Abstract
In healthy children, there is a paucity of information on the growth of the brainstem and thalamus measured by anatomical magnetic resonance imaging. The relationships of age, gender, and age by gender with brainstem and thalamus volumes were analyzed from magnetic resonance brain images of 122 healthy children and adolescents (62 males, 60 females; ages four to seventeen). Results showed that age is a significant predictor of brainstem and thalamus volumes. The volume of the brainstem increases with age, while thalamus volume declines with age. The volumes of right thalami are significantly larger than that of left in both genders with greater rightward asymmetry and greater thalamus/grey matter ratio in females. Males have larger brainstems, but these differences are not significant when covarying for cerebral volumes. Larger thalami were associated with higher verbal IQ. This normative pediatric data is of value to researchers who study these regions in neurodevelopmental disorders.
Keywords: brainstem, thalamus, neurodevelopment
Introduction
The brainstem, which includes the medulla oblongata, midbrain, and pons, shows a characteristic pattern on anatomical magnetic resonance imaging as a white matter structure in children age 4 years and older 1. Magnetic resonance imaging studies of infants and young children up to 5 years postconception demonstrate that the postnatal brainstem, which is derived from the mesencephalon, develops in a well-defined order consisting of 5 distinct maturational stages characterized by myelination and distinct appearances of grey matter nuclei2. The brainstem contains longitudinal white matter ascending and descending tracts, commissural fibres and the middle cerebellar peduncles. Important areas of grey matter include: cranial nerves III to XII; the raphe nuclei; locus coeruleus; reticular formation; and the pontine nuclei of the basis pontis. These nuclei and their subdivisions mediate and control arousal, the sleep cycle, heart rate, breathing, gross axial, limb, head and eye movement, visual, somesthetic, gustatory, and acoustic perception and sound production such as crying (for review see 3). The raphe nuclei are the origin of the brain’s major serotonergic projections, while the locus coeruleus is the origin of the brain’s major noradrenergic projections 4. Neurochemical imbalances between these neurotransmitters can result in psychiatric illness such as depression and posttraumatic stress disorder 5–6. Smaller midsagittal area of the pons is associated with Velocardiofacial Syndrome, a disorder characterized by velopharyngal insufficiency, cardiac abnormalities, and developmental disabilities; these patients are at greater risk for psychosis and schizophrenia 7.
The thalamus is a highly differentiated gray matter structure, comprising many cytoarchitectonically distinct subnuclei, each with different anatomical and specialized functional connections to different cortical, subcortical, and cerebellar sites 8. The thalamus has been characterized as a dynamic conduit linking subcortical with cortical areas. The human thalamus is derived from the diencephalon, and progressive differentiation of its nuclear groups into distinct nuclei is seen in infancy 9. The thalamus is involved in most physiological processes and is affected by many neurological and psychiatric disorders. The function of the thalamus is the integration of sensory input, cortical arousal, memory and language functions 10. Smaller thalami are seen in childhood onset schizophrenia 11 and high-functioning individuals with autism 12.
However, the relationships of age, gender, and IQ with brainstem and thalamus volumes during healthy child development as measured with anatomical magnetic resonance imaging are not well studied. The present study investigates the effects of age, gender, and age by gender with volumes of the brainstem and thalamus, in a large healthy pediatric population of age range four to seventeen years old. The relationship of IQ with these brain structures was also investigated.
Material and method
Subjects
Sixty-two male and 60 female healthy children and adolescents (age range: 4.2 to 17 years) were recruited. The Schedule for Affective Disorders and Schizophrenia for School Aged Children Present and Lifetime Version, which includes a comprehensive posttraumatic stress disorder interview 13, ruled out the presence of DSM-IV Axis I mental disorders or major traumatic life events. Socioeconomic status for each subject was completed using the Hollingshead Four Factor Index 14. An abbreviated version of the Wechsler Intelligence Scale for Children (i.e., Vocabulary, Digit Span, Block Design, and Object Assembly) provided a verbal and performance estimate of Full Scale IQ 15. Handedness was determined using the 12 handedness items from the Revised Physical and Neurological Examination for Subtle Signs Inventory 16 where 8 out of 12 items were defined as right handed. There were no significant gender group differences on age, race, Tanner Stage, socioeconomic status, handedness, child behavioral checklist scores, and Full Scale IQ. Males were significantly taller than females. The majority of subjects were above average on Full Scale IQ (median IQ: 116).
Exclusion criteria were: 1) current or lifetime history of disorders including psychiatric disorders, alcohol, and substance use disorders, 2) significant medical or neurological illness, or history of head injury or loss of consciousness, 3) a history of prenatal confounds that may influence brain maturation such as significant prenatal exposure or birth complications, 4) severe obesity or growth failure, 5) full scale IQ lower than 80, and 6) positive trauma or child maltreatment history. This study was approved by the University Institutional Review Board. Written informed consent and child assent were obtained. Subjects received monetary compensation for participating.
Magnetic Resonance Imaging
Magnetic resonance imaging was performed using GE 1.5 Tesla Unit (Signa System, General Electric Medical Systems, Milwaukee, WI) running version 5.4 software located at the University Magnetic Resonance Research Center. The subject’s head was aligned in a head holder with foam padding and soft towels and chin and forehead straps to minimize head movement. The subject’s nose was positioned at ‘12:00’ for alignment, and a gradient-echo localizing axial slice verified this plane. A sagittal series (echo time = 18 msec, repetition time = 400 msec, flip angle = 90 degrees, acquisition matrix = 256 × 192, number of excitations = 1, field of view = 20 cm, slices = 21) verified patient position, cooperation, and image quality. The midsagittal slice was required to show full visualization of the cerebral aqueduct and the anterior commissure and posterior commissure, in which a line was estimated requiring the anterior and posterior commissure line to be within 3 degrees of 180. If these criteria were not met, the subject was realigned until these criteria were met. Coronal sections were then obtained perpendicular to the anterior and posterior commissure line to provide a more reproducible guide for image orientation. A 3-dimensional spoiled-gradient-recalled acquisition in the steady-state pulse sequence was used to obtain 124 contiguous images with slice thickness of 1.5 mm in the coronal plane (echo time = 5 msec, repetition time = 25 msec, flip angle = 40 degrees, acquisition matrix = 256 × 192, number of excitations = 1, field of view = 24 cm). Axial proton density- and T2-weighted images were obtained to enable exclusion of structural abnormalities on magnetic resonance images. A neuroradiologist reviewed all scans and ruled out clinically significant abnormalities. No sedation was used. Further details of scanning procedure have been described previously 17.
Image Analysis
The imaging data from the coronal sections were transferred from the magnetic resonance scan unit to a computer workstation (Power Macintosh, Apple Computer) and analyzed using IMAGE software (version 1.61) developed at the National Institutes of Health that provides valid and reliable volume measurements of specific structures using a manually operated (hand tracing) approach. Trained and reliable raters who were blind to subject information made all measurements. These methods were described previously by our group 17–18 and methods of measuring volumes of thalamus and brainstem are briefly presented here.
The first slice of the brainstem was measured where the pons first appeared within the suprasellar cistern. The cerebellar peduncles, including the brachium pontis (middle), were included in the brainstem. In the anterior plane, the superior limit of the brainstem was a straight line connecting the ambient cisterns from left to right. Posterior measurements included the cerebral aqueduct and the superior colliculus. Because our data acquisition was in the coronal plane, we were unable to distinguish the tegmentum and basis pontis of the pons in volumetric measures. Thus no separate volumetric measurements were made for the midbrain, pons, and medulla oblongata. Every coronal slice that included the brainstem as described above was manually traced and total volume was computed by summing up successive areas and multiplying by slice thickness. Thus, the volumes of the brainstem were calculated by summing up areas of successive coronal slices after tracing the region of interest and excluding cerebral spinal fluild as previously described 19. Further details including figures describing these structural measures can be found in 18.
The boundaries of the thalamus were described previously 20. Briefly, the mammillary bodies and the interventricular foramen defined the anterior boundary; the internal capsule defined the lateral boundary; the third ventricle defined the medial boundary; the hypothalamus defined the inferior boundary; the lateral ventricle defined the superior boundary; and the crus fornix defined the posterior boundary of the thalamus. Every coronal slice that included the thalamus and excluded cerebral spinal fluild was manually traced and total volume was computed by summing up successive areas and multiplying by slice thickness.
Intraclass correlation for independent designation of regions on segmented images obtained from 20 subjects were 0.99 for interrater and 0.99 for intrarater reliability for cerebral volume, total cerebral gray matter volume, and total cerebral white matter volume respectively; and were 0.96 and 0.98, for right, left, and total thalamus volumes respectively; and 0.89 and 0.94, for brainstem respectively.
Statistical analysis
Shapiro-Wilk W Test was used to test goodness of fit. All magnetic resonance imaging volume data fit the assumptions of normal goodness of fit. An analysis of covariance was performed to test if the volume of the brainstem differs between gender, age, and their interaction. The ratio of brainstem to white matter was also used as a response variable in the second analysis of covariance to determine if age, gender, and their interaction are significant predictors for the ratio. To adjust for known gender differences in cerebral size, we include cerebral volume as additional covariates into the models to examine if gender and age are still significant predictors.
In a similar manner, an analysis of covariance was performed to test if volume of thalamus differs between gender, age, and their interaction. Due to potential asymmetry, separate analysis of covariance was performed for left thalamus and right thalamus. The ratio of thalamus to grey matter was used to estimate the thalamus volume relative to cerebral gray matter volumes as a whole. In the second analysis of covariance, the response variable is the ratio of thalamus/grey matter while all the predictors remain the same, i.e., gender, age, and their interaction. We include cerebral volume as additional covariates into the models.
A paired t-test was performed to test asymmetry of right and left thalamus volume, quantified as the volume difference between right and left thalamus. Furthermore, a one-way analysis of variance was performed to test if there was any asymmetry due to gender differences.
Spearman correlations were used because of the non-normal distribution of IQ measures. All significance testing was two-tailed with alpha = 0.05 (P≤.1 constituted a trend). All data are presented as mean ± standard deviation unless otherwise specified.
Results
Brainstem
Results show both age (F1, 118 = 25.55, p <10−4) and gender (F1,118 = 10.05, p = 0.002) are significant predictors for the volume of the brainstem, but not the gender-by-age interaction (F1,118 = 0.05, p = 0.82). The brainstem volume increases linearly with age and males have larger brainstems than females (mean males, 24.55± 3.2 cm3, mean females, 22.59 ±3.2 cm3; Table 1 and Figure 1).
Table 1.
Means, Standard Deviations (SD) and Linear Regression of Magnetic Resonance Imaging Measured Brainstem and Thalamus Volumes of Healthy Male (n=62) and Female (n=60) Children and Adolescents.
| MRI Structural Volumes (cm3) | Males Means ±SD |
Females Means ±SD |
Group (Male>female) | Age | Group × Age |
|---|---|---|---|---|---|
| Brainstem (range) | 24.55±3.2 (16.6 – 31.9) | 22.59±3.2 (13.6 – 29.8) | F1, 118= 10.0A P=. 002 |
F1, 118= 25.0A P<. 0001 |
F1, 118= .05A P=. 82 |
| Brainstem/white matter Ratio (range) | 0.051±0.008 (0.036 – 0.067) | 0.056±0.009 (0.037 – 0.084) | F1, 118= 8.5A P=. 004 |
F1, 118= .83A P=. 36 |
F1, 118= 1.2A P=. 28 |
| Thalamus (range) | 7.59±1.83 (3.54 – 12.32) | 7.64±1.88 (3.32 – 11.27) | F1, 118= .01A P=. 91 |
F1, 118= 7.2A P=. 008 |
F1, 118= 1.4A P=. 24 |
| Right Thalamus (range) | 3.82±0.91 (1.87 – 6.06) | 3.99±1.0 (1.65 – 6.0) | F1, 118= .56A P=. 46 |
F1, 118= 8.1A P=. 005 |
F1, 118= 1.7A P=. 2 |
| Left Thalamus (range) | 3.77±0.96 (1.67 – 6.59) | 3.64±. 96 (1.56 – 5.75) | F1, 118= .91A P=. 34 |
F1, 118= 5.4A P=. 02 |
F1, 118= .93A P=. 34 |
| Thalamus/grey matter Ratio (range) | 0.009±0.002 (0.005 – 0.013) | 0.01±0.002 (0.005 – 0.017) | F1, 118= 8.4A P=. 004 |
F1, 118= 4.8A P=. 03 |
F1, 118= .01A P=. 92 |
F value for linear regression model adjusting for gender, age and gender by age interaction.
Figure 1.
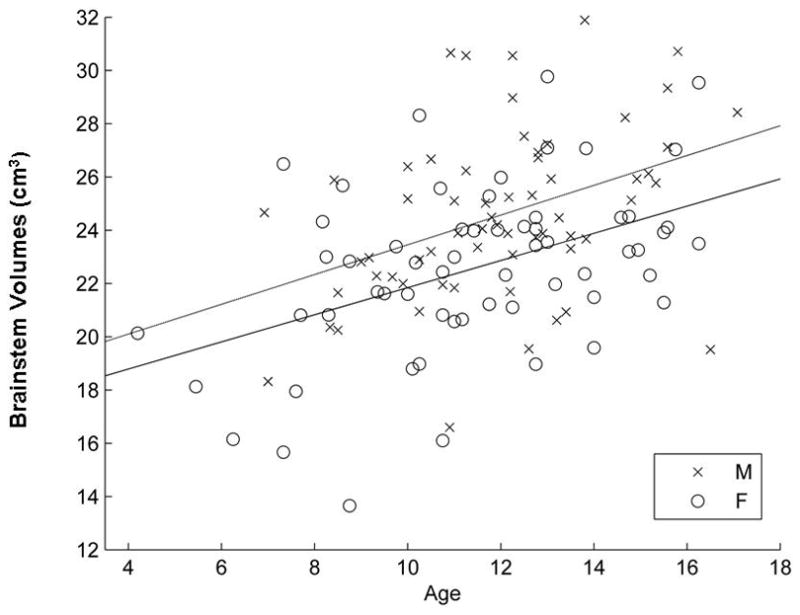
Magnetic resonance imaging volumes of brainstem in males and females plotted against age. Both gender and age are significant predictors of brainstem volumes. The volume of the pediatric male brainstem is significantly larger than that of females (F1, 118 = 10.05, p = 0.002), and the volumes of brainstem increase significantly with age (F1, 118 = 25.55, p < 10−4). The interaction between age and gender is not significant (F1, 118 = 0.05, p = 0.82).
When including cerebral volume as an additional covariate into the models, age is still significant (F1,117 =23.1, p < 0.0001, respectively) for prediction of brainstem volumes. However, the gender effect is no longer significant after cerebral volume (F1,117=0.01, p = 0.92) was included in the model.
Gender is a significant predictor for the brainstem/white matter ratio (F1,118 = 8.5, p = 0.004) but age is not (F1, 118 = 0.83, p = 0.36). Healthy girls have greater brainstem/white matter ratio than healthy boys (See Table 1). Our final model for the brainstem/white matter ratio includes the sole significant predictor, gender (F1, 120 = 9.1, p = 0.003). There was no significant gender-by-age interaction on the volume of brainstem or brainstem/white matter ratio (Table 1).
Thalamus
Age is the sole significant predictor for the thalamus volume (F1,120 = 7.2, p = 0.008) when including age, gender, and gender-by-age interaction in the model (Table 1). We then include the sole significant predictor, age, in the final model (Figure 2). This age related volume decrease is observed bilaterally in both genders (F1, 120 = 6.29, p = 0.014).
Figure 2.
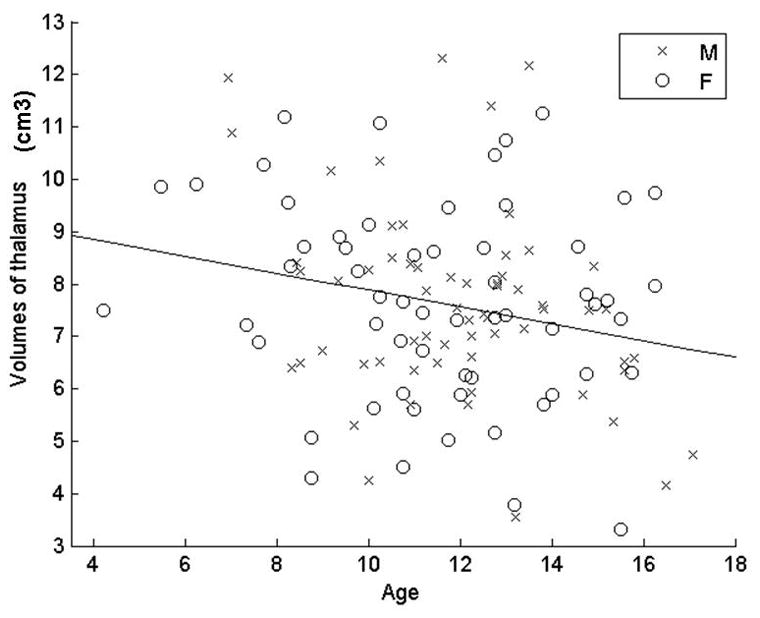
Volumes of Thalami of males and females plotted against age. Our preliminary analysis showed that the volume of thalamus does not differ between genders, and that there are also no significant interactions between gender and age (Table 1). Therefore, we only include one predictor, age, in our final model here. The volume of thalamus decreases significantly with age (F1, 120 = 6.29, p = 0.014).
After adjusting for cerebral volume, there are trends for volume decrease with age for total thalamus, right thalamus, and left thalamus; however, the gender difference becomes marginally significant.
Both age (F1,118 = 4.8, p = 0.03) and gender (F1,118 = 8.4, p = 0.004) are significant predictors for the ratio of thalamus/grey matter, but their interaction is not (F1,118 = 0.01, p = 0.92). Girls have greater ratio of thalamus/grey matter than boys.
The paired t-test shows that the right thalamus volume is significantly larger than that of left thalamus (t = 4.36, p = 2.78 × 10−5). The results of the analysis of covariance further show that females have significantly greater rightward thalamus asymmetry than males (F1, 120 = 11.81, p = 0008; Figure 3).
Figure 3.
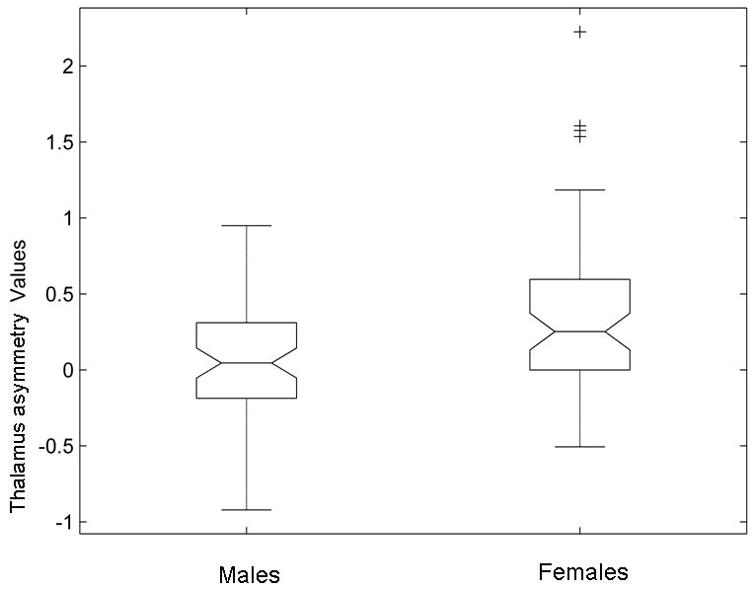
Gender effect on the thalamus asymmetry. Thalamus asymmetry is significantly greater in females than males (F1, 120 = 11.81, p = 0.0008).
The relationship between brainstem, thalamus, and IQ
We found that the verbal IQ (but not Full scale or performance IQ) was positively correlated with total thalamus volume adjusted for cerebral volume to control for gender and age (rs=.195, p=.03). No relationships were seen for Full scale, performance IQ or verbal IQ and brainstem volume.
Discussion
To our knowledge, this is the first volumetric study on the brainstem and thalamus in a large healthy pediatric sample. The study shows that the volume of the brainstem, a subcortical structure, increases with age in boys and girls. This age related change is consistent with the findings of age related increase of volumes of cerebral white matter structures in a previous publication of this sample 17 and other cross sectional and longitudinal studies of cortical white matter 21–24 which did not report data on brainstem or thalamus structures. The volume increase of brainstem in children and adolescents may reflect the structural change from ongoing myelination, increase of axonal size, glial proliferation or a combination of these that support healthy pediatric neurodevelopment. Most studies in adult populations showed that volumes of the brainstem are stable across the adult age span (20–85 years) in both males and females 25. One study showed the volumes of the brainstem decrease after 40 years in males and 50 years in females 26. Combining adult with pediatric data suggests that brainstem volume may not fully mature until young adulthood.
However, certain neurodevelopmental disorders such as Velocardiofacial Syndrome is characterized by smaller midsagitial areas of the pons and vermal lobules VI–VII. 7 Velocardiofacial Syndrome is associated with a 3-Mb deletion on 22q11.2, and includes a Goosecoid-like (GSCL) gene that is highly expressed during early embryogenesis and in brain tissue (e.g., pons and dorsal thalamus) that is probably necessary for normal brain development.27 Smaller brainstem tissue was also seen in a small sample study of 14 young adult patients with William’s syndrome, a genetic disorder resulting from a contiguous deletion on the long arm of chromosome 7 28. William’s syndrome is characterized by uneven neuropsychiatric development, mild to moderate mental retardation, and cardiac or vascular malformations 29–30. Thus, the trajectory of brainstem development may begin early on. Further study focused on child, adolescent, and early adult populations will help to delineate the transitional point of the development of the brainstem in typical development and in patients with developmental disorders.
We found gender differences in the volume of brainstem, specifically that males have smaller brainstem/white matter ratio than females despite the fact that the raw volumes of the brainstem in males are larger than females. This finding adds to the data from previously published data (which did not report measures of brainstem or thalamus) from this same sample showing the rate of cortical white matter increases significantly more in boys than that of girls 17. The brainstem was not associated with verbal, performance or Full Scale IQ in our study.
The study indicates that in this pediatric sample, age and verbal IQ are significant predictors of thalamic volume. The thalamus is derived from the diencephalon, an embryonic central nervous system structure that gives rise to the hypothalamus, subthalamus, epithalamus, retinae optic nerves and tracts in addition to the thalamus proper. Similar to the cerebral cortex, the number of neurons in the mediodorsal nucleus of the thalamus decreases with age; while mediodorsal nucleus glial cell numbers increase from infancy to adulthood 31. The volume of the thalamus, which is measured as a gray matter structure on a 1.5 Tesla magnetic resonance imaging scan, decreases with age in the pediatric sample reported here. This is consistent with a previous study in a smaller sample size of subjects aged 7 to 16 years 32. Research in adults also suggests that the thalamic volumes decrease linearly with age 25. The results of the current study suggest that the volume of the thalamus reaches its maximum in young childhood (before the age of 4–6 years). This finding is consistent with the evidence suggesting that the overall volume of grey matter in children undergoes a general net decrease with most of the neuronal proliferation and selective cell death taking place in utero 33. The age related volumetric decrease of the thalamus in pediatric population follows the pattern of other basal ganglia structures such as caudate, putamen, and globus pallidus 24, 34–36, but is opposite to the changes of other temporal lobe structures such as amygdala and hippocampus 37.
Functionally, the thalamus is associated with complex functions of basal ganglia, sensory integration, cortical arousal, memory and language. Cortico-thalamic networks process syntactic and semantic language violations 38. Some but not all studies have found a decrease in the mediodorsal nucleus of the thalamus of schizophrenic patients 39 while an increase in cell number was seen in adults with depression 40. Accordingly we found a significant and positive relationship between verbal IQ and thalamus volume in children and adolescents. In another study of adolescents and young adults, thalamic gray matter positively correlated with Full-Scale IQ 41. Our finding may suggest that cortical connectivity patterns in the thalamus involving language mature prior to those connections involving prefrontal executive functions.
Significant rightward asymmetry in thalamus volume is observed in this study. This rightward asymmetry has been noted in a relatively small sample of boys age 7–14 years 42. It was also observed in adult populations by two groups 25, 43 but a leftward asymmetry was seen in one adult study where larger left nucleus accumbens, putamen and thalamus were measured with spatial normalization to stereotaxic space 44. This latter pilot study of n=30 adults was undertaken to produce a probabilistic atlas of the adult human basal ganglia and thalamus and did not report gender differences in thalamus asymmetry 44. Clinical studies revealed interesting findings in thalamus asymmetry. One study showed that the rightward asymmetry is decreased in Tourette’s syndrome secondary to the increase of left thalamus 42. Another study shows an exaggerated rightward asymmetry in schizophrenia patients 43. Treatment-naive patients with obsessive compulsive disorder have significantly greater thalamus than that of controls and the increase of thalamus seemed to be prominent on the right side 45.
A more prominent rightward asymmetry of thalamus in girls than boys was detected. Significantly larger right thalamus in females seems to attribute to this gender difference. So far, very few studies have evaluated the gender effect on the thalamus asymmetry. Our result indicates thalamus/grey matter ratio is greater in females than males. Females also have larger total thalamus and right thalamus than males when we control for cerebral volume. The gender effect is consistent with the finding of Sowell’s group 32, which showed greater thalamus size in girls than boys after total brain volume was co-varied. One adult study showed the asymmetry of thalamus is greater in males than in females 46. Besides the age difference of our data here and the later study, other factors such as study method (volumetric measurement in our study vs. 3D texture analysis) and study focuses (on specific anatomical structure in our study vs. global asymmetry of hemispheres), could explain the discrepancy in the results. Differences in hemispheric morphology have been suggested to originate from a complex interplay between evolutionary, hereditary, developmental, experimental, and pathological factors 47. Studies have revealed anatomic findings that correspond with functional lateralization 48. The meaning of thalamus asymmetry is not well understood and the observed patterns of thalamus asymmetry in this understudied literature have not been consistent. However, these data show a more prominent rightward asymmetry of thalamus in females versus males at a relatively young age and in a large sample.
Clinically, it is interesting to see that males have less rightward asymmetry but have higher risk for Tourette’s syndrome, which seems to mainly affect the left thalamus, while females have greater rightward asymmetry but have higher risk for obsessive compulsive disorder, which seems to mainly affect the right thalamus. No differences in volumes of the thalamus were seen in pediatric bipolar disorder, an Axis I mental disorder that is equally common in males and females 49. Taken together, these results indicate that maturation of thalamus rightward asymmetry might differ in a variety of neuro-psychiatric illnesses and influence the known gender bias for these illnesses.
This study has several limitations. It is a cross sectional study, and so has inherent limitations when addressing maturation, a reorganization or a change in function to more complexity in the same organism. Future longitudinal studies using different types of anatomical and functional imaging modes are needed to confirm the developmental patterns of these brain structures. We acquired the data using coronal volumes rather than axial orientations which limited our ability to further divide the brainstem volumes. Our results are also limited by sampling highly functional individuals with above average IQs, which will potentially affect the generalizability of these results. However, this study is one of the few studies focused on developmental patterns in the brainstem and thalamus during human development and adds important healthy developmental data to the literature that will be of value to those who study developmental and neuropsychiatric disorders.
Acknowledgments
Financial disclosure/funding
This work was supported by the National Institute of Health: K24MH71434, K24 DA028773, and K08 MH01324 (PI: De Bellis) and National Alliance for Research on Schizophrenia and Depression Young Investigator Awards-(PI: De Bellis)
Footnotes
Conflict of Interest: All authors do not have any commercial, financial, or other associations that could pose a conflict of interest in connection with this submitted article.
Ethical Approval
The study was approved by the University Medical Center Institutional Review Board.
Author contributions
Dr. Xie wrote the original draft of the manuscript.
Dr. Chen ran the statistical analyses and made the figures with Dr. Xie.
Dr. De Bellis funded the study, supervised all data collection, revised the manuscripts and acted overall as a mentor to Dr. Xie. Dr. De Bellis contributed equally or more to this work.
References
- 1.Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nerous system myelination in human infancy 2 Patterns of myelination in autopsied infants. Journal of Neuropathology & Experimental Neurology. 1988;47:217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Martin E, Krassnitzer S, Kaelin P, Boesch CH. MR imaging of the brainstem: normal postnatal development. Neuroradiology. 1990;33:391–395. doi: 10.1007/BF00598609. [DOI] [PubMed] [Google Scholar]
- 3.Joseph R. Fetal Brain Behavior and Cognitive Development. Developmental Review. 2000;20:81–98. [Google Scholar]
- 4.Emson PC, Lindvall O. Distribution of putative neurotransmitters in the cortex. Neuroscience. 1979;4:1–30. doi: 10.1016/0306-4522(79)90215-x. [DOI] [PubMed] [Google Scholar]
- 5.Garlow SJ, Musselman DL, Nemeroff CB. The neurochemistry of mood disorders: clinical studies. In: Charney DS, Nestler EJ, Bunney BS, editors. Neurobiology of Mental Illness. New York NY: Oxford University Press; 1999. pp. 348–364. [Google Scholar]
- 6.De Bellis MD. Developmental traumatology: the psychobiological development of maltreated children and its implications for research, treatment, and policy. Development & Psychopathology. 2001;13(3):539–564. doi: 10.1017/s0954579401003078. [DOI] [PubMed] [Google Scholar]
- 7.Eliez S, Schmitt JE, White CD, Wellis VG, Reiss AL. A quantitative MRI study of posterior fossa development in velocardiofacial syndrome. Biological Psychiatry. 2001;49:540–546. doi: 10.1016/s0006-3223(00)01005-2. [DOI] [PubMed] [Google Scholar]
- 8.Morel A, Magnin M, Jeanmonod D. Multiarchitectonic and stereotactic atlas of the human thalamus. Journal of Comparative Neurology. 1997;387:588–630. doi: 10.1002/(sici)1096-9861(19971103)387:4<588::aid-cne8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Dekaban A. Human Thalamus: An anatomical, developmental, and pathological study II Development of the human thalmic nuclei. The Journal of Comparative Neurology. 1954;100(1):63–97. doi: 10.1002/cne.901000105. [DOI] [PubMed] [Google Scholar]
- 10.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 11.Rapoport JL, Giedd JN, Kumra S, et al. Childhood-onset schizophenia Progressive ventricular change during adolescence. Archives of General Psychiatry. 1997;54:897–903. doi: 10.1001/archpsyc.1997.01830220013002. [DOI] [PubMed] [Google Scholar]
- 12.Tsatsanis KD, Rourke BP, Klin A, Volkmar FR, Cicchetti D, Schultz RT. Reduced thalamic volume in high-functioning individuals with autism. Biological Psychiatry. 2003;53:121–129. doi: 10.1016/s0006-3223(02)01530-5. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective Disorders and Schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Hollingshead A. Four Factor index of social status. Department of Sociology, Yale University; New Haven, connecticus: 1975. [Google Scholar]
- 15.Wechsler D. Manual for the Wechsler Intelligence Scale for Children-Revised. New York: The Psychological corp; 1974. [Google Scholar]
- 16.Denckla M. Revised physical and neurological exam for subtle signs. Psychopharmacol Bull. 1985;21:773–800. [PubMed] [Google Scholar]
- 17.De Bellis MD, Keshavan MS, Beers SR, et al. Sex differences in brain maturation during childhood and adolescence. Cerebral Cortex. 2001 Jun;11(6):552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- 18.De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcoholism: Clinical & Experimental Research. 2005 Sep;29(9):1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- 19.Hardan AY, Minshew NJ, Harenski K, Keshavan MS. Posterior Fossa Magnetic Resonance Imaging in Autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(6):666–672. doi: 10.1097/00004583-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS. Thalamic volumes in patients with first-episode schizophrenia. American Journal of Psychiatry. 2001 Apr;158(4):618–624. doi: 10.1176/appi.ajp.158.4.618. [DOI] [PubMed] [Google Scholar]
- 21.Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children A volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- 22.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 23.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999 Oct;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 24.Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan EV, Rosenbloom M, Serventi KL, Pfefferbaum A. Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiology of Aging. 2004;25:185–192. doi: 10.1016/s0197-4580(03)00044-7. [DOI] [PubMed] [Google Scholar]
- 26.Oguro H, Okada K, Yamaguchi S, Kobayashi S. Sex differences in morphology of the brain stem and cerebellum with normal ageing. Neuroradiology. 1998 Dec;40(12):788–792. doi: 10.1007/s002340050685. [DOI] [PubMed] [Google Scholar]
- 27.Gottlieb S, Hanes SD, Golden JA, Oakey RJ, Budarf ML. Goosecoid-like, a gene deleted in DiGeorge and velocardiofacial syndromes, recognizes DNA with a bicoid-like specificity and is expressed in the developing mouse brain. Human Molecular Genetics. 1998;7:1497–1505. doi: 10.1093/hmg/7.9.1497. [DOI] [PubMed] [Google Scholar]
- 28.Reiss AL, Eliez S, Schmitt JE, et al. Neuroanatomy of Williams Syndrome: A high-resolution MRI study. Journal of Cognitive Neuroscience. 2000;12( supplement):65–73. doi: 10.1162/089892900561986. [DOI] [PubMed] [Google Scholar]
- 29.Bellugi U, Wang P, Jernigan T, editors. Williams Syndrome: An unusual neuropsychological profile. Hillsdale NJ: Erlbaum; 1993. [Google Scholar]; Broman S, Grafman J, editors. Atypical Cognitive Deficits in Developmental Disorders: Implications for Brain Function. [Google Scholar]
- 30.Morris CA, Demsey SA, Leonard CO, Dilts C, Blackburn BL. Natural history of Williams Syndrome: Physical characteristics. Journal of Pediatrics. 1988;113:318–326. doi: 10.1016/s0022-3476(88)80272-5. [DOI] [PubMed] [Google Scholar]
- 31.Abitz M, Nielsen RD, Jones EG, Laursen H, Graem N, Pakkenber B. Excess of neurons in the human newborn mediodorsal thalamus compared with that of the adult. Cerebral Cortex. 2007;17:2573–2578. doi: 10.1093/cercor/bhl163. [DOI] [PubMed] [Google Scholar]
- 32.Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002 Jan;44(1):4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- 33.Rabinowicz T. The Differentiated Maturation of the Cerebral Cortex: Postnatal growth Neurobiology. New York: Plenum Press; 1986. [Google Scholar]
- 34.Giedd JN, Snell JW, Lange N, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral Cortex. 1996 Jul-Aug;6(4):551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 35.Giedd JN, Vaituzis AC, Hamburger SD, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. Journal of Comparative Neurology. 1996 Mar 4;366(2):223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 36.Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1997 Nov;21(8):1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- 37.Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: what have we learned? Journal of the American Academy of Child & Adolescent Psychiatry. 2001 Sep;40(9):1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Wahl M, Marzinzik F, Friederici AD, et al. The human thalamus processes syntactic and semantic language violations. Neuron. 2008;59:695–707. doi: 10.1016/j.neuron.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Danos P, Schmidt A, Baumann B, et al. Volume and neuron number of the mediodorsal thalamic nucleus in schizophrenia: a replication study. Psychiatry Research. 2005;140:281–289. doi: 10.1016/j.pscychresns.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Young KA, Holcomb LA, Yazdani U, Hicks PB, German DC. Elevated neuron number in the limbic thalamus in major depression. American Journal of Psychiatry. 2004;61:1270–1277. doi: 10.1176/appi.ajp.161.7.1270. [DOI] [PubMed] [Google Scholar]
- 41.Frangou S, Chitins X, Williams SCR. Mapping IQ and gray matter density in healthy young people. NeuroImage. 2004;23:800–805. doi: 10.1016/j.neuroimage.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 42.Lee J-S, Yoo S-S, Cho S-Y, Ock S-M, Lim M-K, Panych LP. Abnormal thalamic volume in treatment na ve boys with Tourette syndrome. Acta Psychiatrica Scandinavica. 2006;113:64–67. doi: 10.1111/j.1600-0447.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- 43.Csernansky JG, Csernansky K, Splinter NR, et al. Abnormalities of Thalamic Volume and Shape in Schizophrenia. American Journal of Psychiatry. 2004;161:896–902. doi: 10.1176/appi.ajp.161.5.896. [DOI] [PubMed] [Google Scholar]
- 44.Ahsan RL, Allom R, Gousias IS, et al. Volumes, spatial extents and a probabilistic atlas of the human basal ganglia and thalamus. NeuroImage. 2007;38:261–270. doi: 10.1016/j.neuroimage.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Gilbert AR, Moore GJ, Keshavan MS, et al. Decrease in thalamic volumes of pediatric patients with obsessive-compulsive disorder who are taking paroxetine. Archives of General Psychiatry. 2000 May;57(5):449–456. doi: 10.1001/archpsyc.57.5.449. [DOI] [PubMed] [Google Scholar]
- 46.Kovalev VA, Kruggel F, von Cramon DY. Gender and age effects in structural brain asymmetry as measured by MRI texture analysis. NeuroImage. 2003;19:895–905. doi: 10.1016/s1053-8119(03)00140-x. [DOI] [PubMed] [Google Scholar]
- 47.Toga AW, Thompson PM. Mapping brain asymmetry. Nature Reviews Neuroscience. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- 48.Jancke L, Steinmetz H, Benilow S, Ziemann U. Slowing fastest finger movements of the dominant hand with low-frequency rTMS of the hand area of the primary motor cortex. Exp Brain Res. 2004 Mar;155(2):196–203. doi: 10.1007/s00221-003-1719-7. [DOI] [PubMed] [Google Scholar]
- 49.Monkul ES, Nicoletti MA, Spence D, et al. MRI study of thalamus volumes in juvenile patients with bipolar disorder. Depression and Anxiety. 2006;23:347–352. doi: 10.1002/da.20161. [DOI] [PubMed] [Google Scholar]


