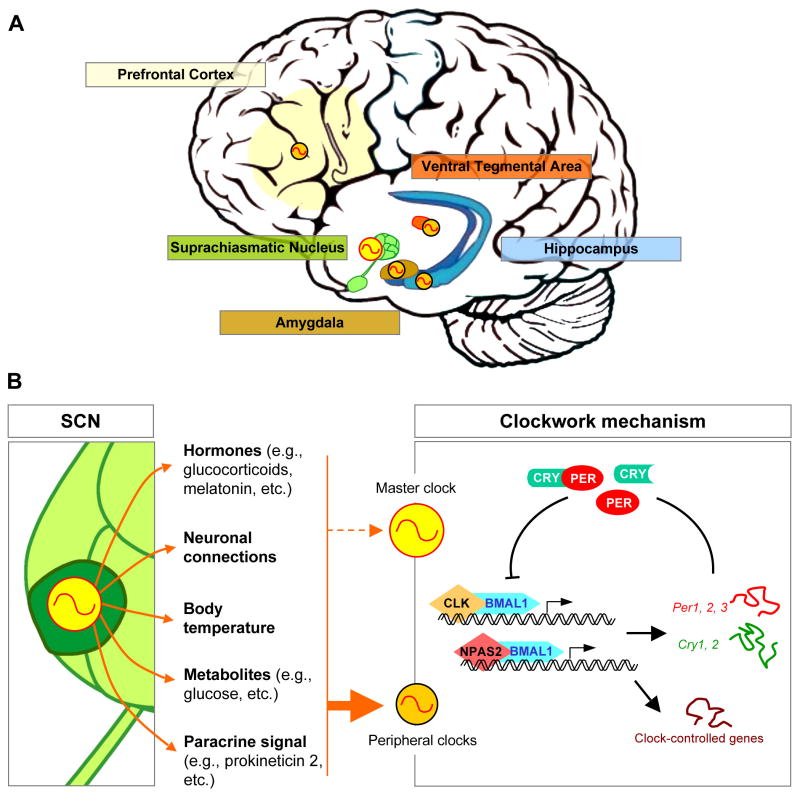
The mammalian circadian system is organized into cell-autonomous molecular oscillators not only in many brain locations but also throughout the body, where they temporally control biological and physiological functions. Cell-autonomy refers to the fact that these clocks continue to run when disconnected to the system, i.e, when incubated in vitro. At the molecular level, all of these oscillators appear to rely on the same mechanism, i.e., transcriptional feedback loops [reviewed in 1,2]. The transcription factor BMAL1 acts as a dimer with either CLOCK (CLK) or Neuronal PAS domain protein 2 (NPAS2) to activate the transcription of many genes including the transcriptional repressors Period (Per1, Per2 and Per3) and Cryptochrome (Cry1 and Cry2) (figure 1). The PERs and CRYs are expressed, post-translationally modified, and feedback to inhibit their own transcription. Rhythmic degradation of the repressor proteins leads to a new round of BMAL1/CLK or BMAL1/NPAS2-mediated transcription. This major feedback loop is accompanied by regulatory interlocked loops, which involve for example the BMAL1 target gene Nr1d1 (Rev-erbα), mediating transcriptional oscillations of Bmal1. These transcriptional rhythms, which are believed to occur similarly in all clock-cells, cycle with a period of about 24h; hence the term circadian.
Figure 1. Multi-levels organization of the circadian system in the human brain.
A/ Clocks are found in many different cerebral structures, including those involved in the neuropsychiatric disorders. These are called peripheral clocks (orange), and contrast with the master circadian clock (yellow) located in the suprachiasmatic nucleus (SCN) of the hypothalamus.
B/ As a master clock, the SCN generates and synchronizes many biological rhythms such as hormones, neuronal connections, body temperature, metabolites and paracrine signals. These circadian signals in turn entrain the molecular clockwork within peripheral clocks, so that their biological and physiological functions are optimal at the most appropriate times of day (plain, thick arrow). The SCN is, in contrast, resistant to most rhythmic signals it synchronizes (dashed, thin arrow).
The molecular clockwork in humans relies on transcriptional feedback loops in which the transcriptions factors BMAL1, CLK and NPAS2 rhythmically activate the expression of their transcriptional repressors Per and Cry genes, as well as many other “clock-controlled genes”. Clock-controlled genes are the output of the clocks and contribute to their rhythmic physiology.
External factors that desynchronize rhythmic signals within peripheral oscillators, as well as genetic mutation of clock genes, provoke improper clock function, which impacts on overall gene expression. Such a clock malfunction within brain oscillators accounts for some of the symptoms observed in patients suffering from neuropsychiatric disorders.
The brain location that has been studied in the most detail is the suprachiasmatic nucleus (SCN) of the hypothalamus, which contains the mammalian master circadian clock (figure 1). The SCN integrates daily environmental signals such as the light:dark cycle (via direct retinal input) and as a master clock, acts as a conductor and orchestrates these other (“peripheral”) rhythms so that they are in an appropriate phase relationship with each other and with the SCN as well as with the daily environmental variations that feed into the SCN [reviewed in 3,4]. The SCN uses a combination of neuronal connections and paracrine signals (e.g., prokineticin 2) to control, directly or indirectly, many endocrine (e.g., melatonin and glucocortcoid rhythms) and behavioral output rhythms (rest activity cycle, daily rhythm of body temperature) [reviewed in 4; figure 1]. In turn, these rhythms act as synchronizing cues.
Importantly, animals with ablated SCN are behaviorally arrhythmic and exhibit a desynchronization and/or loss of these rhythmic signals. Indeed, lesions of the SCN do not abolish all circadian rhythms but instead cause phase desynchrony between the tissues of an individual animal [5].
The circadian clock has been studied in considerable detail in many non-brain tissues, especially in the liver where the clock plays a major role in metabolism. Results show that it is responsible for the rhythmic expression of up to 10% of all mRNAs, most of them being involved in metabolic functions. As a consequence, many biological functions within non-brain tissues are rhythmically regulated, which account for physiology optimized to the best time of the day. The synchronization of circadian gene expression to environmental cues is complex and relies on many different factors that function as systemic cues [reviewed in 6].
1.2 Clock genes are expressed within structures relevant for psychiatric disorders
Expression of clock genes within brain structures relevant for psychiatric disorders, such as the hippocampus, the prefrontal cortex, the ventral tegmental area (VTA) and the amygdala, has been described [4,7–9]. Although details of this expression are beyond the scope of the present review, we will highlight some relevant functional aspects.
First and more broadly (i.e., beyond the confines of the nervous system and psychiatric disorders), there is extensive evidence indicating that morbidity associated with the circadian system is due to a deregulation of peripheral rather than central oscillator function (i.e., SCN-independent). For example, the arthropathy developed by Bmal1−/− mice (ossification of ligaments and tendons) is not induced by deregulation of gene expression within the SCN but rather where the ossification occurs [10]. Likewise in the central nervous system, links between deregulation of circadian rhythms and psychotic disorders are likely due to problems within the peripheral clocks in the hippocampus, prefrontal cortex, amygdala or VTA rather than within the SCN.
Second, the phase of circadian oscillations differs among peripheral oscillators and even within some subdivisions of the same structure. This is for example the case of PER2 rhythmic expression in the amygdala, which peaks in the evening in the central nuclear region (CEA) but in the morning in the basolateral region (BLA) [11]. Moreover, these structure and substructure-specific phases are almost identical in each animal. This strongly suggests that circadian gene expression within peripheral oscillators is set so that they best suit the biological and physiological functions they subserve.
Finally, the phase of circadian rhythms within peripheral clocks is determined, at least in part, by organ-specific synchronizers downstream of the SCN. They include many molecules and systems like hormones, neuronal connections, paracrine signals, metabolites and body temperature (figure 1). It is interesting to speculate that the circadian phase in peripheral oscillators is determined by the phase of the synchronizer as well as the sensitivity of each structure to the different synchronizers. This would depend in turn on the relative expression of each specific “receptor”. For example, higher expression of glucocorticoid receptors or Hsf1 (heat shock transcription factor 1) may confer higher sensitivity to cortisol or temperature, respectively.
Many experiments have examined the effects of “synchronizers” on rhythmic gene expression within different oscillators. In addition to tissue-specificity, one additional characteristic arose: the SCN exhibits low sensitivity to tissue-specific synchronizers (figure 1). Indeed, clock gene expression within the SCN is insensitive to glucocorticoid [12], melatonin [13], temperature [14], food entrainment [15] and its own paracrine signal prokineticin 2 [16,17].
The relative insensitivity of the SCN to tissue-specific synchronizers contrasts with the plethora of strong effects observed within other brain structures, including those important for the development of neuropsychiatric disorders. For example, glucorticoids may be important for the entrainment and function of the hippocampus and amygdala, since these two structures are highly enriched in glucocorticoid receptors [18]. Indeed, Per1 expression in the hippocampus can be directly induced by a pulse of corticosterone in rats [19], and clamping the daily levels of corticosterone suppresses Per1-mediated luciferase expression in the dentate gyrus of Per1-luciferase rats [20]. Moreover, PER2 rhythms in the CEA, but not in the BLA and dentate gyrus, disappear in adrenalectomized rats [11]. Many other reports also indicate that forebrain clock gene expression is affected by various stimuli [e.g., 21,22,23] and overall show that the synchronization of these oscillators is complex and incorporates many factors (figure 1).
Altogether, these results demonstrate that the rhythmic signals orchestrated by the SCN set the phases of peripheral clocks, including those in the brain. The importance of this synchronization process is highlighted by the problems arising in its absence.
2. Internal desynchronization alters brain functions
Aspects of modern life such as shift work lead to activity or food intake during what should be the resting phase. This causes internal signals to be generated at inappropriate circadian times, which results in turn in a conflictual timing between the internal signals and the still properly timed external (environmental) signals generated by the SCN [reviewed in 24]. In the most pronounced cases, all circadian rhythms of hormones, neuronal outputs and metabolites throughout the body are desynchronized [25].
Studies aiming at creating animal models of night work have reported this phenomenon. For instance, rats forced to be active for 8 hours during their sleeping phase (light phase), 5 days a week for 4 weeks, exhibit alterations of many endogenous rhythms [26 *]. In addition to the forced shift in their activity, rats also shift their food intake. This translates into a disappearance of normal glucose rhythms, an out-of-phase rhythm of triacylglycerols as well as the appearance of an additional corticosterone peak at the beginning of their “work” period during the day -- in addition to the normal endogenous peak occurring at the beginning of the night [26 *]. Strikingly, this shift work schedule is accompanied by alterations of rhythms in several hypothalamic structures but not the SCN [27 *].
In a more recent paper, Karatsoreos and collaborators produced internal desynchronization by exposing mice to a 10h-light:10h-dark(LD10:10) schedule for several weeks. Mice are unable to entrain their activity rhythms to such a light:dark regime and therefore exhibit circadian locomotor behavior reflecting their endogenous period of about 24h [28 **]. Although hormonal rhythms were not assayed, their alteration may be responsible for the decreased complexity of the neurons in the medial prefrontal cortex; they show a prominent shortening of apical dendrites. Mice subjected to this protocol also manifested behavioral defects known to be dependent on the prefrontal cortex, as they exhibit a reduced ability to modify a learned behavior and also have a decreased latency to enter a novel environment [28 **]. Strikingly, these are similar to behaviors observed in animal with medial prefrontal cortex lesions [29].
These and other data converge on a model in which internal desynchronization results from conflicts between SCN-driven environmental rhythms and internal rhythms. It affects the entrainment of peripheral clocks and results in altered gene expression within forebrain structures (see below). Interestingly, the internal desynchronization observed after SCN ablation [5; see above] can be overcome by treating the animals with stimulation that provide a strong and rhythmic internal signal. For example, restricted food access or methamphetamine exposure can synchronize the circadian phase of peripheral brain regions of SCN-lesioned arrhythmic mice [30 *]. It is also noteworthy that treatments of mice [31] and human [32] aimed at reinforcing biological rhythms reduce the neuropathological symptoms associated with some psychotic disorders. This presumably occurs by reinforcing peripheral oscillations and implies that some disorders are intimately linked to circadian difficulties, which may even be a more proximate cause of these symptoms.
3. Alteration of sleep in neuropsychiatric disorders: a wider role of clock genes?
Sleep is controlled by the interaction of two components: a circadian component, which controls the timing of sleep, and a homeostasis component, which reflects sleep need [reviewed in 33]. Deterioration of sleep is often associated with neuropsychiatric disorders like depression [34], bipolar disorders [35], schizophrenia [36] and attention deficit hyperactivity disorder [37]. The relationship between sleep and these illnesses appears intimate. Indeed, treatments aimed at ameliorating disease often improve sleep quality. Moreover, sleep deprivation has a spectacular but transient antidepressant effect in humans [reviewed in 38]. It remains unresolved whether the deterioration of sleep associated with mental illnesses is another consequence of circadian dysfunction or whether it is a different manifestation of the disorder. Further research should provide insight into the links between sleep and mental illness, and the possible role of circadian clocks.
4. Association of clock gene polymorphims/SNPs with psychotic disorders
Single-nucleotide polymorphisms (SNPs) in clock genes have been associated with almost all neuropsychiatric disorders [reviewed in 39,40]. Although this may constitute additional evidence linking the circadian clock to psychotic disorders, this remains uncertain as the literature often reports conflicting results. SNPs in clock genes represent only a small fraction (~5–10%) of all SNPs linked to neuropsychiatric disorders and the effects they have on gene expression are still unknown because experimental validation is lacking. These issues should be addressed in the future.
5. Mutations/alterations of gene expression of clock genes and psychotic disorders
Cloning of mammalian clock genes almost 15 years ago was quickly followed by the generation of knockout mice in which their biological functions were assayed. Circadian phenotypes were addressed first and indicated that clock genes are necessary and sufficient for the generation of biological rhythms [reviewed in 1]. Follow up experiments, however, revealed a much broader role of these genes: knock-out mice were more prone to develop a wide range of illnesses including metabolic diseases, cancer, arthropathy, and hypertension. These results collectively highlight the importance of clock gene expression within peripheral clocks as well as how their impairment can lead to physiological disorders. Strikingly, clock mutant mice also develop symptoms similar to those seen in human neuropsychiatric disorders. We will discuss below the three best-characterized examples.
5.1 Clock and mania-like behavior
The first clock-mutant mouse was reported after a N-ethyl-N-nitrosourea mutagenesis screen [41]. Characterization of the point mutation revealed the generation of a dominant negative protein within the gene Clock in which exon 19 was skipped, hence CLKΔ 19 [42]. In addition to circadian [41], metabolic [43], reproductive [44] and sleep disorders [45], ClkΔ19 mice also exhibit behavioral alterations that are symptomatic of mania-like behavior in human [46]. They 1) are hyperactive, 2) sleep less than wild-type littermates, 3) show reduced anxiety and depression-like behavior, 4) show less helplessness, and 5) exhibit an increased propensity for drug abuse [46].
Importantly, most of these effects seem to depend on Clk function within the VTA. Indeed, rescue of ClkΔ19 with functional CLK protein only in this region restores wild-type hyperactivity and anxiety-related behavior [46]. In addition, specific RNA interference knockdown of Clk only in the VTA of wild-type mice causes many of the above-mentioned symptoms, such as a hyperactive response to novelty and less anxiety-related behavior [47 **]. These effects appear to be accompanied by increased dopaminergic response since both the ClkΔ19 mutation and the Clk knockdown enhance the firing rates of dopaminergic cells in the VTA [46,47 **].
The role of CLOCK as a transcription factor seems to be important for these behavioral changes, as the Clk knockdown within the VTA also leads to changes in the expression of several genes. Importantly, many of them encode for channels and channel-associated proteins as well as genes involved in the dopamine synthesis, regulation or metabolism. Expression of these genes may therefore make major contributions to the behavioral and physiological defects observed in both ClkΔ19 and VTA-specific Clk knockdown mice [47 **]. It is however still unknown whether these genes are directly or indirectly regulated by CLK. Some of them are, such as the gene monoamine oxidase A (Maoa), which encodes an enzyme that degrades amine neurotransmitters like dopamine [48]. MAO activity in the VTA and the nucleus accumbens (NAC) is decreased in Per2Brdm1 mutant mice and dopamine levels increased [48].
A recent paper reported altered neuronal functions in the NAC of ClkΔ19 mice, which may also contribute to aspects of the mania-like behavior [49 **]. The authors show that the phase coupling of low-gamma to delta oscillations in the NAC is negatively correlated with the extent to which wild-type mice explore a novel environment. ClkΔ19 mice, which become hyperactive when placed in a novel environment, exhibit a profound alteration of this phasic entrainment. These physiological and behavioral phenotypes may be explained by the complex changes in dendritic morphology of NAC neurons as well as reduced GluR1 expression in the mutant relative to wild-type mice [49 **]. Importantly, chronic lithium treatment, which is frequently prescribed to patients suffering from bipolar disorder, suppresses the exploratory drive of ClkΔ19 mice and ameliorates several of these morphological effects as well as neurophysiological deficits.
Because there is no NAC-specific study, it is not clear whether these effects are due to Clk loss-of-function within the NAC or whether they originate elsewhere, for example with the altered dopaminergic signaling from the VTA. In any case, these papers highlight the numerous molecular, cellular and physiological alterations within the mesolimbic brain regions of ClkΔ19 mice, which may be directly responsible for the development of mania-like behaviors.
5.2 Clock genes and sensitivity to drug of abuse
Neuropsychiatric disorders carry with them an increased risk of drug abuse. Co-occurrence of both serious mental illness and substance dependence, or abuse, was found in 4 millions adults in the USA in 2002 (source from the National Drug Intelligence Center). The mania-like behavior phenotype of ClkΔ19 mice along with their sensitivity to drug of abuse [50] is reminiscent of this co-occurrence. The relationship between clock genes and drugs of abuse is even more widespread and extends to Drosophila where it was first observed [51]. Since this has been recently reviewed in detail [52,53], we will just summarize here the major conclusions.
First, many circadian mutant mice exhibit an altered response to drugs of abuse such as alcohol, cocaine, metamphetamine and morphine. In the case of cocaine for example, ClkΔ19 [50] and mPer2 [54] mutant mice have a hypersensitized reponse. mPer1 [54] and Npas2 [53] knockout mouse respond in the opposite way, i.e., they lack a sensitized behavioral response. Understanding why different clock gene mutants display opposite cocaine sensitivity should provide insight into how clock genes are involved in drug addiction.
Second, chronic or acute administrations of drugs of abuse such as cocaine can directly induce clock genes expression (mainly Per1 and Per2) within the striatum [22,55], nucleus accumbens [56] and hippocampus [22]. There are obvious similarities with the classical light-induced expression of Per1 and Per2 within the SCN [e.g., 4], making it tempting to speculate that drug-induced Per1 and/or Per2 expression in peripheral clocks may perturb the timing of downstream gene expression.
Third, effects on clock gene expression are also observed after withdrawal of drugs of abuse. For example, expression of Per1 and Per2 after morphine withdrawal in rats seems to be out-of-phase within several mesolimbic structures despite no phase change within the SCN [23]. The phase alterations could explain, at least in part, why Per2Brdm1 mutant mice show attenuated withdrawal symptoms compared to wild-type [57].
In conclusion, these results demonstrate that drugs of abuse can affect clock gene expression and therefore induce out of phase molecular oscillations within the mesolimbic system. Because clock genes regulate the expression of many output genes (see below), they may have an important role in the etiology of addiction. This may be relevant to the finding that Per2Brdm1 mutant mice show an alteration of their glutamatergic system, which may be responsible for the increase in alcohol intake in this strain [58].
5.3 Alteration of memory formation and consolidation in clock-impaired animals
Patients suffering from emotional and affective disorders often exhibit a reduced ability to access specific memories of life events [reviewed in 59]. These memory defects involve the mesolimbic system and particularly the hippocampus, which plays an important role in the consolidation of information from short-term to long-term memory.
Several reports have highlighted the importance of the circadian system in hippocampal-dependent memory function [reviewed in 60,61]. For example, lesions of the SCN or light-induced phase-shifts (i.e., jet-lag) impair hippocampus-dependent long-term memory [62–64]. Long-term potentiation (LTP), which is the long-term enhancement in synaptic response occurring after strong, repetitive stimulation, may be linked to these defects; LTP amplitude varies with the time of the day in rodents [65]. Moreover, and despite some conflicting results [66], clock gene mutant mice exhibit defects in hippocampus-dependent memory formation [67–70]. Similarly, wild-type hamsters rendered arrhythmic (by a combination of nighttime light treatments for two consecutive nights) also show altered hippocampus-dependent long-term spatial learning [71]. Importantly, a recent paper demonstrated that hippocampal circadian oscillations are directly required for memory formation and persistence: local pharmacological inhibition of circadian rhythms of MAPK activity only within the hippocampus blocks long-term memory formation [72 **].
Dentate gyrus neurogenesis constitutes another notable aspect of the circadian control of the hippocampus biology. Neurogenesis is associated with learning and memory and the number of newborn hippocampal neurons increases after a hippocampus-dependent learning task [reviewed in 73]. Interestingly, the well-described effect of exercise (e.g., wheel-running activity) on neurogenesis is modulated by the circadian system. Indeed, running activity significantly increases cell proliferation, cell survival, and the total number of new neurons only when animals have access to the wheel for 3hrs in the middle of the dark (active) period [74]. Experimental jet-lag (a 6hrs phase advance every 3 days for 25 days) results in internal desynchronization and also inhibits cell proliferation and neurogenesis in adult hamsters [75 **]. The effect on cell proliferation is dependent on a jet-lag increase in glucocorticoids levels. This circadian disruption results in pronounced deficits in learning and memory, which parallel the marked reductions in hippocampal cell proliferation and neurogenesis. Significantly, deficits in hippocampal-dependent learning and memory also persisted well after the cessation of jet lag, suggesting long-lasting negative consequences on brain function [75 **]. Although long-lasting internal desynchronization has not yet been shown to affect neurogenesis in humans, this may reveal new links between circadian biology and learning and memory mechanisms. Because glucocorticoids have been strongly involved in the regulation of memory in humans [reviewed in 76], they may represent the primary target for further investigation.
6. Regulation of gene expression by clock genes
Whether it occurs because of internal desynchronization or because of a mutation, misexpression or mistiming of clock genes within brain clocks likely affects the expression of many downstream genes, called clock-controlled genes (figure 1). In turn, these changes may account for some of the symptoms observed in patients suffering from psychotic disorders. The current model for the generation of molecular rhythms posits that clock genes interact in transcriptional feedback loops to generate rhythms of about 24hrs and entrain widespread expression of rhythmic transcripts (figure 1; see also §1.1). This has been recently reviewed in detail [e.g., 77,78,79].
Despite considerable work in multiple systems, the molecular events leading to the generation of circadian rhythms are still not very well understood. Moreover, the current model of feedback loops is probably over-simplified with many more regulatory mechanisms yet to be described. One general question also arises: are the symptoms observed after circadian challenge only due to improper rhythmic transcription (i.e., disappearance or improper expression of rhythmic transcripts)? Or are they resulting from a more widespread alteration in gene expression that extends beyond rhythmic transcription? Until now, most of the genome-wide studies investigating dysfunction of peripheral clocks have mainly considered rhythmic transcripts. Future experiments should address whether this kind of analysis is the most relevant.
7. Conclusions
The discovery that autonomous clocks exist in virtually all organs of the human body has led to the concept that biological rhythms within these tissues were important to optimize biological functions with daily environmental fluctuations. However, malfunctions of these peripheral clocks are associated with more than just improper temporal organization. The brain clocks are no exception, as deregulation of oscillators within the mesolimbic system is associated with many symptoms observed in patients suffering from neuropsychiatric disorders. Importantly, this deregulation occurs at both the systems and molecular levels.
It is apparent that brain clock dysfunction leads to a wide range of symptoms. Our current understanding will undoubtedly be deepened by recent advances in mammalian genetic tools, which now allow better temporal and spatial controls of gene expression. Importantly, the emerging notion that well-synchronized internal rhythms reduce the incidence and severity of symptoms associated with human mental illnesses holds considerable therapeutic promise and may even contribute to an enhanced understanding of disease etiology.
Highlights.
Deregulation of brain clocks is associated with neuropsychiatric disorders
Deregulation occurs at both the system and molecular levels
Misexpression of the gene Clk in the mesolimbic system induces mania-like behavior
Mutation of clock genes affect sensitization to drugs of abuse and addiction
Deregulation of clocks in the hippocampus alters memory formation and consolidation
Acknowledgments
We would like to thank Hugues Dardente (INRA, Nouzilly, France), Christine Merlin (University of Massachusetts Medical School, Worcester, MA), Stephanie Perreau-Lenz (University of Heidelberg, Mannheim, Germany) and our colleagues Katherine Abruzzi and Julie Vienne for comments on the manuscript. M.R. is an Investigator at the Howard Hughes Medical Institute (HHMI). This work was supported in part by NIH grants NS44232, NS45713 to M.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jerome S Menet, Email: menet@brandeis.edu.
Michael Rosbash, Email: rosbash@brandeis.edu.
References
2 stars, of outstanding interest:
1 star, of special interest:
- 1.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15 (Suppl 2):R271–277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 2.Dardente H, Cermakian N. Molecular circadian rhythms in central and peripheral clocks in mammals. Chronobiol Int. 2007;24:195–213. doi: 10.1080/07420520701283693. [DOI] [PubMed] [Google Scholar]
- 3.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendoza J, Challet E. Brain clocks: from the suprachiasmatic nuclei to a cerebral network. Neuroscientist. 2009;15:477–488. doi: 10.1177/1073858408327808. [DOI] [PubMed] [Google Scholar]
- 5.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maury E, Ramsey KM, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res. 2010;106:447–462. doi: 10.1161/CIRCRESAHA.109.208355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilding C, Piggins HD. Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain? Eur J Neurosci. 2007;25:3195–3216. doi: 10.1111/j.1460-9568.2007.05581.x. [DOI] [PubMed] [Google Scholar]
- 8.Webb IC, Baltazar RM, Lehman MN, Coolen LM. Bidirectional interactions between the circadian and reward systems: is restricted food access a unique zeitgeber? Eur J Neurosci. 2009;30:1739–1748. doi: 10.1111/j.1460-9568.2009.06966.x. [DOI] [PubMed] [Google Scholar]
- 9.Amir S, Stewart J. Motivational Modulation of Rhythms of the Expression of the Clock Protein PER2 in the Limbic Forebrain. Biol Psychiatry. 2009;65:829–834. doi: 10.1016/j.biopsych.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Bunger MK, Walisser JA, Sullivan R, Manley PA, Moran SM, Kalscheur VL, Colman RJ, Bradfield CA. Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis. 2005;41:122–132. doi: 10.1002/gene.20102. [DOI] [PubMed] [Google Scholar]
- 11.Lamont EW, Robinson B, Stewart J, Amir S. The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc Natl Acad Sci U S A. 2005;102:4180–4184. doi: 10.1073/pnas.0500901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 13.Poirel VJ, Boggio V, Dardente H, Pevet P, Masson-Pevet M, Gauer F. Contrary to other non-photic cues, acute melatonin injection does not induce immediate changes of clock gene mRNA expression in the rat suprachiasmatic nuclei. Neuroscience. 2003;120:745–755. doi: 10.1016/s0306-4522(03)00344-0. [DOI] [PubMed] [Google Scholar]
- 14.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330:379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 16.Prosser HM, Bradley A, Chesham JE, Ebling FJ, Hastings MH, Maywood ES. Prokineticin receptor 2 (Prokr2) is essential for the regulation of circadian behavior by the suprachiasmatic nuclei. Proc Natl Acad Sci U S A. 2007;104:648–653. doi: 10.1073/pnas.0606884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li JD, Hu WP, Boehmer L, Cheng MY, Lee AG, Jilek A, Siegel JM, Zhou QY. Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. J Neurosci. 2006;26:11615–11623. doi: 10.1523/JNEUROSCI.3679-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joels M, de Kloet ER. Mineralocorticoid and glucocorticoid receptors in the brain. Implications for ion permeability and transmitter systems. Prog Neurobiol. 1994;43:1–36. doi: 10.1016/0301-0082(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 19.Conway-Campbell BL, Sarabdjitsingh RA, McKenna MA, Pooley JR, Kershaw YM, Meijer OC, De Kloet ER, Lightman SL. Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the clock gene period 1 in rat hippocampus. J Neuroendocrinol. 2010;22:1093–1100. doi: 10.1111/j.1365-2826.2010.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilhooley MJ, Pinnock SB, Herbert J. Rhythmic expression of per1 in the dentate gyrus is suppressed by corticosterone: implications for neurogenesis. Neurosci Lett. 2011;489:177–181. doi: 10.1016/j.neulet.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Feillet CA, Mendoza J, Albrecht U, Pevet P, Challet E. Forebrain oscillators ticking with different clock hands. Mol Cell Neurosci. 2008;37:209–221. doi: 10.1016/j.mcn.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Uz T, Ahmed R, Akhisaroglu M, Kurtuncu M, Imbesi M, Dirim Arslan A, Manev H. Effect of fluoxetine and cocaine on the expression of clock genes in the mouse hippocampus and striatum. Neuroscience. 2005;134:1309–1316. doi: 10.1016/j.neuroscience.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Li SX, Liu LJ, Jiang WG, Lu L. Morphine withdrawal produces circadian rhythm alterations of clock genes in mesolimbic brain areas and peripheral blood mononuclear cells in rats. J Neurochem. 2009;109:1668–1679. doi: 10.1111/j.1471-4159.2009.06086.x. [DOI] [PubMed] [Google Scholar]
- 24.Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 25.Haus E, Smolensky M. Biological clocks and shift work: circadian dysregulation and potential long-term effects. Cancer Causes Control. 2006;17:489–500. doi: 10.1007/s10552-005-9015-4. [DOI] [PubMed] [Google Scholar]
- 26*.Salgado-Delgado R, Angeles-Castellanos M, Buijs MR, Escobar C. Internal desynchronization in a model of night-work by forced activity in rats. Neuroscience. 2008;154:922–931. doi: 10.1016/j.neuroscience.2008.03.066. In these two studies, the authors created a model of night work in rats, and showed that it altered endogenous rhythms of locomotor activity and food intake. This translated into altered rhythms of glucose, triacylglycerols and corticosterone, as well as desynchronization of rhythms within several hypothalamic structures (but not the SCN) [DOI] [PubMed] [Google Scholar]
- 27*.Salgado-Delgado R, Angeles-Castellanos M, Saderi N, Buijs RM, Escobar C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology. 2010;151:1019–1029. doi: 10.1210/en.2009-0864. In these two studies, the authors created a model of night work in rats, and showed that it altered endogenous rhythms of locomotor activity and food intake. This translated into altered rhythms of glucose, triacylglycerols and corticosterone, as well as desynchronization of rhythms within several hypothalamic structures (but not the SCN) [DOI] [PubMed] [Google Scholar]
- 28**.Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A. 2011;108:1657–1662. doi: 10.1073/pnas.1018375108. The authors produced internal desynchronization in mice and showed that it induces loss of dendritic length and decreased complexity of neurons in the prelimbic prefrontal cortex. These neuroanatomical changes are associated with behavioral phenotypes known to be dependant from the the prelimbic prefrontal cortex and mice exhibit decreased cognitive flexibility and changed emotionality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacroix L, White I, Feldon J. Effect of excitotoxic lesions of rat medial prefrontal cortex on spatial memory. Behav Brain Res. 2002;133:69–81. doi: 10.1016/s0166-4328(01)00442-9. [DOI] [PubMed] [Google Scholar]
- 30*.Pezuk P, Mohawk JA, Yoshikawa T, Sellix MT, Menaker M. Circadian organization is governed by extra-SCN pacemakers. J Biol Rhythms. 2010;25:432–441. doi: 10.1177/0748730410385204. Demonstration that the internal desynchronization observed after SCN ablation can be overcome by treating the animals with stimulation that provide a strong and rhythmic internal signal (e.g., restricted food access or metamphetamine exposure) [DOI] [PubMed] [Google Scholar]
- 31.Maywood ES, Fraenkel E, McAllister CJ, Wood N, Reddy AB, Hastings MH, Morton AJ. Disruption of peripheral circadian timekeeping in a mouse model of Huntington's disease and its restoration by temporally scheduled feeding. J Neurosci. 2010;30:10199–10204. doi: 10.1523/JNEUROSCI.1694-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frank E, Swartz HA, Kupfer DJ. Interpersonal and social rhythm therapy: managing the chaos of bipolar disorder. Biol Psychiatry. 2000;48:593–604. doi: 10.1016/s0006-3223(00)00969-0. [DOI] [PubMed] [Google Scholar]
- 33.Franken P, Dijk DJ. Circadian clock genes and sleep homeostasis. Eur J Neurosci. 2009;29:1820–1829. doi: 10.1111/j.1460-9568.2009.06723.x. [DOI] [PubMed] [Google Scholar]
- 34.Benca RM, Peterson MJ. Insomnia and depression. Sleep Med. 2008;9(Suppl 1):S3–9. doi: 10.1016/S1389-9457(08)70010-8. [DOI] [PubMed] [Google Scholar]
- 35.Murray G, Harvey A. Circadian rhythms and sleep in bipolar disorder. Bipolar Disord. 2010;12:459–472. doi: 10.1111/j.1399-5618.2010.00843.x. [DOI] [PubMed] [Google Scholar]
- 36.Cohrs S. Sleep disturbances in patients with schizophrenia : impact and effect of antipsychotics. CNS Drugs. 2008;22:939–962. doi: 10.2165/00023210-200822110-00004. [DOI] [PubMed] [Google Scholar]
- 37.Weiss MD, Salpekar J. Sleep problems in the child with attention-deficit hyperactivity disorder: defining aetiology and appropriate treatments. CNS Drugs. 2010;24:811–828. doi: 10.2165/11538990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 38.Hemmeter UM, Hemmeter-Spernal J, Krieg JC. Sleep deprivation in depression. Expert Rev Neurother. 2010;10:1101–1115. doi: 10.1586/ern.10.83. [DOI] [PubMed] [Google Scholar]
- 39.Lamont EW, Coutu DL, Cermakian N, Boivin DB. Circadian rhythms and clock genes in psychotic disorders. Isr J Psychiatry Relat Sci. 2010;47:27–35. [PubMed] [Google Scholar]
- 40.Kennaway DJ. Clock genes at the heart of depression. J Psychopharmacol. 2010;24 :5–14. doi: 10.1177/1359786810372980. [DOI] [PubMed] [Google Scholar]
- 41.Vitaterna MH, King DP, Chang A-M, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TDL, Vitaterna MH, Kornhauser JM, Lowrey PL, et al. Positional Cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004;14:1367–1373. doi: 10.1016/j.cub.2004.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naylor E, Bergmann BM, Krauski K, Zee PC, Takahashi JS, Vitaterna MH, Turek FW. The circadian clock mutation alters sleep homeostasis in the mouse. J Neurosci. 2000;20:8138–8143. doi: 10.1523/JNEUROSCI.20-21-08138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47**.Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, Graham A, Gordon E, Enwright JF, 3rd, DiLeone RJ, et al. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol Psychiatry. 2010;68:503–511. doi: 10.1016/j.biopsych.2010.04.031. The authors performed a knockdown of Clk specifically in the VTA of mice (by RNA interference). This procedure recapitulates many of the mania-like symptoms observed in ClkΔ19 mutant mice and therefore highlights the important role of Clock in the VTA in the regulation of dopaminergic activity as well as manic and depressive-like behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, Brunk I, Spanagel R, Ahnert-Hilger G, Meijer JH, et al. Regulation of monoamine oxidase A by circadian-clock components implies clock influence on mood. Curr Biol. 2008;18:678–683. doi: 10.1016/j.cub.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 49**.Dzirasa K, Coque L, Sidor MM, Kumar S, Dancy EA, Takahashi JS, McClung CA, Nicolelis MA. Lithium ameliorates nucleus accumbens phase-signaling dysfunction in a genetic mouse model of mania. J Neurosci. 2010;30:16314–16323. doi: 10.1523/JNEUROSCI.4289-10.2010. This study reports altered phase-coupling between neurons of the nucleus accumbens (NAC) of ClkΔ19 mice, which could be explained by the reduced GluR1 expression and changes in the dendritic morphology. Moreover, chronic lithium treatment, which is frequently prescribed to patients suffering from bipolar disorder, suppresses the exploratory drive of ClkΔ19 mice and ameliorates several of these morphological effects as well as neurophysiological deficits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andretic R, Chaney S, Hirsh J. Requirement of circadian genes for cocaine sensitization in Drosophila. Science. 1999;285:1066–1068. doi: 10.1126/science.285.5430.1066. [DOI] [PubMed] [Google Scholar]
- 52.Perreau-Lenz S, Spanagel R. The effects of drugs of abuse on clock genes. Drug News Perspect. 2008;21:211–217. doi: 10.1358/dnp.2008.21.4.1213350. [DOI] [PubMed] [Google Scholar]
- 53.Falcon E, McClung CA. A role for the circadian genes in drug addiction. Neuropharmacology. 2009;56 (Suppl 1):91–96. doi: 10.1016/j.neuropharm.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A. 2002;99 :9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuferov V, Kroslak T, Laforge KS, Zhou Y, Ho A, Kreek MJ. Differential gene expression in the rat caudate putamen after "binge" cocaine administration: advantage of triplicate microarray analysis. Synapse. 2003;48 :157–169. doi: 10.1002/syn.10198. [DOI] [PubMed] [Google Scholar]
- 56.McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- 57.Perreau-Lenz S, Sanchis-Segura C, Leonardi-Essmann F, Schneider M, Spanagel R. Development of morphine-induced tolerance and withdrawal: involvement of the clock gene mPer2. Eur Neuropsychopharmacol. 2010;20 :509–517. doi: 10.1016/j.euroneuro.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 58.Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- 59.Dere E, Pause BM, Pietrowsky R. Emotion and episodic memory in neuropsychiatric disorders. Behav Brain Res. 215:162–171. doi: 10.1016/j.bbr.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 60.Eckel-Mahan KL, Storm DR. Circadian rhythms and memory: not so simple as cogs and gears. EMBO Rep. 2009;10:584–591. doi: 10.1038/embor.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gerstner JR, Yin JC. Circadian rhythms and memory formation. Nat Rev Neurosci. 2010;11:577–588. doi: 10.1038/nrn2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stephan FK, Kovacevic NS. Multiple retention deficit in passive avoidance in rats is eliminated by suprachiasmatic lesions. Behav Biol. 1978;22:456–462. doi: 10.1016/s0091-6773(78)92565-8. [DOI] [PubMed] [Google Scholar]
- 63.Tapp WN, Holloway FA. Phase shifting circadian rhythms produces retrograde amnesia. Science. 1981;211:1056–1058. doi: 10.1126/science.7193351. [DOI] [PubMed] [Google Scholar]
- 64.Devan BD, Goad EH, Petri HL, Antoniadis EA, Hong NS, Ko CH, Leblanc L, Lebovic SS, Lo Q, Ralph MR, et al. Circadian phase-shifted rats show normal acquisition but impaired long-term retention of place information in the water task. Neurobiol Learn Mem. 2001;75:51–62. doi: 10.1006/nlme.1999.3957. [DOI] [PubMed] [Google Scholar]
- 65.Chaudhury D, Wang LM, Colwell CS. Circadian regulation of hippocampal long-term potentiation. J Biol Rhythms. 2005;20:225–236. doi: 10.1177/0748730405276352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zueger M, Urani A, Chourbaji S, Zacher C, Lipp HP, Albrecht U, Spanagel R, Wolfer DP, Gass P. mPer1 and mPer2 mutant mice show regular spatial and contextual learning in standardized tests for hippocampus-dependent learning. J Neural Transm. 2006;113:347–356. doi: 10.1007/s00702-005-0322-4. [DOI] [PubMed] [Google Scholar]
- 67.Jilg A, Lesny S, Peruzki N, Schwegler H, Selbach O, Dehghani F, Stehle JH. Temporal dynamics of mouse hippocampal clock gene expression support memory processing. Hippocampus. 2009 doi: 10.1002/hipo.20637. [DOI] [PubMed] [Google Scholar]
- 68.Kondratova AA, Dubrovsky YV, Antoch MP, Kondratov RV. Circadian clock proteins control adaptation to novel environment and memory formation. Aging (Albany NY) 2010;2:285–297. doi: 10.18632/aging.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang LM, Dragich JM, Kudo T, Odom IH, Welsh DK, O'Dell TJ, Colwell CS. Expression of the circadian clock gene Period2 in the hippocampus: possible implications for synaptic plasticity and learned behaviour. ASN Neuro. 2009:1. doi: 10.1042/AN20090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garcia JA, Zhang D, Estill SJ, Michnoff C, Rutter J, Reick M, Scott K, Diaz-Arrastia R, McKnight SL. Impaired cued and contextual memory in NPAS2-deficient mice. Science. 2000;288:2226–2230. doi: 10.1126/science.288.5474.2226. [DOI] [PubMed] [Google Scholar]
- 71.Ruby NF, Hwang CE, Wessells C, Fernandez F, Zhang P, Sapolsky R, Heller HC. Hippocampal-dependent learning requires a functional circadian system. Proc Natl Acad Sci U S A. 2008;105:15593–15598. doi: 10.1073/pnas.0808259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72**.Eckel-Mahan KL, Phan T, Han S, Wang H, Chan GC, Scheiner ZS, Storm DR. Circadian oscillation of hippocampal MAPK activity and cAmp: implications for memory persistence. Nat Neurosci. 2008;11:1074–1082. doi: 10.1038/nn.2174. This paper constitutes the first demonstration that circadian oscillations in the hippocampus are directly required for memory formation and persistence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holmes MM, Galea LA, Mistlberger RE, Kempermann G. Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J Neurosci Res. 2004;76:216–222. doi: 10.1002/jnr.20039. [DOI] [PubMed] [Google Scholar]
- 75**.Gibson EM, Wang C, Tjho S, Khattar N, Kriegsfeld LJ. Experimental 'jet lag' inhibits adult neurogenesis and produces long-term cognitive deficits in female hamsters. PLoS One. 2010;5:e15267. doi: 10.1371/journal.pone.0015267. This study shows that experimental jet-lag in adult hamster produces an internal desynchronization and inhibits cell proliferation and neurogenesis. The effect on cell proliferation is dependent on a jet-lag increase in glucocorticoids levels. Moreover, the experimental jet-lag results in pronounced deficits in learning and memory, which persists well after the cessation of jet lag. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Quervain DJ, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Front Neuroendocrinol. 2009;30 :358–370. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 77.Zhang EE, Kay SA. Clocks not winding down: unravelling circadian networks. Nat Rev Mol Cell Biol. 2010;11:764–776. doi: 10.1038/nrm2995. [DOI] [PubMed] [Google Scholar]
- 78.Baggs JE, Hogenesch JB. Genomics and systems approaches in the mammalian circadian clock. Curr Opin Genet Dev. 2010;20:581–587. doi: 10.1016/j.gde.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doherty CJ, Kay SA. Circadian control of global gene expression patterns. Annu Rev Genet. 2010;44:419–444. doi: 10.1146/annurev-genet-102209-163432. [DOI] [PMC free article] [PubMed] [Google Scholar]