Abstract
Background
We previously developed two etomidate analogs that retain etomidate’s favorable hemodynamic properties, but whose adrenocortical effects are reduced in duration or magnitude. Methoxycarbonyl-etomidate (MOC-etomidate) is rapidly metabolized and ultra-short acting whereas (R)-ethyl 1-(1-phenylethyl)-1H-pyrrole-2-carboxylate (carboetomidate) does not potently inhibit 11β-hydroxylase. We hypothesized that MOC-etomidate’s labile ester could be incorporated into carboetomidate to produce a new agent that possesses favorable properties individually found in each agent. We describe the synthesis and pharmacology of methoxycarbonyl-(R)-ethyl 1-(1-phenylethyl)-1H-pyrrole-2-carboxylate (MOC-carboetomidate), a “soft” analog of carboetomidate.
Methods
MOC-carboetomidate’s octanol:water partition coefficient was determined chromatographically and compared with those of etomidate, carboetomidate, and MOC-etomidate. MOC-carboetomidate’s EC50 and ED50 for loss of righting reflexes (LORR) were measured in tadpoles and rats, respectively. Its effect on gamma-aminobutyric acid A (GABAA) receptor function was assessed using two-microelectrode voltage clamp electrophysiological techniques and its metabolic stability was determined in pooled rat blood using high performance liquid chromatography. Its duration of action and effects on arterial blood pressure and adrenocortical function were assessed in rats.
Results
MOC-carboetomidate’s octanol:water partition coefficient was 3300 ± 280, whereas those for etomidate, carboetomidate, and MOC-etomidate were 800 ± 180, 15000 ± 3700, and 190 ± 25, respectively. MOC-carboetomidate’s EC50 for LORR in tadpoles was 9 ± 1 µM and its EC50 for LORR in rats was 13 ± 5 mg/kg. At 13 µM, MOC-carboetomidate enhanced GABAA receptor currents by 400 ± 100%. Its metabolic half-life in pooled rat blood was 1.3 minutes. The slope of a plot of the duration of LORR in rats versus the logarithm of the hypnotic dose was significantly shallower for MOC-carboetomidate than for carboetomidate (4 ± 1 vs. 15 ± 3, respectively; p = 0. 0004123). At hypnotic doses, the effects of MOC-carboetomidate on arterial blood pressure and adrenocortical function were not significantly different from those of vehicle alone.
Conclusions
MOC-carboetomidate is a GABAA receptor modulator with potent hypnotic activity that is more rapidly metabolized and cleared from the brain than carboetomidate, maintains hemodynamic stability similar to carboetomidate, and does not suppress adrenocortical function.
Introduction
Etomidate is an imidazole-based IV sedative-hypnotic that is commonly used to induce general anesthesia in elderly, critically ill, and hemodynamically unstable patients. 1–6 Unfortunately, etomidate binds with high affinity to 11b-hydroxylase, suppressing the adrenocortical synthesis of steroids (i.e., cortisol, corticosterone, and aldosterone) that are important regulators of immune function, glucose homeostasis, and water and electrolyte balance. 7–9 Such suppression precludes etomidate administration as a prolonged continuous infusion and has raised concerns regarding the administration of even a single bolus dose for anesthetic induction. 10–14
We previously developed two etomidate analogs that retain etomidate’s favorable hemodynamic properties, but whose effects on adrenocortical function are significantly reduced in duration or magnitude (Figure 1). Methoxycarbonyl-etomidate (MOC-etomidate) is a soft analog of etomidate that, similar to remifentanil and esmolol, contains a metabolically labile ester group that is rapidly hydrolyzed by esterases. 15 In rats, MOC-etomidate induces hypnosis of extremely short duration and does not produce prolonged suppression of adrenocortical function because it is rapidly metabolized. 15 (R)-ethyl 1-(1-phenylethyl)-1H-pyrrole-2-carboxylate (carboetomidate) is a pyrrole analog of etomidate that was designed not to bind with high affinity to 11β-hydroxylase. 16 It is three orders of magnitude less potent an inhibitor of in vitro cortisol synthesis and does not suppress steroid synthesis in rats. 16
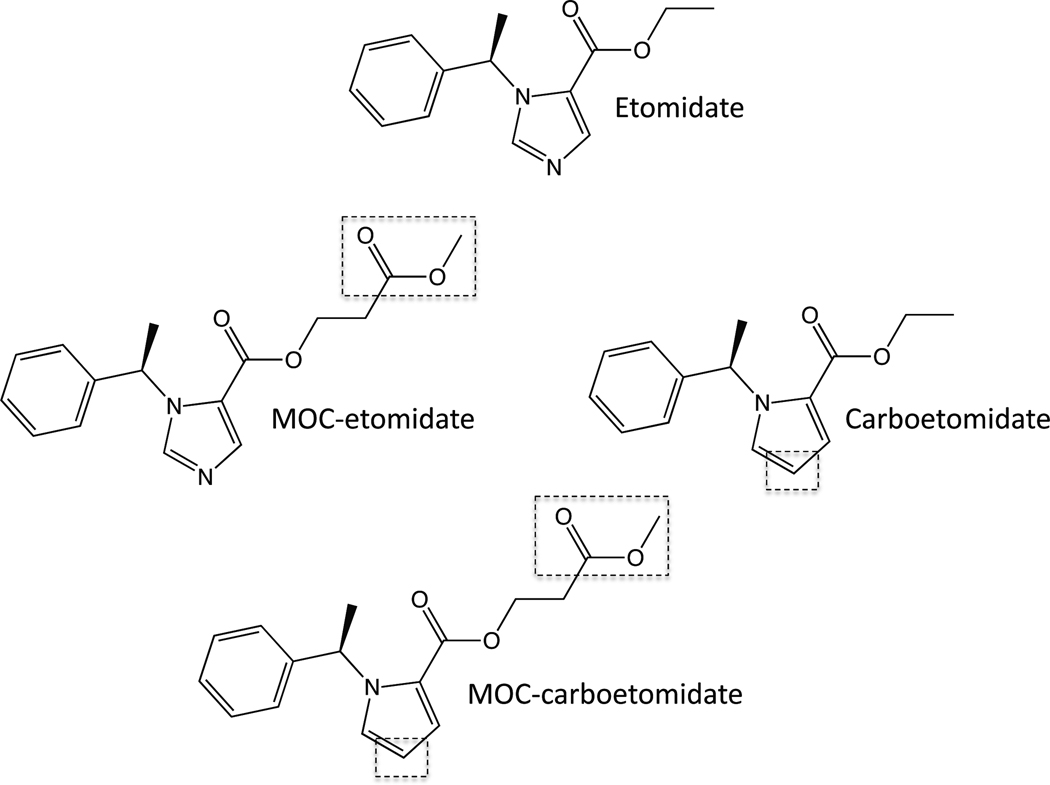
Figure 1.
Chemical structures of etomidate, MOC-etomidate, carboetomidate, and MOC-carboetomidate. Their structural differences are highlighted by the dashed boxes.
Respectively, MOC-etomidate and carboetomidate represent pharmacokinetic and pharmacodynamic solutions to the problem of etomidate-induced adrenocortical suppression. They were produced by modifying distinct regions of the etomidate molecular scaffold in different ways. We hypothesized that these two modifications could be combined to produce a single hypnotic drug that would possess advantageous properties found individually in each agent. We describe the synthesis and pharmacology of methoxycarbonyl-(R)-ethyl 1-(1-phenylethyl)-1H-pyrrole-2-carboxylate (MOC-carboetomidate), a soft analog of carboetomidate.
Methods
Animals
All animal studies were conducted with the approval of the Subcommittee on Research Animal Care at the Massachusetts General Hospital, Boston, Massachusetts. Xenopus laevis tadpoles (early pre-limb stage) and adult female Xenopus laevis frogs were purchased from Xenopus One (Ann Arbor, MI) and housed in our laboratory (tadpoles) or in the Massachusetts General Hospital Center for Comparative Medicine animal care facility (frogs). Adult male Sprague-Dawley rats (300–420g) were purchased from Charles River Laboratories (Wilmington, MA) and housed in the Massachusetts General Hospital Center for Comparative Medicine animal care facility. All drugs were administered via a femoral venous catheter and all blood was drawn from a femoral venous or femoral arterial catheter. Arterial blood pressure was measured using a femoral arterial catheter. All femoral venous and arterial catheters were pre-implanted by the vendor.
Synthesis of Carboetomidate and MOC-carboetomidate
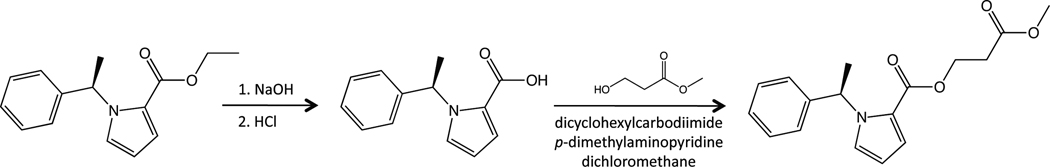
Carboetomidate was synthesized as previously described. 16 MOC-carboetomidate was synthesized from carboetomidate starting material (Figure 2). A solution of carboetomidate (5.85 g, 24 mmol) in MeOH (40 mL) was added to NaOH (6N, 12 ml). The resulting mixture was heated to 50° C for 24h and then concentrated. The residue was acidified with 5N HCl and purified by silica gel column chromatography with dichloromethane/methanol to give the acid (4.4g, yield 86%). MS [M+1] = 216, 1HNMR (CDCl3): δ 7.23–7.34 (m, 3H), 7.15–7.18 (m, 3H), 7.05 (t, 1H, J = 2 Hz), 6.61 (q, 1H, J = 7.2Hz), 6.24 (dd, 1H, J = 4 Hz, J = 2.6Hz), 1.84 (d, 3H, J = 7.2 Hz).
Figure 2.
Synthesis of MOC-carboetomidate.
To a mixture of the acid (4.4g, 20.5 mmol) and methyl-3-hydroxypropanoate (2.34g, 22.6 mmol) in anhydrous dichloromethane (100 ml), dicyclohexylcarbodiimide (4.66g, 22.6 mmol) and p-dimethylaminopyridine (2.76 g, 25.1 mmol) was added. The solution was stirred at room temperature for 60 h. The precipitate was removed by filtration, and the clear solution was applied to a silica gel column with dichloromethane/ether as eluents to give the product with purity ~89%. The crude product was further purified by pre-high performance liquid chromatography. The purified product was treated with HCl in dioxane to obtain MOC-carboetomidate hydrochloride as a yellow oil (3.12g, 45% yield, purity>97%). MS [M+1] = 302, 1HNMR (CDCl3): δ 7.24–7.30 (m, 3H), 7.15–7.17 (m, 2H), 7.02–7.03 (m, 1H), 7.01 (t, 1H, J = 2 Hz), 6.57 (q, 1H, J = 7.2Hz), 6.24 (dd, 1H, J = 4 Hz, J = 2.6Hz), 4.43–4.50 (m, 2H), 3.72 (s, 3H), 2.73 (t, 2H, J = 6.4Hz), 1.82 (d, 3H, J = 7.2 Hz).
Octanol:Water Partition Coefficients
One mg of MOC-carboetomidate, MOC-etomidate, carboetomidate, or etomidate was added to 10 ml of water buffered with 10 mM Tris (pH 7.4) and 0.5 ml or 1 ml of octanol. The mixture was stirred overnight and then centrifuged to more fully separate the organic and aqueous phases. The relative concentrations of sedative-hypnotic in each phase (i.e., the partition coefficient) was determined by high performance liquid chromatography using a Varian Prostar system with a 4.6 × 250 mm Proto 300 C18 column (NEST Group, Southborough, MA) with the UV detector set at 240nm. The mobile phase consisted of water and acetonitrile with 0.05% trifluoroacetic acid (Thermo Scientific, Rockford, IL). A linear gradient of 20% to 90% acetonitrile in water over 30 minutes was used with a flow rate of 1 ml/min.
Loss of Righting Reflex (LORR) in Tadpoles
Groups of 5 Xenopus laevis tadpoles were placed in room temperature water buffered with 2.5 mM Tris HCl (pH=7.4) containing a concentration of MOC-carboetomidate that ranged from 1 to 40 µM. Every 5 minutes, tadpoles were tipped with a flame-polished pipette until the response stabilized. A tadpole was determined to have LORR if it failed to right itself within 5 seconds after being turned supine. At the end of each study, tadpoles were returned to fresh water to ensure reversibility. Tadpoles that did not regain their righting reflexes in fresh water were excluded from the analysis. MOC-carboetomidate’s EC50 for LORR was then determined from the concentration dependence of LORR using the quantal method of Waud. 17
GABAA Receptor Electrophysiology
Adult female Xenopus laevis frogs were anesthetized with 0.2% tricaine (ethyl-m-aminobenzoate) and hypothermia. Ovary lobes were then excised through a small laparotomy incision and placed in OR-2 solution (82 mm NaCl, 2 mM KCl, 2 mM MgCl2, 5 mM HEPES, pH=7.5) containing collagenase 1A (1 mg/ml) for 3 hours to separate oocytes from connective tissue.
Stage 4 and 5 oocytes were injected with messenger RNA encoding the α1, β2 (or β2M286W), and γ2l subunits of the human gamma-aminobutyric acid A (GABAA) receptor (−40 ng of messenger RNA total at a subunit ratio of 1:1:2). This messenger RNA was transcribed from complementary DNA encoding for GABAA receptor α1, β2 (or β2M286W), and γ2l subunits using the mMESSAGE mMACHINE High Yield Capped RNA Transcription Kit (Ambion, Austin, TX). Injected oocytes were incubated in ND-96 buffer solution (96 mM NaCl, 2 mM KCl, 1 mMCaCl2, 0.8 mM MgCl2, 10 mm HEPES, pH=7.5) containing 50 U/ml of penicillin and 50 µg/ml of streptomycin at 17°C for at least 18 hours before electrophysiologic experiments.
All electrophysiologic recordings were performed using the whole cell two-electrode voltage-clamp technique. Oocytes were placed in a 0.04-ml recording chamber and impaled with capillary glass electrodes filled with 3 M KCl and possessing open tip resistances less than 5 MΩ. Oocytes were then voltage clamped at −50 mV using a GeneClamp 500B amplifier (Axon Instruments, Union City, CA) and perfused with ND-96 buffer at a rate of 4–6 ml/min. Buffer perfusion was controlled using a six-channel valve controller (Warner Instruments, Hamden, CT) interfaced with a Digidata 1322A data acquisition system (Axon Instruments) and driven by a Dell personal computer (Round Rock, TX). Current responses were recorded using Clampex 9.2 software (Axon Instruments) and processed using a Bessel (8-pole) low-pass filter with a cutoff at 50 Hz using Clampfit 9.2 software (Axon Instruments).
For each oocyte, the concentration of GABA that produced 5–10% of the maximal current response (EC5–10 GABA) was determined by measuring the peak current responses evoked by a range of GABA concentrations (in ND-96 buffer) and comparing them with the maximal peak current response evoked by 1 mM GABA. The effect of MOC-carboetomidate on EC5–10 GABA-evoked currents was then assessed by perfusing the oocyte with EC5–10 GABA for 90 seconds and then measuring the control peak evoked current. After a 5-minute recovery period, the oocyte was perfused with MOC-carboetomidate for 90 seconds and then with EC5–10 GABA plus MOC-carboetomidate for 90 seconds, and the peak evoked current was measured again. After a 15-minute recovery period to allow MOC-carboetomidate washout, the control experiment (i.e., no MOC-carboetomidate) was repeated to assure reversibility. The peak current response in the presence of MOC-carboetomidate was then normalized to the average peak current response of the two control experiments. MOC-carboetomidate-induced enhancement was quantified from the normalized current responses in the presence versus absence of carboetomidate.
LORR in Rats
Rats were restrained in a 3-inch diameter, 9-inch long acrylic chamber. The desired dose of MOC-carboetomidate or carboetomidate in dimethyl sulfoxide (DMSO) vehicle was injected through the femoral venous catheter followed by a 1-ml normal saline flush. After injection, rats were removed from the restraint device and turned supine. A rat was judged to have LORR if it failed to right itself (onto all four paws) after drug administration. A stopwatch was used to measure the duration of LORR, which was defined as the time from hypnotic injection until the animal spontaneously righted itself. The ED50 for LORR was determined from the dose dependence of LORR using the method of Waud. 17 In a separate study, the induction time was measured in rats by administering MOC-carboetomidate or carboetomidate (in DMSO vehicle) via the femoral venous catheter followed by a 1-ml normal saline flush, immediately removing from the restraint device, and repeatedly turning them supine until they no longer spontaneously righted. The induction time was defined as the time from injection until LORR occurred.
In Vitro Metabolism in Pooled Rat Blood
Whole blood from 3 Sprague-Dawley rats (2.5 ml/rat) was drawn, immediately pooled, anticoagulated with heparin (38 U), and aliquoted into 0.2 ml samples. Each pooled blood sample was warmed at 37°C for 5 minutes and then sedative-hypnotic (20mM in DMSO) was added to a final concentration of 100µM. After the desired incubation time, the metabolic reaction was quenched with 0.2 ml acetonitrile (Sigma-Aldrich, St. Louis, MO). The samples were centrifuged and the resultant plasma separated and stored at −20°C until analyzed. Sedative-hypnotic concentrations were determined in thawed plasma samples by high-pressure liquid chromatography as described above for partition coefficient studies.
Rat Adrenocortical Suppression
Rats were restrained in a 3-inch diameter, 9-inch long acrylic chamber and given dexamethasone (0.2 mg/kg; American Regent, Shirley, NY) IV to suppress endogenous adrenocorticotropic hormone (ACTH) release and corticosterone production. Two hours later, blood was drawn (to measure the baseline serum corticosterone concentration), and a second dose of dexamethasone (0.2 mg/kg) was administered along with MOC-carboetomidate, etomidate, or DMSO vehicle as a control. The concentrations of MOC-carboetomidate and etomidate in DMSO were 38 and 2.9 mg/ml. Immediately after hypnotic or vehicle administration, ACTH1–24 (25 µg/kg; Sigma-Aldrich Chemical Co, St. Louis, MO) was given to stimulate corticosterone production followed by 1-ml normal saline flush. Thirty minutes after ACTH1–24 administration, a second blood sample was drawn to measure the ACTH1–24-stimulated serum corticosterone concentration. Rats in all three groups (MOC-carboetomidate, etomidate, and vehicle control) received the same volume of DMSO (0.7 ml/kg).
All blood draws were approximately 0.4 ml in volume. Corticosterone concentrations in blood serum were determined as reported previously. Briefly, blood samples were allowed to clot at room temperature (30–60 min) and then centrifuged at 3,500g for 5 min. Serum was expressed from the resulting superficial fibrin clot and the sample was centrifuged again at 3,500g for 5 min. After the second centrifugation step, the clot-free serum layer was transferred to a fresh vial for final, high-speed centrifugation (16,000g, for 5min). The serum was transferred to a clean vial and frozen (−20°C) pending corticosterone measurement. After thawing and heat inactivation of corticosterone-binding globulins (65°C for 20 min), serum baseline and ACTH1–24-stimulated corticosterone concentrations were quantified using an enzyme-linked immunosorbent assay (Immunodiagnostic Systems Inc, Fountain Hills, AZ) and a 96-well plate reader (Molecular Devices, Sunnyvale, CA).
Hemodynamic Effects
Rats were restrained in a 3-inch diameter, 9-inch long acrylic chamber and allowed to acclimate for approximately 10–20 minutes before study. Arterial blood pressure was recorded using a Propaq Encore 206EL monitor (Welsh Allyn Inc., Skaneateles Falls, NY) every 30 s beginning 5 minutes before MOC-carboetomidate or DMSO vehicle administration and then for 15 min thereafter. To keep the total volume of DMSO constant at 0.7 ml/kg for all rats, the concentrations of MOC-carboetomidate in DMSO were 38 mg/ml and 76 mg/ml when given at doses of 27 mg/kg and 54 mg/kg, respectively.
Statistical Analysis
All data are reported as mean ± SD unless otherwise noted. Statistical analysis and curve fitting were performed using either Prism v5.0 for the Macintosh (GraphPad Software, Inc., LaJolla, CA) or Igor Pro 6.1 (Wavemetrics, Lake Oswego, OR). P < 0.05 indicates statistical significance unless otherwise indicated. For multiple comparisons of biochemical and physiological data derived from rats, we performed a one-way or two-way ANOVA followed by a Tukey Multiple Comparison Test.
Results
MOC-carboetomidate is viscous oil at room temperature. Its octanol:water partition coefficient was 3300 ± 280, whereas those for etomidate, carboetomidate, and MOC-etomidate were 800 ± 180, 15000 ± 3700, and 190 ± 25, respectively.
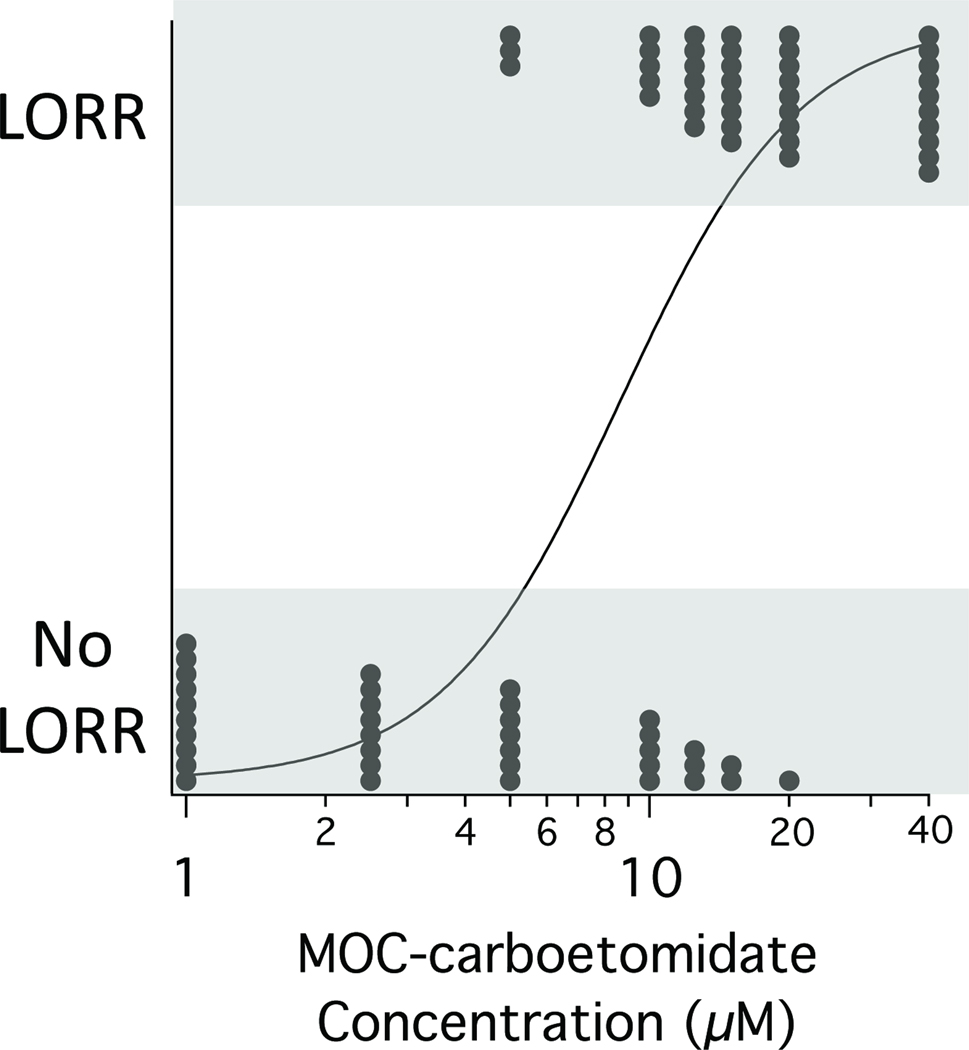
As an initial screen of MOC-carboetomidate’s hypnotic activity, we assessed its ability to produce LORR in tadpoles. We found that MOC-carboetomidate increased the fraction of tadpoles that had LORR in a concentration-dependent manner and at the highest concentration studied (40 µM), it produced LORR in all 10 tadpoles (Figure 3). This LORR was reversible as 42 of 44 tadpoles that had LORR in our studies recovered their righting reflexes when returned to fresh water. From the MOC-carboetomidate concentration-dependence of LORR, we calculated an EC50 of 9 ± 1 µM.
Figure 3.
MOC-carboetomidate concentration-response curve for loss of righting reflexes (LORR) in tadpoles. Each point represents data from a single tadpole. The curve is a fit of the concentration-response data to a logistic equation using the method of Waud. 17 The calculated EC50 for LORR was 9 ± 1 µM.
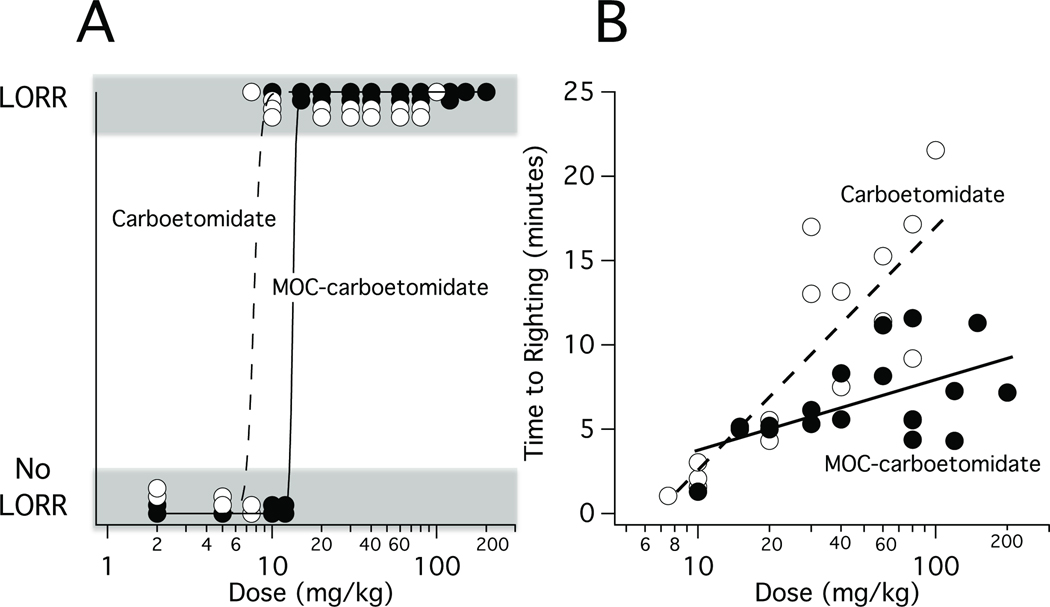
MOC-carboetomidate also increased the fraction of rats that had LORR in a dose-dependent manner and at the highest doses studied (20–200 mg/kg), it produced reversible LORR in all rats. From the MOC-carboetomidate dose-response curve for LORR shown in Figure 4A, we calculated MOC-carboetomidate’s ED50 to be 13 ± 5 mg/kg (n=27). For comparison, this figure also shows the carboetomidate dose-response curve for LORR in rats, which yielded an ED50 of 7.7 ± 0.8 mg/kg. Figure 4B plots the duration of LORR as a function of MOC-carboetomidate or carboetomidate dose and demonstrates that for both sedative-hypnotics, the duration of LORR increased with dose. However the slope of this relationship was significantly shallower for MOC-carboetomidate than for carboetomidate (4 ± 1 vs. 15 ± 3, respectively; p = 0. 0004123). We also determined the induction time upon administration of the two hypnotics at doses equal to 4X their respective ED50s for LORR and found that they were not significantly different at 16 ± 3 s (range: 10 – 18 s) for MOC-carboetomidate and 19 ± 6 s (range: 11 – 27 s) for carboetomidate.
Figure 4.
Loss of righting reflexes (LORR) in rats. (A) MOC-carboetomidate and carboetomidate dose-responses curves for LORR in rats. The curve is a fit of dose-response data to a logistic equation using the method of Waud. 17 The calculated ED50s for LORR were 13 ± 4 mg/kg for MOC-carboetomidate and 7.7 ± 0.8 mg/kg for carboetomidate. (B) Time to righting after bolus administration of MOC-carboetomidate or carboetomidate at the indicated doses. For both drugs, the time to righting increased logarithmically with dose. However, the slope of this relationship was significantly shallower for MOC-carboetomidate than for carboetomidate (4 ± 1 vs. 15 ± 3, respectively; p = 0. 0004123).
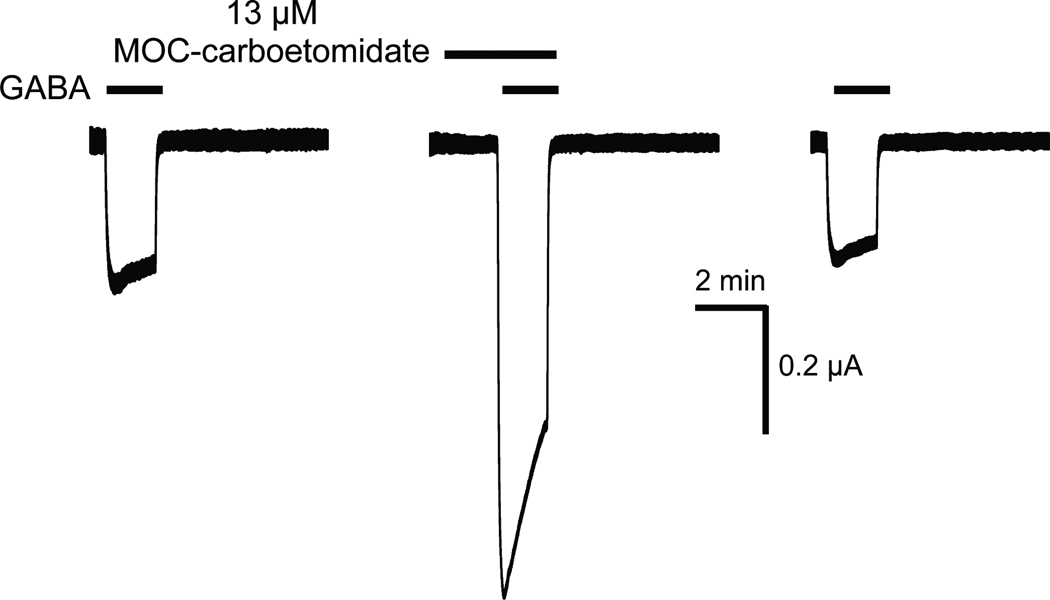
To test whether enhanced GABAA receptor function might mediate this LORR, we assessed MOC-carboetomidate’s ability to increase currents elicited by an EC5–10 GABA and mediated a1b2g2L GABAA receptors. Figure 5 shows representative current traces obtained from a single oocyte experiment and demonstrates that MOC-carboetomidate increased such currents. At a MOC-carboetomidate concentration of 13 µM, the current magnitude measured in 5 separate oocytes increased by 400 ± 100%.
Figure 5.
Representative traces showing the enhancing effect of MOC-carboetomidate on human γ-aminobutyric acid type A receptor function. The first and last traces show the control electrophysiological responses elicited with 3 µM γ-aminobutyric acid alone. The middle trace demonstrates the enhancing effect of 13 µM MOC-carboetomidate on currents elicited with 3 µM γ-aminobutyric acid in the same oocyte.
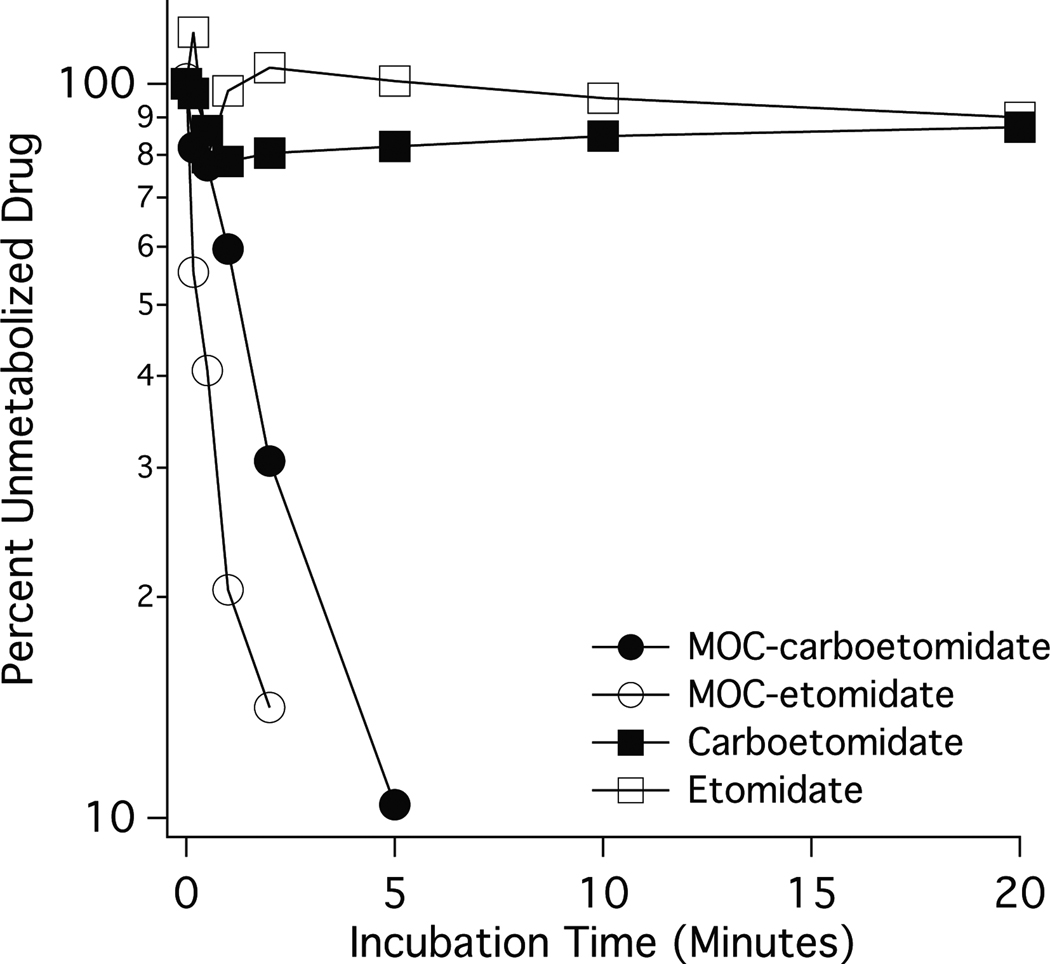
MOC-carboetomidate was designed as a rapidly metabolized “soft” analog of carboetomidate. We compared the metabolic stability of MOC-carboetomidate with those of carboetomidate, MOC-etomidate, and etomidate by adding each sedative-hypnotic to pooled rat blood and measuring the incubation time-dependent reduction in sedative-hypnotic concentration. Figure 6 shows the percentage of unmetabolized drug remaining as a function of incubation time in rat blood and reveals that neither carboetomidate nor etomidate was significantly metabolized even after 20 minutes, our longest incubation time. In contrast, MOC-carboetomidate and MOC-etomidate were metabolized in an approximately first-order fashion with metabolic half-lives of 1.3 minutes (95% confidence intervals 1.0 – 1.6 min) and 0.35 minutes (95% confidence intervals 0.22 – 0.84 min), respectively. In blood samples containing MOC-carboetomidate and MOC-etomidate (but not carboetomidate or etomidate), we also detected a higher polarity compound (as indicated by its shorter chromatographic retention time) whose concentrations increased before reaching a plateau of 0.82 minutes (95% confidence intervals 0.60 – 1.3 min) and 0.40 minutes (95% confidence intervals 0.32 – 0.54 min), respectively (data not shown).
Figure 6.
Metabolic stability of sedative-hypnotics in rat blood. Each point is the average value determined in two separate experiments. Each experiment used blood pooled from a different group of three rats. The metabolic half-lives of MOC-carboetomidate and MOC-etomidate were 1.3 min and 0.35 min, respectively.
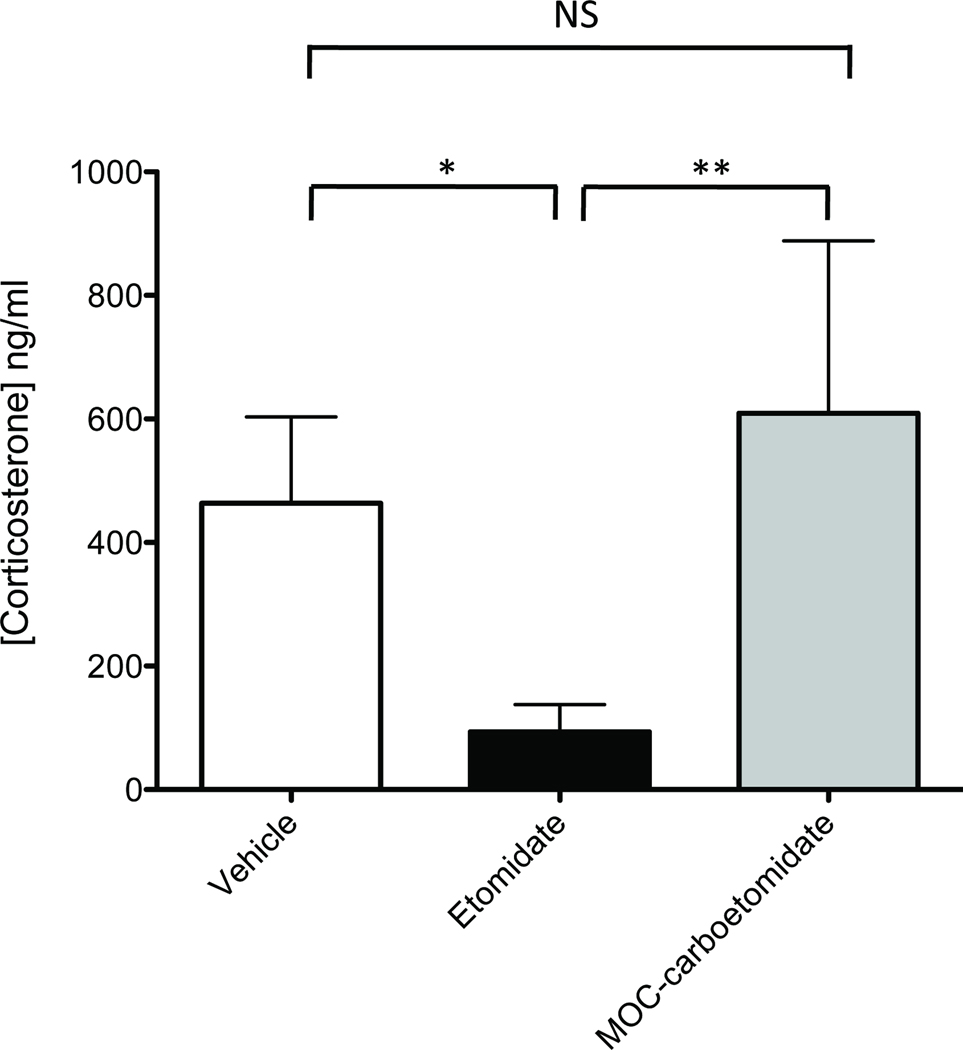
The effects of MOC-carboetomidate and etomidate on adrenocortical function were compared by measuring the serum corticosterone concentrations in rats thirty minutes after simultaneous administration of ACTH1–24 and either MOC-carboetomidate (27 mg/kg), etomidate (2mg/kg), or DMSO vehicle alone. These doses correspond to twice their respective ED50s for producing LORR in rats. Baseline corticosterone concentrations before ACTH1–24 administration were not significantly different among groups and averaged 26 ± 59 ng/ml. After ACTH1–24 administration, the average serum corticosterone concentration of rats that received etomidate was 90 ± 44 ng/ml, which was significantly lower than that in rats that received DMSO vehicle (460 ± 140 ng/ml; Figure 7). In contrast, the serum corticosterone concentration of rats that received MOC-carboetomidate was 610 ± 280 ng/ml. This was significantly higher than the serum corticosterone concentration in rats that received etomidate and not significantly different from the concentration in rats that received DMSO vehicle.
Figure 7.
The differential effects of MOC-carboetomidate (27 mg/kg) and etomidate (2 mg/kg) on adrenocortical function in the rat. These doses correspond to twice the respective ED50s for loss of righting reflexes. Adrenocorticotropic hormone1–24 was given simultaneously with sedative-hypnotic to stimulate corticosterone production and serum corticosterone concentrations were measured 30 minutes later. Four rats were studied in each group. Average corticosterone concentrations (±SD) were 460 ± 140 ng/ml in the vehicle control group, 90 ± 44 ng/ml in the etomidate group, and 610 ± 280 ng/ml in the MOC-carboetomidate group. *, P < 0.05; **, P < 0.01; NS, no significant difference.
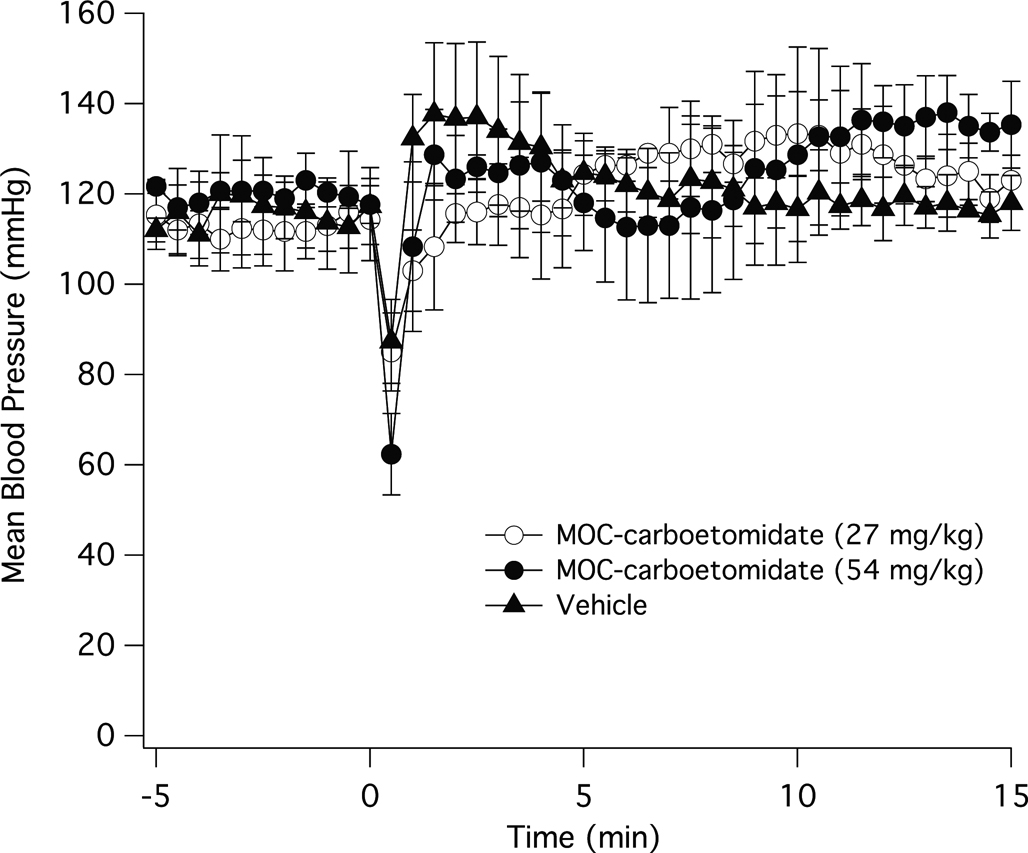
To determine whether the hemodynamic stability that is characteristic of etomidate, MOC-etomidate, and carboetomidate is preserved in MOC-carboetomidate, we measured the effect of MOC-carboetomidate at doses corresponding to 2× and 4× their ED50s for LORR in rats. Figure 8 shows the change in mean arterial blood pressure after a single IV bolus of 27 mg/kg MOC-etomidate, 54 mg/kg MOC-carboetomidate, or DMSO vehicle alone. Although there was a decrease in mean arterial blood pressure in all three groups 30 seconds after injection, the pressure recovered within one minute and was not significantly different at any time point among groups during the entire experiment.
Figure 8.
The effects of MOC-carboetomidate on mean arterial blood pressure in the rat. MOC-carboetomidate (27 mg/kg or 54 mg/kg) in dimethyl sulfoxide vehicle or vehicle alone was administered IV at time 0. In all three groups, there was a significant decrease in mean arterial blood pressure 30 s after injection. However among the three groups, the mean blood pressure was not significantly different at any time point during the experiment.
Discussion
The current study describes the synthesis and pharmacological activity of MOC-carboetomidate, an etomidate analog that combines the two distinct structural features of MOC-etomidate and carboetomidate into a single chemical entity. Its development was motivated by the observation that MOC-etomidate and carboetomidate individually possess pharmacological properties that could be desirable if present in a single drug. MOC-etomidate is rapidly metabolized and produces hypnosis of extremely short duration because it contains a metabolicallylabile ester moiety that renders it highly susceptible to ester hydrolysis. 15 Carboetomidate is longer-acting, but does not suppress in vivo adrenocortical function because the imidazole ring thought to be necessary for high affinity etomidate binding to 11b-hydroxylase has been replaced with a pyrrole ring. 16 In the current studies, we tested the hypothesis that MOC-etomidate’s metabolicallylabile ester group could be added to carboetomidate to produce a new chemical entity that possessed features common to both agents (i.e., GABAA receptor modulatory ability, potent hypnotic activity, and hemodynamic stability) and the key desirable properties described above that each possesses individually.
The current studies show that MOC-carboetomidate retains the GABAA receptor modulatory ability and potent hypnotic activity that are characteristic of etomidate and the two etomidate analogs upon which its structure is based. 15, 16, 18 With single bolus injection, it also maintains hemodynamic stability similar to carboetomidate and better than propofol, although we note that the DMSO vehicle that we used to formulate MOC-carboetomidate (and carboetomidate in our previous studies) produced a brief reduction in arterial blood pressure immediately after injection. However unlike etomidate and carboetomidate (which were not detectably metabolized in rat blood even after 20 minutes), MOC-carboetomidate was rapidly metabolized in blood. Its 1.3 min in vitro metabolic half-life, while significantly slower than that of MOC-etomidate (0.35 min), was similar to that reported for esmolol (2.27 min) 19, which also contains a metabolicallylabile ester moiety. The modestly slower metabolism of MOC-carboetomidate as compared to MOC-etomidate might be advantageous because it should reduce the quantity of drug required to maintain anesthesia while retaining sufficiently rapid elimination to produce fast recovery.
The slope of a plot of the duration of LORR versus the logarithm of the hypnotic dose depends upon the rate of drug clearance from the brain and is independent of hypnotic potency. 20, 21 We previously reported that this slope is shallower for MOC-etomidate than for etomidate, which is consistent with faster MOC-etomidate brain clearance due to more rapid metabolism. 15 This slope was also shallower for MOC-carboetomidate than for carboetomidate; however, the difference (4-fold) was not as large as we had previously measured when comparing MOC-etomidate versus etomidate (10-fold). In addition, the duration of LORR was ~4-fold longer after administering MOC-carboetomidate than we had previously observed upon administering equihypnotic doses of MOC-etomidate. 15 These results suggest that while MOC-carboetomidate is cleared from the brain more rapidly than carboetomidate, it is not cleared as quickly as MOC-etomidate perhaps because it is metabolized more slowly.
We administered MOC-carboetomidate dissolved in the organic solvent DMSO. This is a common approach with hydrophobic drugs during preclinical development because defining an optimal IV formulation can be time-consuming and expensive. However, it should be noted that formulation may affect the actions of very hydrophobic drugs. For example, Dutta and Ebling found that LORR occurred significantly more slowly and electroencephalographic burst suppression required higher doses when propofol was dissolved in an organic solvent (ethanol) rather than formulated as an emulsion. 22 This group subsequently measured 300-fold higher peak lung propofol concentrations in rats that received brief infusions of nonemulsified versus emulsified propofol and concluded that pulmonary uptake and release significantly slows the increase and decrease of arterial and brain concentrations of nonemulsified propofol and reduces peak brain concentrations. 23 If MOC-carboetomidate (or carboetomidate) administered in DMSO is similarly sequestered by the lung, then optimal formulation may reduce induction time and increase hypnotic potency. Alternately, it might be possible to develop less hydrophobic analogues of MOC-carboetomidate (or carboetomidate) that do not require formulation in an emulsion and have more rapid onset of action.
In conclusion, MOC-carboetomidate combines the unique structural features of MOC-etomidate and carboetomidate into a single chemical entity. It is a GABAA receptor modulator with potent hypnotic activity that is more rapidly metabolized and cleared from the brain than carboetomidate, maintains hemodynamic stability, and does not suppress adrenocortical function.
Acknowledgments
Funding: Supported by grants R01-GM087316, R21-DA029253, and K08-GM083216 from the National Institutes of Health, Bethesda, MD and the Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
Name: Ervin Pejo, B.S.
Contribution: EP led in conduct of study and manuscript preparation. He attests to the integrity of the data and analysis.
Conflicts: EP has no conflicts of interest to declare.
Name: Joseph F. Cotten M.D., Ph.D.
Contribution: JFC assisted in study design, data interpretation, and manuscript preparation.
Conflicts: JFC is a co-inventor on a patent application submitted by the Massachusetts General Hospital. He, his department, his laboratory, and his institution could receive royalties relating to the development of methoxycarbonyl-carboetomidate or related analogs.
Name: Elizabeth W. Kelly
Contribution: EWK performed the electrophysiology experiments
Conflicts: EWK has no conflicts of interest to declare.
Name: Ri Le Ge, M.D., Ph.D.
Contribution: RLG assisted in study conduct.
Conflicts: RLG has no conflicts of interest to declare.
Name: Gregory D. Cuny, Ph.D.
Contribution: GDC designed the synthetic pathways for the syntheses of methoxycarbonyl-carboetomidate and carboetomidate.
Conflicts: GDC is a co-inventor on a patent application submitted by the Massachusetts General Hospital. He, his department, his laboratory, and his institution could receive royalties relating to the development of methoxycarbonyl-carboetomidate or related analogs.
Name: Joydev K. Laha Ph.D.
Contribution: JKL help to design the synthetic pathways for the syntheses of methoxycarbonyl-carboetomidate and carboetomidate.
Conflicts: JKL has no conflicts of interest to declare.
Name: Jifeng Liu, Ph.D.
Contribution: JL oversaw the synthesis and purification of methoxycarbonyl-carboetomidate and carboetomidate.
Conflicts: JL has no conflicts of interest to declare.
Name: Xiang Jie Lin, MSc
Contribution: XJL performed the synthesis and purification of methoxycarbonyl-carboetomidate and carboetomidate.
Conflicts: XJL has no conflicts of interest to declare.
Name: Douglas E. Raines, M.D.
Contribution: DER conceived of methoxycarbonyl-carboetomidate, assisted in study design, data interpretation, and manuscript preparation.
Attestation: Dr. Raines attests to the integrity of the data and analysis.
Conflicts: DER is a co-inventor on a patent application submitted by the Massachusetts General Hospital. He, his department, his laboratory, and his institution could receive royalties relating to the development of methoxycarbonyl-carboetomidate or related analogs. DER holds an equity position in Annovation BioPharma, a pharmaceutical company that seeks to develop technologies covered by that patent.
This manuscript was handled by: Marcel E. Durieux, MD, PhD
Contributor Information
Ervin Pejo, Massachusetts General Hospital, Boston, MA
Joseph F. Cotten, Massachusetts General Hospital, Boston, MA
Elizabeth W. Kelly, Massachusetts General Hospital, Boston, MA
Ri Le Ge, Massachusetts General Hospital, Boston, MA
Gregory D. Cuny, Brigham and Women’s Hospital, Boston, MA
Joydev K. Laha, Brigham and Women’s Hospital, Boston, MA
Jifeng Liu, Aberjona Laboratories, Inc, Beverly, MA
Xiang Jie Lin, Aberjona Laboratories, Inc, Beverly, MA.
Douglas E. Raines, Massachusetts General Hospital, Boston, MA
References
- 1.Janssen PA, Niemegeers CJ, Schellekens KH, Lenaerts FM. Etomidate, R-(+)-ethyl-1-(-methyl-benzyl)imidazole-5-carboxylate (R 16659), a potent, short-acting and relatively atoxic intravenous hypnotic agent in rats. Arzneimittelforschung. 1971;21:1234–1243. [PubMed] [Google Scholar]
- 2.Vinson DR, Bradbury DR. Etomidate for procedural sedation in emergency medicine. Ann Emerg Med. 2002;39:592–598. doi: 10.1067/mem.2002.123695. [DOI] [PubMed] [Google Scholar]
- 3.Guldner G, Schultz J, Sexton P, Fortner C, Richmond M. Etomidate for rapid-sequence intubation in young children: hemodynamic effects and adverse events. Acad Emerg Med. 2003;10:134–139. doi: 10.1111/j.1553-2712.2003.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 4.Zed PJ, Abu-Laban RB, Harrison DW. Intubating conditions and hemodynamic effects of etomidate for rapid sequence intubation in the emergency department: an observational cohort study. Acad Emerg Med. 2006;13:378–383. doi: 10.1197/j.aem.2005.11.076. [DOI] [PubMed] [Google Scholar]
- 5.Dhawan N, Chauhan S, Kothari SS, Kiran U, Das S, Makhija N. Hemodynamic responses to etomidate in pediatric patients with congenital cardiac shunt lesions. J Cardiothorac Vasc Anesth. 2010;24:802–807. doi: 10.1053/j.jvca.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Siedy J, Knapik P, Saucha W, Gross M. Comparison of propofol and etomidate anaesthesia for elective electrical cardioversion. Kardiol Pol. 2010;68:1249–1255. [PubMed] [Google Scholar]
- 7.de Jong FH, Mallios C, Jansen C, Scheck PA, Lamberts SW. Etomidate suppresses adrenocortical function by inhibition of 11 beta-hydroxylation. J Clin Endocrinol Metab. 1984;59:1143–1147. doi: 10.1210/jcem-59-6-1143. [DOI] [PubMed] [Google Scholar]
- 8.Wagner RL, White PF, Kan PB, Rosenthal MH, Feldman D. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N Engl J Med. 1984;310:1415–1421. doi: 10.1056/NEJM198405313102202. [DOI] [PubMed] [Google Scholar]
- 9.Duthie DJ, Fraser R, Nimmo WS. Effect of induction of anaesthesia with etomidate on corticosteroid synthesis in man. Br J Anaesth. 1985;57:156–159. doi: 10.1093/bja/57.2.156. [DOI] [PubMed] [Google Scholar]
- 10.Jackson WL., Jr. Should we use etomidate as an induction agent for endotracheal intubation in patients with septic shock?: a critical appraisal. Chest. 2005;127:1031–1038. doi: 10.1378/chest.127.3.1031. [DOI] [PubMed] [Google Scholar]
- 11.Fengler BT. Should etomidate be used for rapid-sequence intubation induction in critically ill septic patients? Am J Emerg Med. 2008;26:229–232. doi: 10.1016/j.ajem.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 12.Hildreth AN, Mejia VA, Maxwell RA, Smith PW, Dart BW, Barker DE. Adrenal suppression following a single dose of etomidate for rapid sequence induction: a prospective randomized study. J Trauma. 2008;65:573–579. doi: 10.1097/TA.0b013e31818255e8. [DOI] [PubMed] [Google Scholar]
- 13.Cuthbertson BH, Sprung CL, Annane D, Chevret S, Garfield M, Goodman S, Laterre PF, Vincent JL, Freivogel K, Reinhart K, Singer M, Payen D, Weiss YG. The effects of etomidate on adrenal responsiveness and mortality in patients with septic shock. Intensive Care Med. 2009 doi: 10.1007/s00134-009-1603-4. [DOI] [PubMed] [Google Scholar]
- 14.Kulstad EB, Kalimullah EA, Tekwani KL, Courtney DM. Etomidate as an induction agent in septic patients: red flags or false alarms? West J Emerg Med. 2010;11:161–172. [PMC free article] [PubMed] [Google Scholar]
- 15.Cotten JF, Husain SS, Forman SA, Miller KW, Kelly EW, Nguyen HH, Raines DE. Methoxycarbonyl-etomidate: a novel rapidly metabolized and ultra-short-acting etomidate analogue that does not produce prolonged adrenocortical suppression. Anesthesiology. 2009;111:240–249. doi: 10.1097/ALN.0b013e3181ae63d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotten JF, Forman SA, Laha JK, Cuny GD, Husain SS, Miller KW, Nguyen HH, Kelly EW, Stewart D, Liu A, Raines DE. Carboetomidate: a pyrrole analog of etomidate designed not to suppress adrenocortical function. Anesthesiology. 2010;112:637–644. doi: 10.1097/ALN.0b013e3181cf40ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waud DR. On biological assays involving quantal responses. J Pharmacol Exp Ther. 1972;183:577–607. [PubMed] [Google Scholar]
- 18.Rusch D, Zhong H, Forman SA. Gating allosterism at a single class of etomidate sites on alpha1beta2gamma2L GABA A receptors accounts for both direct activation and agonist modulation. J Biol Chem. 2004;279:20982–20992. doi: 10.1074/jbc.M400472200. [DOI] [PubMed] [Google Scholar]
- 19.Quon CY, Stampfli HF. Biochemical properties of blood esmolol esterase. Drug Metab Dispos. 1985;13:420–424. [PubMed] [Google Scholar]
- 20.Shafer SL. Principles of Pharmacokinetics and Pharmacodynamics. In: Longnecker DE, Tinker JH, Morgan GE, editors. Anesthesiology. St. Louis: 1998. pp. 1159–1210. [Google Scholar]
- 21.Liao M, Sonner JM, Husain SS, Miller KW, Jurd R, Rudolph U, Eger EI., 2nd R (+) etomidate and the photoactivable R (+) azietomidate have comparable anesthetic activity in wild-type mice and comparably decreased activity in mice with a N265M point mutation in the gamma-aminobutyric acid receptor beta3 subunit. Anesth Analg. 2005;101:131–135. doi: 10.1213/01.ANE.0000153011.64764.6F. table of contents. [DOI] [PubMed] [Google Scholar]
- 22.Dutta S, Ebling WF. Emulsion formulation reduces propofol's dose requirements and enhances safety. Anesthesiology. 1997;87:1394–1405. doi: 10.1097/00000542-199712000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Dutta S, Ebling WF. Formulation-dependent brain and lung distribution kinetics of propofol in rats. Anesthesiology. 1998;89:678–685. doi: 10.1097/00000542-199809000-00018. [DOI] [PubMed] [Google Scholar]