Abstract
Human immunodeficiency virus (HIV-1) infection causes chronic inflammation. COX-2 derived prostaglandin E2 (PGE2) has been linked to both inflammation and carcinogenesis. We hypothesized that HIV-1 could induce COX-2 in cervical tissue and increase systemic PGE2 levels and that these alterations could play a role in AIDS-related cervical cancer. Levels of cervical COX-2 mRNA and urinary PGE-M, a biomarker of systemic PGE2 levels, were determined in 17 HIV-negative women with a negative cervical HPV test, 18 HIV-infected women with a negative HPV test, and 13 HIV-infected women with cervical HPV and high-grade squamous intraepithelial lesions on cytology. Cervical COX-2 levels were significantly associated with HIV and HPV status (P=0.006 and 0.002, respectively). Median levels of urinary PGE-M were increased in HIV-infected compared to uninfected women (11.2 ng/mg creatinine vs. 6.8 ng/mg creatinine, P=0.02). Among HIV infected women, urinary PGE-M levels were positively correlated with plasma HIV-1 RNA levels (P<0.001). Finally, levels of cervical COX-2 correlated with urinary PGE-M levels (P=0.005). This study demonstrates that HIV-1 infection is associated with increased cervical COX-2 and elevated systemic PGE2 levels. Drugs that inhibit the synthesis of PGE2 may prove useful in reducing the risk of cervical cancer or systemic inflammation in HIV infected women.
INTRODUCTION
Human immunodeficiency virus (HIV-1) infection causes chronic inflammation, which is beneficial for HIV-1 replication but detrimental for the human host leading to premature senescence of the immune system, cardiovascular disease, organ fibrosis, and cancer (1–3). The exact mechanisms underlying HIV induced chronic inflammation and resulting disease are not known.
The inflammatory molecule prostaglandin E2 (PGE2) has been linked to carcinogenesis in a number of tumor types including cervical cancer. PGE2 is secreted from cells and stimulates carcinogenesis by multiple mechanisms (4). PGE2 promotes angiogenesis (5,6), suppresses apoptosis (7), increases cell proliferation (8), enhances cell invasiveness (9), and suppresses antitumor cell mediated immunity (10).
The enzyme cyclooxygenase (COX) catalyzes the synthesis of PGs from arachidonic acid and is rate limiting in PGE2 synthesis. COX-2 is over-expressed in transformed cells (11) and in various tumor types (12) including cervical intraepithelial neoplasia (CIN) and cervical cancer (13–15). Notably, human papillomavirus (HPV) oncoproteins E6 and E7 stimulate cervical carcinogenesis, activate COX-2 transcription, and enhance PGE2 production (16). Elevated levels of COX-2 correlate with poor prognosis for patients with cervical cancer (17–19). HIV-1 infection induces COX-2 in a number of cell typesincluding circulating monocytes, tissue macrophages, lymphocytes, and neuronal cells (20–25). The up-regulation of COX-2 has been related to several AIDS defining illnesses including HIV associated dementia and HIV cardiomyopathy (23–25).
We hypothesized that HIV-1 could up-regulate COX-2 in cervical tissue and also increase systemic levels of PGE2 and that these alterations in PG metabolism could play a role in HIV-1-related diseases including cervical cancer. Cervical cancer is an AIDS defining illness, and HIV-1 infected women are five times more likely to develop invasive cervical cancer than HIV negative women (26). We quantified levels of COX-2 in the cervix and systemic PGE2 levels in HIV infected vs. uninfected women. The study was conducted in Haiti, where cervical cancer is a leading cause of death in HIV-1 infected women (27).
METHODS
Study Site
Study participants were recruited at the GHESKIO clinical and research center in Port au Prince, Haiti. GHESKIO provides free HIV voluntary counseling and testing, AIDS care, reproductive health services, and management of sexually transmitted infections. In 2009, the GHESKIO clinic provided HIV voluntary counseling and testing to ~ 25,000 people, and antiretroviral therapy to 6,000 patients with AIDS. Clinical samples were shipped to Weill Cornell Medical College (New York, NY), Columbia University College of Physicians and Surgeons (New York, NY) and Vanderbilt University School of Medicine (Nashville, TN) for laboratory analyses.
Study design
COX-2 mRNA and urinary PGE metabolite (PGE-M) levels, a biomarker of systemic PGE2, were quantified in three groups: 1) HIV negative women with normal cervical Pap test and a negative test for cervical high-risk HPV DNA (HIV−/HPV−); 2) HIV-1 infected women with a normal cervical Pap test and a negative test for cervical HPV DNA (HIV+/HPV−); and 3) HIV-1 infected women with a high grade squamous intraepithelial lesion (HSIL) by Pap test and a positive test for cervical HPV DNA (HIV+/HPV+). We hypothesized that there would be a stepwise increase in cervical COX-2 mRNA expression in the three groups. We also hypothesized that HIV-1 infected women would have higher systemic levels of PGE2 than HIV-1 negative women. The number of subjects enrolled in each group was determined by feasibility and logistics.
The study was approved by the institutional review boards (IRBs) of the participating institutions. All women in the study provided written informed consent (28).
Patient Population
HIV infected women were recruited from a research cohort examining the effect of early vs. deferred antiretroviral therapy (ART) (29). These women were well characterized with CD4 counts and plasma HIV-1 RNA levels measured every six months and annual cervical cancer screening with Pap test and HPV testing. Women with a normal cervical Pap test and a negative test for cervical high-risk HPV DNA were recruited. HIV infected women with high grade intraepithelial lesions on Pap test and a positive test for cervical high-risk HPV DNA were also recruited. The study visit was scheduled after the screening Pap and HPV test results were available and prior to treatment for the high grade lesion by cryotherapy or loop electrosurgical excision procedure (LEEP).
HIV uninfected women were recruited from a cohort of HIV negative women of reproductive age at high risk for HIV infection followed at GHESKIO. We recruited women with normal cervical Pap test and a negative test for cervical HPV DNA.
Women who converted from HPV negative to HPV positive with low grade squamous intraepithelial lesions between their annual screening visit and the study visit were not included in the analysis.
Study visit and sample collection
At a single study visit, the Pap test and the HPV test were repeated, urine collected for measurement of PGE-M, and cervical cells collected for quantification of COX-2 mRNA. The study visit was scheduled mid-menses so that cervical samples did not contain menstrual blood. Women with signs or symptoms of a sexually transmitted infection other than HPV were treated and scheduled for their study visit during their next menstrual cycle. Women were asked not to take nonsteroidal anti-inflammatory drugs (NSAIDs) or aspirin for two weeks prior to their study visit. Women takings NSAIDs chronically were excluded.
At the single study visit, women were asked to urinate in a specimen collection container. Urine was aliquoted into three 2 mL cryovials and stored at −70°C. A gynecologist collected cervical cells with a cytobrush for HPV and Pap testing in 20 mL Cytyc Thinprep bottles. The gynecologist also collected cervical cells for COX-2 mRNA analysis with a cytobrush by passing it in a complete 360 degree circle around the endocervical junction. The cells were immediately suspended in a cryovial with 2 mL of RNAlater (Qiagen Inc.), placed on dry ice in the clinic, and then transported to a freezer for storage at −70°C. The 20 mL Cytyc bottles for Pap test and high-risk HPV testing were shipped to the United States and subjected to analysis as detailed below. The urine and cervical cell suspensions were shipped on dry ice to the United States for analysis.
Laboratory tests
Clinical laboratory tests
The GHESKIO laboratory quantified CD4 T cell counts by flow cytometry (Becton, Dickinson, Franklin Lakes, NJ). HIV-1 viral load was quantified by plasma HIV-1 RNA levels measured by the NucliSens EasyQ HIV-1 PCR Test (BioMérieux, Lyons, France).
Liquid-based cytology specimens were routinely processed in the Division of Obstetric, Gynecologic, and Cytologic Pathology at Columbia University Medical Center under the supervision of Dr. Thomas C. Wright. Results were reported follong the Bethesda 2001 classification system as normal, atypical squamous cells of unknown significance (ASC-US), low grade squamous intraepithelial lesion (LSIL), or high grade squamous intraepithelial lesion (HSIL) (30).
High risk HPV testing was performed on cervical samples with the digene Hybrid Capture II HPV DNA Assay (Qiagen Corporation) following manufacturer’s instructions. The HC II test is a nucleic acid hybridization assay with signal amplification used to detect and quantify high-risk HPV types in cervical specimens. The test detects 13 high-risk HPV types, (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) and is reported as positive or negative for high-risk HPV DNA.
Quantification of COX-2 mRNA
Cervical samples stored in RNAlater were thawed, centrifuged, and the supernatant discarded. Total RNA was isolated using RNeasy Mini Kit (QIAGEN Inc.). RNA quantification and quality assessment were performed using a 2100 Bioanalyzer (Agilent Technologies). 200 ng of total RNA was reverse transcribed using murine leukemia virus reverse transcriptase (Roche Applied Science) and oligo d(T)16 primer. The resulting cDNA was then used for amplification. Each PCR reaction was 20 μL and contained 5 μL cDNA, 2× SYBR Green PCR master mix, and forward and reverse primers. Primer sequences for amplification were: COX-2, forward 5′-CCCTTGGGTGTCAAAGGTAA-3′, reverse 5′-GCCCTCGCTTATGATCTGTC-3′; β-actin, forward 5′-AGAAAATCTGGCACCACACC-3′, reverse 5′-AGAGGCGTACAGGGATAGCA-3′. Experiments were performed using a 7500 real time PCR system (Applied Biosystems). The standard curve method was used to determine levels of COX-2 mRNA using a known copy number of COX-2 mRNA /10 ng RNA. β-actin served as an endogenous normalization control.
Urinary PGE-M
Urinary PGE-M is an index of systemic PGE2 production (31, 32). Catabolism of PGE2 results in a stable end metabolite, 11-〈-hydroxy-9,15-dioxo-2,3,4,5-tetranor-prostane- 1,20-dioic acid (PGE-M) that is excreted in the urine (33–36). PGE-M in urine is stable for prolonged periods of time when stored at –70° (32). Measurement of urine PGE-M is a better measure of systemic PGE2 than plasma measurements, because PGE2 in plasma is rapidly metabolized in the lungs and consequently may not accurately reflect endogenous PG production (37).
PGE-M concentrations in urine were measured in the Eicosanoid Core Laboratory directed by Dr. Ginger Milne at Vanderbilt University. Urine (1 mL) was acidified to pH 3 with HCl and treated with methyloxime HCl to convert PGE-M to the O-methyloxime derivative. The methoximated PGE-M was extracted, applied to a C-18 Sep-Pak, and eluted with ethyl acetate. An [2H6]-O-methyloxime PGE-M deuterated internal standard was then added. Liquid chromatography was performed using a Waters Acquity UPLC fitted with an Acquity BEH C18 UPLC column (2.0 × 50mm, 1.7 μm) coupled to a Thermo Scientific Quantum Vantage triple quadrupole mass spectrometer. For the subject’s urine, the precursor ion of endogenous-formed PGE-M is m/z 385 and [2H6]-PGE-M internal standard is m/z 391 with the expected predominant product ions being m/z 336 and m/z 339, respectively. Quantification of subject’s PGE-M was calculated by ratiometric determinations of unlabeled:labeled peak areas corresponding to both precursor and product ions. The lower limit of detection of PGE-M is in the range of 40 pg, which is approximately 100-fold below levels in normal human urine. Urinary creatinine levels are measured using a test kit from Enzo Life Sciences. The urinary PGE-M level in each sample are normalized using the urinary creatinine level of the sample and expressed in ng/mg creatinine.
Statistical analyses
Subject characteristics including age and smoking status are described for all study participants. For HIV infected women, ART use, plasma HIV-1 RNA level, and CD4+ T cell counts at the time of study visit were also summarized and compared among groups defined by HIV serostatus and cervical HPV status. For continuous variables, ANOVA and Kruskal-Wallis methods were used to compare the means and medians, respectively. For categorical variables, Fisher’s exact test was used to compare the differences in proportions.
Distribution of the two primary study endpoints cervical COX-2 mRNA and urinary PGE-M levels in study groups were summarized graphically using box-plots. We first used univariate analysis to examine the association between the primary outcome variable and each of the covariates including HIV serostatus, cervical HPV status, age, and smoking status. For the categorical covariates, the association was assessed using the non-parametric Wilcoxon rank-sum test. For age, the association was assessed using univariate linear regression where the outcome variable was log transformed to ensure the underlying model assumptions were satisfied.
We used multivariate linear regression analysis to adjust for potential confounding and effect modification. A backward variable selection procedure based on the Akaike Information criteria (AIC) was used to identify the multiple linear regression model that best fit the outcome data (38). The strength of association between each covariate in the best model and the outcome variable was quantified using P-values. Similar analyses were then performed for HIV positive subjects but with additional covariates, including ART, HIV-1 RNA level, and CD4 counts included in the analysis. P-values that are less than 0.05 are considered statistically significant. All the statistical tests are two-sided.
RESULTS
Study Population
Women were recruited between November 2008 and June 2009. Fifty five-women were recruited. Seven women were not included in the study analysis. One HIV negative and two HIV positive women were excluded because their cervical HPV test converted from negative to positive with LSIL at the time of enrollment. Two HIV positive/HPV positive, one HIV negative/HPV negative, and one HIV positive/HPV negative women had insufficient cervical cells collected for analysis. No one was excluded for chronic aspirin or NSAID use. Complete data are available for 48 women. The characteristics of the three groups of study subjects are shown in Table 1. The HIV negative women were younger than the HIV positive women; smoking history was similar among groups.
Table 1.
Characteristics of study population stratified by HIV status and Pap test results
| HIV negative Normal Pap test 1 (n=17) | HIV positive Normal Pap test (n=18) | HIV positive HSIL on Pap test 2 (n=13) | P-value | |
|---|---|---|---|---|
| Mean Age ± sd 3 | 32 ± 12 | 43 ± 11 | 39 ± 8 | 0.006 |
| Smoking | 1 (6%) | 1 (6%) | 2 (15%) | 0.66 |
|
| ||||
| Receiving ART 4 | - | 15 (83%) | 9 (69%) | 0.41 |
| Median nadir CD4 T cells per mm3 (range) 5 | - | 228 (31 - 301) | 224 (80 - 302) | 0.89 |
| Median CD4 T cells per mm3 at time of study(range) | - | 402 (181 - 815) | 327 (183 - 660) | 0.07 |
| Median plasma HIV-1 RNA copies per ml at time of study (range) | - | 50 (50–240,000) | 23,000 (50- 630,000) | 0.03 |
Women with normal Pap test had normal cervical cytology by Bethesda Classification system and negative Digene HCII test for high risk HPV.
Women with high grade squamous intraepithelial lesions (HSIL) by Bethesda Classification system also had a positive test by Digene HCII for high risk HPV.
Standard deviation (sd)
Antiretroviral therapy (ART)
The nadir CD4 T cell count is the lowest documented CD4 T cell count for an individual patient
Levels of cervical COX-2 are increased in HIV and HIV/HPV infected women
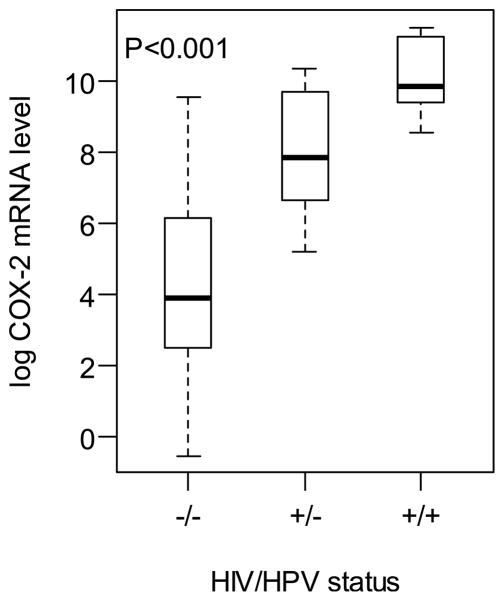
Cervical cells were collected by cytobrush and stored in RNAlater. Microgram quantities of high quality RNA were prepared. COX-2 mRNA levels were significantly associated with HIV and HPV status. Distribution of the log COX-2 levels stratified by HIV and HPV status are shown with box-plots in Figure 1. Levels of COX-2 mRNA were elevated in the HIV-positive/HPV-negative group compared with the HIV-negative/HPV negative group (P<0.001). A further increased in COX-2 mRNA levels was observed in the HIV-positive/HPV-positive group compared with the HIV-positive/HPV-negative group (P<0.001). In the HIV infected women, COX-2 levels were also associated with CD4 counts in multivariate analysis controlling for age. Lower CD4 counts were significantly associated with higher COX-2 expression levels (P=0.02).
Figure 1.
Box-plot of the log COX-2 mRNA levels in cervical cells, stratified by human immunodeficiency virus (HIV) and human papillomavirus (HPV) status.
Urinary PGE-M levels are increased in HIV infected women
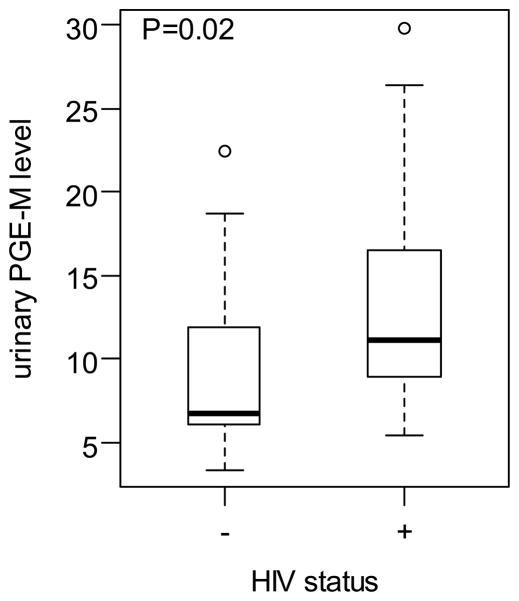
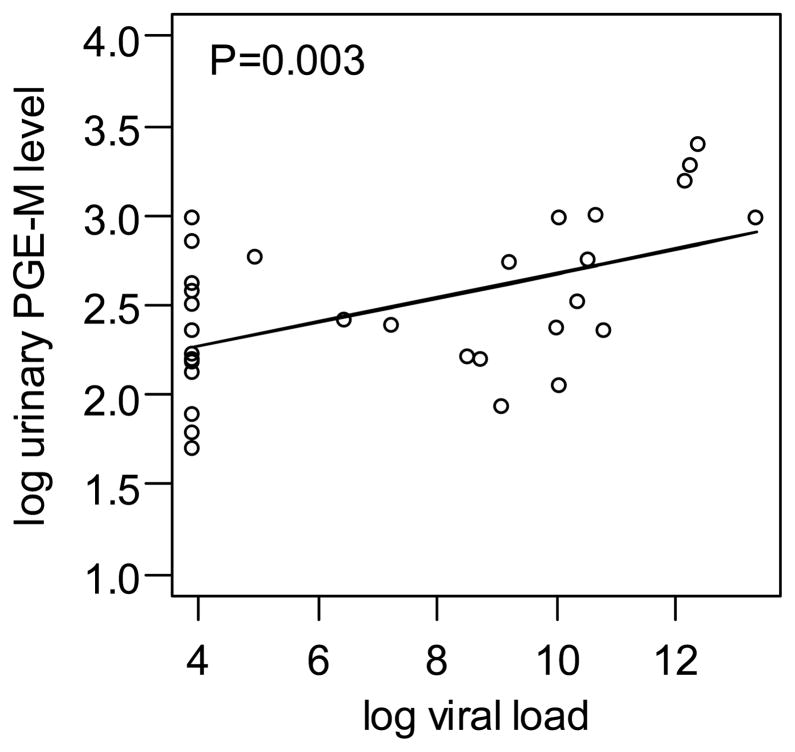
The median and range PGE-M values in ng/mg creatinine for the three groups were: HIV-negative/HPV-negative group, 6.81 (3.39,22.48); HIV-positive/HPV-negative 11.05 (5.42,29.86); and HIV-positive/HPV-positive 12.37 (6.6,24.07). HIV status was the only statistically significant predictor of urinary PGE-M levels in both univariate and multivariate analyses (Figure 2). HPV infection was not significantly associated with PGE-M levels. Among the HIV infected women, subjects with higher plasma HIV-1 viral load had significantly higher urinary PGE-M levels after controlling for age and HPV status (P=0.003) (Figure 3).
Figure 2.
Box-plot of urine PGE-M levels in ng/mg in HIV-positive and negative subjects.
Figure 3.
Correlation between log urinary PGE-M levels and log plasma HIV-1 RNA levels in HIV infected women. We used natural logarithm, so that log(630,000 copies HIV-1 RNA) =13.35.
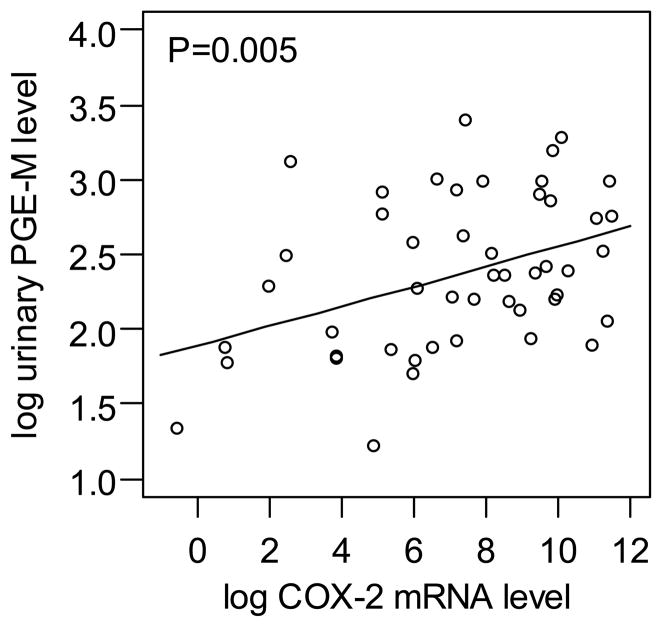
Because elevated levels of COX-2 have been associated with increased levels of urinary PGE-M (39,40), we next correlated levels of cervical COX-2 and urinary PGE-M. Of note, cervical COX-2 mRNA and urine PGE-M levels were positively correlated (P=0.005) (Figure 4).
Figure 4.
Correlation between log urinary PGE-M levels and log cervical COX-2 mRNA levels.
DISCUSSION
This study validates prior evidence that HIV-1 increases COX-2 expression, although to our knowledge this has not been previously reported in the cervix. Prior studies have focused upon HIV-1 effects on COX-2-mediated inflammation in neural cells and early onset dementia. This study extends these findings to cervical cells and systemic PGE2 levels and suggests a possible link between HIV-1 induced increase in PGE2 levels and AIDS-related malignancies. The strong correlation between plasma HIV-1RNA levels and urinary PGE-M levels provides evidence that HIV-1 infection increases systemic production of PGE2, an immunomodulatory molecule linked to carcinogenesis (10).
Studies have shown that HIV-1 induces COX-2 in neuronal cells, circulating monocytes, tissue macrophages, and lymphocytes (20–23). The up-regulation of COX-2 has been implicated in AIDS-related dementia and general immune activation in chronic HIV disease (21–25, 41). We extend these findings to the cervix, where HIV-mediated induction of COX-2 may play a role in cervical carcinogenesis. Notably, COX-2 levels were higher in cervical cells from dual HIV-1/HPV infected women with squamous intraepithelial neoplasia than cervical cells from women infected with HIV alone. Whether this increase in COX-2 levels reflects cell transformation, a direct effect of HPV E6 and E7 oncoproteins on COX-2 transcription or both is uncertain (11, 16).
We demonstrate that HIV-1 infection is associated with increased levels of urinary PGE-M, which is a measure of systemic PGE2 levels. This finding is consistent with prior evidence that HIV-1 infection causes chronic inflammation (1–3). In a recent study, increased urinary PGE-M levels correlated with future risk of colorectal cancer (42). In a study of patients with squamous cell head and neck cancer, elevated urinary PGE-M levels correlated with poor prognosis (43). Importantly in our study, HIV-1 plasma viral load was directly correlated with this potential cancer biomarker. Future studies are warranted to determine whether suppression of HIV-1 replication results in a normalization of urinary PGE-M levels.
HIV-1 infection may increase COX-2 expression and systemic PGE2 levels through a number of mechanisms. HIV-1 increases COX-2 expression in the T lymphocytes and macrophages that it infects (20,21,23,25). The HIV-1 transcription factor Tat stimulates COX-2 transcription (44). HIV-1 infection can also increase plasma cytokines including IL-6 and TNFα (45, 46). Each of these pro-inflammatory cytokines can induce COX-2 and PGE2 synthesis (12). Destruction of the gut associated lymphoid tissue by HIV-1 results in translocation of bacteria and elevations of blood lipopolysaccharide (LPS) levels (47). LPS is a potent inducer of COX-2.
Further studies are warranted to elucidate the mechanism(s) by which HIV-1 infection induces COX-2 in cervical cells and enhances systemic PGE2 synthesis. Although a strong correlation was found between levels of cervical COX-2 and urinary PGE-M, it is highly unlikely that cervical inflammation is responsible for increased systemic PGE2 levels. It is much more likely that the findings in the cervix reflect a systemic inflammatory process. The correlation between COX-2 expression and systemic PGE2 levels suggests that elevated PGE2 results from increased COX-2 activity, likely in systemic lymphocytes and macrophages, but this needs to be verified. Further, prospective studies will need to determine if elevated COX-2 expression and PGE2 levels predict future HPV disease progression and treatment outcomes. This report provides useful biomarkers for these future studies; samples for cervical COX-2 expression and urine PGE-M can be collected non-invasively in settings with high rates of HIV and HPV infection, stored at −70°C, and shipped to reference laboratories for analysis.
It’s possible that HIV-1-mediated induction of systemic PGE2 levels contributes to a variety of HIV-related disease processes. For example, COX-2-derived PGE2 can induce matrix metalloproteinase-9 (48), a proteinase linked to aging-related diseases including coronary artery disease, cancer, and early emphysema in HIV-infected individuals (49). Studies are warranted to determine if urinary PGE-M levels predict future occurrence of diseases related to chronic inflammation in HIV-1 infected people (1–3).
This study enrolled relatively small numbers of women and did not enroll women with HPV and low grade dysplasia. Therefore, we do not know the independent effect of HPV infection versus cervical cell transformation on COX-2 levels. Larger studies are needed to validate our findings and to examine cervical COX-2 in women with HPV infection and different grades of dysplasia.
This study demonstrates that HIV-1 infection is associated with increased amounts of cervical COX-2 and elevated systemic PGE2 levels. Drugs that inhibit the synthesis of PGE2, including aspirin, selective COX-2 inhibitors, and traditional non-steroidal anti- inflammatory drugs, may prove useful in reducing the risk of cervical cancer and systemic inflammation in HIV infected women.
Acknowledgments
The authors thank the clinical staff and volunteers at the GHESKIO clinic in Haiti.
Grant Suppport:
The project was supported by grants CA142422 from the National Cancer Institute, TW 00018 and TW007988 from the Fogarty International Center and the Flight Attendant Medical Research Institute.
Footnotes
Disclosure of Potential Conflicts of Interest:
Andrew J. Dannenberg is a member of the Scientific Advisory Board of Tragara Pharmaceuticals, Inc., a company that is developing a selective COX-2 inhibitor. Daniel W. Fitzgerald supervises a scholarship program for Tanzanian medical students sponsored by Pfizer Inc. The other authors disclosed no potential conflicts of interest.
References
- 1.Dubé MP, Sattler FR. Inflammation and complications of HIV disease. J Infect Dis. 2010;201:1783–5. doi: 10.1086/652751. [DOI] [PubMed] [Google Scholar]
- 2.Giorgi JV, Liu Z, Hultin LE, Cumberland WG, Hennessey K, Detels R. Elevated levels of CD38+ CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. The Los Angeles Center, Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 1993;6:904–12. [PubMed] [Google Scholar]
- 3.Tien PC, Choi AI, Zolopa AR, Benson C, Tracy R, Scherzer R, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr. 2010;55:316–22. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rigas B, Goldman IS, Levine L. Altered eicosanoid levels in human colon cancer. J Lab Clin Med. 1993;122:518–523. [PubMed] [Google Scholar]
- 5.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Av P, Crofford LJ, Wilder RL, Hla T. Induction of vascular endothelial growth factor expression in synovial fibroblasts by prostaglandin E and interleukin-1: a potential mechanism for inflammatory angiogenesis. FEBS Lett. 1995;372:83–87. doi: 10.1016/0014-5793(95)00956-a. [DOI] [PubMed] [Google Scholar]
- 7.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 8.Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem. 2001;276:18075–18081. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- 9.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–20. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 11.Subbaramaiah K, Telang N, Ramonetti JT, Araki R, DeVito B, Weksler BB, et al. Transcription of cyclooxygenase-2 is enhanced in transformed mammary epithelial cells. Cancer Res. 1996;56:4424–4429. [PubMed] [Google Scholar]
- 12.Dannenberg AJ, Subbaramaiah K. Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell. 2003;4:431–436. doi: 10.1016/s1535-6108(03)00310-6. [DOI] [PubMed] [Google Scholar]
- 13.Ryu H-S, Chang K-H, Yang H-W, Kim M-S, Kwon H-C, Oh K-S. High cyclooxygenase-2 expression in stage 1B cervical cancer with lymph node metastasis or parametrial invasion. Gynecol Oncol. 2000;76:320–325. doi: 10.1006/gyno.1999.5690. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni S, Rader JS, Zhang F, Liapis H, Koki AT, Masferrer JL, et al. Cyclooxygenase-2 is overexpressed in human cervical cancer. Clin Cancer Res. 2001;7:429–434. [PubMed] [Google Scholar]
- 15.Sales KJ, Katz AA, Davis M, Hinz S, Soeters RP, Hofmeyr MD, et al. Cyclooxygenase-2 expression and prostaglandin E(2) synthesis are up-regulated in carcinomas of the cervix: a possible autocrine/paracrine regulation of neoplastic cell function via EP2/EP4 receptors. J Clin Endocrinol Metab. 2001;86:2243–2249. doi: 10.1210/jcem.86.5.7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subbaramaiah K, Dannenberg AJ. Cyclooxygenase-2 transcription is regulated by human papillomavirus 16 E6 and E7 oncoproteins. Evidence of a corepressor/coactivator exchange. Cancer Res. 2007;67:3976–85. doi: 10.1158/0008-5472.CAN-06-4273. [DOI] [PubMed] [Google Scholar]
- 17.Gaffney DK, Holden J, Zempolich K, Murphy KJ, Dicker AP, Dodson M. Elevated COX-2 expression in cervical carcinoma: reduced cause-specific survival and pelvic control. Am J Clin Oncol. 2001;24:443–446. doi: 10.1097/00000421-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Ferrandina G, Lauriola L, Distefano MG, Zannoni GF, Gessi M, Legge F, et al. Increased cyclooxygenase-2 expression is associated with chemotherapy resistance and poor survival in cervical cancer patients. J Clin Oncol. 2002;20:973–981. doi: 10.1200/JCO.2002.20.4.973. [DOI] [PubMed] [Google Scholar]
- 19.Kim YB, Kim GE, Cho NH, Pyo HR, Shim SJ, Chang SK, Park HC, Suh CO, Park TK, Kim BS. Overexpression of cyclooxygenase-2 is associated with a poor prognosis in patients with squamous cell carcinoma of the uterine cervix treated with radiation and concurrent chemotherapy. Cancer. 2002;95:531–539. doi: 10.1002/cncr.10684. [DOI] [PubMed] [Google Scholar]
- 20.Longo N, Zabay JM, Sempere JM, Navarro J, Fernández-Cruz E. Altered production of PGE2, IL-1 beta and TNF-alpha by peripheral blood monocytes from HIV-positive individuals at early stages of HIV infection. J Acquir Immune Defic Syndr. 1993;6:1017–23. [PubMed] [Google Scholar]
- 21.Ramis I, Roselló-Catafau J, Gómez G, Zabay JM, Fernández Cruz E, Gelpí E. Cyclooxygenase and lipoxygenase arachidonic acid metabolism by monocytes from human immune deficiency virus-infected drug users. J Chromatogr. 1991;557:507–13. doi: 10.1016/s0021-9673(01)87159-4. [DOI] [PubMed] [Google Scholar]
- 22.Barreto-de-Souza V, Pacheco GJ, Silva AR, Castro-Faria-Neto HC, Bozza PT, Saraiva EM, et al. Increased Leishmania replication in HIV-1-infected macrophages is mediated by tat protein through cyclooxygenase-2 expression and prostaglandin E2 synthesis. J Infect Dis. 2006;194:846–54. doi: 10.1086/506618. [DOI] [PubMed] [Google Scholar]
- 23.Liu QN, Reddy S, Sayre JW, Pop V, Graves MC, Fiala M. Essential role of HIV type 1-infected and cyclooxygenase 2-activated macrophages and T cells in HIV type 1 myocarditis. AIDS Res Hum Retroviruses. 2001;17:1423–33. doi: 10.1089/088922201753197097. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez S, Serramía MJ, Fresno M, Muñoz-Fernández MA. HIV-1 envelope glycoprotein 120 induces cyclooxygenase-2 expression in astrocytoma cells through a nuclear factor-kappaB-dependent mechanism. Neuromolecular Med. 2007;9:179–93. doi: 10.1007/BF02685891. [DOI] [PubMed] [Google Scholar]
- 25.Pereira CF, Boven LA, Middel J, Verhoef J, Nottet HS. Induction of cyclooxygenase-2 expression during HIV-1-infected monocyte-derived macrophage and human brain microvascular endothelial cell interactions. J Leukoc Biol. 2000;68:423–8. [PubMed] [Google Scholar]
- 26.Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus associated cancers in patients with HIV and AIDS. J Natl Canc Inst. 2000;92:1500–1510. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- 27.Fink VI, Shepherd BE, Cesar C, Krolewiecki A, Wehbe F, Cortés CP, et al. Cancer in HIV-infected Persons from the Caribbean, Central and South America. J Acquir Immune Defic Syndr. 2010;56:467–73. doi: 10.1097/QAI.0b013e31820bb1c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzgerald DW, Marotte C, Verdier RI, Johnson WD, Jr, Pape JW. Comprehension during informed consent in a less-developed country. Lancet. 2002;360:1301–2. doi: 10.1016/S0140-6736(02)11338-9. [DOI] [PubMed] [Google Scholar]
- 29.Severe P, Jean Juste MA, Ambroise A, Eliacin L, Marchand C, Apollon S, et al. Early Versus Standard Antiretroviral Therapy for HIV Infected Adults in Haiti. N Engl J Med. 2010;363:257–65. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solomon D, Davey D, Kurman R, Moriarity A, O’Connor D, Prey M, et al. The 2001 Bethesda System: Terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 31.Ferretti A, Flanagan VP, Roman JM. Quantitative analysis of 11 alpha-hydroxy-9,15-dioxo-2,3,4,5,20-pentanor-19-carboxyprostanoic acid, the major urinary metabolite of E prostaglandins in man. Ann Biochem. 1983;128:351–8. doi: 10.1016/0003-2697(83)90385-8. [DOI] [PubMed] [Google Scholar]
- 32.Murphey LJ, Williams MK, Sanchez SC, Byrne LM, Csiki I, Oates JA, et al. Quantification of the major urinary metabolite of PGE2 by a liquid chromatographic/mass spectrometric assay: determination of cyclooxygenase-specific PGE2 synthesis in healthy humans and those with lung cancer. Ann Biochem. 2004;334:266–75. doi: 10.1016/j.ab.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 33.Hamberg M, Samuelsson B. On the metabolism of prostaglandins E 1 and E 2 in man. J Biol Chem. 1971;246:6713–21. [PubMed] [Google Scholar]
- 34.Hamberg M, Samuelsson B. The structure of the major urinary metabolite of prostaglandin E2 in man. J Am Chem Soc. 1969;91:2177–8. doi: 10.1021/ja01036a092. [DOI] [PubMed] [Google Scholar]
- 35.Oates JA, FitzGerald GA, Branch RA, Jackson EK, Knapp HR, Roberts LJ., 2nd Clinical implications of prostaglandin and thromboxane A2 formation. N Engl J Med. 1988;319:761–7. doi: 10.1056/NEJM198809153191106. [DOI] [PubMed] [Google Scholar]
- 36.Schweer H, Meese CO, Seyberth HW. Determination of 11 alpha-hydroxy-9,15-dioxo-2,3,4,5,20-pentanor-19-carboxyprostanoic acid and 9 alpha,11 alpha-dihydroxy-15-oxo-2,3,4,5,20-pentanor-19-carboxyprostanoic acid by gas chromatography/negative ion chemical ionization triple-stage quadrupole mass spectrometry. Ann Biochem. 1990;189:54–8. doi: 10.1016/0003-2697(90)90043-9. [DOI] [PubMed] [Google Scholar]
- 37.Piper PJ, Vane JR, Wyllie JH. Inactivation of prostaglandins by the lungs. Nature. 1970;225:600–4. doi: 10.1038/225600a0. [DOI] [PubMed] [Google Scholar]
- 38.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- 39.Duffield-Lillico AJ, Boyle JO, Zhou XK, Ghosh A, Butala GS, Subbaramaiah K, et al. Levels of prostaglandin E metabolite and leukotriene E4 are increased in the urine of smokers. Evidence that celecoxib shunts arachidonic acid into the 5-lipoxygenase pathway. Cancer Prev Res. 2009;2:322–9. doi: 10.1158/1940-6207.CAPR-09-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Csiki I, Morrow JD, Sandler A, Shyr Y, Oates J, Williams MK, et al. Targeting cyclooxygenase-2 in recurrent non-small cell lung cancer: a phase II trial of celecoxib and docetaxel. Clin Cancer Res. 2005;11:6634–40. doi: 10.1158/1078-0432.CCR-05-0436. [DOI] [PubMed] [Google Scholar]
- 41.Rahmouni S, Aandahl EM, Nayjib B, Zeddou M, Giannini S, Verlaet M, et al. Cyclo-oxygenase type 2-dependent prostaglandin E2 secretion is involved in retrovirus-induced T-cell dysfunction in mice. Biochem J. 2004;384:469–76. doi: 10.1042/BJ20031859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai Q, Gao YT, Chow WH, Shu XO, Yang G, Ji BT, et al. Prospective study of urinary prostaglandin E2 metabolite and colorectal cancer risk. J Clin Oncol. 2006;24:5010–6. doi: 10.1200/JCO.2006.06.4931. [DOI] [PubMed] [Google Scholar]
- 43.Kekatpure VD, Boyle JO, Zhou XK, Duffield-Lillico AJ, Gross ND, Lee NY, et al. Elevated Levels of Urinary Prostaglandin E Metabolite Indicate a Poor Prognosis in Ever Smoker Head and Neck Squamous Cell Carcinoma Patients. Cancer Prev Res. 2009;2:957–965. doi: 10.1158/1940-6207.CAPR-09-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blanco A, Alvarez S, Fresno M, Muñoz-Fernández MA. Extracellular HIV-Tat induces cyclooxygenase-2 in glial cells through activation of nuclear factor of activated T cells. J Immunol. 2008;180:530–40. doi: 10.4049/jimmunol.180.1.530. [DOI] [PubMed] [Google Scholar]
- 45.Neuhaus J, Jacobs DR, Jr, Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–95. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cozzi-Lepri A, French MA, Baxter J, Okhuysen P, Plana M, Neuhaus J, et al. Resumption of HIV replication is associated with monocyte/macrophage derived cytokine and chemokine changes: results from a large international clinical trial. AIDS. 2011;25:1207–17. doi: 10.1097/QAD.0b013e3283471f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 48.Steenport M, Khan KMF, Du B, Barnhard SE, Dannenberg AJ, Falcone DJ. Matrix metalloproteinase (MMP)-1 and MMP-3 induce macrophage MMP-9: Evidence for the role of TNF-α and cyclooxygenase-2. J Immunol. 2009;183:8119–8127. doi: 10.4049/jimmunol.0901925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaner RJ, Santiago F, Crystal RG. Up-regulation of alveolar matrix metalloproteinases in HIV+ smokers with early emphysema. J Leukoc Biol. 2009;86:913–922. doi: 10.1189/jlb.0408240. [DOI] [PMC free article] [PubMed] [Google Scholar]