Abstract
Background
Neuropeptide Y (NPY) is involved in stress regulation. Genetic variations predict plasma NPY and neural correlates of emotion and stress. We examined whether the functional NPY haplotype modulates stress-induced NPY and anxiety responses, and if plasma NPY stress responses are associated with substance dependence outcomes.
Methods
Thirty-seven treatment-engaged, abstinent substance dependent patients (SD) and 28 controls (HC) characterized on NPY diplotypes (HH: high expression; HLLL: intermediate/low expression) were exposed to stress, alcohol/drug cues and neutral relaxing cues, using individualized guided imagery, in a 3-session laboratory experiment. Plasma NPY, heart rate and anxiety were assessed. Patients were prospectively followed for 90-days post-treatment to assess relapse outcomes.
Results
HH individuals showed significantly lower stress-induced NPY with greater heart rate and anxiety ratings, while the HLLL group showed the reverse pattern of NPY, anxiety and heart rate responses. This differential genetic modulation of NPY stress response was suppressed in the SD group, who showed no stress-related increases in NPY and higher heart rate and greater anxiety, regardless of diplotype. Lower NPY predicted subsequent higher number of days and greater amounts of post-treatment drug use.
Conclusion
These preliminary findings are the first to document chronic drug abuse influences on NPY diplotype expression where NPY diplotype modulation of stress-related plasma NPY, heart rate and anxiety responses was absent in the substance abuse sample. The finding that lower stress-related NPY is predictive of greater relapse severity provides support for therapeutic development of neuropeptide Y targets in the treatment of substance use disorders.
Keywords: Neuropepetide Y, Functional Haplotype, Stress, Substance Use Disorders
Introduction
Converging evidence from animal and human studies suggests that neuropeptide Y (NPY) regulates stress response and has anxiolytic-like action (Broqua et al. 1995; Heilig et al. 1989). Animal studies show central effects of NPY in the amygdala and cortical regions that are important in stress regulation (Heilig 2004; Karl et al. 2008a; Sajdyk et al. 2008; Thorsell et al. 1999; Thorsell et al. 2000a). Human studies indicate basal NPY levels from cerebrospinal fluid (CSF) and plasma NPY are significantly decreased in patients with major depression (Heilig et al. 2004), patients with suicide attempts (Westrin et al. 1999) and survivors of military combat with post-traumatic stress disorder (Rasmusson et al. 2000). These results suggest that plasma NPY may contribute to the pathophysiology of major depression and stress related disorders (Heilig 2004). However, no previous research has examined the effects of NPY genetic variation on peripheral NPY levels during acute stress manipulation in healthy control (HC) and substance dependent (SD) patients, and examined its effects on subsequent alcohol and drug use behaviors.
Stress mechanisms increase addiction vulnerability and also significantly increase addiction relapse risk. Chronic alcohol and drug abuse are associated with altered functioning of stress and reward pathways (Koob 2008; Sinha 2008). Recovering alcohol and cocaine dependent individuals present with altered autonomic responses, poor sympathetic and parasympathetic balance, high levels of anxiety and greater alcohol/drug craving and compulsive drug seeking as compared to healthy individuals (Fox et al. 2008; Koob et al. 2004; Sinha 2009; Sinha et al., 2009). Furthermore, high levels of anxiety and drug craving and altered hypothalamic-pituitary- adrenal (HPA) responses to stress contribute to increased relapse risk in addiction (Majewska 2002; Sinha 2007; Walter et al. 2006). There is growing data from animal studies that NPY levels are highly sensitive to acute and chronic ethanol administration and NPY administration suppresses ethanol drinking in ethanol abstinent rats (Gilpin et al. 2008). Infusion of NPY attenuates ethanol withdrawal symptoms in rats (Woldbye et al. 2002) and NPY gene expression is decreased during alcohol withdrawal in rats (Olling et al. 2007). In human study, plasma NPY level was negatively correlated with heroin craving and anxiety in the first post-treatment heroin dependent population (Shi et al, 2009). Interestingly, a recent study reported that NPY injections increased cocaine seeking behavior and heroin self-administration behavior (Maric et al. 2008a; Maric et al. 2008b). Together, these findings suggest that high levels of anxiety and autonomic dysregulation in substance abusing individuals could be associated with dysfunctional NPY responses, which may, in turn, contribute to addiction relapse and poor substance abuse outcomes.
Growing evidence indicates that genetic and environmental interaction contributes to individual differences in stress responses. Genetic variation confers significant risk for stress-related disorders such as addiction (Goldman 1995; Goldman et al. 2005; Kreek et al. 2005). A functional NPY polymorphism (Leu7Pro) has been directly studied for association with prevalence of substance dependence with inconsistent findings (Lappalainen et al. 2002; Mottagui-Tabar et al. 2005; Zhu et al. 2003; Zill et al. 2008). However, we recently reported that the functional NPY haplotype (combination of genetic variations) predicted both NPY mRNA expression in human postmortem brain tissue and lymphoblasts and plasma NPY levels, and it was inversely associated with high trait anxiety and amygdala activity in response to threatening stimuli or a pain/stress challenge (Zhou et al. 2008). Variation in this functional NPY haplotype has also been recently associated with brain response to stress and increased risk of major depression (Mickey et al., 2011). In healthy controls, individuals with low-expression NPY haplotype reported more negative effects under a painful stress condition. Furthermore, low-expression NPY haplotype presented higher frequency in major depression subjected than healthy controls (Mickey et al, 2011). These data suggest that genetic variation in this functional NPY haplotype modulates baseline peripheral NPY and neural responses to stressful and aversive situations. Some individuals with low-expression NPY haplotype are more vulnerable to stress and with greater risk of stress related pathological conditions. As discussed above, stress is associated with increased vulnerability to addiction (Sinha, 2008) and increased risk of relapse. Central injection NPY reduces alcohol intake in animals and plasma NPY level is associated with higher anxiety and craving in post-treatment heroin addiction in human (Shi et al, 2009). Thus, we hypothesize that NPY plays a role in modulating dysfunctional stress responses and autonomic arousal associated with addictive disorders. We further hypothesized that dysfunctional NPY response in addicted individuals would be predictive of greater relapse severity and poor clinical outcomes.
Therefore, using the well-validated guided imagery method to provoke emotional stress and drug craving in controls and patient samples (see Sinha, 2009 for review), we examined whether plasma NPY and anxiety responses are modulated by the functional NPY haplotype in an experiment involving exposure to stress, alcohol/drug cue and neutral imagery on consecutive days in recently abstinent, inpatient treatment engaged substance dependent (SD) individuals and in healthy controls (HC). The SD patients were also prospectively followed to assess post-treatment drug use behaviors after discharge from inpatient treatment. We hypothesized that stress-induced plasma NPY and anxiety responses will be influenced by the NPY diplotype in controls (HC), but that this normal variation in stress-related NPY will be altered in the addicted sample, and the level of alteration will affect subsequent substance use and relapse severity.
Method
Participants
Treatment-seeking individuals between the ages of 21–50 (n = 37, male/female 24/13) meeting DSM-IV criteria for current substance dependence (SD) as assessed by the Structured Clinical Interview for DSM-IV (SCID-I, First et al., 1995) conducted by trained masters level research associate staff were included in the study. These addicted patients were admitted to the Clinical Neuroscience Research Unit (CNRU) of the Connecticut Mental Health Center (CMHC) at Yale University for 5 weeks of inpatient treatment and research participation. Urine and breathalyzer testing were conducted regularly to ensure continued abstinence. All patients participated in specialized substance abuse treatment for 4 weeks prior to the laboratory sessions. Healthy individuals in the 21–50 age range (n = 28, male/female 15/13) who were light social drinkers (up to 6 drink weekly) and did not meet current or lifetime DSM-IV criteria for any substance use disorders (as per the SCID-I) were recruited from the community via local advertisements. In addition, controls and addicted individuals on medications for current medical or psychiatric problems, including those receiving medications for current mood and anxiety disorders, were excluded from the study. However, because of high comorbidity of lifetime mood disorders with addiction and increased current mood and anxiety symptoms in substance dependence during early abstinence, patients who met lifetime DSM-IV criteria of anxiety and mood disorders were not excluded from the study. Substance abusing individuals with current or past opioid abuse were also excluded. The study was approved by the Human Investigation Committee of the Yale University School of Medicine.
During the first two weeks of the inpatient stay, SD patients underwent an initial medical evaluation and completed demographic, psychiatric, drug use and psychosocial assessments. Subjects then completed preparation for individualized guided imagery (see description below) in week 3. To control for any residual alcohol or drug withdrawal/abstinence effects, the laboratory experiment was only conducted in week 5 of their inpatient stay (as described in previous reports (Fox et al. 2008; Sinha 2007; 2009). Healthy control subjects completed demographic, diagnostic, and alcohol/drug-related assessments in two to three assessment appointments and were then admitted for a 3-day hospital stay to the Yale General Clinical Research Center (GCRC) at Yale-New Haven Hospital for participation in the experimental study procedures as the SD patients.
Procedures and measurements
Imagery Script Development Procedures
Prior to the laboratory sessions, individualized guided imagery scripts for stressful situations, alcohol/drug-related stimuli and neutral relaxing states were developed using well-established and previously validated procedures (Fox et al. 2007; Sinha 2009; Sinha et al. 1992; Sinha et al. 2008; Sinha and O’Malley 1999; Sinha and Parsons 1996; Sinha et al. 2003). Stress imagery scripts were developed from subjects’ descriptions of recent personal stressful events that were experienced as “most stressful”, which was determined by having the subjects rate their distress level for each stressful situation on a 10-point Likert scale where “10=the most stress they felt recently in their life”. Only situations rated as 8 or above were accepted as appropriate for script development such as divorce and loss of family members. Alcohol/drug cue scripts were developed based on situations that included alcohol or drug-related stimuli and resulted in subsequent alcohol/drug use (e.g. buying alcohol or drugs, being at a bar, watching others drink alcohol or use drugs). Alcohol/drug-related situations that occurred in the context of negative affect or psychological distress were not allowed. Neutral scripts were developed from the participants’ individual experiences of commonly experienced neutral-relaxing situations, such as a summer day relaxing at the beach or a fall day reading at the park. Details of each elicited situation were described using the Scene Construction Questionnaires, and a script was developed for each of the three types of situations described, based on methods developed by Lang and colleagues (Lang et al. 1980) and further adapted in our previous work with healthy and addicted samples (Fox et al. 2007; Fox et al. 2008; Sinha 2007; 2009; Sinha et al. 1992; Sinha et al. 2008; Sinha and O’Malley 1999; Sinha and Parsons 1996; Sinha et al. 2003). The stress, drug cue and neutral relaxing script was audiotaped for presentation in the laboratory sessions. The stress, drug cue and neutral relaxing imagery conditions were randomized and counterbalanced across subjects to account for any possible order effects. Only one script was presented per day and in each laboratory session and research staff conducting the experiment were blind to the imagery condition on each day.
Habituation and Imagery Training Session
On a day prior to the laboratory sessions, subjects were brought into the testing room where they were acclimatized to specific aspects of the study procedures, such as intravenous (IV) insertion, and training in completing the subjective rating forms and specific training in relaxation and imagery procedures (see Sinha 2009 for review).
Laboratory Sessions
On each day of the experiment, all subjects were allowed an initial smoke break at 7:30 AM to prevent potential nicotine abstinence symptoms from affecting the experiment. A heparin-treated catheter was inserted by the research nurse in order to periodically obtain repeated blood samples during the laboratory sessions. A pulse sensor was also attached to the subject’s index figure. At 9:00 AM, subjects were provided headphones and the audiotape presented the instructions for the imagery procedure and the script for guided imagery. After imagery, subjects remained in the testing room for an additional 75 minutes for repeated measurements to examine recovery from the imagery exposure. After the last assessment, the subject was disconnected from the apparatus.
Plasma NPY measurement
To assess plasma NPY levels, 4 ml of whole blood were collected at two baseline timepoints (−20 and −5), immediately following imagery (0 timepoint) and at +15, +30, +45, +60 and +75 minutes after imagery for all three experimental sessions. Within 30 minutes of collection, the blood was centrifuged at 4°C and 2 ml of plasma were pooled, aliquoted and stored at −80°C until shipment to the Hauger laboratory at the University of California, San Diego for analysis.
Plasma NPY were measured using our previously well-characterized double-antibody radioimmunoassay using 125I-NPY as the tracer (Allen et al. 1991) (Rasmusson et al. 1998). Plasma samples are prepared by completing an acid ethanol extraction (recovery ~70%). After lyophilized extracts are reconstituted in assay buffer, NPY is detected using a highly sensitive and specific NPY antibody (Allen et al. 1991). The NPY assay working range is 19.5 to 1250 pg/ml, and the assay sensitivity is ~15 pg/ml. The assay intra- and inter-assay coefficients of variation are ~4% and ~9%, respectively. To more carefully account for variability in the plasma NPY levels across days and timepoints, the NPY data were normalized to standardized z scores and these were used in the final analysis.
Heart rate
A pulse sensor was attached to the subject’s finger and connected to the Dinamap Monitor to provide a continuous measure of pulse. Heart rate was averaged for the 5 minutes prior to imagery (as a baseline measurement), during the 5-minute imagery period (imagery time-point) and then at each of the +15, +30, +45, +60 and +75 minutes after imagery.
Subjective Anxiety
The anxiety subscale from the Differential Emotions Scale-Revised short form (DES-R; Izard, 1972) was used to assess subjective anxiety at each time point before and after exposure to stress, drug/alcohol cues and neutral imagery. The anxiety subscale is made up of 5 adjectives describing distress, anxiety and arousal (tense, anxious, jittery, aroused, nervous). Participants rate on a 5-point scale the extent to which each word describes the way they feel at the present moment. Assessments were made at the same time point as described above.
Prospective Follow-up of Substance Abuse Post-inpatient Treatment
All SD patients participated in three face-to-face follow-up interviews at day 14, 30 and 90 days following discharge from the inpatient treatment research, where they provided urine and breath alcohol samples and a detailed daily assessment of alcohol and cocaine use using the Time-line Follow-back method on the Substance Use Calendar (SUC). This is a well-established and reliable instrument for assessing self-reported alcohol and drug use outcome measures in treatment studies (Scheurich et al. 2005). As in our previous studies (Paliwal et al. 2008; Sinha et al. 2006), the urine and the SUC data were matched for corroboration, and number of days of alcohol and drug use (frequency of days of using alcohol or cocaine or both) and the average weekly and total number of alcohol drinks and total number of grams of cocaine (0.1 gram, dime bags) consumed (quantity sum) in the 90-day follow-up period was calculated as measures of alcohol and drug use.
NPY SNP genotyping and diplotype identification
All participants were genotyped as per procedures described previously (Zhou et al. 2008). Briefly, a total of six SNPs (rs3037354; rs17149106; rs16147; rs16139; rs5573; rs5574) from the NPY gene were genotyped in this study population by using 5′-nuclease assay.
Our previous study found that one haplotype block defined by six SNPs showed strong pairwise linkage disequilibrium in a Finnish population (Zhou et al. 2008). The haplotype configurations are: H1 Ins/G/C/T/A/T; H2: Del/G/T/T/G/C; H3: Ins/G/T/T/G/C; H4: Ins/G/C/T/A/C; H5: Ins/T/T/C/G/C. Three common haplotypes (H1, H2, H3) were previously found to predict NPY mRNA expression in lymphoblasts among 47 healthy Finnish individuals, which represented six common diplotypes: H1/H1, H1/H2, H1/H3, H2/H2, H2/H3, H3/H3 (Zhou et al. 2008). Individual diplotype was determined based on haplotype configuration on each chromosome. On the basis of lymphoblast NPY mRNA levels, the expression value for each haplotype was calculated by regression analysis. Diplotypes were clustered into three NPY expression groups: predicted high NPY expression (HH) including diplotype H2/H3 and H2/H2, predicted intermediate expression (HL) including diplotype H1/H3, H3/H3 and H1/H2 and predicted low expression (LL) including diplotype H1/H1.
Diplotype distributions for the 65 subjects were HH: 20; HL: 35, and LL: 10. As in previous studies (Hariri et al., 2002; Pezwas et al., 2005; Colzato et al., 2010), low frequency of expression (LL) in one group (HC: LL=2; SD: LL=8) were combined with the intermediate (HL) expression group for statistical analyses (HLLL Group: SD=25; HC=20; HH Group: SD=12; HC=8).
Statistical Analysis
Chi-square analysis and t-tests were conducted to assess both demographic and individual characteristics and diplotype frequency differences in the SD and HC groups. Significant differences between groups and/or diplotype on any of these variables led to the inclusion of the measure as a covariate in all final analyses.
Linear Mixed Effect (LME; Laird & Ware, 1982) models were implemented to analyze the data, using the SAS software package (Version 9, 2006; SAS Institute, Cary, NC). Between-subjects factors of Group 2 (SD vs. HC), predictive NPY Expression group (HH vs. HLLL) and within-subjects factors of Condition 3 (neutral, stress, drug/alcohol cue) and Time-point (varying levels) were the Fixed effects while Subject was the Random effect in the models predicting plasma NPY (z-NPY), heart rate and anxiety responses. In the case of baseline differences for any particular measure, the LME models included the baseline values as a covariate. Otherwise, baseline timepoints were included in the LME model to ensure baseline variation was accounted for in the zNPY, heart rate and subjective anxiety responses to stress, drug/alcohol cue and neutral imagery. To illustrate the findings, baseline adjusted means across the timepoints in each condition are presented in figures. Finally, the association between z-NPY and number of days of alcohol/drug use and amounts of drug used post-discharge was examined using multiple regression analyses.
Results
Demographic and Individual Characteristics of Participants
There were no significant differences on race, gender and prevalence of lifetime mood and anxiety disorders across the SD and HC groups and by NPY genotype. However, the SD groups were older, somewhat less educated, and included more cigarette smokers than the HC groups (p’s <0.05). All analyses presented below included age, years of education and cigarette smoking as covariates.
Baseline Differences
No baseline differences between conditions were seen for plasma NPY, heart rate and subjective anxiety in the control and the substance abusing group. This indicates that the randomizing and counterbalancing of the condition order across the 3 days of testing was successful.
Plasma NPY
There were no significant differences between groups on baseline NPY levels. In experimental stress, cue and neutral provocation, a significant 2-way (Diplotype X Condition) (F[2,107]=12.28, p<0.0001; effect size f=.48) and a significant 3-way (Diplotype X Group X Condition) interaction (F[2,107]=4.03, p<0.02; effect size f=.28) was found. The Diplotype X Condition interaction resulted from the HH group showing significant reduction in plasma NPY in stress versus the neutral condition (p=0.0023), while the HLLL group showed significant increase in NPY during stress versus the neutral condition (p<0.0001). There were no significant differences in plasma NPY in cue versus neutral condition in either HH or HLLL diplotype groups.
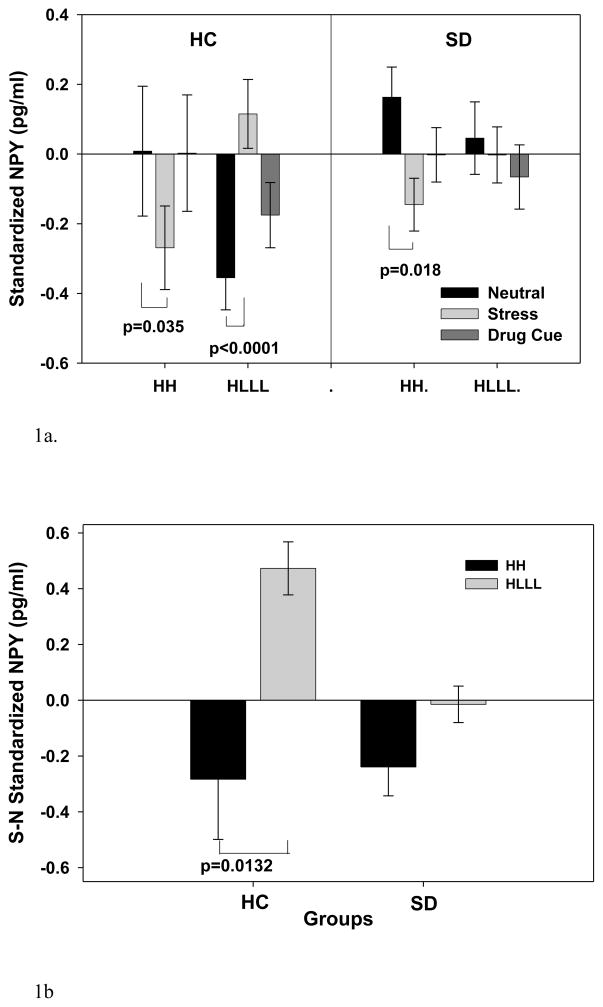
However, the Diplotype X Group X Condition interactions revealed that the differential effect of NPY in stress condition by diplotype was only significant in the controls (HC) and not present in the SD group (Figure 1a, 1b). Thus, in the HC group, the HH individuals showed significantly lower plasma NPY during stress compared to neutral condition (p=0.035), while the HLLL individuals showed significantly higher NPY in comparison of stress and neutral (p<0.0001) conditions (Figure 1a, 1b). The HLLL group also showed greater NPY in stress versus the cue (p<0.0009), as well as cue compared to neutral condition (p < 0.01). The SD individuals presented NPY reduction in HH group (0.02) and no NPY up-regulation in HLLL groups with stress provocation compared to the neutral condition (Figure 1a, shown with averaged NPY level across all timepoints in each condition). A summary of plasma NPY changes in stress condition across all time points is presented in the supplement figure 1. As a follow-up to these analyses, stress relative to neutral change in NPY was computed for the HH and HLLL diplotypes in the HC and the SD groups and as shown in figure 1b, the HLLL diplotype showed significant higher level of plasma NPY in stress than the HH diplotype individuals in the HC group but this difference was not present in the SD group (Figure 1b). All addicted subjects regardless of NPY diplotype showed no significant changes of plasma NPY in alcohol/drug cue compared with neutral condition.
Figure 1.
(1a) Baseline adjusted mean and SE for plasma NPY for the SD and HC groups with the HH and HLLL diplotypes (averaged across time points in each condition). The significant effect of Diplotype x Condition x Group (p=0.0206) is shown. In HC group, HH individuals showed reduced NPY (p=0.035) while HLLL individuals showed increased plasma NPY (p<0.0001) in the stress relative to neutral condition. In the SD group, HH individuals showed significantly lower NPY (p=0.0176), while HLLL individuals showed no differences in plasma NPY (p=0.703) during stress relative to neutral condition. No plasma NPY difference between diplotypes for either group was observed in the cue versus neutral conditions.
(1b) Baseline adjusted plasma NPY changes in stress (S) relative to the neutral (N) condition expressed as a subtraction in response values for neutral from the stress condition (S-N NPY) in SD and HC groups for the HH and HLLL diplotypes is shown. Relative NPY increases in the HLLL subjects but decreases in the HH subjects in the HC group (p<0.0132) is shown, but no such effect of diplotype on NPY stress response is seen in the SD group.
Heart Rate
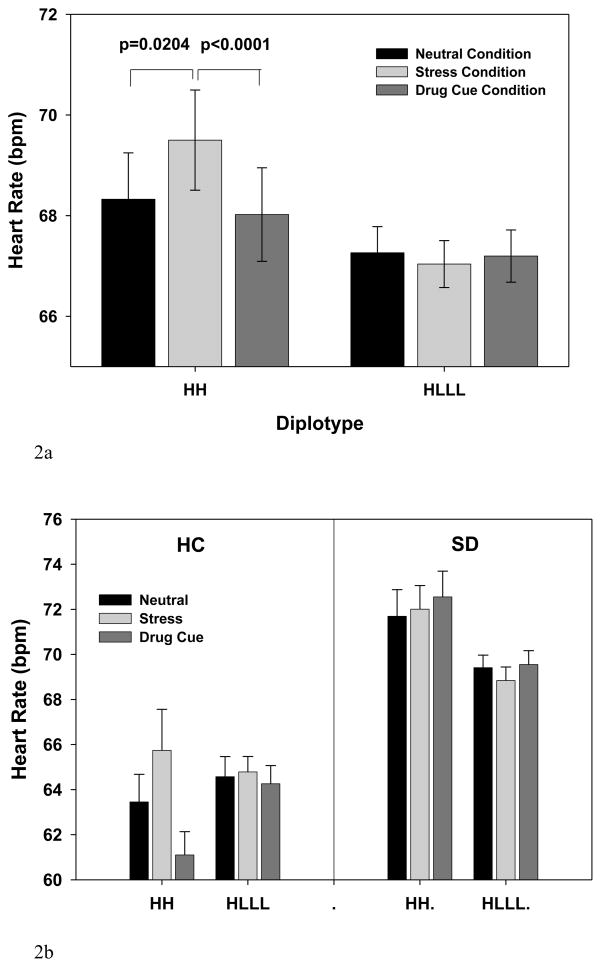
As expected based on previous studies ((Fox et al. 2007; Ingjaldsson et al. 2003; Sinha 2009), there was an overall main effect of Group (F[1,59]=7.14, p<0.001) and Condition (F[2,122]=5.38, p<0.006), and Group X Condition interaction (F[2,122]=15.08, p<0.0001). These data indicated greater basal heart rate in the SD vs the HC group (p=0.0035), higher heart rate responses in the stress relative to the neutral condition, but only in the HC group (p=0.0047) and not in the SD group (p=0.3927).
More importantly, there was a significant 2-way (Diplotype X Condition) (F[2,122]=6.63, p<0.002; effect size f=.33) and a significant 3-way (Diplotype X Group X Condition) interaction (F[2,122]=5.55, p<0.005; effect size f=.30) (see Fig 2a, 2b showing averaged timepoints in each condition). The findings indicated that the HH individuals showed increased heart rate in response to stress relative to neutral (p=0.0204) and relative to the cue condition (p<0.0001), but no such increases were observed in the HLLL group (p=0.56 stress vs neutral; p= 0.77 stress vs cue), and higher overall heart rate was seen in the SD versus HC group (p<0.001), irrespective of diplotype group and condition (also see supplemental figure 2).
Figure 2.
Baseline adjusted mean heart rate response (and SE) during stress, drug cue and neutral conditions for the HH and HLLL diplotypes and the HC and SD groups are presented (averaged across time points in each condition). (2a) A significant diplotype X condition (p<0.002) effect is shown with increased heart rate during stress relative to neutral (p=0.0204) and relative to the drug cue condition (p<0.0001) in the HH group, but no stress-related increases in heart rate were seen in the HLLL individuals.
(2b) Higher overall heart rate was observed in the SD versus the HC group (p<.0.001). A diplotype X group X condition interaction (p<0.005) was seen due to HH and not the HLLL individuals showing stress related increases in heart rate, as shown in 4a, but this difference by diplotype was only present in the HC and not in the SD group, who showed no condition related increases in heart rate.
Subjective Anxiety
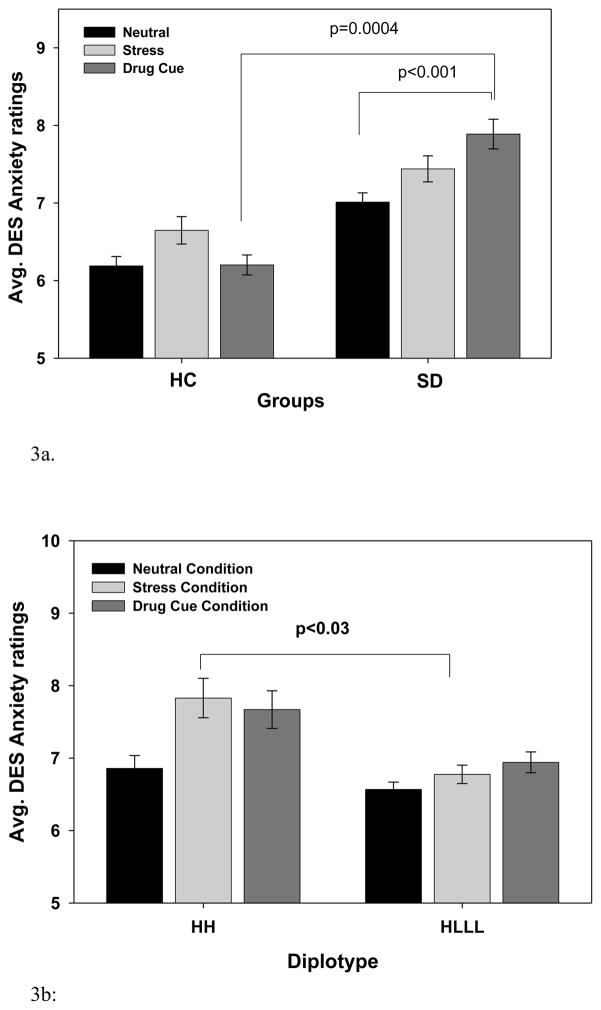
As expected, an overall main effect of Condition (F[2,122]=16.08, p<0.0001), and Group X Condition interaction (F[2,122]=11.07, p<0.0001) was observed, indicating greater overall anxiety in the stress (p <0.0001) and cue (p < 0.0001) versus neutral and in the stress versus cue (p<0.001) conditions. The Group X Condition interaction resulted from significant higher anxiety during stress versus neutral in both the SD and HC group (p’s <0.0001), but significantly increased anxiety during alcohol/drug cue exposure only in the SD group (p<0.0001) but not in the HC group (p=0.58) (see Figure 3a, shown averaged across timepoints in each condition). More interestingly, a significant 2-way Diplotype X Condition interaction (F[2,122]=5.25, p=0.0065; effect size f=.30) was also observed, resulting from the HH diplotype group showing greater anxiety ratings relative to the HLLL diplotype group during stress (p=0.03), but no differences in anxiety ratings by diplotype in the neutral and the cue conditions (Fig 3b, also see supplemental figure 3).
Figure 3.
Baseline adjusted mean and SE for subjective anxiety ratings during the stress, drug cue and neutral conditions (averaged across time points in each condition). (3a) Significantly higher anxiety ratings were observed in the stress (p<0.0001) and drug cue (p<0.0001) relative to the neutral and in the stress relative to cue (p<0.001) conditions. A significant Group X Condition effect (p<0.0001) was observed with both HC and SD groups showing higher stress-related anxiety relative to neutral condition (p’s<0.001), but significantly higher anxiety during drug cue exposure in the SD and not in the HC group (p<0.0004). In cue relative to neutral, anxiety level was significantly increased in SD group (p<0.001) but not in the HC group (p=0.582).
(3b). A significant Diplotype X Condition effect (p=0.0065) was observed with greater anxiety ratings in the stress (HH: p<0.0001; HLLL: p<.07) and in cue (HH: p<0.0004; HLLL: p<.007) versus to the neutral conditions, but the HH individuals showed significantly higher stress-related anxiety than the HLLL individuals (p<0.03), and no differences between diplotypes in the neutral and cue conditions.
Association between Plasma NPY and Post-treatment Alcohol/Drug Use
Plasma NPY during stress, alcohol/drug cue and neutral conditions were not associated with baseline levels of alcohol and cocaine use. However, lower plasma NPY during both the stress and the alcohol/drug cue condition was negatively associated with higher number of days of alcohol and/or drug consumed post-treatment (Stress: R2=0.18, r = −0.42, p<0.02; Cue: R2=0.11, r = −0.33, p<0.08) and higher total amounts of alcohol and drug consumed during the 90-day follow-up period (Stress: R2=0.40, r = −0.63, p<0.0003; Cue: R2=0.26, r = −0.51, p<0.006).
Discussion
The current findings demonstrate that genetic variations in NPY haplotype which predicts NPY mRNA expression modulated stress-induced plasma NPY levels, heart rate arousal and subjective anxiety. While on the basis of our previous work (Sinha et al., 2003; Fox et al., 2008; Chaplin et al., 2008; Sinha et al., 2009), emotional stress exposure significantly increased heart rate and anxiety levels relative to the alcohol cue and the neutral imagery condition, current data indicate genetic variation in NPY haplotype predicted individual differences in these responses. Differential plasma NPY and anxiety responses to stress provocation were observed in healthy controls with the HH vs HLLL NPY variants, but not in the recovering addicted patients. Stress-induced NPY release was low, while anxiety and heart rate responses were increased in the addicted individuals, particularly in those with the HLLL genotype compared to HLLL controls. This suppressed NPY response to stress was also predictive of greater alcohol/drug use in the subsequent post-treatment period.
The current results are consistent with previous animal studies revealing that pro-NPY mRNA and NPY peptide levels typically increase in the amygdala of laboratory rats in response to restraint stress (Krysiak et al. 1999; Thorsell et al. 1999). In rhesus macaques, a genetic variant on the promoter region of NPY resulted in lower mRNA expression in the amygdala and lower cerebrospinal fluid NPY level. These rhesus macaques exhibited higher level of arousal during stress exposure (Lindell et al., 2010). Furthermore, while mice with a deletion of the NPY gene exhibit higher anxiety and increased alcohol consumption (Karl et al., 2008) (Thiele et al. 1998), transgenic rats with NPY over expression have a remarkably decreased reaction to anxiogenic-like stimuli and fail to develop fear-induced behavioral suppression (Thorsell et al., 2000). Relevant to the present study, NPY-overexpressing transgenic mice display hypersensitivity to alcohol intoxication and reduced alcohol intake (Thiele et al. 1998). Although the above basic science findings indicate central NPY effects and there is no evidence of similar central effects in humans, the current findings extend this previous preclinical literature, and are the first in humans to show functional genetic variations in NPY haplotype (i.e., HH vs HLLL) influences individual differences in peripheral NPY stress response and behavioral adaptation to stress in healthy subjects. On the other hand, suppressed NPY levels during stress in recovering addicted patients raise the possibility that epigenetic mechanisms regulating NPY gene expression could play a role in stress dysregulation of plasma NPY and anxiety responses in addicted individuals, which in turn significantly impacts subsequent alcohol/drug abuse.
Significantly lower stress-induced NPY release with greater heart rate responses and higher anxiety were observed in individuals with the diplotype predicting higher mRNA expression (HH), while those with the NPY diplotype predicting low-moderate mRNA expression (HLLL) showed up-regulated plasma NPY responses to stress, with lower heart rate and subjective anxiety responses during stress relative to the neutral condition. The genetic influence on the inverse response in plasma NPY on the one hand, and heart rate and anxiety on the other, indicates that the regulatory role of peripheral NPY stress responses is modulated by variation in the NPY haplotype. The results suggest that the anti-anxiety effect of plasma NPY could function in an inverted “U” manner, where individuals with a HH diplotype, known to have higher basal plasma NPY levels (Zhou et al. 2008), show lower peripheral NPY reactivity to stress, while individuals with HLLL diplotype, known to have lower basal plasma NPY (Zhou et al., 2008), show increased NPY reactivity to stress stimuli. In this way, normal genetic variation in NPY mRNA expression could modulate plasma NPY regulation during stress and contribute to individual differences in stress adaptation in healthy controls.
The same functional NPY haplotype was previously linked to higher amygdala and hippocampus activation in fMRI responses to negative emotional faces in individuals with lower NPY expression diplotype (LL) than in those with HH diplotype. Lower NPY expression diplotype also showed higher anxiety traits as measured by TPQ. Although these findings may appear to be inconsistent with the current data, there are key differences between the previous and current study in methodology and measures representing anxiety or distress. For example, the Zhou et al (2008) study included data from post-mortem brains, a large epidemiological sample, smaller samples for basal plasma NPY, and assessed brain responses in limbic regions associated with emotions and stress, while the current study examined peripheral NPY and self reported anxiety and heart rate responses. A one-to-one correspondence in central and peripheral NPY action cannot be assumed. Furthermore, our findings indicate that peripheral NPY, which is co-released with norepinephrine from the sympathetic nerve terminals during stress and arousal (Gehlert 2004), plays a role in modulating subjective anxiety and heart rate. One possible mechanism for this pattern of responses is the sympathetic arousal during stress along with activation of NPY receptor signaling in the arcuate nucleus, which projects to the hypothalamus and influences neurotransmission in lower and mid-brain limbic regions (Chronwall et al. 1985) involved in emotional arousal and regulation, thereby exerting central anxiolytic effects via autonomic pathways. Despite the differences in methods and measures, together, the Zhou et al paper and the current study indicate that genetic variants in the functional NPY haplotype modulate stress responses via regulation of both central and peripheral NPY pathways.
A significant finding of clinical relevance is that the addicted patients showed suppressed NPY in the stress and alcohol/drug cue conditions, regardless of NPY diplotype. This was accompanied by enhanced stress- and cue-induced anxiety responses, with elevated basal heart rate and blunted stress and cue related heart rate reactivity in the addicted patients as compared to controls. Chronic alcohol-related autonomic dysregulation has been previously documented (Bar et al. 2006; Fox et al. 2008; Paliwal et al. 2008; Pandey et al. 2008; Sinha 2009), and these changes co-occur with increased anxiety and drug craving in recently abstinent, treatment engaged alcohol and cocaine dependent individuals (Fox et al. 2008; Sinha et al., 2009). Suppression of NPY diplotype expression in the addicted patients raises the possibility that stress and/or alcohol related epigenetic mechanisms may be involved in NPY stress dysregulation. Substance abusing patients report higher cumulative stress and adversity, show reciprocal effects of stress and chronic alcohol/drug use (Sinha, 2008), and stress and drug-related epigenetic changes have been documented in a number of addiction relevant signaling pathways (Renthal & Nestler, 2008). Some evidence also suggests chronic alcohol-related epigenetic changes involving DNA methylation and histone modification-induced chromatin remodeling in several signaling systems, including significant effects in decreasing central NPY expression, which in turn, is associated with increased alcohol intake and higher anxiety like behaviors in alcohol dependent animals (Pandey et al. 2004; Pandey et al. 2008). Thus, it is possible that stress and/or alcohol/drug-related decreases in NPY gene expression contributes to the autonomic dysregulation and suppressed NPY diplotype effects, resulting in lower plasma NPY, which in turn predicts higher levels of subsequent alcohol and drug intake as observed in the current study. These data suggest that increasing NPY signaling and NPY levels could decrease substance use in addicted samples, thereby lending support to preclinical research indicating that therapeutic strategies that selectively target Y1, Y2 and/or Y5 receptors and activate NPY signaling may be effective in the addiction treatment (Pandey et al. 2008; Prakash et al. 2008).
As discussed earlier, association studies of the functional NPY Leu7Pro (rs16139) variants with alcohol/cocaine dependence in epidemiological samples have been inconsistent, with positive association in some studies (Kauhanen et al., 2000; Lappalainen et al., 2002; Mottagui-Tabar et al., 2005) but not in the others (Zhu et al., 2003; Hu et al. 2005; Mottagui-Tabar et al., 2005; Zill et al., 2008). Limited sample size, small gene effect size, and population stratification may contribute to these inconsistent findings in epidemiologically based case-control populations. On the other hand, the current study used a well-defined stress provocation procedure to induce stress responses and assess it as an intermediate endophenotype to examine the effects of the NPY functional haplotype (different from the one previously studied), and showed significant effects of NPY haplotype and environmental influences of alcohol and drug use history on plasma NPY responses and their association with future clinical outcomes. These data suggest that using stress-related endophenotypes may provide greater sensitivity to assess functional NPY gene expression changes in substance abuse.
This study was limited by the small sample size of control and addicted patients in each diplotype group, and thus the reported findings should be considered preliminary. However, it is important to note that the effect sizes for the significant Diplotype X Condition and Diplotype X Group X condition interaction effects for plasma NPY, heart rate and anxiety were in the medium (<.25) to large (<.40) range (Cohen, 1988), indicating that there was adequate power to detect differences in the two diplotypes across the conditions and the HC and SD groups, as hypothesized. Despite this drawback, this is the first study in humans to document functional NPY haplotype influences on stress-related plasma NPY, heart rate and subjective responses in controls, and show suppressed genetic influences on plasma NPY stress responses in recently abstinent, recovering addicted patients. Furthermore, lower plasma NPY stress response was also associated with subsequent poor clinical outcomes, providing preliminary support for developing NPY targets in the treatment of substance use disorders.
Supplementary Material
NPY diplotype modulation of plasma NPY level across all time points (including baseline (−5), immediately following imagery (0), and post-imagery, recovery timepoints (+15, +30, +45, +60, +75) during the stress condition in substance dependence group (SD) and healthy control (HC) group. In HC group, plasma NPY level in subjects with HLLL diplotype was significantly higher compared to subjects with HH diplotype background (p<0.001), suggesting HLLL healthy control subjects show increased plasma NPY in response to stress. However, in SD group, there was no significant difference of plasma NPY between subjects with HH and HLLL, indicating SD subjects showed no increases and variation in plasma NPY after stress exposure, resulting in a significant 3-way interaction.
Heart rates across all time points (including baseline (−5), immediately following imagery (0), and post-imagery, recovery timepoints (+15, +30, +45, +60, +75) in the stress condition in substance dependent subjects (SD) and healthy control (HC) subjects. Overall heart rate in SD subjects were significantly higher compared to HC group (p<0.001). In HC group, subjects with HH diplotype presented higher heart rate compared to subjects with HLLL diplotype (p< 0.01); however, in SD group, heart rate was significant high regardless diplotype background.
Subjective anxiety ratings across all time points (including baseline (−5), immediately following imagery (0), and post-imagery, recovery timepoints (+15, +30, +45, +60, +75) in the stress condition in substance dependence (SD) and health controls (HC). In SD group, anxiety level was significantly increased compared to HC group, regardless diplotype background (p<0.001). In HC group, subjects with HH diplotype showed greater anxiety level compared to subjects with HLLL diplotype (p=0.03)
Table 1.
Demographic and clinical characteristics of the substance dependent (SD) and healthy control (HC) subjects
| Subject Variable | SD/HH (N=12) | SD/HLLL (N=25) | HC/HH (N=8) | HC/HLLL (N=20) |
|---|---|---|---|---|
| Race | ||||
| Caucasian | 4 (33.3%) | 17 (68%) | 6 (75%) | 11 (55%) |
| African American | 6 (50%) | 6(24%) | 1(12.5%) | 6(30%) |
| Hispanic | 0 | 2(8%) | 1 (12.5%) | 2 (10%) |
| Other | 2 (16.7%) | 0 | 0 | 1 (5%) |
| Gender (Male) | 7 (58.3%) | 17 (68%) | 6 (75%) | 9 (45%) |
| Age * | 37.6 (7.06) | 37.6 (5.85) | 34.8 (9.85) | 29.1 (9.85) |
| Average Years of Education * | 13.1 (1.78) | 12.6 (1.23) | 14.5 (1.97) | 14.9 (1.77) |
| Average Years of Alcohol Use | 12.4 (8.32) | 13.7 (8.13) | 8.9 (8.41) | 7.5 (8.46) |
| Average Days of Alcohol Use/Month* | 14.8 (9.04) | 15.9 (11.97) | 3.2 (3.35) | 5.8 (8.33) |
| Total Amount (drinks) of Alcohol Use /Month* | 187.7 (210.18) | 242.3 (237.5) | 8.0 (6.93) | 11.5 (10.35) |
| Average Years of Cocaine Use | 9.54 (4.72) | 8.00 (7.26) | - | - |
| Average Days of Cocaine Use/Month | 21.00 (10.43) | 14.24 (13.23) | - | - |
| Total Amount (g) of Cocaine Use/Month | 44.80 (38.22) | 33.88 (23.14) | - | - |
| Average Days of Marijuana Use/Month | 9.5 (12.94) | 0.22 (0.52) | - | - |
| Total Amount (g) of Marijuana Use/Month | 23.52 (26.60) | 0.6 (0.94) | - | - |
| Regular Cigarette Smokers: N(%)* | 8 (66.67%) | 23 (92%) | 4 (50%) | 5 (25%) |
| Lifetime Prevalence of PTSD | 2 (16.7%) | 2 (8%) | 0 | 2 (10%) |
| Lifetime Other Anxiety Disorders | 3 (25%) | 3 (12%) | 0 | 0 |
| Lifetime Major Depression | 1 (8.3%) | 7 (28%) | 2 (25%) | 1 (5%) |
Note:
SD significantly different from HC group, p<0.05, but no differences between HH and HLLL groups; HH: NPY Diplotype predicting high NPY mRNA expression; HLLL: NPY diplotype predicting moderate and low NPY mRNA expression; PTSD: post-traumatic stress disorder
Acknowledgments
This research was supported by the National Institutes of Health grants R01-AA013892 (RS), R01-AG022982 (RLH), R01-MH074697 (RLH), T32-MH019961, R25-MH071584 and the NIH Roadmap Fund for Medical Research grants: UL1-DE019586 (RS) and UL1-RR024139 (Yale CTSA) as well as the Department of Mental Health and Addiction Services of the State of Connecticut. Dr. Hauger was supported by a Merit Review grant from the Department of Veterans Affairs and the VISN22 VA Center of Excellence for Stress and Mental Health (CESAMH). We thank the staff at the Clinical Neuroscience Research Unit of the Connecticut Mental Health Center and the Hospital Research Unit of the Yale Center for Clinical Investigation for their assistance in completing this study.
Footnotes
Author Disclosures: All of the authors report that they have no conflicts of interest to report as related to the subject of the report. Dr. Sinha is on the Scientific Advisory Board for Embera Neurotherapeutics and is also a consultant for Glaxo-Smith Kline, Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen R, Boublik J, Hauger R, Scott H, Rivier J, Brown MB. Neuropeptide Y radioimmunassay: Characterization and application. Clin Exp Pharmacol Physiol. 1991;18:825–833. doi: 10.1111/j.1440-1681.1991.tb01402.x. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Boettger MK, Neubauer R, Groteluschen M, Jochum T, Baier V, Sauer H, Voss A. Heart rate variability and sympathetic skin response in male patients suffering from acute alcohol withdrawal syndrome. Alcoholism, Clinical and Experimental Research. 2006;30:1592–8. doi: 10.1111/j.1530-0277.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- Broqua P, Wettstein JG, Rocher MN, Gauthier-Martin B, Junien JL. Behavioral effects of neuropeptide Y receptor agonists in the elevated plus-maze and fear-potentiated startle procedures. Behav Pharmacol. 1995;6:215–222. [PubMed] [Google Scholar]
- Chaplin TM, Hong K, Bergquist K, Sinha R. Gender differences in response to emotional stress: an assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcoholism, Clinical and Experimental Research. 2008;32:1242–50. doi: 10.1111/j.1530-0277.2008.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronwall BM, DiMaggio DA, Massari VJ, Pickel VM, Ruggiero DA, O’Donohue TL. The anatomy of neuropeptide-Y-containing neurons in rat brain. Neuroscience. 1985;15:1159–81. doi: 10.1016/0306-4522(85)90260-x. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for behavioral sciences. 2. Lawrence Elrbaum Associates; New Jersey: 1988. [Google Scholar]
- Colzato LS, van den Wildenberg WP, Van der Does AJ, Hommel B. Genetic markers of striatal dopamine predict individual differences in dysfunctional, but not functional impulsivity. Neuroscience. 2010 Oct 27;170(3):782–8. doi: 10.1016/j.neuroscience.2010.07.050. Epub 2010 Aug 1. [DOI] [PubMed] [Google Scholar]
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology. 2005;30:880–91. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol dependent individuals. Alcoholism: Clinical and Experimental Research. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz K, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33:796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR. Introduction to the reviews on neuropeptide Y. Neuropeptides. 2004;38:135–40. doi: 10.1016/j.npep.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Badia-Elder NE. Neuropeptide Y suppresses ethanol drinking in ethanol-abstinent, but not non--ethanol-abstinent, Wistar rats. Alcohol. 2008;42:541–51. doi: 10.1016/j.alcohol.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. Candidate genes in alcoholism. Clin Neurosci. 1995;3:174–81. [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–32. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002 Jul 19;297(5580):400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–24. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Heilig M, Soderpalm B, Engel JA, Widerlov E. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology (Berl) 1989;98:524–9. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Fox HC, Hong KA, Doebrick CA, Sinha R. Stress and drug cue-induced craving in opiate dependent individuals in naltrexone treatment. Experimental and Clinical Psychopharmacology. 2007;15:134–143. doi: 10.1037/1064-1297.15.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: relationship with negative mood, chronic thought suppression, and compulsive drinking. Biol Psychiatry. 2003;54:1427–36. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Izard C. Patterns of emotions: A new analysis of anxiety and depression. Academic Press; New York: 1972. [Google Scholar]
- Karl T, Duffy L, Herzog H. Behavioural profile of a new mouse model for NPY deficiency. Eur J Neurosci. 2008;28:173–80. doi: 10.1111/j.1460-9568.2008.06306.x. [DOI] [PubMed] [Google Scholar]
- Kauhanen J, Karvonen M, Pesonen U, Koulu M, Tuomainen T, Uusitupa M, Salonen J. Neuropeptide Y polymorphism and alcohol consumption in middle-aged men. American Journal of Medical Genetics. 2000;93:117–121. doi: 10.1002/1096-8628(20000717)93:2<117::aid-ajmg7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–49. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–7. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Krysiak R, Obuchowicz E, Herman ZS. Interactions between the neuropeptide Y system and the hypothalamic-pituitary-adrenal axis. European Journal of Endocrinology / European Federation of Endocrine Societies. 1999;140:130–6. doi: 10.1530/eje.0.1400130. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lang PJ, Kozak MJ, Miller GA, Levin DN, McLean A., Jr Emotional imagery: Conceptual structure and pattern of somatovisceral response. Psychophysiology. 1980;17:179–192. doi: 10.1111/j.1469-8986.1980.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Kranzler HR, Malison R, Price LH, Van Dyck C, Rosenheck RA, Cramer J, Southwick S, Charney D, Krystal J, Gelernter J. A functional neuropeptide Y Leu7Pro polymorphism associated with alcohol dependence in a large population sample from the United States. Arch Gen Psychiatry. 2002;59:825–31. doi: 10.1001/archpsyc.59.9.825. [DOI] [PubMed] [Google Scholar]
- Lindell SG, Schwandt ML, Sun H, Sparenborg JD, Björk K, Kasckow JW, Sommer WH, Goldman D, Higley JD, Suomi SJ, Heilig M, Barr CS. Functional NPY variation as a factor in stress resilience and alcohol consumption in rhesus macaques. Arch Gen Psychiatry. 2010 Apr;67(4):423–31. doi: 10.1001/archgenpsychiatry.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD. HPA axis and stimulant dependence: an enigmatic relationship. Psychoneuroendocrinology. 2002;27:5–12. doi: 10.1016/s0306-4530(01)00033-6. [DOI] [PubMed] [Google Scholar]
- Maric T, Cantor A, Cuccioletta H, Tobin S, Shalev U. Neuropeptide Y augments cocaine self-administration and cocaine-induced hyperlocomotion in rats. Peptides. 2008;30:721–6. doi: 10.1016/j.peptides.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Maric T, Tobin S, Quinn T, Shalev U. Food deprivation-like effects of neuropeptide Y on heroin self-administration and reinstatement of heroin seeking in rats. Behav Brain Res. 2008;194:39–43. doi: 10.1016/j.bbr.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Mayberg H, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mickey BJ, Zhou Z, Heitzeg MM, Heinz E, Hodgkinson CA, Hsu DT, Langenecker SA, Love TM, Peciña M, Shafir T, Stohler CS, Goldman D, Zubieta JK. Emotion processing, major depression, and functional genetic variation of neuropeptide Y. Arch Gen Psychiatry. 2011 Feb;68(2):158–66. doi: 10.1001/archgenpsychiatry.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonat S, Starkman BG, Sakharkar A, Pandey SC. Neuroscience of alcoholism: molecular and cellular mechanisms. Cell Mol Life Sci. 2010;67:73–88. doi: 10.1007/s00018-009-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottagui-Tabar S, Prince JA, Wahlestedt C, Zhu G, Goldman D, Heilig M. A novel single nucleotide polymorphism of the neuropeptide Y (NPY) gene associated with alcohol dependence. Alcohol Clin Exp Res. 2005;29:702–7. doi: 10.1097/01.alc.0000164365.04961.b1. [DOI] [PubMed] [Google Scholar]
- Olling JD, Ulrichsen J, Haugbol S, Glenthoj B, Hemmingsen R, Woldbye DP. Decreased gene expression of neuropeptide Y and its receptors in hippocampal regions during ethanol withdrawal in rats. Neurosci Lett. 2007;424:160–4. doi: 10.1016/j.neulet.2007.07.050. [DOI] [PubMed] [Google Scholar]
- Orr SP, Pitman RK, Lasko NB, Herz LR. Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. Journal of Abnomal Psychology. 1993;102:152–159. doi: 10.1037//0021-843x.102.1.152. [DOI] [PubMed] [Google Scholar]
- Paliwal P, Hyman SM, Sinha R. Craving predicts time to cocaine relapse: further validation of the Now and Brief versions of the cocaine craving questionnaire. Drug and Alcohol Dependence. 2008;93:252–9. doi: 10.1016/j.drugalcdep.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H, Xu T. Partial deletion of the cAMP response element-binding protein gene promotes alcohol-drinking behaviors. Journal of Neuroscience. 2004;24:5022–30. doi: 10.1523/JNEUROSCI.5557-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28:3729–37. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005 Jun;8(6):828–34. doi: 10.1038/nn1463. Epub 2005 May 8. [DOI] [PubMed] [Google Scholar]
- Prakash A, Zhang H, Pandey SC. Innate differences in the expression of brain-derived neurotrophic factor in the regions within the extended amygdala between alcohol preferring and nonpreferring rats. Alcoholism, Clinical and Experimental Research. 2008;32:909–20. doi: 10.1111/j.1530-0277.2008.00650.x. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Hauger RL, Morgan CA, Bremner JD, Charney DS, Southwick SM. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol Psychiatry. 2000;47:526–39. doi: 10.1016/s0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Southwick SM, Hauger RL, Charney DS. Plasma neuropeptide Y (NPY) increases in humans in response to the alpha 2 antagonist yohimbine. Neuropsychopharmacology. 1998;19:95–8. doi: 10.1016/S0893-133X(97)00199-1. [DOI] [PubMed] [Google Scholar]
- Renthal W, Nestler E. Epigenetic mechanisms in drug addiction, Trends in Molecular Medicine. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, Morin M, Gehlert DR, Urban JH, Shekhar A. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J Neurosci. 2008;28:893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheurich A, Muller MJ, Anghelescu I, Lorch B, Dreher M, Hautzinger M, Szegedi A. Reliability and validity of the form 90 interview. European Addiction Research. 2005;11:50–6. doi: 10.1159/000081417. [DOI] [PubMed] [Google Scholar]
- Shi J, Li SX, Zhang XL, Wang X, Le Foll B, Zhang XY, Kosten TR, Lu L. Time-dependent neuroendocrine alterations and drug craving during the first month of abstinence in heroin addicts. Am J Drug Alcohol Abuse. 2009;35(5):267–72. doi: 10.1080/00952990902933878. [DOI] [PubMed] [Google Scholar]
- Shively CA Shively CA, Mietus JE, Grant KA, Goldberger AL, Bennett AJ, Willard SL. Effects of chronic moderate alcohol consumption and novel environment on heart rate variability in primates (Macaca fascicularis) Psychopharmacology. 2007;192:183–91. doi: 10.1007/s00213-007-0709-z. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lovallo WR, Parsons OA. Cardiovascular differentiation of emotions. Psychosomatic Medicine. 1992;54:422–435. doi: 10.1097/00006842-199207000-00005. [DOI] [PubMed] [Google Scholar]
- Sinha R, Parsons OA. Multivariate response patterning of fear and anger. Cognition and Emotion. 1996;10:173–198. [Google Scholar]
- Sinha R, Catapano D, O’Malley SS. Stress-induced craving and stress responses in cocaine dependent individuals. Psychopharmacology. 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Renee-Aubin L, O’Malley SS. Psychological stress, drug cues and cocaine craving. Psychopharmacology. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Anderson GA, Cooney N, Kreek M. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–30. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced Negative Emotion and Alcohol Craving, and Altered Physiological Responses Following Stress and Cue Exposure in Alcohol Dependent Individuals. Neuropsychopharmacology. 2009;34:1198–208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Archives of General Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R. Manual for imagery script development procedures. Unpublished manuscript 2001 [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Bio. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Curr Psychiatry Rep. 2007;9:388–95. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–9. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Carlsson K, Ekman R, Heilig M. Behavioral and endocrine adaptation, and up-regulation of NPY expression in rat amygdala following repeated restraint stress. Neuroreport. 1999;10:3003–7. doi: 10.1097/00001756-199909290-00024. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Michalkiewicz M, Dumont Y, Quirion R, Caberlotto L, Rimondini R, Mathe AA, Heilig M. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12852–7. doi: 10.1073/pnas.220232997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Gerhard U, Gerlach M, Weijers HG, Boening J, Wiesbeck GA. Cortisol concentrations, stress-coping styles after withdrawal and long-term abstinence in alcohol dependence. Addict Biol. 2006;11:157–62. doi: 10.1111/j.1369-1600.2006.00018.x. [DOI] [PubMed] [Google Scholar]
- Westrin A, Ekman R, Traskman-Bendz L. Alterations of corticotropin releasing hormone (CRH) and neuropeptide Y (NPY) plasma levels in mood disorder patients with a recent suicide attempt. Eur Neuropsychopharmacol. 1999;9:205–11. doi: 10.1016/s0924-977x(98)00026-1. [DOI] [PubMed] [Google Scholar]
- Woldbye DP, Nanobashvili A, Husum H, Bolwig TG, Kokaia M. Neuropeptide Y inhibits in vitro epileptiform activity in the entorhinal cortex of mice. Neurosci Lett. 2002;333:127–30. doi: 10.1016/s0304-3940(02)01024-8. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu XZ, Hodgkinson CA, Xu K, Buzas B, Yuan Q, Shen PH, Ferrell RE, Manuck SB, Brown SM, Hauger RL, Stohler CS, Zubieta JK, Goldman D. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Pollak L, Mottagui-Tabar S, Wahlestedt C, Taubman J, Virkkunen M, Goldman D, Heilig M. NPY Leu7Pro and alcohol dependence in Finnish and Swedish populations. Alcohol Clin Exp Res. 2003;27:19–24. doi: 10.1097/01.ALC.0000050642.62233.44. [DOI] [PubMed] [Google Scholar]
- Zill P, Pruess UW, Koller G, Bondy B, Soyka M. Analysis of single nucleotide polymorphisms and haplotypes in the neuropeptide Y gene: no evidence for association with alcoholism in a German population sample. Alcohol Clin Exp Res. 2008;32:430–4. doi: 10.1111/j.1530-0277.2007.00586.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NPY diplotype modulation of plasma NPY level across all time points (including baseline (−5), immediately following imagery (0), and post-imagery, recovery timepoints (+15, +30, +45, +60, +75) during the stress condition in substance dependence group (SD) and healthy control (HC) group. In HC group, plasma NPY level in subjects with HLLL diplotype was significantly higher compared to subjects with HH diplotype background (p<0.001), suggesting HLLL healthy control subjects show increased plasma NPY in response to stress. However, in SD group, there was no significant difference of plasma NPY between subjects with HH and HLLL, indicating SD subjects showed no increases and variation in plasma NPY after stress exposure, resulting in a significant 3-way interaction.
Heart rates across all time points (including baseline (−5), immediately following imagery (0), and post-imagery, recovery timepoints (+15, +30, +45, +60, +75) in the stress condition in substance dependent subjects (SD) and healthy control (HC) subjects. Overall heart rate in SD subjects were significantly higher compared to HC group (p<0.001). In HC group, subjects with HH diplotype presented higher heart rate compared to subjects with HLLL diplotype (p< 0.01); however, in SD group, heart rate was significant high regardless diplotype background.
Subjective anxiety ratings across all time points (including baseline (−5), immediately following imagery (0), and post-imagery, recovery timepoints (+15, +30, +45, +60, +75) in the stress condition in substance dependence (SD) and health controls (HC). In SD group, anxiety level was significantly increased compared to HC group, regardless diplotype background (p<0.001). In HC group, subjects with HH diplotype showed greater anxiety level compared to subjects with HLLL diplotype (p=0.03)