Abstract
Interactions between naringenin and the cytochrome P450 (CYP) system have been of interest since the first demonstration that grapefruit juice reduced CYP3A activity. The effects of naringenin on other CYP isoforms have been less investigated. In addition, it is well known that interactions with enzymes are often stereospecific, but due to the lack of readily available, chirally pure naringenin enantiomers, the enantioselectivity of its effects has not been characterized. We isolated pure naringenin enantiomers by chiral HPLC and tested the ability of (R)-, (S)-and rac-naringenin to inhibit several important drug-metabolizing CYP isoforms using recombinant enzymes and pooled human liver microsomes. Naringenin was able to inhibit CYP19, CYP2C9 and CYP2C19 with IC50 values below 5 μM. No appreciable inhibition of CYP2B6 or CYP2D6 was observed at concentrations up to 10 μM. While (S)-naringenin was 2-fold more potent as an inhibitor of CYP19 and CYP2C19 than (R)-naringenin, (R)-naringenin was 2-fold more potent for CYP2C9 and CYP3A. Chiral flavanones like naringenin are difficult to separate into their enantiomeric forms, but enantioselective effects may be observed that ultimately impact clinical effects. Inhibition of specific drug metabolizing enzymes by naringenin observed in vitro may be exploited to understand pharmacokinetic changes seen in vivo.
Keywords: flavonoid, drug metabolism, CYP19, CYP2C9, CYP2C19, CYP3A, enantioselectivity, food-drug interaction
INTRODUCTION
Naringenin (Figure 1, Structure 1) is a chiral flavanone belonging to the flavonoid class that has potential health related properties. It is contained in some Citrus species, in particular grapefruit, that contain large amounts of its 7-O-neohesperidoside, naringin (Figure 1, Structure 2). Upon ingestion, naringin undergoes cleavage of the sugar moiety, leaving the free aglycone, naringenin in the gastrointestinal tract1. In a seminal paper, naringenin was found to inhibit the cytochrome P450 (CYP) 3A4-mediated oxidation of two dihydropyridine cardiodepressive drugs, more markedly than naringin2. Later, naringenin was found inter alia to inhibit the activity of CYP isoforms that activate the potent environmental carcinogen, nicotine-derived nitrosamine ketone (NNK)3. Naringenin also inhibited quinine 3-hydroxylation mediated by CYP3A4, although it is recognized that other components of grapefruit juice are more active4. It may also act as a potent immunomodulator in mice with pulmonary fibrosis5, and the antioxidant properties of naringenin and other polyphenols have been well studied6.
Fig. 1.
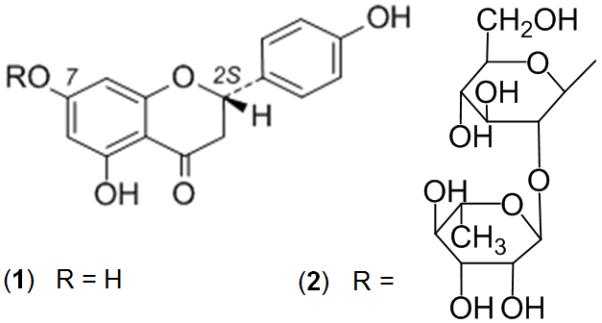
Structures of naringenin (1, R= H) and naringin (2, R= neohesperidosyl).
Despite a volume of research over many years, the relevance of stereochemistry at the C-2 stereogenic center of naringenin has not been carefully evaluated. It is well known that interactions between an enzyme system and a substrate are frequently stereospecific7, and often influence the potency of, and the response to single enantiomer8. For example, quinidine is a clinically relevant and potent inhibitor of CYP2D6, while its diastereomer quinine is not9, (S)-lansoprazole is a more potent inhibitor of CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4-mediated hydroxylation than (R)-lansoprazole10 and many other chiral drugs have been well documented to exibit enantioslective inhibition of P450-mediated metabolism11-13. Difficulties in separating enantiomers and the resulting absence of readily available chirally pure enantiomers have limited research in this area until now. In one report, inhibition of recombinant CYP3A4 by specific components of grapefruit juice were described for single enantiomers of naringenin (50 μM) but details on the separation of the enantiomers and their purity were not given14.
The inhibition of human drug metabolism by flavonoids like naringenin may be of medical importance. Since the demonstration that grapefruit juice can inhibit the metabolism of the CYP3A substrate felodipine15, it has become widely appreciated that the ingestion of a number of fruit juices can slow drug metabolism16, and thereby increase the concentrations of important medications such as cyclosporine17 and methadone18. The biochemical basis for these interactions involves the interaction of flavonoids with specific cytochrome P450 isoforms. Although CYP3A has been most frequently studied, a number of different isoforms may interact in this way. It is important to study interactions of naringenin with multiple CYP isoforms in order to be aware of any potential clinically relevant interactions between naringenin and medications.
For these reasons, we set out to develop an efficient procedure for the isolation of single naringenin enantiomers (R and S) in sizeable amounts. We used rac-naringenin and the purified (R)- and (S)- naringenin preparations to test the ability of naringenin to inhibit a series of key drug metabolizing cytochrome P450 isoforms in vitro, with a focus on the enantioselectivity of the interaction.
MATERIALS AND METHODS
Chemicals and Reagents
Rac-naringenin, testosterone, 6-β hydroxytestosterone, desmethyldiazepam, β-NADP, glucose-6-phosphate dehydrogenase, and glucose-6-phosphate were purchased from Sigma-Aldrich (St. Louis, MO). Bupropion, 4-hydroxybupropion and nevirapine were purchased from Toronto Research Chemicals Inc. (North York, ON, Canada). Magnesium chloride was purchased from Fisher Scientific (Pittsburgh PA). All drug solutions were prepared by dissolving each compound in methanol or acetonitrile, and were stored at −20 °C. All high-performance liquid chromatography (HPLC)-grade reagents and chemicals used for mobile phase and buffers were obtained as previously described19. Pooled human liver microsomes (HLMs) and the cytochrome P450 inhibitor screening kits for CYP19, 2C9, 2C19 and 2D6 were purchased from BD Biosciences (San Jose, CA). All microsomal preparations were stored at −80°C.
Separation, Isolation and Purification of Individual Enantiomers of Naringenin
The HPLC apparatus used for separation of the (R)- and (S)-enantiomers consisted of a Jasco pump PU 980 with Rheodyne 20 or 200 μL sample loops, a low pressure mixer LG-1580-02 and a line degasser 1550-54, a Uvidec 100-IIII spectrophotometric detector operating at 292 nm (Jasco, Tokyo, Japan). Chromatograms were acquired and processed using computer based Jasco Borwin 2 software. Circular dichroism (CD) spectra were recorded on a Jasco 810 spectropolarimeter using a 1 mm cell. Chromatographic separations were performed on a Lux Cellulose 2 column, 250 mm × 4.6 mm i.d., whose chiral selector was tris-3-chloro-4-methylphenylcarbamate coated on 5 μm silica gel (Phenomenex Italia, Bologna). A column in line filter with 0.5 μm stainless steel frit of 3 mm diameter from Rheodyne was used to protect the HPLC column. Disposable PTFE filters of 0.2 μm pore size were used for filtration of sample solutions. HPLC chromatographic parameters α and Rs were calculated according to the usual procedure20.
Inhibition of Recombinant Human CYP450 Isoforms
The activity of each recombinant human CYP450 isoform was determined by measuring the conversion rate of a fluorometric substrate to its fluorescent metabolite. CYP19 and CYP2C9 activities were determined using the metabolism of 7-methoxy-4-trifluoromethylcoumarin (MFC) to 7-hydroxytrifluoromethylcoumarin. CYP2C19 activity was determined using the metabolism of 3-cyano-7-ethoxycoumarin (CEC) to 3-cyano-7-hydroxycoumarin. CYP2D6 activity was determined using the metabolism of 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methylcoumarin (AMMC) to 3-[2-(N,N-diethylamino)ethyl]-7-methoxy-4-methylcoumarin. Experimental procedures were consistent with the published methodology21. All incubations were carried out using incubation times and protein concentrations that were within the linear range for reaction velocity. Substrates and inhibitors were prepared in acetonitrile. A series of concentrations of inhibitor in a volume of 4 μl were mixed with 96 μl of NADPH-Cofactor Mix (16.3 μM NADP, 828 μM glucose-6-phosphate, 828 μM MgCl2, and 0.4 U/ml glucose 6-phosphate dehydrogenase), and prewarmed for 10 min at 37°C. Enzyme/Substrate Mix was prepared with fluorometric substrate, recombinant human CYP450 isoform and 0.1 M potassium phosphate buffer (pH 7.4). Reactions were initiated by adding 100 μl of Enzyme/Substrate Mix to bring the incubation volume to 200 μl. The optimal final recombinant enzyme concentrations, substrate concentrations and incubation times were: 7.5 nM CYP19 + 25 μM MFC for 30 min, 15 nM CYP2C9 + 150 μM MFC for 45 min, 7.5 nM CYP2C19 + 25 μM CEC for 30 min, and 7.5 nM CYP2D6 + 1.5 μM AMMC for 30 min. All reactions were stopped by adding 75 μl of acetonitrile/0.1 M tris base. The generation of fluorescent metabolites was determined immediately by measuring fluorescent response using a BioTek (Winooski, VT) Synergy 2 fluorometric plate reader. Excitation-emission wavelengths were 400-540 nm for the MFC metabolite or 400-460 nm for the CEC and AMMC metabolites. Standard curves were constructed using the appropriate fluorescent metabolite standards. Quantification of samples was carried out by applying the linear regression equation of the standard curve to the fluorescence response. The limits of quantification for the metabolites of MFC, CEC and AMMC were 4 pmol, 0.1 pmol and 0.8 pmol in a final volume of 200 μl respectively, with intra- and inter-assay coefficients of variation of less than 10%.
Inhibition of Specific CYP450 Isoforms Using Pooled HLMs
A single isoform-specific substrate concentration at the respective Km value (10 μM testosterone or 100 μM bupropion) was incubated at 37°C in duplicate with pooled HLMs and the a NADPH-generating system (1.3 mM NADP, 3.3 mM glucose-6-phosphate, 3.3 mM MgCl2, and 0.4 U/ml glucose 6-phosphate dehydrogenase) in the absence or the presence of a range of (R)-, (S)- or rac-naringenin (up to 50 μM). The activity of CYP2B6 was determined by measuring the formation rate of 4-hydroxybupropion from bupropion in pooled HLMs, while the activity of CYP3A was determined by measuring the formation rate of 6-β hydroxytestosterone from testosterone. All incubations were carried out using incubation time and protein concentrations that were within the linear range for reaction velocity. An incubation mixture that consisted of substrate probes, HLMs, and 100 mM phosphate reaction buffer (pH 7.4) was pre-warmed for 5 min at 37°C. The reaction was initiated by the addition of the NADPH-generating system, and incubated at 37°C for 15 min. The final protein concentration of pooled HLMs was 0.25 mg/ml. All reactions were terminated by the addition of 500 μl of acetonitrile, followed by immediate vortex and placement of the tubes on ice.
Quantification of 4-Hydroxybupropion and 6β-Hydroxytestosterone Formation
All samples were extracted immediately after the incubations were carried out. First, an internal standard was added to each sample. The incubation mixture was then centrifuged at 14,000 rpm for 5 min at room temperature. The supernatant layer was made alkaline by adding 500 μl of 1 M glycine-NaOH buffer (pH 11.3) and extracted by adding 6 ml of ethyl acetate. This mixture was vortex-mixed for 10 min and then centrifuged at 36,000 rpm for 15 min. The organic layer was transferred to 13×100-mm glass culture tubes and evaporated to dryness. The resulting residue was reconstituted in mobile phase. HPLC assays with ultraviolet (UV) detection were developed for the quantification of 4-hydroxybupropion and 6β-hydroxytestosterone. The HPLC system consisted of a Shimadzu LC-10AT pump, SIL-10AD auto-sampler, SCL-10A system controller and SPD-10A UV-VIS detector (Shimadzu Scientific Instruments Inc., Columbia, MD). 1) 4-hydroxybupropion and nevirapine (internal standard) were separated using a Zorbax SB-C18 column (150 × 4.6 mm, 3.5 μm particle size; Phenomenex, Torrance, CA), a Luna C18 Guard column (30 × 4.6 mm, 5 μm; Phenomenex) and mobile phase consisting of 85% (v/v) 10mM KH2PO4 (pH adjusted to 3) and 15% acetonitrile (flow rate, 1 ml/min). UV detection was set at 214 nm for 4-hydroxybupropion (retention time: 14.2 min) and 282 nm for nevirapine (retention time: 29 min). 2) 6β-hydroxytestosterone and desmethyldiazepam (internal standard) were separated using the same column but with a mobile phase consisting of 40% 30 mM ammonium acetate (pH adjusted to 6.3) and 60% methanol (flow rate, 1 ml/min). UV detection was set at 254nm for 6β-hydroxytestosterone (retention time: 5 min) and desmethyldiazepam (retention time: 10.4 min). Peak areas for each peak were obtained from an integrator, and peak area ratios with internal standard were calculated. Formation rate of metabolite from the respective probe substrate was quantified by using the appropriate standard curve. Intra- and inter-day coefficients of variation of the assays were less than 15%.
Kinetic Analyses
The rates of metabolite formation from substrate probes in the presence of the test inhibitors were compared with those for control in which the inhibitor was replaced with vehicle. The extent of CYP450 inhibition was expressed as percent enzyme activity remaining compared to control. IC50 values were determined as the inhibitor concentration that brought about a 50% reduction in enzyme activity by fitting all the data to a one-site competition equation using Prism version 5.01 for Windows (GraphPad Software Inc., San Diego, CA).
RESULTS
Separation, Isolation and Purification of Naringenin Enantiomers
An improved separation of the enantiomers of naringenin was accomplished by chiral HPLC on a polysaccharide-derived22 chiral stationary phase (Lux Cellulose 2) using a mobile phase n-hexane/ethanol with 0.5 % of trifluoroacetic acid (TFA) 80:20 at 1.2 ml/min. We obtained good values for enantioselectivity (α) and resolution factor (Rs), of 1.97 and 8.3 respectively. This is a large improvement in the resolution factor with respect to previous enantioseparation reported on a different chiral stationary phase23. A typical chromatogram is shown in Figure 2A with retention times of 6.87 min and 11.19 min for the (S)- and (R)-enantiomers respectively. Isolation of the single enantiomers of naringenin was performed using a composition 70:30 of the same mobile phase without TFA, at a flow rate 1.5 ml/min. The absence of TFA avoided problems in the solvent elimination from the eluates although some tailing of the peaks was present. These conditions are a compromise between short elution times (t1 = 3.38, t2 = 4.18 min) and adequate resolution factors. The isolation was accomplished by repeated injections (about 160) of 70 μL of a solution (5 mg/mL) of rac-naringenin in n-hexane/ethanol (1:1). Collection of the eluates after filtration and rotoevaporation yielded 20 mg of each enantiomer. The enantiomeric purity (e.p.) was checked using the same analytical conditions and it was 98.0 and 96.5 % for the first and the second eluted enantiomer, respectively. Due to the high values of α and Rs, the method can be scaled up using a larger column with the same chiral selector (not available to us) for isolating a greater quantity amount of single enantiomers with a much minor number of injections.
Fig. 2.
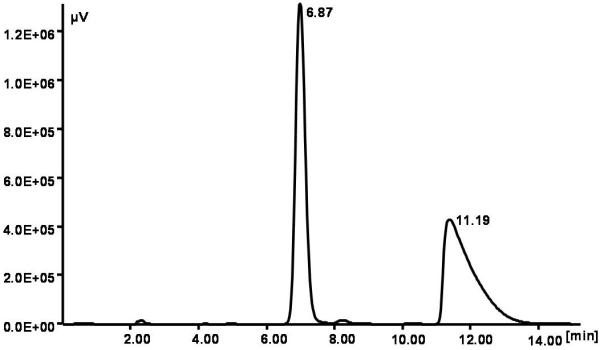
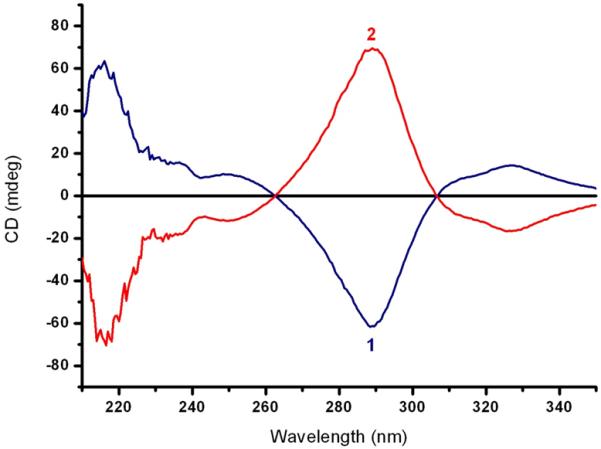
Separation of naringenin enantiomers. (A) Typical HPLC separation of the (S) and (R) enantiomers of naringenin with retention times of 6.87 min and 11.19 min respectively. Conditions: chiral stationary phase Lux Cellulose 2; mobile phase: n-hexane/ethanol with 0.5 % TFA, 80:20 at 1.2 ml/min. (B) Circular dichroism (CD) spectra (ethanol, 22 °C) of the enantiomers of naringenin obtained from the first (1) and the second (2) HPLC eluted peaks. The negative CD band centred at 288 nm corresponds to the (S)-configuration.
The circular dichroism (CD) spectra of the enantiomers were mirror images of each other indicating the enantiomeric nature of the collected peaks, as shown in Figure 2B. The absolute configuration of the enantiomers was established taking into account that the negative CD band centred at 288 nm corresponds to the (S)-configuration23,24. Thus, the first eluted enantiomer, shown in Figure 2A, is the (S)-enantiomer and the second eluted enantiomer is the (R)-enantiomer. Remarkably, the elution order of the enantiomers is reversed with respect to the elution order observed using different polysaccharide-based chiral stationary phases23-24.
Using this efficient procedure the (R)– and (S)-enantiomers of naringenin were isolated in sizeable amounts (15 mg) and used for subsequent experiments.
Inhibition of Cytochrome P450 Isoform Activity
Naringenin was able to inhibit a number of cytochrome P450 isoforms. Specifically, dose-dependent inhibition of CYP19, CYP2C9, CYP2C19, and CYP3A was demonstrated (Figure 3-6), while no appreciable inhibition of CYP2B6 or CYP2D6 was observed at concentrations up to 10 μM (data not shown). When the enantiomers of naringenin were tested, enantioselective inhibition of CYP19, CYP2C9, CYP219 and CYP3A was shown. While the (S)-enantiomer was approximately 2-fold more potent than the (R)-enantiomer as an inhibitor of CYP19 and CYP2C19, the (R)-enantiomer was about 2-fold more potent as an inhibitor of CYP2C9 and CYP3A (Figure 3-6, Table I). In each case, the IC50 value for rac-naringenin was between that for the (R)-enantiomer and that for the (S)-enantiomer.
Fig. 3.
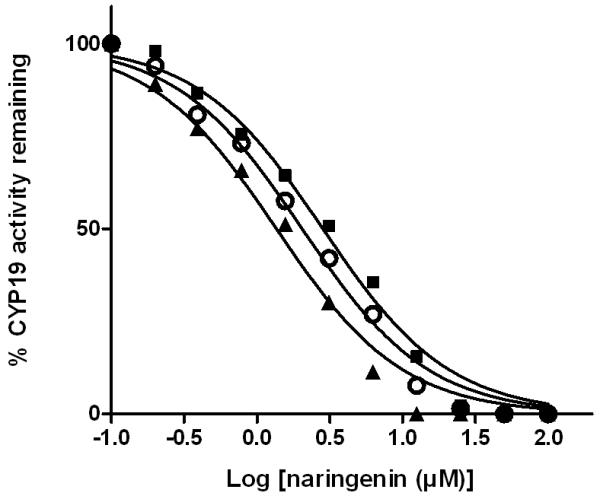
Enantioselective inhibition of human CYP19 by naringenin. A range of concentrations of rac-naringenin (open circle), (R)-naringenin (dark square), or (S)-naringenin (dark triangle) was incubated with recombinant human CYP19 (7.5 nM) and substrate MFC at 37°C for 30 min and enzyme activity was determined in three independent experiments. Individual points represent the mean of duplicate incubations. MFC, 7-methoxy-4-trifluoromethylcoumarin.
Fig. 6.
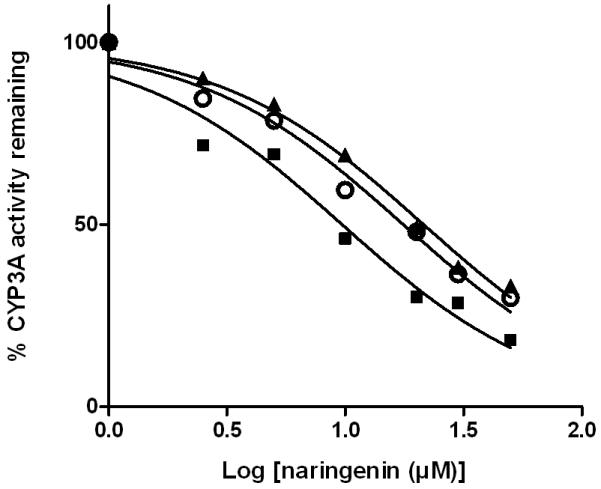
Enantioselective inhibition of human CYP3A by naringenin. A range of concentrations of rac-naringenin (open circle), (R)-naringenin (dark square), or (S)-naringenin (dark triangle) was incubated with pooled human liver microsomes (protein concentration 0.25 mg/ml) and probe substrate testosterone at 37°C for 15 min and enzyme activity was determined in two independent experiments. Individual points represent the mean of duplicate incubations.
TABLE I. IC50 values for enantioselective inhibition of multiple human CYP450 isoforms by naringenin.
| μM | CYP19 | CYP2C9 | CYP2C19 | CYP3A |
|---|---|---|---|---|
| rac-naringenin | 2.0 (1.7, 2.4) | 2.6 (2.0, 3.4) | 2.6 (2.4, 2.9) | 17.6 (14.8, 20.8) |
| (R)-naringenin | 2.8 (2.4, 3.4) | 1.6 (1.2, 2.1) | 4.9 (4.4, 5.5) | 9.7 (7.3, 12.8) |
| (S)-naringenin | 1.4 (1.1, 1.7) | 3.4 (2.6, 4.5) | 1.2 (1.1, 1.4) | 21.4 (18.5, 24.7) |
IC50 values are expressed as mean (95% confidence interval)
DISCUSSION
In this study we describe an efficient and effective separation of the chiral enantiomers of naringenin. This has allowed the testing of these enantiomers as inhibitors of cytochrome P450 isoforms, but their use need not be limited to this study. Many other naturally occurring flavonoids and isoflavanoids exist as stereoisometric mixtures, in which one enantiomer predominates. The enantioselective separation and purification of chiral flavonoids such as that described here should allow researchers to address biological questions with clarity, and to understand biochemical mechanisms with precision.
The data presented here demonstrate the ability of naringenin to inhibit multiple CYP450 isoforms in vitro. The IC50 concentrations of naringenin we observed all fall in the low micromolar range (Table I) consistent with previous studies that have shown maximal plasma concentrations of naringenin after ingestion of grapefruit juice to be ~ 6 μM25. These data suggest that naringenin may have effects in vivo on the activity of multiple enzymes. Such inhibitory effects may contribute to the clinically observed food-drug interactions brought about by grapefruit juice and other fruit products26. These data are consistent with those reported on inhibition of CYP 3A, but whether interactions with CYP19, CYP2C9, or CYP2C19 carry similar clinical relevance must await further research.
Studies presented here with purified (R)- and (S)-naringenin showed that the individual enantiomers were selective. While the (R)-enantiomer was a more potent inhibitor of some CYP isoforms (e.g CYP2C9 and CYP3A), remarkably the (S)-enantiomer was more potent on others (CYP2C19 and CYP19). These data shed light on the enantioselective preferences of specific CYP isoforms. While this enantioselective inhibition is clear in vitro, the predominant enantiomer of naringenin in natural fruits is unknown and so the vulnerability of specific enzymes to naringenin-related drug interactions in vivo cannot be determined. While a large literature exists on the effects of grapefruit and other juices on CYP3A16,27, few attempts have been made to study effects on other isoforms. The data presented here now suggest that interactions with substrates of CYP2C9 such as (S)-warfarin28, of CYP2C19, such as clopidogrel29, and of aromatase, such as methadone19 may also be important.
Of note, naringin, the natural precursor of naringenin, is present as (2S)- and (2R)-diastereomers in grapefruit, sour orange and pummelo with the (2S)-naringin being predominant. Naringin is responsible for the bitter taste of juice and marmalade and the ratio (2S)/(2R) of the concentrations of the diastereomers markedly decreases during maturation due to a nonenzymatic racemization at the C-2 position via, ring opening to a chalcone 30-32. Since our data showed that the inhibitory effects of naringenin on drug-metabolizing enzymes are pleiotropic and enantioselective, the well known grapefruit juice-drug interaction33 may be affected by the degree of maturation of the fruits. In addition, other chiral flavonoids contained in fruits may also posess enantioselective pharmacologic and biological effects.
A limitation of this study is that inhibitory potencies for different CYP isoforms were obtained using different microsomal systems. Four microsomal preparations of different recombinant cytochrome P450 isoforms and one preparation of pooled human liver microsomes were used. As a result the observed IC50 values in any individual enzyme system should not be directly compared with those obtained in any other system. The question of whether or not naringenein inhibits CYP19, CYP2C9 or CYP2C19 clinically, as has been reported for CYP3A, requires further investigation. Despite this limitation, it is clear that the inhibition of these cytochrome P450 enzymes by naringenin is enantioselective in every case.
CONCLUSIONS
These studies have characterized for the first time the different pharmacologic properties of the enantiomers of naringenin, specifically the enantioselective inhibition of CYP19, CYP2C9, CYP2C19 and CYP3A. The data indicate that inhibition of these enzymes by naringenin can occur with potency in the physiologic range. These findings allow more precise estimates of the potential risks for food-drug interactions to be made and identify interactions between specific CYP450 isoforms and naringenin that deserve further study. Lastly, the enantioselective properties exhibited by naringenin here could provide insight into the use of naringenin and other flavonoids as novel, selective therapeutic agents.
Fig. 4.
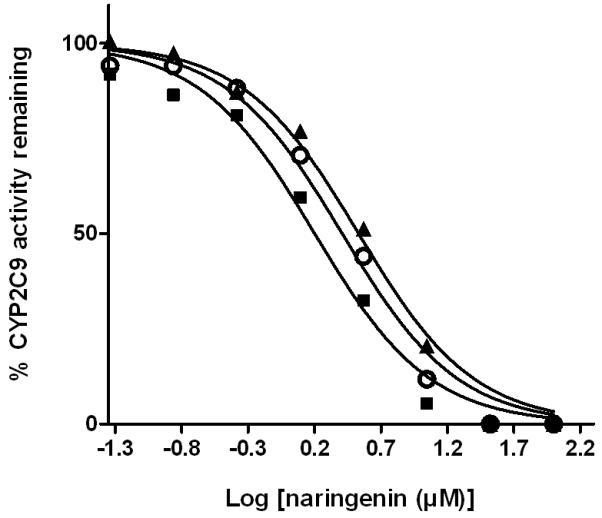
Enantioselective inhibition of human CYP2C9 by naringenin. A range of concentrations of rac-naringenin (open circle), (R)-naringenin (dark square), or (S)-naringenin (dark triangle) was incubated with recombinant human CYP2C9 (15 nM) and substrate MFC at 37°C for 45 min and enzyme activity was determined in three independent experiments. Individual points represent the mean of duplicate incubations. MFC, 7-methoxy-4-trifluoromethylcoumarin.
Fig. 5.
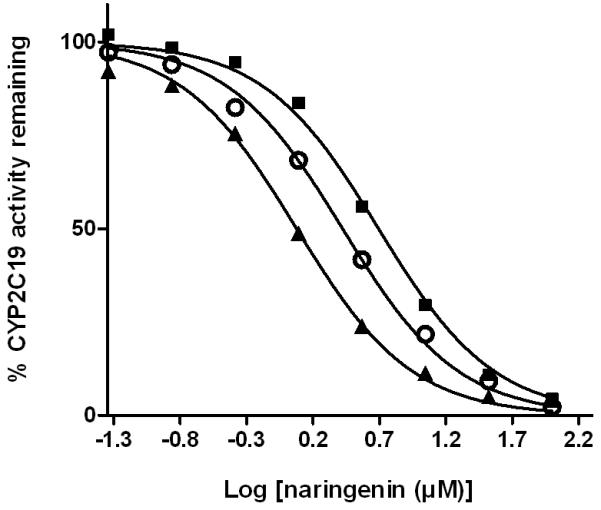
Enantioselective inhibition of human CYP2C19 by naringenin. A range of concentrations of rac-naringenin (open circle), (R)-naringenin (dark square), or (S)-naringenin (dark triangle) was incubated with recombinant human CYP2C19 (7.5 nM) and substrate CEC at 37°C for 30 min and enzyme activity was determined in three independent experiments. Individual points represent the mean of duplicate incubations. CEC, 3-cyano-7-ethoxycoumarin.
Acknowledgments
Contract grant sponsor: National Institutes of Health National Center for Research Resources; Contract grant number: K24RR020815. (DAF)
Contract grant sponsor: National Institute for General Medical Sciences; Contract grant number: T32GM008425, U01GM061373. (DAF)
Contract grant sponsor: Department of Defense Breast Cancer Research Program; Contract grant number: W81XWH-11-1-0016. (WJL)
Footnotes
All the authors declare that they have no conflict of interest.
LITERATURE CITED
- 1.Yanez JA, Davies NM. Stereospecific high-performance liquid chromatographic analysis of naringenin in urine. J Pharm Biomed Anal. 2005;39(1-2):164–9. doi: 10.1016/j.jpba.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 2.Guengerich FP, Kim DH. In vitro inhibition of dihydropyridine oxidation and aflatoxin B1 activation in human liver microsomes by naringenin and other flavonoids. Carcinogenesis. 1990;11(12):2275–9. doi: 10.1093/carcin/11.12.2275. [DOI] [PubMed] [Google Scholar]
- 3.Bear WL, Teel RW. Effects of citrus phytochemicals on liver and lung cytochrome P450 activity and on the in vitro metabolism of the tobacco-specific nitrosamine NNK. Anticancer Res. 2000;20(5A):3323–9. [PubMed] [Google Scholar]
- 4.Ho PC, Saville DJ, Wanwimolruk S. Inhibition of human CYP3A4 activity by grapefruit flavonoids, furanocoumarins and related compounds. J Pharm Pharm Sci. 2001;4(3):217–27. [PubMed] [Google Scholar]
- 5.Du G, Jin L, Han X, Song Z, Zhang H, Liang W. Naringenin: a potential immunomodulator for inhibiting lung fibrosis and metastasis. Cancer Res. 2009;69(7):3205–12. doi: 10.1158/0008-5472.CAN-08-3393. [DOI] [PubMed] [Google Scholar]
- 6.Manthey JA, Grohmann K, Guthrie N. Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr Med Chem. 2001;8(2):135–53. doi: 10.2174/0929867013373723. [DOI] [PubMed] [Google Scholar]
- 7.Crossley R. The relevance of chirality to the study of biological activity. Tetrahedron. 1992;48:8155–8178. [Google Scholar]
- 8.Hutt A. Drug chirality and its pharmacological consequences. In: Smith H, editor. Introduction to the Principles of Drug Design and Action. CRC Press; Boca Raton FL: 2006. pp. 117–183. [Google Scholar]
- 9.Muralidharan G, Hawes EM, McKay G, Korchinski ED, Midha KK. Quinidine but not quinine inhibits in man the oxidative metabolic routes of methoxyphenamine which involve debrisoquine 4-hydroxylase. Eur J Clin Pharmacol. 1991;41(5):471–4. doi: 10.1007/BF00626372. [DOI] [PubMed] [Google Scholar]
- 10.Liu KH, Kim MJ, Shon JH, Moon YS, Seol SY, Kang W, Cha IJ, Shin JG. Stereoselective inhibition of cytochrome P450 forms by lansoprazole and omeprazole in vitro. Xenobiotica. 2005;35(1):27–38. doi: 10.1080/00498250400026472. [DOI] [PubMed] [Google Scholar]
- 11.Carabaza A, Cabre F, Garcia AM, Rotllan E, Garcia ML, Mauleon D. Stereoselective inhibition of rat brain cyclooxygenase by dexketoprofen. Chirality. 1997;9(3):281–5. doi: 10.1002/(SICI)1520-636X(1997)9:3<281::AID-CHIR13>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 12.Shin JG, Kane K, Flockhart DA. Potent inhibition of CYP2D6 by haloperidol metabolites: stereoselective inhibition by reduced haloperidol. Br J Clin Pharmacol. 2001;51(1):45–52. doi: 10.1046/j.1365-2125.2001.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dilmaghanian S, Gerber JG, Filler SG, Sanchez A, Gal J. Enantioselectivity of inhibition of cytochrome P450 3A4 (CYP3A4) by ketoconazole: Testosterone and methadone as substrates. Chirality. 2004;16(2):79–85. doi: 10.1002/chir.10294. [DOI] [PubMed] [Google Scholar]
- 14.Bailey DG, Dresser GK, Kreeft JH, Munoz C, Freeman DJ, Bend JR. Grapefruit-felodipine interaction: effect of unprocessed fruit and probable active ingredients. Clin Pharmacol Ther. 2000;68(5):468–77. doi: 10.1067/mcp.2000.110774. [DOI] [PubMed] [Google Scholar]
- 15.Bailey DG, Arnold JM, Munoz C, Spence JD. Grapefruit juice--felodipine interaction: mechanism, predictability, and effect of naringin. Clin Pharmacol Ther. 1993;53(6):637–42. doi: 10.1038/clpt.1993.84. [DOI] [PubMed] [Google Scholar]
- 16.Bailey DG, Malcolm J, Arnold O, Spence JD. Grapefruit juice-drug interactions. Br J Clin Pharmacol. 2004;58(7):S831–40. doi: 10.1111/j.1365-2125.2004.02305.x. 1998. discussion S841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee M, Min DI, Ku YM, Flanigan M. Effect of grapefruit juice on pharmacokinetics of microemulsion cyclosporine in African American subjects compared with Caucasian subjects: does ethnic difference matter? J Clin Pharmacol. 2001;41(3):317–23. doi: 10.1177/00912700122010131. [DOI] [PubMed] [Google Scholar]
- 18.Benmebarek M, Devaud C, Gex-Fabry M, Powell Golay K, Brogli C, Baumann P, Gravier B, Eap CB. Effects of grapefruit juice on the pharmacokinetics of the enantiomers of methadone. Clin Pharmacol Ther. 2004;76(1):55–63. doi: 10.1016/j.clpt.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Lu WJ, Bies R, Kamden LK, Desta Z, Flockhart DA. Methadone: a substrate and mechanism-based inhibitor of CYP19 (aromatase) Drug Metab Dispos. 2010;38(8):1308–13. doi: 10.1124/dmd.110.032474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allenmark S. Chromatographic Enantioseparation: Methods and Applications. Ellis Horwood; Chichester, UK: 1991. [Google Scholar]
- 21.Stresser DM. High-throughput screening of human cytochrome P450 inhibitors using fluorometric substrates. In: Yan Z, Caldwell GW, editors. Optimization in drug discovery: in vitro methods, Methods in pharmacology and toxicology. 2004. pp. 215–230. [Google Scholar]
- 22.Chen X, Yamamoto C, Okamoto Y. Polysaccharide derivatives as useful chiral stationary phases in high-performance liquid chromatography. Pure and Applied Chemistry. 2007;79:1561–1573. [Google Scholar]
- 23.Giorgio E, Parrinello N, Caccamese S, Rosini C. Non-empirical assignment of the absolute configuration of (−)-naringenin, by coupling the exciton analysis of the circular dichroism spectrum and the ab initio calculation of the optical rotatory power. Org Biomol Chem. 2004;2(24):3602–7. doi: 10.1039/b411110a. [DOI] [PubMed] [Google Scholar]
- 24.Caccamese S, Caruso C, Parrinello N, Savarino A. High-performance liquid chromatographic separation and chiroptical properties of the enantiomers of naringenin and other flavanones. J Chromatogr A. 2005;1076(1-2):155–62. doi: 10.1016/j.chroma.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 25.Erlund I, Meririnne E, Alfthan G, Aro A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J Nutr. 2001;131(2):235–41. doi: 10.1093/jn/131.2.235. [DOI] [PubMed] [Google Scholar]
- 26.Dresser GK, Bailey DG. The effects of fruit juices on drug disposition: a new model for drug interactions. Eur J Clin Invest. 2003;33(Suppl 2):10–6. doi: 10.1046/j.1365-2362.33.s2.2.x. [DOI] [PubMed] [Google Scholar]
- 27.Bailey DG, Dresser GK, Bend JR. Bergamottin, lime juice, and red wine as inhibitors of cytochrome P450 3A4 activity: comparison with grapefruit juice. Clin Pharmacol Ther. 2003;73(6):529–37. doi: 10.1016/S0009-9236(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 28.Rettie AE, Korzekwa KR, Kunze KL, Lawrence RF, Eddy AC, Aoyama T, Gelboin HV, Gonzalez FJ, Trager WF. Hydroxylation of warfarin by human cDNA-expressed cytochrome P-450: a role for P-4502C9 in the etiology of (S)-warfarin-drug interactions. Chem Res Toxicol. 1992;5(1):54–9. doi: 10.1021/tx00025a009. [DOI] [PubMed] [Google Scholar]
- 29.Kreutz RP, Stanek EJ, Aubert R, Yao J, Breall JA, Desta Z, Skaar TC, Teagarden JR, Frueh FW, Epstein RS, et al. Impact of proton pump inhibitors on the effectiveness of clopidogrel after coronary stent placement: the clopidogrel Medco outcomes study. Pharmacotherapy. 2010;30(8):787–96. doi: 10.1592/phco.30.8.787. [DOI] [PubMed] [Google Scholar]
- 30.Caccamese S, Manna L, Scivoli G. Chiral HPLC separation and CD spectra of the C-2 diastereomers of naringin in grapefruit during maturation. Chirality. 2003;15(8):661–7. doi: 10.1002/chir.10262. [DOI] [PubMed] [Google Scholar]
- 31.Caccamese S, Bianca S, Santo D. Racemization at C-2 of naringin in sour oranges with increasing maturity determined by chiral high-performance liquid chromatography. J Agric Food Chem. 2007;55(10):3816–22. doi: 10.1021/jf063355w. [DOI] [PubMed] [Google Scholar]
- 32.Caccamese S, Chillemi R. Racemization at C-2 of naringin in pummelo (Citrus grandis) with increasing maturity determined by chiral high-performance liquid chromatography. J Chromatogr A. 2010;1217(7):1089–93. doi: 10.1016/j.chroma.2009.10.073. [DOI] [PubMed] [Google Scholar]
- 33.Fuhr U. Drug interactions with grapefruit juice. Extent, probable mechanism and clinical relevance. Drug Saf. 1998;18(4):251–72. doi: 10.2165/00002018-199818040-00002. [DOI] [PubMed] [Google Scholar]


