Abstract
Background/Objectives
The results of short-term studies in humans suggest that, compared with glucose, acute consumption of fructose leads to increased postprandial energy expenditure and carbohydrate oxidation and decreased postprandial fat oxidation. The objective of this study was to determine the potential effects of increased fructose consumption compared to isocaloric glucose consumption on substrate utilization and energy expenditure following sustained consumption and under energy-balanced conditions.
Subjects/Methods
As part of a parallel arm study, overweight/obese male and female subjects, 40–72 y, consumed glucose- or fructose-sweetened beverages providing 25% of energy requirements for 10 weeks. Energy expenditure and substrate utilization were assessed using indirect calorimetry at baseline and during the 10th week of intervention.
Results
Consumption of fructose, but not glucose, led to significant decreases of net postprandial fat oxidation and significant increases of net postprandial carbohydrate oxidation (P < 0.0001 for both). Resting energy expenditure decreased significantly from baseline values in subjects consuming fructose (P = 0.031) but not in those consuming glucose.
Conclusions
Increased consumption of fructose for 10 weeks leads to marked changes of postprandial substrate utilization including a significant reduction of net fat oxidation. In addition, we report that resting energy expenditure is reduced compared to baseline values in subjects consuming fructose-sweetened beverages for 10 weeks.
Keywords: fructose, fat oxidation, carbohydrate oxidation, energy expenditure, metabolic rate, humans
Introduction
An increase in the use of sweeteners containing fructose has occurred in parallel with the increasing prevalence of overweight and obesity over the past three decades in the U.S. (Bray et al 2004), suggesting that increased consumption of fructose, high fructose corn syrup, and/or sucrose may contribute to the current epidemic of obesity and the increased incidence of metabolic syndrome (Bray et al 2004, Havel 2005). In animal studies, consumption of diets high in fructose produces obesity, insulin resistance and dyslipidemia (Bezerra et al 2000, Elliott et al 2002, Havel 2005, Martinez et al 1994, Okazaki et al 1994, Storlien et al 1993). In humans, moderate fructose consumption has no apparent health concerns (Dolan et al 2010, Rizkalla 2010), but the health consequences of fructose consumption in large amounts are less clear, particularly its effects on substrate utilization and body weight regulation.
Recently we reported that consumption of fructose-sweetened beverages for 10 weeks, at 25% of energy requirements, increased hepatic de novo lipogenesis (DNL), promoted accumulation of intra-abdominal fat, produced a more atherogenic lipid profile, and reduced insulin sensitivity in older, overweight and obese adults compared with isocaloric consumption of glucose (Stanhope et al 2009). We hypothesized that the increased rate of DNL following consumption of fructose leads to an accumulation of hepatic lipid, which promotes dyslipidemia and decreases in insulin sensitivity (Stanhope et al 2009). McGarry observed that increases of DNL led to a concomitant reduction in fat oxidation, which may contribute to an accumulation of hepatic lipid (McGarry 1995). Therefore, to determine if the increases of DNL associated with increased fructose consumption were accompanied by decreased fat oxidation, we measured resting and postprandial substrate utilization and energy expenditure using indirect calorimetry in the overweight and obese adults during their participation in the study mentioned above (Stanhope et al 2009).
Previous investigations of the effects of fructose consumption on substrate utilization and energy expenditure are limited to acute or short-term studies (Chong et al 2007, Couchepin et al 2008, Markov et al 2000, Schwarz et al 1989, Schwarz et al 1992a, Schwarz et al 1992b, Tappy et al 1986). Our results from this 10 week study indicate that in overweight/obese adults, 40–72 years of age, sustained consumption of fructose-sweetened beverages, at 25% of energy requirements, leads to decreased net postprandial fat oxidation and increased net postprandial carbohydrate oxidation, similar to what has been observed in short-term studies. In addition, we found that resting energy expenditure decreased significantly after 10 weeks in subjects consuming fructose-sweetened beverages, despite increases of body weight.
Subjects and methods
Study design
This was a parallel arm study with 3 phases: 1) a 2-wk inpatient baseline period; 2) an 8-wk outpatient intervention period; and 3) a 2-wk inpatient intervention period. The details of the study design and diet interventions have been described (Stanhope, et al. 2009). Briefly, during baseline, subjects resided as inpatients and consumed an energy-balanced diet. Procedures included: indirect calorimetry, dual energy x-ray absorptiometry (DXA), and a computerized tomography (CT) scan of the abdomen. Subjects then began the 8-wk outpatient intervention and consumed either fructose- or glucose-sweetened beverages at 25% of energy requirements with self-selected ad libitum diets. Subjects returned as inpatients for the final 2 wks of intervention, during which the procedures were repeated while the subjects consumed their assigned glucose- or fructose-sweetened beverages as part of an energy-balanced diet.
Subjects
Participants were recruited through advertisements and underwent a telephone and an in-person interview to assess eligibility. Inclusion criteria included age 40–72 y, BMI 25–35 kg/m2, and stable body weight during the prior six months. Women were post-menopausal. Exclusion criteria included: evidence of diabetes, renal or hepatic disease, fasting serum TG concentrations >400 mg/dL, blood pressure >140/90 mmHg, and surgery for weight loss. Individuals who smoked, reported exercise of more than 3.5 hrs/wk at a level more vigorous than walking, or having used thyroid, lipid-lowering, glucose-lowering, anti-hypertensive, anti-depressant, or weight loss medications were also excluded. Diet-related exclusion criteria included habitual ingestion of more than one sugar-sweetened beverage/d or more than two alcoholic beverages/d. The UC-Davis Institutional Review Board approved the study, and subjects provided informed consent to participate. Initially 39 subjects enrolled, but seven subjects did not complete the study due to personal or work-related conflicts, and one subject was opted out of the indirect calorimetry protocol. Thus, a total of 31 subjects completed the study: n=15 for the glucose group and n=16 for the fructose group. As previously reported (Stanhope et al 2009), baseline characteristics between the two experimental sugar groups were not different (Table 1).
Table 1.
| Glucose | Fructose | |||
|---|---|---|---|---|
|
| ||||
| Parameter | Male (n=7) | Female (n=8) | Male (n=9) | Female (n=7) |
| Age (yr) | 54 ± 3 | 56 ± 2 | 52 ± 4 | 53 ± 3 |
| Weight (kg) | 88.4 ± 2.9 | 84.0 ± 4.5 | 89.3 ± 2.9 | 80.5 ± 4.6 |
| BMI (kg/m2) | 29.3 ± 1.1 | 29.4 ± 1.3 | 28.4 ± 0.7 | 30.0 ± 1.2 |
| Body Fat (%) | 29.4 ± 1.1 | 43.2 ± 1.5 | 28.5 ± 1.3 | 40.5 ± 2.1 |
| Fat Free Mass (kg) | 63.3 ± 1.4 | 48.2 ± 2.1 | 64.8 ± 2.0 | 47.6 ± 2.4 |
| Triglycerides (mg/dL) | 148 ± 31 | 145 ± 23 | 131 ± 21 | 151 ± 36 |
| Cholesterol (mg/dL) | 179 ± 14 | 193 ± 10 | 176 ± 6 | 197 ± 14 |
| LDL-cholesterol (mg/dL) | 124 ± 5 | 123 ± 11 | 107 ± 7 | 125 ± 10 |
| Glucose (mg/dL) | 89 ± 2 | 89 ± 3 | 88 ± 1 | 90 ± 2 |
Values are means± SEM. Clinical chemistry values are fasting values. There were no significant differences between sugar × gender groups.
Data were published previously (Stanhope, et al. 2009).
Diets
During the inpatient metabolic phases, subjects consumed diets designed to maintain energy balance providing 15% of energy as protein, 30% as fat, and 55% as carbohydrate. Daily energy intake was calculated at baseline using the Mifflin equation to estimate resting energy expenditure (REE) (Mifflin et al 1990) and adjusted for activity using a multiplication factor of 1.5. During baseline, the carbohydrate content consisted primarily of complex carbohydrates and contained 8.8 ± 1.2 g of dietary fiber/1000 kcal. For the final 2-wk inpatient intervention period, subjects consumed diets at the baseline energy level and macronutrient composition except that 30% of energy was from complex carbohydrates and 25% was provided by fructose- or glucose-sweetened beverages. Additional details about the diet intake for inpatient and outpatient phases have been described previously (Stanhope et al 2009).
Indirect calorimetry
An automated metabolic measuring cart (Truemax 2400 Metabolic Measurement System, Parvomedics, Salt Lake City, UT) was used to measure rates of O2 consumption (VO2) and CO2 production (VCO2). Gas analyzers were calibrated using a certified gas mixture of known O2 and CO2 concentrations, and the flowmeter was calibrated using a 3 L syringe, 4 times daily (07:00h, 11:00h, 15:00h, and 18:00h). The protocol was conducted at the CCRC at baseline-wk 0, and at intervention-wk 10 and was preceded by a minimum of 5 days of controlled diet. REE was measured on two separate days during wk 0 and again at wk 10, whereas postprandial energy expenditure (PPEE) was measured only once during wk 0 and once during wk 10, following a REE measurement. Respiratory gases were collected while subjects were in a semi-reclined position and wore a facemask fitted securely, covering the nose and mouth. The facemask was attached to tubing connected to the cart’s mixing chamber. Through the facemask, subjects inhaled room air, and all expired breath was trapped by the mask and directed to the cart’s mixing chamber for volume and gas analyses. Prior to the test, subjects fasted overnight for 13.5 h and rested quietly for at least 10 min before measurements commenced. REE was measured at 07:30 h and 08:30 h for 20 min periods. For PPEE, respiratory gases were collected for 15 min every hour over the next 14 h; the most stable 10-min interval of each 15-min collection was selected to represent PPEE for that hour. During the protocol subjects consumed the controlled breakfast (09:00 h), lunch (13:00 h), and dinner (18:00 h) meals. They were permitted to perform light activities associated with living in a metabolic ward, but rested in a semi-reclined position for at least 5 min before each measurement. Indirect calorimetry data were analyzed for all 31 subjects for REE, but only for 30 subjects for PPEE (n=15 in the fructose group and n=15 in the glucose group) due to procedure scheduling conflicts for one female in the fructose group.
Calculation of energy expenditure and substrate oxidation rates
Energy expenditure was calculated using the Weir equation (Weir 1990):
Net carbohydrate oxidation (CHO-Ox) and net fat oxidation (FAT-Ox) were calculated using the following formulas (Frayn 1983):
For all equations, units for VO2 and VCO2 are in L/min. To estimate urinary nitrogen excretion, in g/min, a constant rate of protein catabolism was assumed, equivalent to the 24-h protein intake, as reported by Bingham (Bingham 2003).
Measurements of body composition
Total body fat and fat free mass were determined by DXA, and intra- and extra-abdominal fat were measured by CT scan as described previously (Stanhope et al 2009).
Data analysis
REE, PPEE and corresponding net substrate oxidation values for baseline and intervention were calculated using minute-by-minute values for VO2 and VCO2 (L/min). Overall PPEE and postprandial substrate oxidation rates were estimated by averaging values for the 14 postprandial time periods. Statistical tests were performed with SAS 9.2. The absolute or percent change for each outcome was analyzed in a 3-factor (type of sugar, gender, and + or − metabolic syndrome) mixed procedure (PROC MIXED) ANOVA model with adjustment for the change in fat-free mass (FFM). The model was also run using baseline REE and PPEE as covariates, and the changes in REE and PPEE were still significant and comparable to those obtained when the model was adjusted for FFM. Metabolic syndrome (MetSyn) was defined as having at least 3 MetSyn risk factors as defined by the American Heart Association/National Heart Lung and Blood Institute (Grundy et al. 2004). There were nine subjects with MetSyn (Fructose n= 5, Glucose n = 4) and 22 subjects without MetSyn (Fructose n=11, Glucose n=11). Outcomes with least squares means (LS means) of the change (10 wk versus 0 wk) significantly different than zero were identified. Statistical tests with P values <0.05 were considered significant. Data are presented as mean ± SEM.
Results
Body weight and composition
As reported previously (Stanhope et al 2009), despite comparable weight gain (~1–2% of initial body weight) following the 8-wk outpatient intervention, subjects consuming fructose primarily exhibited increases of visceral adipose tissue (VAT), while in subjects consuming glucose subcutaneous adipose tissue (SAT) was preferentially increased.
Net substrate oxidation rates
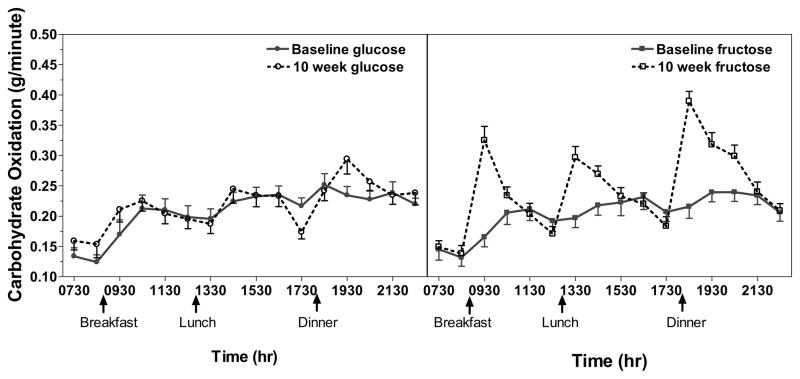
Overall, fasting CHO-Ox did not change in response to glucose (P = 0.29) or fructose (P = 0.11) consumption, however subjects with metabolic syndrome consuming fructose exhibited significant increases of fasting CHO-Ox (P = 0.02) after 10 weeks of intervention (Table 2). Postprandial CHO-Ox increased significantly from baseline in subjects consuming fructose-sweetened beverages (P < 0.0001), but not in those consuming glucose-sweetened beverages (P = 0.54) (Table 2, PFigure 1). Similar to the fasting condition, subjects with metabolic syndrome consuming fructose exhibited marked percent increases of postprandial CHO-ox ( < 0.0001), whereas in subjects consuming fructose without metabolic syndrome postprandial CHO-ox rates were not changed from baseline values (P = 0.34) (sugar x MetSyn interaction: P = 0.01). There was also an effect of gender on the change of postprandial CHO-Ox (P = 0.05), such that male subjects in both sugar groups exhibited greater increases (21.35 ± 9.8%; P < 0.001) than female subjects (5.75 ± 3.9%; P = 0.81).
Table 2.
Net carbohydrate and fat oxidation rates and percent change after consumption of glucose- or fructose-sweetened beverages for 10 wks1
| Fructose | Fructose | Fructose | Glucose | Glucose | Glucose | 2 sugar | 2 sugar x MetSyn | ||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Baseline | Week 10 | % change | Baseline | Week 10 | % change | |||
| Carbohydrate Oxidation Rate, g/min | |||||||||
| Fasting | All | 0.16 ± 0.06 | 0.17 ± 0.01 | 14.8 ± 17.6 | 0.16 ± 0.01 | 0.18 ± 0.07 | 23.6 ± 11.5 | 0.745 | 0.047 |
| Fasting | (−)MetSyn | 0.16 ± 0.02 | 0.15 ± 0.01 | −2.2 ± 8.5 | 0.16 ± 0.02 | 0.20 ± 0.02 | 30.4 ± 14.7 | ||
| Fasting | (+)MetSyn | 0.17 ± 0.04 | 0.20 ± 0.03 | 52.0 ± 52.9* | 0.14 ± 0.02 | 0.14 ± 0.02 | 5.2 ± 13.9 | ||
| Postprandial | All | 0.24 ± 0.02 | 0.29 ± 0.01 | 23.5 ± 8.6*** | 0.25 ± 0.01 | 0.26 ± 0.01 | 3.8 ± 3.3 | 0.005 | 0.012 |
| Postprandial | (−)MetSyn | 0.26 ± 0.02 | 0.28 ± 0.01 | 11.9 ± 4.8 | 0.25 ± 0.02 | 0.26 ± 0.02 | 5.2 ± 4.2 | ||
| Postprandial | (+)MetSyn | 0.21 ± 0.03 | 0.29 ± 0.02 | 46.6 ± 21.8*** | 0.23 ± 0.02 | 0.23 ± 0.02 | −0.04 ± 8.3 | ||
| Fat Oxidation Rate, g/min | |||||||||
| Fasting | All | 0.06 ± 0.01 | 0.05 ± 0.01 | −13.1 ± 8.0 | 0.06 ± 0.01 | 0.05 ± 0.01 | −14.2 ± 14.1 | 0.366 | 0.037 |
| Fasting | (−)MetSyn | 0.06 ± 0.01 | 0.05 ± 0.01 | −7.0 ± 7.6 | 0.06 ± 0.01 | 0.05 ± 0.01 | −26.7 ± 15.5* | ||
| Fasting | (+)MetSyn | 0.06 ± 0.01 | 0.04 ± 0.03 | −26.5 ± 19.8 | 0.05 ± 0.01 | 0.05 ± 0.01 | 20.3 ± 27.4 | ||
| Postprandial | All | 0.05 ± 0.01 | 0.03 ± 0.01 | −38.2 ± 4.8*** | 0.05 ± 0.01 | 0.04 ± 0.01 | −8.6 ± 6.1 | 0.001 | 0.020 |
| Postprandial | (−)MetSyn | 0.05 ± 0.01 | 0.03 ± 0.01 | −33.7 ± 4.3*** | 0.05 ± 0.01 | 0.04 ± 0.01 | −14.1 ± 7.4* | ||
| Postprandial | (+)MetSyn | 0.06 ± 0.01 | 0.03 ± 0.01 | −47.4 ± 11.0*** | 0.04 ± 0.01 | 0.04 ± 0.01 | 6.3 ± 6.9 | ||
Values are Means ± SEM. Abbreviations: MetSyn=metabolic syndrome: (−) without; (+) with. Sample sizes in sugar x MetSyn groups are: fructose(−)MetSyn, n=11 resting, n=10 postprandial; fructose(+)MetSyn, n=5; glucose(−)MetSyn, n=11; glucose(+)MetSyn, n=4.
PROC MIXED 3-factor ANOVA (sugar, gender, (+) or (−) MetSyn) adjusted for change in fat-free mass.
P < 0.05
P < 0.01
P < 0.001 for changes significantly different from zero.
Figure 1.
Net carbohydrate oxidation rate (g/min) profiles over 15 hours for subjects consuming glucose- and fructose-sweetened beverages. Subjects consumed meals at 09:00, 13:00, and 18:00 as indicated. The first 2 data points represent resting values and the remaining 14 data points represent postprandial values. Data points represent the mean of 10-min measurements ± SEM with n=31 (fructose group n=16; glucose group n=15) for resting values and n=30 (fructose group n=15; glucose group n=15) for postprandial values.
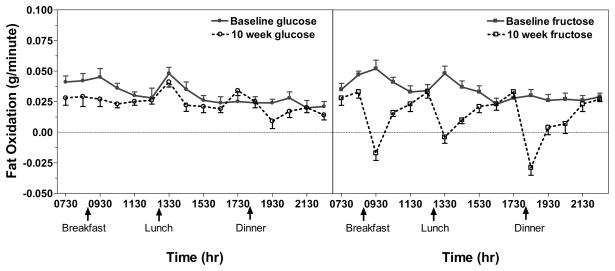
The change of fasting FAT-Ox differed both by sugar and by the presence/absence of MetSyn. While fasting FAT-Ox tended to decrease in the fructose group at 10 weeks (P = 0.15), this trend was driven by the larger decreases that were observed in subjects with metabolic syndrome (P = 0.14) as opposed to those without metabolic syndrome (P = 0.74). Although there was no overall change in fasting FAT-Ox in the glucose group at 10 weeks (P = 0.92), there was a significant decrease in subjects without metabolic syndrome (P < 0.05) (Table 2). In subjects consuming fructose-sweetened beverages postprandial FAT-Ox rates decreased significantly both compared with baseline values (P < 0.0001) and compared with subjects consuming glucose-sweetened beverages (P < 0.0001) (Table 2, PFigure 2). Overall the percent decrease of postprandial FAT-Ox was significant in subjects consuming fructose regardless of the presence ( < 0.0001) or absence (P = 0.0002) of MetSyn, whereas with glucose consumption only subjects without metabolic syndrome exhibited statistically significant decreases of postprandial FAT-Ox (P = 0.03) (Table 2).
Figure 2.
Net fat oxidation rate (g/min) profiles over 15 hours for subjects consuming glucose-and fructose sweetened beverages. Subjects consumed meals at 09:00, 13:00, and 18:00 as indicated. The first 2 data points represent resting values and the remaining 14 data points represent postprandial values. Data points represent the mean of 10-min measurements ± SEM. with n=31 (fructose group n=16; glucose group n=15) for resting values and n=30 (fructose group n=15; glucose group n=15) for postprandial values.
Energy expenditure
REE was significantly decreased from baseline values by wk 10 in subjects consuming fructose (P = 0.03) but not in those consuming glucose (P = 0.86). Postprandial energy expenditure also tended to decrease from baseline values in subjects in the fructose group but the change was not statistically significant (P = 0.19). PPEE was unchanged from baseline values in the glucose group (P = 0.86) (Table 3).
Table 3.
Energy expenditure before and after consumption of glucose- or fructose-sweetened beverages for 10 weeks1
| Fructose Baseline | Fructose 10 wk | Fructose change | Glucose Baseline | Glucose 10 wk | Glucose change | P-value for effect of sugar2 | |
|---|---|---|---|---|---|---|---|
| Energy Expenditure (kcal/min) | |||||||
| 2 Resting | 1.19 ± 0.06 | 1.10 ± 0.04 | −0.09 ± 0.04* | 1.17 ± 0.07 | 1.15 ± 0.05 | −0.02 ± 0.04 | 0.108 |
| Postprandial | 1.41 ± 0.06 | 1.37 ± 0.05 | −0.05 ± 0.02 | 1.40 ± 0.06 | 1.36 ± 0.05 | −0.03 ± 0.03 | 0.445 |
Values are Means ± SEM. Fasting values are based on n=31 (fructose group n=16; glucose group n=15) and postprandial values are based on n=30 (fructose group n=15; glucose group n=15).
PROC MIXED 3-way model (sugar, gender, sugar, gender, (+) or (−) metabolic syndrome) adjusted for change in fat-free mass.
P < 0.05 for changes significantly different from zero.
Discussion
Net substrate oxidation rates
The results of acute and short-term studies of fructose ingestion, ranging from periods of 4 hours to 6 days, indicate that when fructose is consumed in large amounts ranging from 30–50% of total calories, net fat oxidation is decreased and net carbohydrate oxidation is increased (Chong et al 2007, Couchepin et al 2008, Markov et al 2000, Schwarz et al 1989, Schwarz et al 1992a, Schwarz et al 1992b, Tappy et al 1986). However, the majority of these studies only examined the effects of consuming a single meal containing fructose, and those that examined the effects of multiple days of fructose consumption did not do so under energy-balanced conditions, but rather during consumption of 25–50% excess calories. Importantly, our results demonstrate that the acute effects of fructose consumption persist when fructose is consumed over longer periods (10 wks), and in subjects consuming an energy-balanced diet, suggesting that fructose-induced changes of the regulation of key metabolic pathways involved in cellular energy utilization are sustained even in the absence of positive energy balance.
We have previously reported that sustained consumption of fructose-sweetened beverages increased hepatic DNL in these same subjects (Stanhope et al 2009). Schwarz et al reported strong correlations between changes of substrate oxidation rates (increased CHO-ox and decreased FAT-ox) and increases of DNL in subjects consuming 25% or 50% excess energy as carbohydrate (carbohydrate composition not specified) for 5 days (Schwarz et al 1995). Here we demonstrate that, under energy-balanced conditions, consuming 25% of energy from fructose leads to reduced net fat oxidation and increased net carbohydrate oxidation, in addition to previously reported increases of DNL (Stanhope et al 2009). This relationship was not observed with isocaloric glucose consumption suggesting that consumption of fructose, not carbohydrate in general (in this case monosaccharides), leads to these changes in the regulation of substrate oxidation and DNL; however recent evidence suggests that other factors such as differences in the amylose/amylopectin ratio of carbohydrate rich foods may also lead to similar changes (Isken et al 2010).
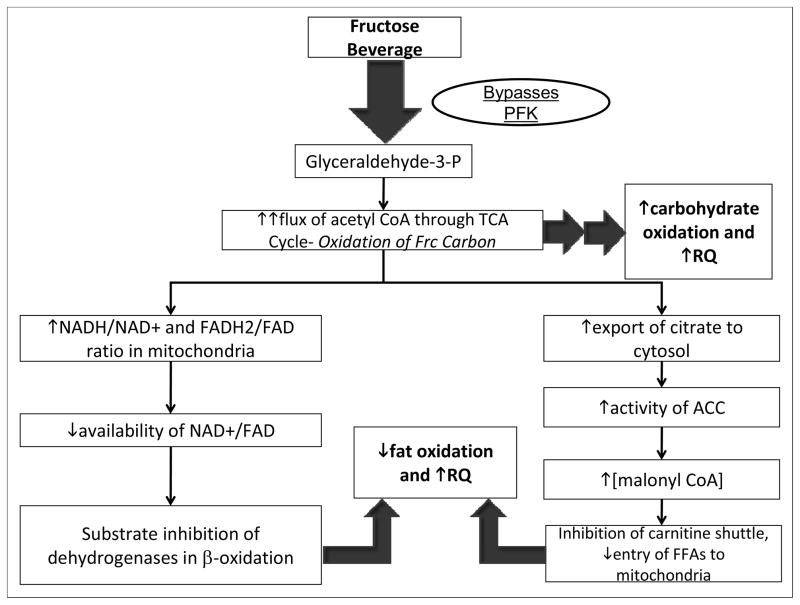
These findings support our hypothesis that decreases of fat oxidation occurring concurrently with fructose-induced upregulation of DNL promotes increases of hepatic lipid content, which may mediate the adverse changes in lipid metabolism and decreased insulin sensitivity we have reported previously (Stanhope et al 2009). These results are also consistent with the mechanism proposed by McGarry and others by which consumption of fructose leads to reductions of fat oxidation and increased carbohydrate oxidation (Figure 3) (Mayes 1993) (McGarry 1995). It must be emphasized that values for substrate utilization derived from indirect calorimetry represent rates of substrate disappearance that may not always equate with rates of substrate oxidation. Determination of actual rates of substrate oxidation would require additional studies using isotopic tracer methodology.
Figure 3.
Proposed mechanisms contributing to observed changes of substrate utilization in subjects consuming fructose-sweetened beverages. In the liver fructose is phosphorylated by fructokinase (which is not regulated by cellular energy status) and largely bypasses phosphofructokinase (PFK), the enzyme catalyzing the rate-limiting step of glycolysis (which is subject to inhibition by ATP and citrate). Ultimately fructose enters the glycolytic pathway as glyceraldehyde-3-phosphate. Following a high-fructose meal, an unregulated flux of fructose (Frc) carbon upregulates carbohydrate metabolism in the liver (increased CHO-Ox), leading to an increased flux of acetyl CoA through the tricarboxylic acid (TCA) cycle and a concomitant increase in cellular energy status (increased ATP/ADP ratio and NADH/NAD+ ratio). A high NADH/NAD+ ratio in the mitochondria results in substrate inhibition of isocitrate dehydrogenase (ICD) in the TCA cycle, leading to increased export of citrate to the cytosol, activation of acetyl-CoA carboxylase (ACC), and increased production of malonyl-CoA, the precursor to fatty acid synthesis (DNL). Elevated cytosolic concentrations of malonyl-CoA inhibit the carnitine shuttle via carnitine palmitoyl transferase (CPT), leading to reduced entry of fatty acids (FAs) into the mitochondria, decreased fat oxidation. The elevation of cellular energy status following a high-fructose meal would also lead to reduced mitochondrial availability of the fixed pool of oxidized cofactors NAD+ and FAD, which are required substrates for β-oxidation, also resulting in reduced fat oxidation (Locke et al 2008, Mayes 1993, McGarry 1995, Williamson and Cooper 1980).
Effects of metabolic syndrome
We observed that subjects with metabolic syndrome consuming fructose-sweetened beverages exhibited the largest decreases of postprandial fat oxidation rates and increases of carbohydrate oxidation rates (Table 2). This relationship was not evident in subjects consuming glucose. It should be noted that the number of subjects entering the study with metabolic syndrome was small, 5 in the fructose group and 4 in the glucose group. Additionally, as we have reported previously (Stanhope et al 2009), 10 weeks of fructose consumption promoted the development of risk factors for MetSyn, such as accumulation of intra-abdominal fat, dyslipidemia and insulin resistance. Hence, it is likely that we are observing a worsening of metabolic function in subjects consuming fructose that is further exacerbated in those who already had evidence of MetSyn prior to the intervention.
Effects of gender
We also observed that men consuming both glucose- and fructose-sweetened beverages exhibited greater increases of postprandial carbohydrate oxidation than women. These findings support those of Couchepin et al. who reported a significant increase of carbohydrate oxidation in male, but not female subjects consuming fructose (Couchepin et al 2008). Together these findings suggest that there is a gender-specific response with respect to changes of substrate utilization following sustained consumption of fructose.
Energy expenditure
The decrease of REE that we observed in subjects consuming fructose was unexpected and conflicts with the findings of several previous short-term studies that reported increased energy expenditure following administration of oral fructose as compared with consumption of glucose (Schwarz et al 1989, Schwarz et al 1992a, Schwarz et al 1992b, Tappy et al 1986). These changes suggest that sustained fructose consumption may contribute to an overall reduction in energy expenditure, which could increase the risk for weight gain if energy intake is not adjusted downward accordingly. For example, if the mean measured decrease of REE associated with 10 weeks of fructose consumption, 0.09 kcal/min, was maintained for one year it could total ~15,000 kcals, assuming that REE reflects metabolism during rest/sleep periods adding to about 8 h/d; potentially, a gain of ~1.6 kg of body fat could result. Additional studies examining the effects of chronic sugar consumption on 24-hour energy expenditure conducted in a whole-room calorimeter are needed to confirm these findings and determine if the observed reductions in metabolic rate are directly related to fructose or to sweetener (i.e. sucrose, high fructose corn syrup, etc.) consumption in general. We are currently performing such measurements.
Conclusions
Consumption of fructose at 25% of energy requirements for 10 weeks, when compared with isocaloric consumption of glucose, leads to significant reductions of net postprandial fat oxidation and increases of net postprandial carbohydrate oxidation. Furthermore, the results of this study demonstrate that these changes are evident even when fructose is consumed under energy-balanced conditions. We also report that resting energy expenditure is reduced compared to baseline values in subjects consuming fructose-sweetened beverages for 10 weeks. These findings may thus have important implications with regard to long-term energy balance in individuals consistently consuming large amounts of dietary fructose.
Acknowledgments
The authors thank Marinelle Nuñez, Brandi Bair, Rebecca Stewart, Sara Wuehler, Barbara Gale, Artem Dyachenko and Patrick Lam for their excellent technical support and Nicole Mullen and the nursing staff at CCRC for their dedicated nursing support. We also thank Janet Peerson for expert advice on the statistical analysis of the data.
Sources of funding:
This research was supported with funding from NIH grant R01HL-075675. The project also received support from the UC Davis Clinical and Translational Science Center (grant UL1 RR024146). Dr. Havel’s laboratory also receives support from NIH grants HL-091333, AT-002599, AT-002993, and AT-003545 and the American Diabetes Association. N.L. Keim’s research is supported by intramural USDA-ARS CRIS 5306-51530-019-00D.
Footnotes
Conflict of interest
None of the authors had any financial or personal conflicts of interest.
References
- Bezerra RM, Ueno M, Silva MS, Tavares DQ, Carvalho CR, Saad MJ. A high fructose diet affects the early steps of insulin action in muscle and liver of rats. J Nutr. 2000;130:1531–1535. doi: 10.1093/jn/130.6.1531. [DOI] [PubMed] [Google Scholar]
- Bingham SA. Urine nitrogen as a biomarker for the validation of dietary protein intake. J Nutr. 2003;133(Suppl 3):921S–924S. doi: 10.1093/jn/133.3.921S. [DOI] [PubMed] [Google Scholar]
- Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr. 2007;85:1511–1520. doi: 10.1093/ajcn/85.6.1511. [DOI] [PubMed] [Google Scholar]
- Couchepin C, Le KA, Bortolotti M, da Encarnacao JA, Oboni JB, Tran C, et al. Markedly blunted metabolic effects of fructose in healthy young female subjects compared with male subjects. Diabetes Care. 2008;31:1254–1256. doi: 10.2337/dc07-2001. [DOI] [PubMed] [Google Scholar]
- Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76:911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63:133–157. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- Isken F, Klaus S, Petzke KJ, Loddenkemper C, Pfeiffer AF, Weickert MO. Impairment of fat oxidation under high- vs. low-glycemic index diet occurs before the development of an obese phenotype. Am J Physiol Endocrinol Metab. 2010;298:E287–295. doi: 10.1152/ajpendo.00515.2009. [DOI] [PubMed] [Google Scholar]
- Locke GA, Cheng D, Witmer MR, Tamura JK, Haque T, Carney RF, et al. Differential activation of recombinant human acetyl-CoA carboxylases 1 and 2 by citrate. Arch Biochem Biophys. 2008;475:72–79. doi: 10.1016/j.abb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Markov AK, Neely WA, Didlake RH, Terry J, 3rd, Causey A, Lehan PH. Metabolic responses to fructose-1,6-diphosphate in healthy subjects. Metabolism. 2000;49:698–703. doi: 10.1053/meta.2000.6249. [DOI] [PubMed] [Google Scholar]
- Martinez FJ, Rizza RA, Romero JC. High-fructose feeding elicits insulin resistance, hyperinsulinism, and hypertension in normal mongrel dogs. Hypertension. 1994;23:456–463. doi: 10.1161/01.hyp.23.4.456. [DOI] [PubMed] [Google Scholar]
- Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. 1993;58:754S–765S. doi: 10.1093/ajcn/58.5.754S. [DOI] [PubMed] [Google Scholar]
- McGarry JD. Malonyl-CoA and carnitine palmitoyltransferase I: an expanding partnership. Biochem Soc Trans. 1995;23:481–485. doi: 10.1042/bst0230481. [DOI] [PubMed] [Google Scholar]
- Okazaki M, Zhang H, Yoshida Y, Ichino K, Nakayama S, Oguchi K. Correlation between plasma fibrinogen and serum lipids in rats with hyperlipidemia induced by cholesterol free-high fructose or high cholesterol diet. J Nutr Sci Vitaminol (Tokyo) 1994;40:479–489. doi: 10.3177/jnsv.40.479. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Schutz Y, Froidevaux F, Acheson KJ, Jeanpretre N, Schneider H, et al. Thermogenesis in men and women induced by fructose vs glucose added to a meal. Am J Clin Nutr. 1989;49:667–674. doi: 10.1093/ajcn/49.4.667. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Acheson KJ, Tappy L, Piolino V, Muller MJ, Felber JP, et al. Thermogenesis and fructose metabolism in humans. Am J Physiol. 1992a;262:E591–598. doi: 10.1152/ajpendo.1992.262.5.E591. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Schutz Y, Piolino V, Schneider H, Felber JP, Jequier E. Thermogenesis in obese women: effect of fructose vs. glucose added to a meal. Am J Physiol. 1992b;262:E394–401. doi: 10.1152/ajpendo.1992.262.4.E394. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Neese RA, Turner S, Dare D, Hellerstein MK. Short-term alterations in carbohydrate energy intake in humans. Striking effects on hepatic glucose production, de novo lipogenesis, lipolysis, and whole-body fuel selection. J Clin Invest. 1995;96:2735–2743. doi: 10.1172/JCI118342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storlien LH, Oakes ND, Pan DA, Kusunoki M, Jenkins AB. Syndromes of insulin resistance in the rat. Inducement by diet and amelioration with benfluorex. Diabetes. 1993;42:457–462. doi: 10.2337/diab.42.3.457. [DOI] [PubMed] [Google Scholar]
- Tappy L, Randin JP, Felber JP, Chiolero R, Simonson DC, Jequier E, et al. Comparison of thermogenic effect of fructose and glucose in normal humans. Am J Physiol. 1986;250:E718–724. doi: 10.1152/ajpendo.1986.250.6.E718. [DOI] [PubMed] [Google Scholar]
- Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. 1949. Nutrition. 1990;6:213–221. [PubMed] [Google Scholar]
- Williamson JR, Cooper RH. Regulation of the citric acid cycle in mammalian systems. FEBS Lett. 1980;117(Suppl):K73–85. doi: 10.1016/0014-5793(80)80572-2. [DOI] [PubMed] [Google Scholar]