Abstract

Introduction
The descriptions of total spondylectomy and further development of the technique for the treatment of vertebral sarcomas offered for the first time the opportunity to achieve oncologically sufficient resection margins, thereby improving local tumor control and overall survival. Today, single level en bloc spondylectomies are routinely performed and discussed in the literature while only few data are available for multi-level resections. However, due to the topographic vicinity of the spinal cord and large vessels, the multisegmental resections are technically demanding, represent major surgery and only few case reports are available. Surgical options are even more limited in cases of revision surgery and local recurrences when en bloc spondylectomy was considered to be not feasible due to high risk of vital complications in expanding resection margins. Deranged anatomy, implants in situ and extensive intra-/paraspinal scar tissue formation resulting from previously performed approaches and/or radiation are considered the principal complicating factors that usually hold back spine surgeons to perform revision for resection leaving the patient to palliative treatment.
Methods
We present two patient cases with previously performed piecemeal vertebrectomy in the thoracic spine due to a solitary high-grade spinal sarcoma. After extensive re-staging, both patients underwent a multi (4)-level en bloc spondylectomy in our department (one patient with combined en bloc lung resection). Except a local wound disturbance, there was no severe intra- or postoperative complication.
Results
After multilevel en bloc spondylectomy both patients showed a good functional outcome without neurological deficits, except those resulting from oncologically scheduled resection of thoracic nerve roots. After a median follow-up of 13 months, there was no local recurrence or distant metastasis. The reconstruction using a posterior screw rod system that is interconnected to an anterior vertebral body replacement with a carbon composite cage showed no implant failure or loosening. In summary, the approach of a multilevel en bloc surgery for revision and oncologically sufficient resection in cases of spinal sarcoma recurrences seems possible. However, interdisciplinary decision making in a tumor board, realistic evaluation of surgical resectability to attain tumor free margins, advanced experiences in spinal reconstructions and involvement of vascular, visceral and thoracic surgical expertise are essential preconditions for acceptable oncological and functional outcome.
Keywords: En bloc spondylectomy, Spinal sarcoma, Solitary metastases, Local recurrence
Case presentation
Case1
A 46-year-old male presented with a history of back pain for 12 months and was hospitalized after acute deterioration in an external hospital. MRI showed osteolytic, destructive tumor growth reaching from the seventh through the ninth thoracic vertebra (Fig. 1). Following acute intralesional decompression surgery of the eight thoracic level and posterior stabilization of the levels T5/6 to T10/11, intraoperative histopathological specimens revealed a low-grade osteosarcoma. In a second surgical approach, intralesional resection of thoracic vertebras seven to nine and partial resection of the sixth and eight ribs have been performed (Fig. 2). Reconstruction of the vertebras was achieved with an expandable cage and an antero-lateral screw/rod-system. All these surgeries have been performed in an external hospital. All surgical margins were considered to be intralesional. A control MRI investigation 4 months after the last operation showed suspicion of local recurrence/progressive residual tumor mass at the corresponding thoracic levels. Then the patient was referred to our center and a later biopsy confirmed the diagnosis of a recurrent giant-cell containing osteosarcoma (Grade I-II). Neo-adjuvant chemotherapy was performed according to the protocol of EURO-BOSS, consisting of Cisplatin 100 mg/m2 and Doxorubicin 60 mg/m2 at week 0, Ifosfamid 3 g/m2 and Cisplatin 100 mg/m2 at week 3 and Ifosfamid 3,000 mg/m2 and Doxorubicin 60 mg/m2 at week 6. Physical examination demonstrated back pain in the area of the seventh, eighth, and ninth thoracic vertebrae and decreased mobility of the thoracic spine without neurological deficit. Computed tomography and MRI scans showed a tumor at the described thoracic levels T7–T9, around the cage and the screws in T7 and T10, but without spinal canal involvement. The cage-system used for reconstruction of the levels seven to nine, as well as the screws used for posterior stabilization showed no signs of breakage, dislocation or loosening. No evidence of distant metastasis was found on PET-CT and bone scintigraphy. The local tumor board recommended a total en bloc spondylectomy of the seventh to tenth thoracic vertebral level.
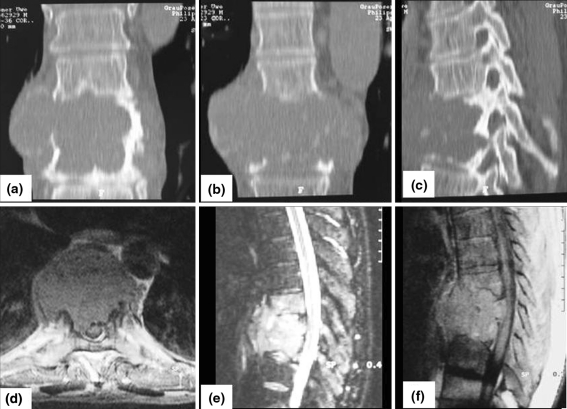
Fig. 1.
Coronar (a, b) and sagittal (c) CT scan of the tumor appearance T7–9 before intralesional resection (external hospital). Axial T2 (d), sagittal T2 (e) and sagittal T1 (f) MRI of a low grade osteosarcoma (case 1)
Fig. 2.
Control X-ray after the second operation with an anterior tumor resection following the emergency decompression and stabilisation from T 5/6 to T10/11 (case 1)
Case 2
A 54-year-old male was referred to our department with a 1-year history of spinal chondrosarcoma T6 and T7. The patient sustained acute sensomotoric paraplegia during a vacation trip abroad. Initial MR-imaging revealed a tumorous destruction of the T6/7 thoracic vertebral body with massive epidural spinal cord compression and intraspinal tumor growth. Therefore, he underwent emergency laminectomy during which adequate tumor tissue was harvested, revealing diagnosis of chondrosarcoma grade 2. During the later 2 weeks, the paraplegia decreased and the patient regained ambulation, full strength and sensibility. Back home he was admitted to an external, non-university hospital without any evidence of distant metastatic disease and underwent anterior intralesional surgery/resection (2-level piecemeal corpectomy) followed by vertebral body replacement using a large expandable cage (Fig. 3). Excisional margins of the corpectomy were considered to be clearly intralesional. Control follow-up CT-staging at 10 months later suspected a tumor relapse/progressive residual tumor growth demonstrating chondroid-like tumor mass around and cranial as well as caudal to the cage involving the adjacent vertebral body levels with partial invasion of the spinal canal and dural contact. For further therapy, the patient was referred to our center. By admission, the patient complained of progressive pain in the mid thoracic spine as well as numbness in his heels. Further neurological deficits were not detectable. For staging purposes, a local MRI and a PET–CT scan were performed. An open biopsy of the relapse lesion confirmed the diagnosis of a recurrent moderately differentiated chondrosarcoma (G II). For determination of the therapeutically strategy, the patient was introduced to the local tumor board with the diagnosis of a recurrent spinal chondrosarcoma of the thoracic spine following previous surgery without an evidence of distant metastases. An oncologically sufficient resection in terms of a 4-level total en bloc spondylectomy T5–T9 by a combined two-stage anterior-posterior approach was recommended.
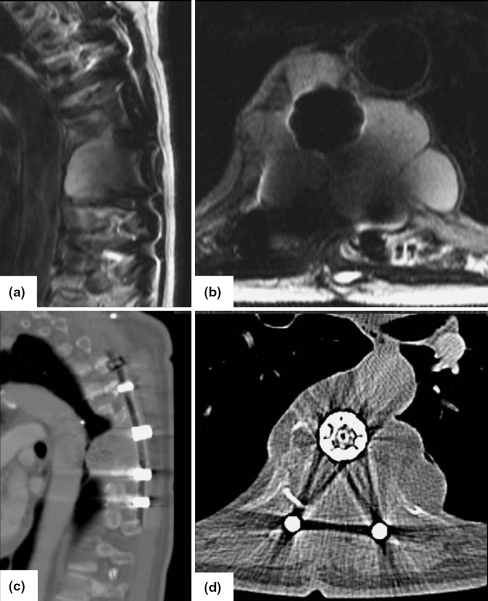
Fig. 3.
Preoperative MRI a sagittal, b axial and CT c sagittal, d axial images of a chondrosarcoma T6 and T7 (case 2)
Historical review/epidemiology
Primary vertebral tumors, i.e. sarcomas of the spinal column, are very rare entities [10]. Among all primary malignant bone tumors about 5–10% arise in the spinal column, but only 25% are allotted to spinal sarcomas [9]. For decades, intralesional resection, e.g. curettage or piecemeal excision was the common surgical practice [4] for spinal sarcoma treatment. The association to a poor oncosurgical outcome, i.e. reduced overall survival is due to surgery-induced uncontrollable tumor cell spread by a disseminating intralesional approach and the limited adjuvant therapy options resulting in rapid development of local recurrence and distant metastases [4, 5].
Pursuing the approach of Enneking’s principles and its revolutionary consequences on the oncological outcome, the concept and understanding of compartment-orientated surgery were successfully transferred to the management of primary spinal bone tumors [7]. The first reports about en bloc resection of the compartment “vertebral body”, later termed “total en bloc spondylectomy” [1] and further development of that technique, especially by Tomita and co-workers at the beginning of the early nineties of the last century, revealed the chance to achieve wide resection margins. Thus, the surgical option to improve both, local tumor control and overall survival [2, 3] was offered for the first time. Today, single level en bloc spondylectomies are routinely performed and discussed in the literature while only few data are available for multi-level resections [11, 12]. However, due to the topographic vicinity of the spinal cord and large vessels the multisegmental resections are technically demanding and represent major surgery with a surgical “tour de force” for the patient [6]. Only few case reports/series reporting multisegmental en bloc excisions are published. Surgical treatment options are even more limited in cases of revision surgery and local recurrences when en bloc spondylectomy was considered to be not feasible due to high risk of vital complications in expanding resection margins. Deranged anatomy, implants in situ and extensive intra-/paraspinal scar tissue formation resulting from previously performed approaches and/or radiation are considered the principal complicating factors, that usually hold back spine surgeons to perform revision for resection, finally leaving the patient to palliative treatment.
Local recurrences of vertebral sarcomas or metastatic lesions with extracompartimental tumor extension around essential neurovascular structures or implanted screws and cages have long been considered as either nonresectable or treatable only with palliative decompressions, intralesional resections and tumor reduction.
Treatment rational
En bloc resection of primary malignant vertebral/spinal tumors with negative surgical margins has attracted major surgical interest as it increases survival rate and improves local tumor control. GI and II osteosarcomas are rather insensitive to chemotherapy and only high dose radiation therapy seems to slow down the progression of disease. According to the available literature, median survival ranges from 6 to 8 months when the spine is involved due to primary or metastatic tumors [14]. In the past, solid malignant tumors of the spine were considered incurable because attaining wide surgical margins was assumed to be surgically impossible. Meanwhile, several techniques of total spondylectomy for spine tumors have been developed. Tomita’s technique allows both resection and stabilization in a single posterior approach [3]. This technique seems to reduce the co-morbidities of a combined approach. In contrast, different authors have reported simultaneous/sequential combined approaches with low to acceptable complications rates [15, 16]. The advantage of these procedures is the control of both the posterior neural structures as well as the anterior vascular, mediastinal and/or visceral structures during the resection. If possible, our group prefers a single posterior approach for standard mono- or bi- segmental en bloc resections. Anterior approach is considered if there is large extracompartimental tumor mass and involvement/encasement of large vessels is present. Moreover, as exemplified by these two cases anterior release and approach is also mandatory in case of adhesions and scar tissue formation resulting from previous surgeries with extensive anterior tumor mass involving the anterior aorta, caval and azygos vein as well as spinal implant systems. In order to attain tumor free margins, en bloc excision of local tumor recurrence at the thoracolumbar spine requires expansion of resection margins/lines including all possible contaminated/adherent and potentially resectable structures when compared with primary surgery. This approach, however, ultimately and considerably increases the risk of surgery in terms of intra-/postoperative morbidity and mortality for the patient.
Increase in complication rates is associated with the number of levels/vertebrae that need to be resected, the necessity to manipulate, release and/or resect essential structures as well as the number, type and extent of surgical approaches. While the incidence of complications is significantly higher in revision surgery as compared to non-preoperated patients the rate of local recurrences is even more significantly enhanced [13], as recognition and attainment of tumor free margins is much more difficult. Various authors described en bloc resections, mostly single or double level resections. Liljenqvist et al. [11] reported four multilevel resections—2, 3 and 4 level resections—out of 21 patients with malignant spine tumors. In case of the widest resection with four vertebras, a marginal resection margin was achieved. Unfortunately, the patient received a complete paraplegia due to spinal cord ischemia. Junming et al. [17] described one case with a 3 level resection in cervicothoracic junction due to a giant cell tumor. The patient also developed a local recurrence.
The reconstruction with a carbon composite cage and 3 level posterior fixation above and below was shown to be stable and to offer good preconditions for anterior bone fusion. Biomechanical studies already showed that the post-implantional stability following en bloc spondylectomy is mainly influenced by the number of pedicle screws placed for posterior fixation [8]. A screw fixation of at least 2, better 3 adjacent levels is recommended. Load distribution to a higher number of screws secures construct stability and decreases the risk of primary implant failure and loosening [8]. As compared to long posterior fixations, short posterior fixations even with an anterolateral plate showed a minor stability [8].
Boriani et al. reported of high complications rates in patients with previously performed surgery. This group showed a complication rate of 46% in patients as compared to 31% in patients primarily treated in a spinal tumor center. Furthermore, a higher risk of major complications was observed. However, as Boriani et al. has correctly emphasized that local recurrence is the worst complication, as this negatively affects quality of life, decreases prognosis and is often not susceptible to another surgical intervention. This fact reflects that the prognosis is mainly related to the type of the first treatment. An infringement of oncosurgical principles may result in preprogrammed local recurrences, which make local control incomparably harder and unlikely more difficult. In other words, if initial treatment of usually radioinsensitive vertebral sarcoma is performed intralesionally, the patient and the surgeon will not have to wait long for a local recurrence which in most instances, determines the patients’ fate [6].
Nevertheless, realistic assessment of the oncological usefulness of surgical interventions and the evaluation of surgical feasibility has to precede any consideration of indications for such type of surgery to justify the risk for the patient. Usually this decision is made in an interdisciplinary approach of a sarcoma board, involving the experience and therapeutic options of all disciplines involved in oncological care of spine tumor patients. Once the indication for surgery is made, careful treatment planning is crucial in these recurrent cases to achieve adequate oncosurgical results and minimize perioperative complications. Each patient suspected of having a recurrent tumor disease undergoes a thorough local and systemic work-up to complete re-staging. Imaging studies include plain radiographs, CT and magnetic resonance imaging of the recurrent tumor region. In addition, CT of the chest and abdomen, MRI of the corresponding spinal levels, nucleid bone scan and more recently positron emission tomography scan complete the systemic imaging. A biopsy of the recurrent lesion is essential before rendering the definitive treatment. Therefore, indication for surgical treatment should be based on a consent decision in a broad community of experts involved in oncological management of spine tumor patients. The careful planning and performance of the biopsy according to the oncosurgical guidelines decreases the likelihood of adverse effects on the prognosis and subsequent treatment options. Anterior approaches and releases are useful and absolutely essential in the presence of encasement of anterior large vessels, mediastinal, retroperitoneal and/or visceral structures. Effective anterior preparation lays the foundation for minimal risk during resection from posterior when surgical control of anterior structures and potential sources for bleeding is minimized.
Summarizing, the presented two cases underscore that by consequently expanding the borders of excision, if necessary with inclusion of dura, large vessels, lung or chest wall into the resection, oncologically sufficient resections with good local tumor control can be reached. However, enormous experience in tumor surgery and reconstructive techniques of the spine as well as thoracic, vascular and visceral surgery in an interdisciplinary approach is an absolute precondition for successful outcome. Therefore, ultimately require therapy in a spine tumor center which provides optimum prerequisites in terms of facilities, interdisciplinary network, postoperative intensive care and rehabilitation to prevent and manage potential serious complications.
Procedure
Case 1
Spinal angiography and selective embolisation of the T7–9 segmental arteries were performed 1 day before surgery. A total en bloc spondylectomy of the seventh to tenth thoracic vertebral level was performed using a sequential anterior and posterior approach. With the patient in supine position, the left thoracic cavity was exposed by thoracotomy. The neighboring anterior structures (i.e. the aorta, azygos vein) were liberated from the anterior aspect of the spine without violation of the tumor. Then, the anterior plate/screws were removed and the intervertebral discs adjacent to the tumor free vertebral segments have been cut. In a second surgical step, the patient was placed in a prone position and a midline skin incision was made from levels T3 through L2. Implants previously used for posterior stabilization except screws and cage between T7 and T10 were removed. New transpedicular screws were inserted at levels T4–T6 and T11–L1. The most lateral extent of each costotransverse process of the seventh through tenth thoracic vertebrae was exposed bilaterally, as were the ribs at the same level. The ribs were transected 5–7 cm lateral to the costotransverse joint and the parietal pleura were bluntly separated from the vertebrae affected by the tumor. Then a circumferential release of the spinal cord via an extension of the previously performed laminectomy was carefully carried out at levels T7–T10. The nerve roots on the right side had to be dissected allowing rotational removal of the vertebrae around the axis of spinal cord. Thereafter, the disc spaces and posterior longitudinal ligaments were transected by scalpel at the level of T6/7 and T10/11. After a unilateral fixation of the seventh through to tenth level, the vertebraes were rotated carefully using the spinal cord as center of rotation using the laminectomy gap as the corridor for passage of the spinal cord (Fig. 4). For reconstruction, a carbon composite vertebral body replacement system (©coLigne AG, Zürich, Switzerland) filled with autologous bone from the iliac crest as well as homologous allograft bone was implanted and rigidly fixed to the posterior stabilization system with artificial pedicle system (Fig. 5). After insertion of two redon drains and one chest tube, the wound was closed.
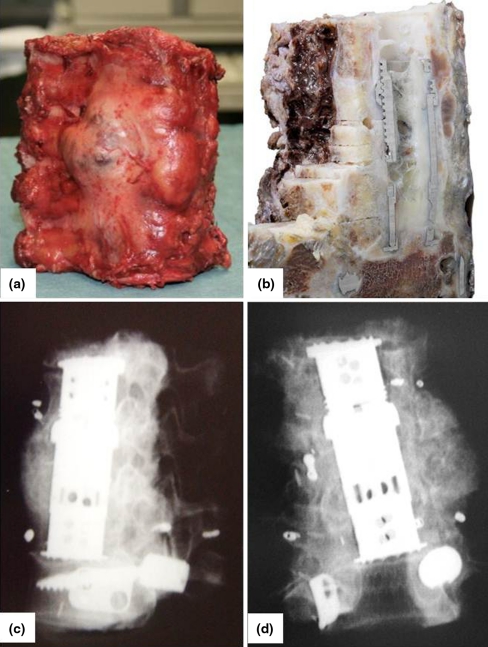
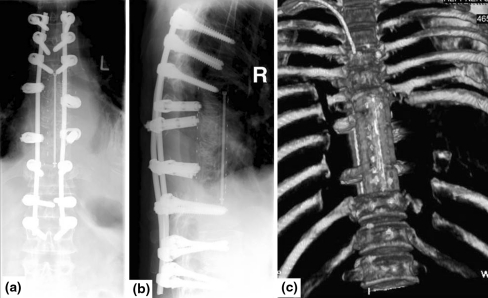
Fig. 4.
Resected specimen a, b intraoperative images, c, d postoperative X-rays showing four resected vertebrae (case 1)
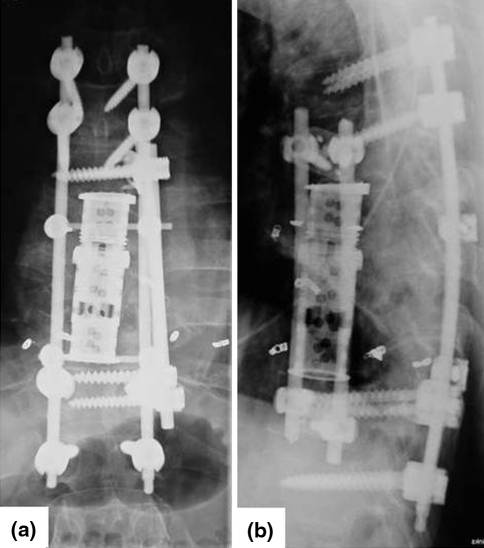
Fig. 5.
Postoperative X-ray in lateral and a.p. view and 3D-computed tomography with good implant positioning (case 1)
Case 2
In the first anterior surgical step, the relapsed tumor (adherent to the visceral pleura and lung) was liberated from the large vessels (aorta, azygos vein, vena cava) by open thoracotomy to reduce the risks and complications of an en bloc resection from posterior in a second step. Since there was broad and tight adhesion of the left lower lung lobe to the anterior aspect of the tumor-affected segments of the thoracic spine, the lower left lung lobe was resected from the lung hilus and left en bloc to the tumor spinal/vertebral levels. On the following day, the 4-level en bloc spondylectomy with the lower left lung lobe adherent to the tumor was completed via the posterior approach. For this the longitudinal incision of the median approach had to be extended in terms of a T-shaped incision to allow the 4-level segment en bloc with the lower lung lobe to pass and be rotated around the longitudinal axis of the spinal cord (Fig. 6). The reconstruction of the resection defect was again performed using a carbon composite VBR system (©coLigne AG, Zürich, Switzerland) that is interconnected by an artificial pedicle to a posterior screw/rod fixation system. Posterior stabilization included three vertebras above and below the resected levels (Fig. 7).
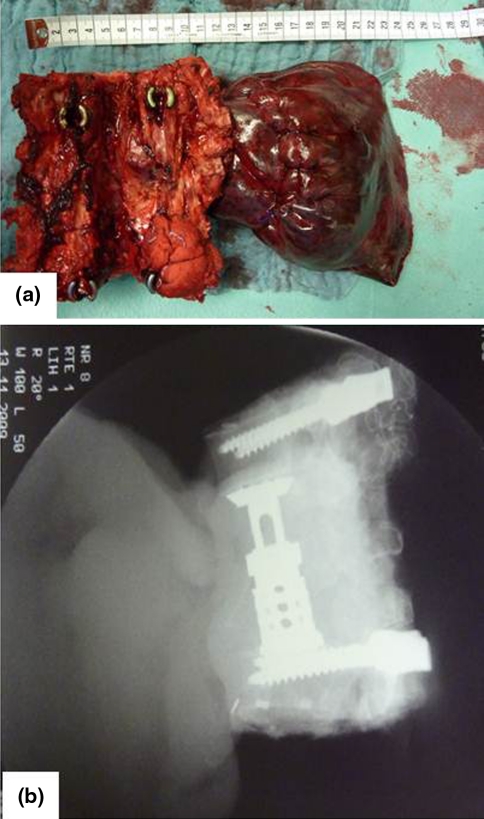
Fig. 6.
Resected specimen en bloc a intraoperative images, b postoperative X-rays showing four resected vertebrae with a partial lung resection (case 2)
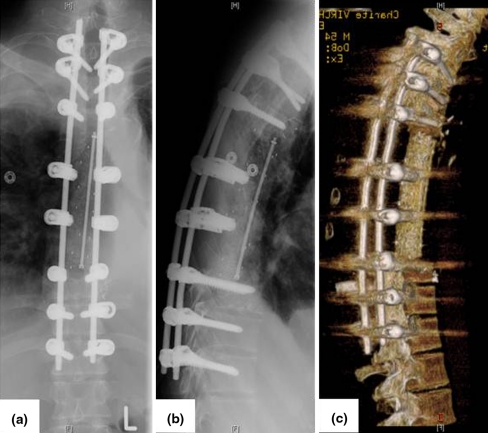
Fig. 7.
Postoperative X-ray images and 3D CT scan (case 2)
Outcome
Case 1
Total operation time was 14 h (anterior and posterior) with overall blood loss volume of approximately 5,000 ml. The patient stayed at the intensive care unit for 1 week and postoperatively there were, apart from temporary subileus symptoms, no further major or minor complications. Histopathological examination demonstrated a specimen with measurements of 13.0 × 13.0 × 8.0 cm, including an 8.0 × 8.0 cm large tumor including the previously used implants. A grade II osteosarcoma (Enneking Stage 2B) was confirmed. No regression of tumor cells was shown (grade VI Salzer/Kuntschik, i.e. non-responder) after neo-adjuvant chemotherapy, rendering the patient as a non-responder to neoadjuvant polychemotherapy. There was tumor infiltration in surrounding lymphatic tissue. Resection margins were considered to be wide, as there was no spinal canal invasion with maximum tumor extension around the cage and anteriorly. Postoperatively, no adjuvant therapy was performed. On a follow up of 17 month, there was no evidence of a local recurrence or distant metastatic lesions.
Case 2
Overall operation time was 15 h. Due to a subsequent pneumonia with reduced respiratory function, the patient stayed at the intensive care unit for nearly 4 weeks. After 5 weeks, a local surgical revision was necessary due to superficial posterior wound healing disturbances. Histopathological examination revealed a specimen with an extensive chondrosarcoma recurrence grade 2. Resection margins were considered to be marginal with thin tumor free margin to the spinal canal/dura. As recommended by the tumor board radiotherapy was scheduled to be performed postoperatively. At a follow-up of 8 month, there was no evidence of local recurrence or distant metastatic lesions.
Conflict of interest
The author has none financial interest/arrangement with one or more organizations that could be perceived as a real or apparent conflict of interest.
References
- 1.Roy-Camille R, Saillant G, Mazel C. Plating of thoracic, thoracolumbar, and lumbar injuries with pedicle screw plates. Orthop Clin North Am. 1986;17(1):147–159. [PubMed] [Google Scholar]
- 2.Talac R, Yaszemski MJ, Currier BL, et al. Relationship between surgical margin and local recurrence in sarcomas of the spine. Clin Orthop Relat R. 2002;397:127–132. doi: 10.1097/00003086-200204000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Tomita K, Kawahara N, Baba H, et al. Total en bloc spondylectomy. A new surgical technique for primary malignant vertebral tumors. Spine. 1997;22:324–333. doi: 10.1097/00007632-199702010-00018. [DOI] [PubMed] [Google Scholar]
- 4.Kawahara N, Tomita K, Murakami H, Demura S, et al. Total en bloc spondylectomy for spinal tumors: surgical techniques and related basic background. Orthop Clin N Am. 2009;40:47–63. doi: 10.1016/j.ocl.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Fujita T, Ueda Y, Kawahara N, et al. Local spread of metastatic vertebral tumors. A histologic study. Spine. 1997;22(16):1905–1912. doi: 10.1097/00007632-199708150-00020. [DOI] [PubMed] [Google Scholar]
- 6.Boriani S, Bandiera S, Donthineni R, Amendola L, Cappuccio M, Iure F, Gasbarrini A. Morbidity of en bloc resections in the spine. Eur Spine J. 2010;19(2):231–241. doi: 10.1007/s00586-009-1137-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer CG, Saravanja D, Boyd M, et al. Surgical management of primary bone tumors of the spine using the enneking principles: a multicenter cohort study. Quebec, Canada: Presented at the Ninth Annual Meeting of the Canadian Spine Society; Gatineau; 2009. [Google Scholar]
- 8.Disch AC, Schaser KD, Melcher I, Luzzati A, Feraboli F, Schmoelz W. En bloc spondylectomy reconstructions in a biomechanical in vitro study. Eur Spine J. 2008;17(5):715–725. doi: 10.1007/s00586-008-0588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley SP, Ashford RU, Rao AS, Dickson RA. Primary bone tumors of the spine: a 42-year survey from the Leeds Regional Bone Tumour Registry. Eur Spine J. 2007;16(3):405–409. doi: 10.1007/s00586-006-0188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melcher I, Disch AC, Khodadadyan-Klostermann C, Tohtz S, Smolny M, Stöckle U, Haas NP, Schaser KD. Primary malignant bone tumors and solitary metastases of the thoracolumbar spine: results by management with total en bloc spondylectomy. Eur Spine J. 2007;16(8):1193–1202. doi: 10.1007/s00586-006-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liljenqvist U, Lerner T, Halm H, Buerger H, Gosheger G, Winkelmann W. En bloc spondylectomy in malignant tumors of the spine. Eur Spine J. 2008;17(4):600–609. doi: 10.1007/s00586-008-0599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boriani S, Biagini R, Iure F. En bloc resections of bone tumors of the thoracolumbar spine. A preliminary report on 29 patients. Spine; 1996;21:1927–1931. doi: 10.1097/00007632-199608150-00020. [DOI] [PubMed] [Google Scholar]
- 13.Boriani S, Bandiera S, Donthineni R, Amendola L, Cappuccio M, De Iure F, Gasbarrini A (2009) Morbidity of en bloc resections in the spine. Eur Spine J (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- 14.Sundaresan N, Rosen G, Huvos AG, et al. Combined treatment of osteosarcoma the spine. Neurosurgery. 1988;23:714–719. doi: 10.1227/00006123-198812000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Abe E, Sato K, Tazawa H, Murai H, Okada K, Shimada Y, Morita H. Total spondylectomy for primary tumor of the thoracolumbar spine. Spinal Cord. 2000;38(3):146–152. doi: 10.1038/sj.sc.3100968. [DOI] [PubMed] [Google Scholar]
- 16.Krepler P, Windhager R, Bretschneider W, Toma CD, Kotz R. Total vertebrectomy for primary malignant tumours of the spine. J Bone Joint Surg Br. 2002;84(5):712–715. doi: 10.1302/0301-620X.84B5.12684. [DOI] [PubMed] [Google Scholar]
- 17.Junming M, Cheng Y, Dong C, Jianru X, Xinghai Y, Quan H, Wei Z, Mesong Y, Dapeng F, Wen Y, Bin N, Lianshun J, Huimin L. Giant cell tumor of the cervical spine: a series of 22 cases and outcomes. Spine (Phila Pa 1976) 2008;33(3):280–288. doi: 10.1097/BRS.0b013e318162454f. [DOI] [PubMed] [Google Scholar]