Abstract
This study examined renal and glycemic effects of chromium picolinate (Cr(pic)3) supplementation in the context of its purported potential for DNA damage. In preventional protocol, male obese diabetic db/db mice were fed diets either lacking or containing 5, 10 or 100 mg/kg chromium as Cr(pic)3 from 6 to 24 weeks of age; male lean nondiabetic db/m mice served as controls. Untreated db/db mice displayed increased plasma glucose and insulin, hemoglobin A1c, renal tissue advanced glycation end (AGE) products, albuminuria, glomerular mesangial expansion, urinary 8-hydroxydeoxyguanosine (8-OHdG, an index of oxidative DNA damage) and renal tissue immunostaining for γH2AX (a marker of double-strand DNA breaks) compared to db/m controls. Creatinine clearance was lower while blood pressure was similar between untreated db/db mice and their db/m controls. High Cr(pic)3 intake (i.e., 100 mg/kg diet) mildly improved glycemic status and albuminuria without affecting blood pressure or creatinine clearance. Treatment with Cr(pic)3 did not increase DNA damage despite marked renal accumulation of chromium. In interventional protocol, effects of diets containing 0, 100 and 250 mg/kg supplemental chromium, from 12 to 24 weeks of age, were examined in db/db mice. The results generally revealed similar effects to those of the 100 mg/kg diet of the preventional protocol. In conclusion, the severely hyperglycemic db/db mouse displays renal structural and functional abnormalities in association with DNA damage. High-dose Cr(pic)3 treatment mildly improves glycemic control and it causes moderate reduction in albuminuria, without affecting histopathological appearance of the kidney and increasing the risk for DNA damage.
Keywords: Diabetes, Chromium picolinate, Kidney, Albuminuria, 8-OHdG, γH2AX
Introduction
Chromium picolinate (Cr(pic)3) is suggested to improve glycemic control in type 2 diabetes [1-6]. The interest in trivalent chromium stems from studies which indicate that chromodulin is capable of binding to the insulin receptor thereby resulting in amplification of insulin signaling; chromodulin is a low molecular weight chromium binding substance or a likely product of its metabolism known as the glucose tolerance factor [1-2, 6]. Improvement in glucose homeostasis per se, in turn, ameliorates oxidative stress, which is a major sequela of chronic hyperglycemia that contributes importantly to the pathogenesis of diabetic complications including nephropathy [7-9].
While the beneficial metabolic effects of Cr(pic)3 have been the focus of a number of investigations, others have raised concerns regarding its safety and toxicity. Of particular concern has been the potential genotoxicity of Cr(pic)3 [10-17]. Accordingly, it has been suggested that reduction of Cr(pic)3 by ascorbate produces [Cr(II)(pic)3]-, which is susceptible to oxidation thereby generating hydroxyl free radicals [14]. Hydroxyl free radicals can cause severe DNA damage through a large and complex set of reactions ranging from oxidation of deoxyribose and base moieties to strand breaks [18].
Chronic Cr(pic)3 supplementation results in distribution of chromium into a variety of tissues including the kidney [19-21]. The kidney is also a principal route of elimination for chromium [22]. Given that nephropathy is a major complication of type 2 diabetes, it is possible that chronic Cr(pic)3 use could be of potential detrimental consequences to the kidney (i.e., with possibility to cause functional dysregulation and/or altered structure) because of its accumulation within this organ [21]. Thus, we examined the effects of preventional and interventional Cr(pic)3 on glycemic control and the kidney of the db/db mouse which is one of the best characterized and most intensively studied animal models of human type 2 diabetic nephropathy; the db/db mouse lacks the leptin receptor due to an autosomal recessive mutation of the diabetic (db) gene [23-27]. The db/db mouse is obese, markedly insulin resistant and severely hyperglycemic; the heterozygous db/m littermate is lean and is spared from the type 2 diabetic condition thereby serving as an ideal genetic control for the db/db mouse. Accordingly, we tested the hypothesis that relatively high-dose chronic Cr(pic)3 increases the risk for DNA damage in association with worsening of renal functional and structural alterations in db/db mice. Assessment of DNA damage included measurement of urinary excretion of 8-hydroxydeoxyguanosine (8-OHdG), an index of oxidative DNA damage, and renal tissue γH2AX immunostaining which is a sensitive marker of double strand DNA (dsDNA) breaks; dsDNA breaks are considered to be the most severe form of DNA injury [28-29].
Materials and Methods
The present studies utilized two major protocols, preventional and interventional. For the preventional protocol, dietary Cr(pic)3 supplementation of db/db mice commenced at 6 weeks of age before the manifestation of the disease (i.e., marked obesity and type 2 diabetes). On the other hand, for the interventional protocol, dietary Cr(pic)3 treatment started at about 12 weeks of age (i.e., after development of obesity and type 2 diabetes). The animals (male db/m and db/db mice) were purchased from the Jackson Laboratories and were housed in the laboratory animal facilities at the Georgia Health Sciences University. All animals had free access to food and water throughout the studies (unless otherwise specified). The use of animals for these studies conformed to the institutional guidelines for the care and use of laboratory animals.
Preventional protocol
At 6 weeks of age, the db/db mice were randomly assigned to either remain on the regular rodent diet (Harlan Teklad diet number 8604) or switched to the 8604-based diet that was supplemented with 5 , 10 or 100 mg/kg of chromium as chromium picolinate (i.e., db/db; 5 Cr, db/db; 10 Cr, and db/db; 100 Cr identified by Harlan Teklad diet numbers 07602, 07603 and 07604, respectively; n=10 animals per group). Lean, non-diabetic db/m controls (n=9) were provided with the 8604 diet (without supplemental chromium).
Prior to sacrifice at 24 weeks of age, tail-cuff hemodynamics were measured and two consecutive 48-hour urine samples collected from each animal; urine samples were used for determination of urinary excretions of albumin and 8-OHdG [30]. Further, hemoglobin A1c levels were measured using a drop of blood from the tail (Bayer HealthCare - Diabetes Care Sunnyvale, California). For determination of plasma glucose and insulin concentrations, the 24-week-old animals were fasted overnight and blood samples were obtained from the retro-orbital plexus of the conscious animal.
Interventional protocol
The interventional protocol utilized 3 groups of 12-week-old db/db mice that were fed either the normal 8604 diet (n=4) or the 8604 diet that was supplemented with either 100 or 250 mg/kg of chromium as Cr(pic)3 (n=6 mice/ group; db/db; 100 Cr and db/db; 250 Cr, respectively). Glycemic and renal parameters were measured before initiation of dietary Cr(pic)3; these measurements were repeated 4 weeks after initiation of dietary regimen as well as before sacrifice and collection of tissues (i.e., 24 weeks of age) as described under the preventional protocol. The 24-week-old db/m mice of the preventional protocol served as “historical controls” for untreated db/db mice of the interventional protocol since these animals (i.e., db/m) were not provided with supplemental chromium.
Tissue collection
After collection of metabolic data and measurement of tail-cuff hemodynamics, the animals were anesthetized with sodium pentobarbital (40 mg/kg) and renal tissue procured for histopathological examination and immunohistochemistry (i.e., 10% formalin-fixed). Slot blot analysis was performed for determination of tissue advanced glycation end (AGE) products (e.g., tissue frozen in liquid nitrogen until assayed) [30].
Histological and immunohistochemistry of kidney
Formalin-fixed and paraffin-embedded kidney blocks were cut in 4 μm sections. For histopathological examination, kidney sections were processed for hematoxylin-eosin (H&E), trichrome and periodic acid Schiff (PAS) staining protocols [30]. Immunohistochemical staining for γH2AX was carried out utilizing a monoclonal anti-γH2AX primary antibody (Cell Signaling Technology, Davers MA) and Biotinylated anti-rabbit IgG (BioGenex, San Ramon CA) as secondary antibody [30]. Assessment of renal tissue γH2AX staining was performed using a BIOQUANT computerized image analysis system (Nashville, TN). After obtaining image, in initial automated steps of the image analysis, digital color thresholds distinguished the immunoreactive objects followed by assignment of numerical values to stained areas. This was followed by calculation of the ratio (in percent) of immunoreactive to nonreactive areas. For each kidney section, five zones (4 corners and one center) were analyzed and the average percent value recorded for each animal; renal tissues from 4 animals per group were subjected to immunohistochemistry and image analysis.
Slot blot analysis
To determine kidney levels of AGE products, frozen renal tissue was pulverized and added to the isolation buffer (10 mM triethanolamine, 250 mM Sucrose, pH 7.6, 1 μg/ml Leupeptin, PMSF (2 mg/ml), sonicated and sodium dodecyl sulfate added to a final concentration of 1% prior to centrifugation; the supernatant was used for protein assay (Biorad protein assay DC kit) [30]. Thereafter, samples were immobilized onto a nitrocellulose membrane using the Slot Blot apparatus (BioRad). After blocking, membranes were reacted with antibody against AGE products (6D12, Trangenic Inc. Kumamoto Japan) followed by determination of optical density. To confirm equal loading, membranes were stained with 0.1% Ponceau S solution (Sigma-Aldrich) and also reprobed for β-actin [30]. The data were normalized to β-actin and expressed as percent of the db/m control for AGE products.
Plasma and urinary parameters
Plasma glucose concentration was measured using a Beckman Glucometer II and plasma insulin concentration determined using a radioimmunoassay Kit (MP Biomedicals, LLC; Solon, OH). The data were used to calculate the insulin resistance Homeostatic Model Assessment (HOMA) index as follows: (FPI × FPG)/22.5 where FPI and FPG denote fasting plasma insulin (μU/ml) and fasting plasma glucose (mmol/l), respectively [30]. In addition, blood hemoglobin A1c values were measured as an index of chronic glycemic status.
Urinary albumin excretion was measured using a murine Microalbuminuria ELISA kit (Exocell, Philadelphia, PA). Similarly, urinary excretion of 8-OHdG was measured using an ELISA kit (Northwest Life Science Specialties, LLC; Vancouver, WA). Urinary and plasma creatinine were measured (Cayman Chemicals and Biovision, respectively) and used to calculate creatinine clearance rate, an index of glomerular filtration rate [30].
Tissue chromium analysis
Analysis of kidney chromium content was carried out by the Wisconsin State Laboratory of Hygiene using Inductively Coupled Plasma Mass Spectrometry (ICP-MS) [30].
Statistics
All data were analyzed by the analysis of variance (ANOVA). Variables that were measured sequentially were analyzed by repeated measure ANOVA. Duncan's post hoc test was used for comparison of mean values (significance of criteria of p<0.05). Data are reported as mean ± SEM.
Results
I. Preventional protocol
Based on measurement of food intake, the diets provided chromium at doses of 460 ± 33, 1, 208 ± 125 and 9,672 ± 704 μg/kg/day for diets which contained 5, 10 and 100 mg/kg of chromium, respectively. Lower Cr(pic)3 intake (e.g., ranging 190 to 410 μg/kg/day) does not influence glycemic control or oxidative DNA damage (indexed by 8-OHdG) in the obese Zucker rat [30]. All db/m control mice reached 24 weeks of age when experiments were carried out. However, 6 untreated db/db mice and 8 animals in each of the 3 Cr(pic)3 supplemented groups reached 24 weeks of age; mortalities occurred between 20-24 weeks of age. It is established that db/db mice die with increasing age and worsening of diabetes- and obesity-related complications [23].
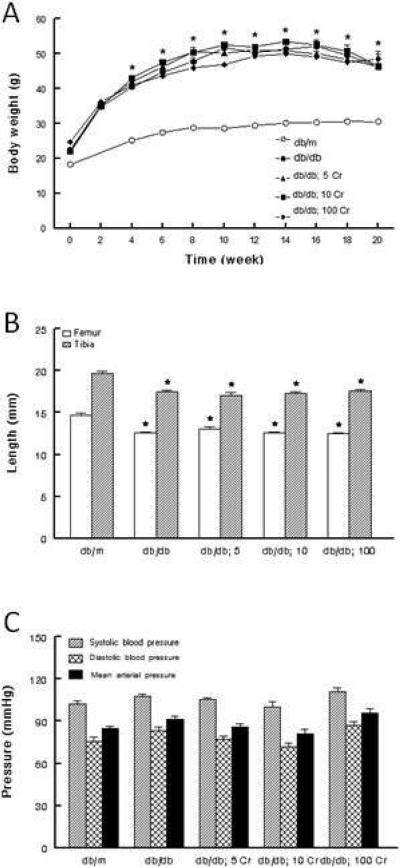
All animals gained significant body weight during the course of the study although the db/db mice gained more weight than their db/m controls (Figure 1A). Dietary Cr(pic)3 treatment did not affect either the course or the extent of gain in body weight in the db/db mice (Figure 1A). Further, the untreated db/db mice displayed significantly reduced length for tibia and femur but Cr(pic)3 did not affect these parameters (Figure 1B) likely suggesting lack of an adverse effect of the supplement on the skeletal system. Kidney weight was greater in the db/db (0.45 ± 0.02 g) than db/m (0.38 ± 0.02 g; p<0.05) but Cr(pic)3 treatment did not affect renal tissue weight (0.48 ± 0.03, 0.48 ± 0.01 and 0.48 ± 0.02 g for db/db mice fed 5, 10 and 100 mg/kg chromium diets, respectively). The untreated db/db group displayed 7-8 mmHg increase, albeit non-significant, in diastolic and mean arterial pressures compared to the db/m group; Cr(pic)3 treatment did not affect blood pressure (Figure 1C). Heart rate was generally similar among the groups (average range of 468-536 beats/min).
Figure 1.
Effects of dietary Cr(pic)3 on body weight, bone length and blood pressure of db/db mice. Panel A shows that db/db mice gained significantly more weight than their db/m controls. However, dietary Cr(pic)3 treatment did not affect body weight of db/db mice. Panel B shows that obesity/diabetes, but not Cr(pic)3 treatment, was associated with reduced bone length. Panel C shows that blood pressure was similar among the groups, irrespective of Cr(pic)3 treatment. The Cr(pic)3-treated db/db mice (e.g., db/db; 5, db/db; 10, and db/db; 100) are identified according to the chromium content of their respective diets (i.e., 5, 10 and 100 mg/kg diet as chromium, respectively). Data are mean ± SEM of 6-9 animals per group.
* p<0.05 compared to their db/m counterparts.
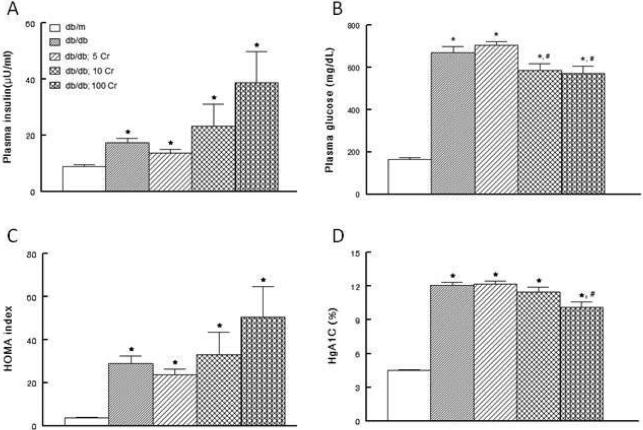
Figure 2 shows that both fasting baseline plasma insulin and glucose concentrations were greater in the db/db than db/m mice (panels A and B). As a result, the HOMA value, an index of insulin resistance, was elevated in the db/db than db/m mice (Figure 2C). These observations are consistent with marked elevation in hemoglobin A1c levels in the db/db compared to db/m mice (Figure 2D). Chronic low dose chromium supplementation (i.e., 5 mg/kg of diet) did not affect the glycemic status of the db/db mice. However, relative to both db/db and db/db; 5Cr groups, db/db; 10 Cr and db/db; 100 Cr groups displayed significant reductions in fasting plasma glucose in association with a tendency for higher fasting insulin concentrations. Interestingly, consistent with reduction in plasma glucose level (Figure 2B), the db/db; 100 Cr group displayed a mild, albeit significant, reduction in HgA1c level compared to db/db and db/db; 5 Cr groups (Figure 2D).
Figure 2.
Effects of Cr(pic)3 on metabolic parameters in db/db mice. Panels A and B show fasting plasma insulin and glucose concentrations while panel C shows the Homeostatic Model Assessment (HOMA) insulin resistance index of experimental groups as described in Figure 1. On the other hand, panel D shows hemoglobin A1c values, an index of chronic glycemic status. Data are mean ± SEM of 6-9 animals per group.
* p<0.05 compared to the db/m group. # p<0.05 compared to untreated db/db and db/db; 5 Cr groups.
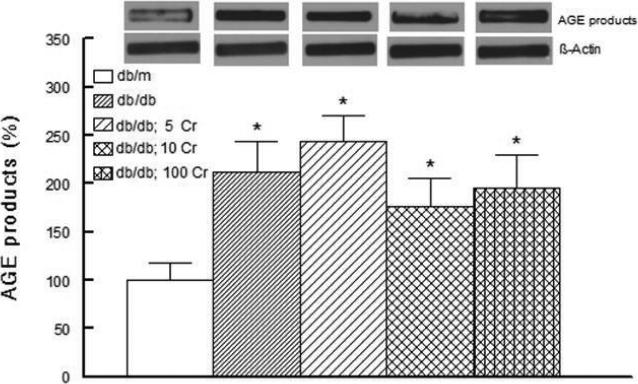
Figure 3 shows increase in renal tissue content of AGE products in the db/db compared to db/m mice. Kidney AGE products remained higher in the chromium-treated db/db mice than lean db/m controls (despite a mild, but non-significant, reduction in renal tissue AGE products of db/db; 10 Cr and db/db; 100 Cr groups).
Figure 3.
Bar graph showing kidney level of advanced glycation end (AGE) products expressed as a percent of the nondiabetic db/m control group in experimental groups and effects of Cr(pic)3 as described in Figure 1. Representative blots are also shown for each group. Data are mean ± SEM of 6-9 animals per group.
* p<0.05 compared to the db/m group.
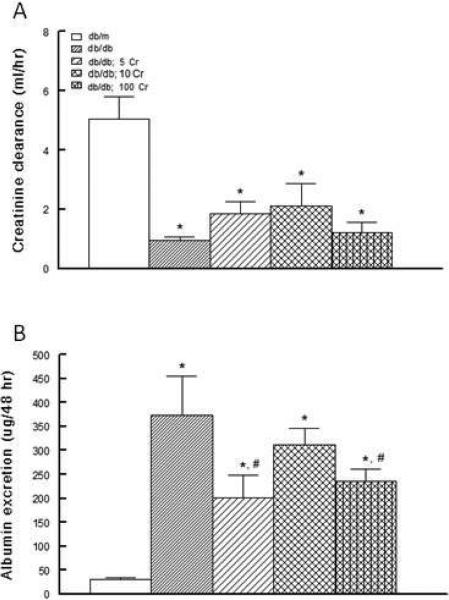
As shown in Figure 4A, creatinine clearance was markedly lower in the untreated db/db mice than their db/m control counterparts. The 5 and 10 mg/kg Cr(pic)3-treated db/db displayed a tendency for improved creatinine clearance relative to the untreated group. On the other hand, urinary albumin excretion was markedly higher in the untreated db/db than their db/m controls (Figure 4B). Dietary Cr(pic)3 treatment partially reduced albuminuria with the differential achieving statistical significance for the db/db; 5 Cr and db/db; 100 Cr groups compared to their untreated db/db counterparts.
Figure 4.
Effects of Cr(pic)3 on creatinine clearance and albumin excretion in experimental animals. Panel A shows that creatinine clearance was markedly lower in the untreated db/db group than db/m controls. Dietary Cr(pic)3 treatment was associated with variable, non-significant, increase in creatinine clearance. On the other hand, panel B shows that untreated db/db mice displayed marked elevation in urinary albumin excretion than their db/m counterparts. Dietary Cr(pic)3 treatment was associated with generally lower albuminuria in experimental groups as described in Figure 1. Data are mean ± SEM of 6-9 animals per group.
* p<0.05 compared to the db/m group.
# p<0.05 compared to the untreated db/db group.
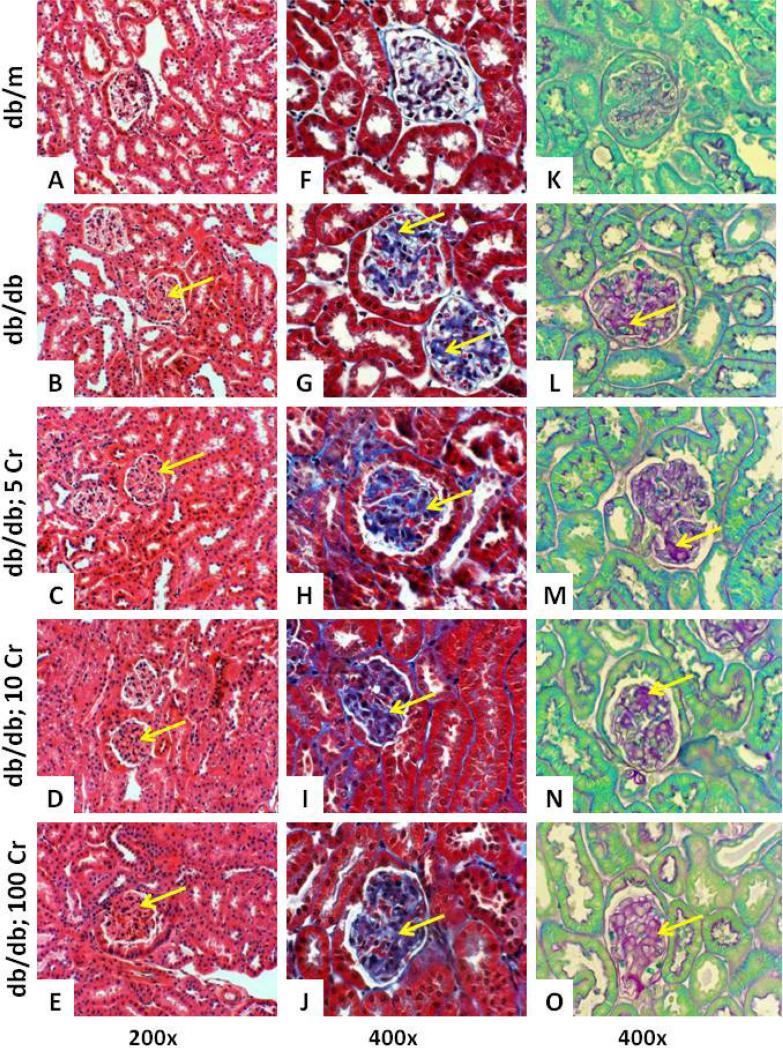
Examination of H&E stained renal tissue demonstrated diffuse glomerular eosinophilic deposition in db/db mice with expansion of glomerular tufts, irrespective of Cr(pic)3 treatment, compared to their db/m controls (Figure 5, panels A-E). Nonetheless, some glomeruli of db/db kidneys morphologically resembled those of the db/m controls. Trichrome and PAS stains were performed to help highlight glomerular deposition (Figure 5, panels F-J and K-O, respectively). These stains revealed diffuse glomerular deposition in renal tissues of db/db mice, irrespective of Cr(pic)3 treatment, compared to those of db/m controls. However, similar to H&E-stained sections, no specific pattern was observed to correlate the extent of the material deposition with progressive increase in dietary Cr(pic)3 intake.
Figure 5.
Histopathological features of experimental groups. Panels show renal tissue staining for H&E (A-E), trichrome (F-J) and PAS (K-O) of experimental groups described in Figure 1. Arrows point to eosinophilic-, trichrome- and PAS-positive materials.
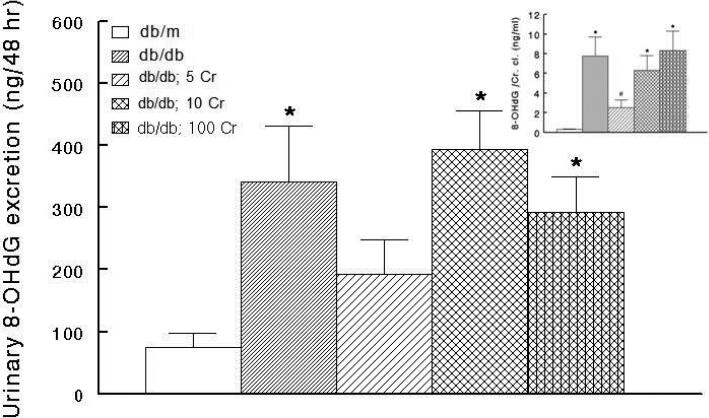
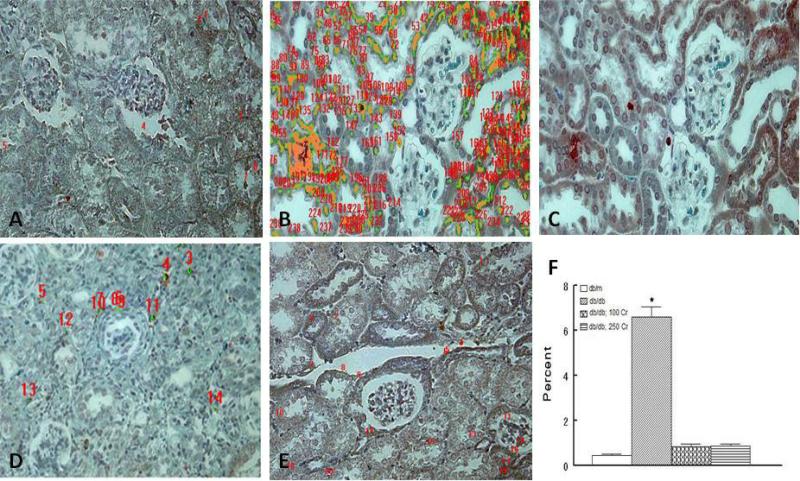
In order to determine whether chronic Cr(pic)3 treatment influences whole body oxidative DNA damage, urinary excretion of 8-OHdG was measured for experimental groups. As shown in Figure 6, the untreated db/db mice displayed marked increase in urinary 8-OHdG excretion relative to their db/m controls. However, Cr(pic)3 treatment did not cause further increase in urinary excretion of 8-OHdG (Figure 6). In light of differences in creatinine clearance among groups (Figure 4A), 8-OHdG excretion data were normalized to respective creatinine clearance data. As shown in Figure 6 inset, a similar pattern to that of absolute 8-OHdG excretion emerged although the db/db; 5 Cr group showed reduced ratio compared to the other three db/db groups. To further explore potential impact of diabetes and Cr(pic)3 on DNA damage, we carried out immunohistochemical assessment of γH2AX, which is a sensitive biomarker of dsDNA breaks [29]. As shown in panels B and C of Figure 7, the untreated db/db mice tissue sections show greater nuclear γH2AX immunostaining compared to their lean controls (Figure 7A). We conjectured that the impact of Cr(pic)3 would more likely be evident in animals with highest Cr(pic)3 intake. Interestingly, however, the 100 mg/kg Cr(pic)3 diet was not associated with more marked immunostaining for γH2AX; rather, renal tissue of high-dose Cr(pic)3-treated db/db mice showed reduced immunostaining than that of untreated db/db mice, resembling that of the db/m group (Figure 7, panels A-C ). Consistent with this notion image analysis revealed higher percent score (i.e., ratio of immunoreactive to nonreactive areas) for the untreated db/db than db/m controls (Figure 7F) while the db/db; 100 Cr group displayed a percent score value lower than untreated db/db but similar to the db/m group.
Figure 6.
Bar graphs showing urinary excretion of 8-hydroxydeoxyguanosine (8-OHdG) of experimental groups and effects of Cr(pic)3 as described in Figure 1. Data are mean ± SEM of 6-9 animals per group. The inset shows urinary 8-OHdG excretion normalized to creatinine clearance (Cr. Cl.).
* p<0.05 compared to the db/m group.
# p<0.06 compared to other db/db groups.
Figure 7.
Effects of Cr(pic)3 on renal DNA damage as assessed by γH2AX immunostaining. Renal tissue of untreated db/db mouse (panels B-C) shows increased nuclear immunostaining compared to its db/m control (panel A). On the other hand, renal tissue of db/db mice treated with 100 mg/kg chromium diet in the preventional protocol (panel D) or the db/db mice treated with 250 mg/kg of chromium diet in the interventional protocol (panel E) shows reduced γH2AX immunostaining. Panels are representative of 4 animals per group; numerical values on panels A-B and D-E relate to image analysis as described in Methods. A-E; 200x. Bar graph shows percent of immunoreactive to nonreactive areas as described in Methods. Data are mean ± SEM of 4 animals per group.
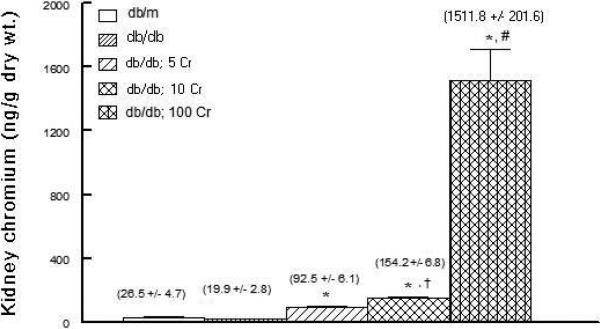
Chromium is known to accumulate substantially in the kidney [15,21]. Therefore, to determine whether the diabetic kidney also accumulates chromium, renal tissue chromium content was measured by ICP-MS. As shown in Figure 8, dietary Cr(pic)3 treatment resulted in dose-related accumulation of chromium in the db/db mouse kidney.
Figure 8.
Bar graph showing kidney chromium content of experimental groups as described in Figure 1.
* p<0.05 compared to the db/m and db/db groups.
† p<0.05 compared to the db/db; 5 Cr group.
# p<0.05 compared to other groups.
Interventional protocol
The preventional studies suggested improvement in glycemic control of db/db mice fed the diet containing 100 mg/kg of chromium without detectable adverse effects on the ability of the animal to grow and thrive. Indeed, the Cr(pic)3-treated db/db mice had better survival than their untreated counterparts (i.e., 80% vs. 60%, above). Thus, for the interventional protocol, db/db mice were fed Cr(pic)3-enriched diets containing 0, 100 and 250 mg/kg of supplemental chromium. The 100 and 250 mg/kg chromium diets provided chromium at doses of 9,430 ± 1,150 and 19,180 ± 3,960 μg/kg/day, respectively. All animals except one in the 100 mg/kg chromium diet group reached 24 weeks of age.
Body weights (ranging 44 to 46 g) and hemoglobin A1c levels (ranging 6.2 to 6.9%) were similar among the three groups of db/db mice (i.e., untreated db/db, db/db; 100 Cr and db/db; 250 Cr) at 12 weeks of age prior to initiation of dietary Cr(pic)3 treatment. By 24 weeks of age, body weight remained similar among the groups (48.8 ± 1.5 g, untreated db/db; 49.0 ± 2.9 g, db/db; 100 Cr and 48.9 ± 1.0g, db/db; 250 Cr). In progression from 12 to 24 weeks of age, all groups showed a significant increase in hemoglobin A1c levels although the increase was less (p< 0.05) for the two Cr(pic)3-treated groups than the untreated db/db mice [(3.8 ± 0.2%, db/db; 250 Cr), (3.2 ± 0.4%, db/db; 100) and (4.5 ± 0.2%; untreated db/db). However, similar to the Cr(pic)3-treated animals of the preventional protocol, renal tissue content of AGE products for the db/db; 250 Cr group (250% ± 20%) was similar to the untreated db/db group (and significantly greater than the 24-week-old db/m controls shown in Figure 3).
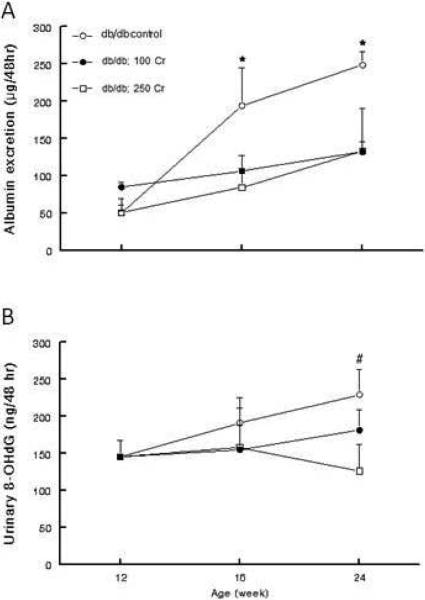
As shown in Figure 9A, baseline (i.e., at 12 weeks of age) albumin excretion was generally similar among the groups. However, while the untreated db/db mice displayed a marked increase in albuminuria with age, the two Cr(pic)3-treated groups showed a milder increase in this parameter thereby resulting in a significant difference compared to the untreated db/db group. Baseline urinary excretion of 8-OHdG was also similar among the three db/db groups (Figure 9B). While the untreated db/db mice showed an increase in urinary 8-OHdG excretion with progression to 24 weeks of age, Cr(pic)3-treated groups showed a mild decrease in this parameter relative to the untreated mice. It is noteworthy that the db/db; 250 Cr group excreted less 8-OHdG than the untreated db/db group by 24 weeks of age. Also, as shown in panel E of Figure 7, renal tissue of db/db; 250 Cr group resembled that of the db/m group with respect to γH2AX immunostaining. Image analysis revealed a percent score value similar to that of the db/m control group but lower (p<0.05) than that of the untreated db/db group (Figure 7F).
Figure 9.
Line graphs showing urinary albumin (A) and 8-OHdG (B) excretions of db/db mice from the interventional protocol. Data are mean ± SEM of 4-6 animals per group.
* p<0.05 compared to other groups at the same age.
# p<0.05 compared to the db/db; 250 Cr group.
Discussion
The present study examined the impact of Cr(pic)3 in db/db mice, with treatment initiated prior to or after the manifestation of obesity and type 2 diabetes (i.e., preventional or interventional protocol, respectively). The results indicate that a) db/db mice display marked hyperglycemia with prominent alterations in renal structure and function in association with marked DNA damage as indexed by urinary 8-OHdG excretion and renal tissue immunostaining for γH2AX, b) Cr(pic)3 treatment, even high dose, did not exert adverse consequences on the ability of db/db mice to grow and thrive, c) high-dose preventive and interventional Cr(pic)3 treatment was associated with a mild improvement in glycemic control but moderate reduction in albuminuria and d) Cr(pic)3 treatment resulted in dose-related, marked renal accumulation of chromium but did not increase urinary excretion of 8-OHdG or renal tissue γH2AX immunostaining. Collectively, the results suggest that Cr(pic)3 used either as a preventive or interventional supplement can exert mild improvement on glycemic control and moderate reduction in albuminuria without increasing the risk for DNA damage in db/db mice.
Previous studies have utilized in vitro cellular and acellular systems or in vivo systems (primarily involving normal animals) [10-17]. However, the present investigation is unique because a) the effects of both preventional and interventional protocols, involving several doses of Cr(pic)3 were examined using a highly relevant animal model of the disease(s) for which the use of the formulation is often advocated (i.e., type 2 diabetes and obesity) and b) the studies focused on the kidney, an organ that accumulates chromium [21,30]. Indeed, utilizing ICP-MS technology, there was dose-related marked accumulation of chromium by the kidney of db/db mouse. Thus we conjectured that any impact of Cr(pic)3 on DNA damage should be most pronounced in animals with highest intake, and accumulation, of the nutritional supplement. Accordingly, urinary 8-OHdG and immunohistochemical assessment of renal tissue γH2AX were used as indices of whole body and local DNA damage, respectively. Of the variety forms of DNA damage, dsDNA breaks are among the most severe forms of DNA injury, thereby compromising genomic stability. An early cellular response to dsDNA breaks is rapid phosphorylation of H2AX, the minor histone variant, leading to generation of γH2AX [29]. The phosphorylation of H2AX has emerged as one of the most well-established chromatin modifications linked to DNA damage and repair [29].
A major sequela of hyperglycemia-induced oxidative stress is DNA injury [8-9]. Accordingly, hyperglycemia increases glucose entry into cells which do not regulate glucose uptake (e.g., endothelial and mesangial cells). Consequently, greater glucose metabolism generates increased superoxide from several sources including the mitochondria. Superoxide anion is dismutated to generate hydrogen peroxide which can be detoxified by the action of catalase. However, hydrogen peroxide, through the Fenton reaction, can generate hydroxyl radical which is one of the most devastating reactive oxygen species [8-9, 18]. For example, hydroxyl radicals can interact with DNA to cause a variety of effects including oxidation of deoxyribose and base moieties and strand breaks [18]. Consistent with the notion of hyperglycemia-induced DNA injury [8-9], the untreated db/db mice showed marked increase in urinary excretion of 8-OHdG and renal tissue γH2AX immunostaining compared to their db/m controls. While urinary excretion of 8-OHdG has been advocated as a sensitive biomarker of whole body oxidative damage, to our knowledge, this is the first report documenting evidence of dsDNA breaks in kidneys of db/db mice. Interestingly, however, treatment with Cr(pic)3, even at high doses, was not associated with increases in either urinary excretion of 8-OHdG or renal tissue γH2AX immunostaining. Rather, the treatment was associated with mild-moderate decline in urinary 8-OHdG (i.e., db/db; 5 Cr) and a pronounced reduction of γH2AX immunostaining. This is an unexpected finding because the high-dose Cr(pic)3-treated db/db mice remained markedly hyperglycemic. Thus, from the stand point of hyperglycemia-induced oxidative stress and DNA damage, and similar to 8-OHdG data, one would expect similar renal immunostaining to that of untreated db/db kidney. Nonetheless, the collective observations suggest that it is unlikely that even high Cr(pic)3 intake exacerbates (local or systemic) DNA damage in db/db mice.
Our observations are consistent with reports of a number of other investigators who concluded lack of adverse genomic consequences of Cr(pic)3 in other experimental models [31-34]. In this context, it is noteworthy that Hepburn and Vincent [19] injected Sprague-Dawley rats with 51Cr(pic)3 and confirmed increased tissue content of Cr(pic)3 (e.g., in liver and kidney) but also showed preferential accumulation of 51Cr in the cytosol of hepatocytes (~ 75%). Further, the authors showed that the 51Cr(pic)3 enters and exists both in the nucleus and the mitochondria, thus these organelles showed very little accumulation of 51Cr. Based on these findings, the authors suggested that the risk of damage to the DNA from generation of any hydroxyl radical is reduced because Cr(pic)3 is preferentially accumulated in the cytosol rather than the nucleus or the mitochondria [19]. The finding that high dose Cr(pic)3 treatment of db/db mice in our study did not increase indices of DNA damage is in general agreement with these observations.
Another noted finding of the study is that, diets containing high amounts of the nutritional supplement did not affect body weight of db/db mice whether the treatment was instituted before or after manifestation of marked obesity. This is consistent with lack of an appreciable effect of the supplement in human subjects [6, 22]. On the other hand, higher doses of the formulation exerted mild-moderate improvement in glycemic status as reflected by lower fasting plasma glucose, but a tendency for higher insulin concentrations, in association with reduced hemoglobin A1c level. It is noteworthy that the chromium intake of for human subjects consuming Cr(pic)3 is in the range of 200-1000 μg/day which corresponds to a dose of 2.9-14.3 μg/kg/day [2, 6, 17]. In the preventional protocol, daily chromium intake ranged from about 460 to 9,670 μg/kg/day while in the interventional protocol it ranged from 9,430 to 19,810 μg/kg/day. Yet, mild-moderate beneficial glycemic effects were seen only with higher chromium intake ranging from 1,370 to 9,670 μg/kg/day (e.g., 10 and 100 mg/kg chromium diets of the preventional protocol). On the other hand, generally similar effects on glycemic control were seen for both the 100 and the 250 mg/kg chromium diet of the interventional protocol which provided chromium in the range of 9,430 to 19,810 μg/kg/day. Taken together, these data suggest that the beneficial influence on glycemic status of db/db mice are near maximal with the 100 μg/kg chromium diet and that greater chromium intake does not further improve glycemic status in this animal model. While variations in species can determine the effectiveness of an agent, it is noteworthy that human studies have also resulted in varied findings which may relate, at least in part, to the dose and duration of treatment [35-37]. Some studies indicate that chromium supplementation (in the range of 200-1,000 μg/day), in the form of Cr(pic)3, particularly at higher doses may improve insulin sensitivity and glucose metabolism in patients with dysglycemia [6]. On the other hand, others have concluded that Cr(pic)3 does not beneficially influence glycemic status of patients manifesting symptoms of metabolic disease and type 2 diabetes [35, 37]. However, it is likely that Cr(pic)3 potentiates the effects of other interventional modalities such as exercise and/or hypoglycemic agents. Indeed, a recent report suggests that Cr(pic)3 treatment increases insulin sensitivity and improves glycemic control in patients on sulfonylurea agents [36].
Hallmark features of diabetic nephropathy include marked albuminuria but a relentless decline in glomerular filtration rate [38]. Consistent with this notion, db/db mice showed marked reduction in creatinine clearance, in association with prominent glomerular mesangial expansion, but significant albuminuria compared to the db/m group. The pathogenesis of the reciprocal relation between albumin excretion and glomerular function is multifactorial. Diabetes mellitus causes major alterations of the components of the glomerular filtration barrier (i.e., podocytes, glomerular basement membrane and fenestrated glomerular endothelial cells) resulting in progressive loss of its permselective property [39]. Consequently, the increased and unrestricted loss of macromolecules (e.g., albumin) initiates a self-perpetuating process of progressive glomerulosclerosis, tubulointerstitial inflammation and scarring thereby contributing to progressive loss of renal function [38]. Indeed, the development of macroalbuminuria is closely associated with a progressive and rapid decline of glomerular function. Among multiple mechanisms, a loss of intrinsic ultrafiltration capacity contributes importantly to the relentless decline in glomerular filtration rate in type 2 diabetic nephropathy [40]. Other contributing mechanisms to functional abnormalities of the diabetic kidney include hemodynamic factors (both systemic and intrarenal) and hyperglycemia-induced activation of a number of pathogenic mechanisms (e.g., formation of AGE products) [9]. Renal tissue content of AGE products was greater in untreated db/db mice than db/m thereby providing a plausible contributing mechanism to the manifested functional abnormalities. On the other hand, blood pressure was mildly higher (7-8 mmHg; p>0.05) in db/db than db/m mice. Nonetheless, it is recognized that diabetic kidney is more sensitive to any elevation in blood pressure and the mild increase in blood pressure of db/db mice possibly along with other factors, could contribute to the decline in creatinine clearance and marked albuminuria. Importantly, however, treatment with Cr(pic)3 did not beneficially impact renal structural alterations (e.g., glomerular mesangial expansion) or creatinine clearance in db/db mice. On the other hand, the nutritional supplement was associated with moderate reduction in albuminuria which was apparently dissociated from glycemic control because the Cr(pic)3-treated db/db mice (e.g., of the preventional protocol) displayed varying reduction in albuminuria while a mild improvement in glycemic control was seen with higher Cr(pic)3 intake. Also, blood pressure was not beneficially influenced by Cr(pic)3 treatment. Thus, factors aside from glycemic control and/or blood pressure likely account for the effect of the beneficial influence of Cr(pic)3 on albuminuria. These may include attenuation of contribution of proinflammatory cytokines and chemokines, among others factors [30, 41].
In conclusion, dietary Cr(pic)3 intake, in doses that far exceed those consumed by human subjects, did not increase the risk for DNA damage, in a highly relevant animal model of type 2 diabetes (i.e., db/db mouse), as indexed by assessment of urinary 8-OHdG excretion and renal γH2AX immunostaining, despite marked accumulation of chromium by the kidney. Rather, the treatment was associated with mild beneficial effect on glycemic control in this animal model in association with a reduction in albuminuria.
Acknowledgements
The financial support for this study was solely provided by a grant from the National Institutes of Health (1R21AT003012-01A2: MSM). The authors thank Noel Stanton (Wisconsin State Laboratory of Hygiene) for chromium analyses and Nutrition 21 for the generous gift of Cr(pic)3.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors do not have any conflicts of interest.
References
- 1.Anderson RA. Chromium in the prevention and control of diabetes. Diabetes Metab. 2000;26:22–27. [PubMed] [Google Scholar]
- 2.Vincent JB. Mechanisms of chromium action: low-molecular-weight chromium-binding substance. J Am Coll Nutr. 1999;18(1):6–12. doi: 10.1080/07315724.1999.10718821. [DOI] [PubMed] [Google Scholar]
- 3.Wang ZQ, Zhang XH, Russell JC, Hulver M, Cefalu WT. Chromium picolinate enhances skeletal muscle cellular insulin signaling in vivo in obese, insulin-resistant JCR:LA-cp rats. J Nutr. 2006;136:415–420. doi: 10.1093/jn/136.2.415. [DOI] [PubMed] [Google Scholar]
- 4.Qiao W, Peng Z, Wang Z, Wei J, Zhou A. Chromium improves glucose uptake and metabolism through upregulating the mRNA levels of IR, GLUT4, GS, and UCP3 in skeletal muscle cells. Biol Trace Elem Res. 2009;131(2):133–42. doi: 10.1007/s12011-009-8357-2. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Yao M. Effects of chromium picolinate on glucose uptake in insulin-resistant 3T3-L1 adipocytes involve activation of p38 MAPK. J Nutr Biochem. 2009;20:982–91. doi: 10.1016/j.jnutbio.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Cefalu WT, Hu FB. Role of chromium in human health and in diabetes. Diabetes Care. 2004;11:2741–2751. doi: 10.2337/diacare.27.11.2741. [DOI] [PubMed] [Google Scholar]
- 7.Sheetz MJ, King GL. Molecular understanding of hyperglycemia's adverse effects of diabetic complications. JAMA. 2002;288(20):2579–2588. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 8.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 9.Mozaffari MS, Abdelsayed R, Schaffer SW. Predictive Diagnostics and Personalized Treatment: Dream or Reality. Golubnitschaja O Nova Publishers; 2009. Diabetic Complications: Pathogenic Mechanisms and Prognostic Indicators. pp. 157–182. [Google Scholar]
- 10.Stearns DM, Wise JP, Sr, Patierno SR, Wetterhahn KE. Chromium (III) picolinate produces chromosome damage in Chinese hamster ovary cells. FASEB J. 1995;9:1643–1648. [PubMed] [Google Scholar]
- 11.Stallings DM, Hepburn DD, Hannah M, Vincent JB, O'Donnell J. Nutritional supplement chromium picolinate generates chromosomal aberrations and impedes progeny development in Drosophila melanogaster. Mutat Res. 2006;610:101–113. doi: 10.1016/j.mrgentox.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Snow ET. Effects of chromium on DNA replication in vitro. Environ Health Perspect. 1994;102:41–44. doi: 10.1289/ehp.94102s341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bridgewater LC, Manning FC, Woo ES, Patierno SR. DNA polymerase arrest by adducted trivalent chromium. Mol Carcinog. 1994;9:122–133. doi: 10.1002/mc.2940090304. [DOI] [PubMed] [Google Scholar]
- 14.Speetjens JK, Collins RA, Vincent JB, Woski SA. The nutritional supplement Chromium(III) Tris(picolinate) cleaves DNA. Chem Res Toxicol. 1999;12:483–487. doi: 10.1021/tx9900167. [DOI] [PubMed] [Google Scholar]
- 15.Coryell VH, Stearns DM. Molecular analysis of hprt mutations induced by chromium picolinate in CHO AA8 cells. Mutat Res. 2006;610:114–123. doi: 10.1016/j.mrgentox.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Bagchi D, Bagchi M, Balmoori J, Ye X, Stohs SJ. Comparative induction of oxidative stress in cultured J774A.1 macrophage cells by chromium picolinate and chromium nicotinate. Res Commun Mol Pathol Pharmacol. 1997;97:335–346. [PubMed] [Google Scholar]
- 17.Stout MD, Nyska A, Collins BJ, Witt KL, Kissling GE, Malarkey DE, Hooth MJ. Chronic toxicity and carcinogenicity studies of chromium picolinate monohydrate administered in feed to F344/N rats and B6C3F1 mice for 2 years. Food Chem Toxicol. 2009;47:729–733. doi: 10.1016/j.fct.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cadet J, Delatour T, Douki T, Gasparutto D, Pouget JP, Ravanat JL, Sauvaigo S. Hydroxyl Radicals and DNA base damage. Mutat Res. 1999;424:9–21. doi: 10.1016/s0027-5107(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 19.Hepburn DD, Vincent JB. In vivo distribution of chromium from chromium picolinate in rats and implications for the safety of the dietary supplement. Chem Res Toxicol. 2002;15(2):93–100. doi: 10.1021/tx010091t. [DOI] [PubMed] [Google Scholar]
- 20.Hepburn DD, Vincent JB. Tissue and subcellular distribution of chromium picolinate with Time after entering the bloodstream. J Inorg Biochem. 2003;94:86–93. doi: 10.1016/s0162-0134(02)00623-2. [DOI] [PubMed] [Google Scholar]
- 21.Lamson DW, Plaza SM. The safety and efficacy of high-dose chromium. Altern Med Rev. 2002;7(3):218–235. [PubMed] [Google Scholar]
- 22.Diaz ML, Watkins BA, Li Y, Anderson RA, Campbell WW. Chromium picolinate and conjugated linoleic acid do not synergistically influence diet- and exercise-induced changes in body composition and health indexes in overweight women. J Nutr Biochem. 2008;19:61–68. doi: 10.1016/j.jnutbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Sharma K, McCue P, Dunn SR. Diabetic kidney disease in db/db mouse. Am J Physiol Renal Physiol. 2003;284:F1138–F1144. doi: 10.1152/ajprenal.00315.2002. [DOI] [PubMed] [Google Scholar]
- 24.Like AA, Lavine RL, Poffenbarger PL, Chick WL. Studies in the diabetic mutant mouse. VI. Evolution of glomerular lesions and associated proteinuria. Am J Pathol. 1972;66:193–224. [PMC free article] [PubMed] [Google Scholar]
- 25.Koya D, Haneda M, Nakagawa H, Isshiki K, Sato H, Maeda S, Sugimoto T, Yasuda H, Kashiwagi A, Ways DK, King GL, Kikkawa R. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. FASEB J. 2000;14(3):439–47. doi: 10.1096/fasebj.14.3.439. [DOI] [PubMed] [Google Scholar]
- 26.Levine DZ, Iacovitti M, Robertson SJ, Mokhtar GA. Modulation of single-nephron GFR in the db/db mouse model of type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol. 2006;290:R975–R981. doi: 10.1152/ajpregu.00693.2005. [DOI] [PubMed] [Google Scholar]
- 27.Chin M, Isono M, Isshiki K, Araki S, Sugimoto T, Guo B, Sato H, Haneda M, Kashiwagi A, Koya D. Estrogen and raloxifene, a selective estrogen receptor modulator, ameliorate renal damage in db/db mice. Am J Pathol. 2005;166(6):1629–1636. doi: 10.1016/s0002-9440(10)62473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu LL, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004;339:1–9. doi: 10.1016/j.cccn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Mah LJ, El-Osta A, Karagiannis TC. γH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24:679–686. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- 30.Mozaffari MS, Abdelsayed R, Liu JY, Wimborne H, El-Remessy A, El-Marakby A. Effects of chromium picolinate on glycemic control and kidney of the obese Zucker rat. Nutr Metab. 2009;6:51. doi: 10.1186/1743-7075-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gudi R, Slesinski RS, Clarke JJ, San RH. Chromium picolinate does not produce chromosome damage in CHO cells. Mutat Res. 2005;587:140–146. doi: 10.1016/j.mrgentox.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Slesinski RS, Clarke JJ, San RH, Gudi R. Lack of mutagenicity of chromium picolinate in the hypoxanthine phosphoribosyltransferase gene mutation assay in Chinese hamster ovary cells. Mutat Res. 2005;585(1-2):86–95. doi: 10.1016/j.mrgentox.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Andersson MA, Petersson Grawé KV, Karlsson OM, Abramsoon-Zetterberg LA, Hellman BE. Evaluation of the potential genotoxicity of chromium picolinate in mammalian cells in vivo and in vitro. Food Chem Toxicol. 2007;45(7):1097–1106. doi: 10.1016/j.fct.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Komorowski JR, Greenberg D, Juturu V. Chromium picolinate does not produce chromosome damage. Toxicol In Vitro. 2008;22:819–826. doi: 10.1016/j.tiv.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Iqbal N, Cardillo S, Volger S, Bloedon LT, Anderson RA, Boston R, Szapary PO. Chromium picolinate does not improve key features of metabolic syndrome in obese nondiabetic adults. Metab Syndr Relat Disord. 2009;7(2):143–50. doi: 10.1089/met.2008.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin J, Wang ZQ, Zhang XH, Wachtel D, Volaufova J, Matthews DE, Cefalu WT. Chromium picolinate supplementation attenuates body weight gain and increases insulin sensitivity in subjects with type 2 diabetes. Diabetes Care. 2006;29:1826–1832. doi: 10.2337/dc06-0254. [DOI] [PubMed] [Google Scholar]
- 37.Kleefstra N, Houweling ST, Jansman FG, Groenier KH, Gans RO, Meyboom-de Jong B, Bakker SJ, Bilo HJ. Chromium treatment has no effect in patients with poorly controlled insulin-treated type 2 diabetes in an obese Western population: a randomized, double-blind, placebo-controlled trial. Diabetes Care. 2006;29(3):521–525. doi: 10.2337/diacare.29.03.06.dc05-1453. [DOI] [PubMed] [Google Scholar]
- 38.Ruggenenti P, Remuzzi G. Time to abandon microalbuminuria? Kidney Int. 2006;70:1214–1222. doi: 10.1038/sj.ki.5001729. [DOI] [PubMed] [Google Scholar]
- 39.Singh A, Satchell SC. Microalbuminuria: causes and implications. Pediatr Nephrol. doi: 10.1007/s00467-011-1777-1. [Epub Ahead of Print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson RG, Bennett PH, Beck GJ, Tan M, Knowler WC, Mitch WE, Hirschman GH, Myers BD. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. Diabetic Renal Disease Study Group. N Engl J Med. 1996;335(22):1636–42. doi: 10.1056/NEJM199611283352203. 1996. [DOI] [PubMed] [Google Scholar]
- 41.Jain SK, Rains JL, Croad JL. Effect of chromium niacinate and chromium picolinate supplementation on lipid peroxidation, TNF-alpha, IL-6, CRP, glycated hemoglobin, triglycerides, and cholesterol levels in blood of streptozotocin-treated diabetic rats. Free Radic Biol Med. 2007;43:1124–1131. doi: 10.1016/j.freeradbiomed.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]