Abstract
Aspirin and celecoxib prevent colorectal adenoma recurrence. Genetic variants in the UGT1A6 enzyme are associated with delayed aspirin metabolism and greater chemopreventive efficacy. We examined the effect of combining aspirin and celecoxib in relation to UGT1A6 T181A and R184S variants among 1,647 patients in the Adenoma Prevention with Celecoxib (APC) trial who were stratified according to the use of low-dose aspirin after removal of adenomas and randomized to placebo, 200 mg-twice-daily, or 400 mg-twice-daily celecoxib for 3 years. Patients underwent follow-up colonoscopies at 1 and 3 years to assess on-treatment efficacy. At 5 years, 538 patients underwent a colonoscopy to assess risk of recurrence after treatment was discontinued for at least 1 year. During treatment, the relative risk (RR) of recurrent adenoma was 0.68 (95%CI,0.59–0.79) for 200 mg-twice-daily celecoxib and 0.54 (95%CI,0.46–0.64) for 400 mg-twice-daily celecoxib compared with placebo. Aspirin use was not independently associated with recurrent adenoma (RR, 0.98, 95%CI,0.86–1.15). These results did not vary according to UGT1A6 genotype. However, among those with a variant UGT1A6 genotype on aspirin, the RR of adenoma was 1.60 (95%CI,0.81–3.15) after withdrawal of 200 mg-twice-daily and 1.98 (95%CI,1.06–3.70) after withdrawal of 400 mg-twice-daily celecoxib compared to withdrawal of placebo. In contrast, there was no increased risk associated with discontinuing celecoxib among any other groups. Concurrent use of low-dose aspirin does not influence the efficacy of celecoxib in adenoma prevention. However, discontinuing celecoxib among aspirin-using individuals that initially developed adenoma despite a UGT1A6 variant genotype resulted in rapid re-emergence of disease.
Keywords: Adenoma, celecoxib, UGT1A6, inflammation, chemoprevention
Introduction
Randomized, placebo-controlled trials have shown that aspirin and celecoxib each reduce the risk of recurrent colorectal adenoma (1–6). In the Adenoma Prevention with Celecoxib (APC) trial, patients who had recently undergone colonoscopic removal of an adenoma were randomly assigned to receive placebo, low-dose (200 mg, twice daily) or high dose (400 mg, twice daily) celecoxib and underwent follow-up colonoscopies at 1 and 3 years. The relative risk (RR) of the detection of one or more new adenomas by year 3 compared with placebo was 0.67 (95% confidence interval [CI] 0.59–0.77) for those receiving low-dose celecoxib and 0.55 (95% CI, 0.48–0.64) for those receiving high-dose celecoxib (5). Unfortunately, in a separate, adjudicated safety analysis, the APC trial also revealed unexpected dose-related cardiovascular toxicity (7).
Aspirin is principally glucuronidated by the UDP-glucuronyosyltransferase isoenzyme 1A6 (UGT1A6) (8). Two common variant alleles -- a tandem mutation in amino acids 181 and 184 (T181A+R184S) and a single mutation in amino acid 184 (R184S) -- are present in 30% and 2% of Caucasians, respectively. Compared with wild-type, these polymorphisms are associated with 30–50% lower enzyme activity (9). We, and others, have previously shown that variant UGT1A6 genotypes significantly enhance the effect of aspirin on adenoma risk (10, 11).
All patients enrolled on the APC Trial had a high risk of adenoma development due to a history of large (≥6mm) or multiple adenomas. Approximately one third of those randomized on the study was using aspirin at enrollment, and therefore likely had developed adenomas while taking aspirin. Aspirin use was a stratification factor for randomization to celecoxib versus placebo. Aspirin use continued for all of these participants during the trial. Because the APC trial was a placebo-controlled, randomized trial of celecoxib, and use of low-dose aspirin was carefully monitored, we had a unique opportunity to examine the joint influence of these drugs and UGT1A6 genotype in relation to risk of adenoma and adverse events. Moreover, in a 5-year extension study, a subset of patients in the APC trial underwent a colonoscopy after discontinuing celecoxib for at least 1 year. Thus, we were also able to evaluate the effect of drug withdrawal on risk of recurrent disease (12).
Methods
Study Population
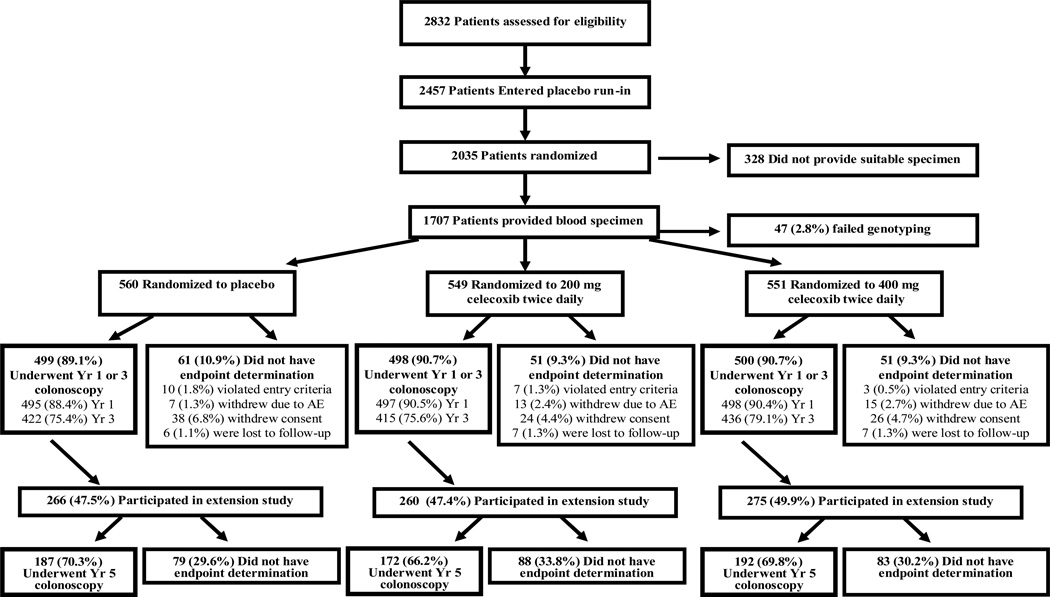
The APC trial was an NCI-sponsored, randomized, placebo-controlled trial which enrolled patients who had undergone colonoscopic removal of multiple colorectal adenomas or a single adenoma ≥6mm in diameter within 6 months of study entry (ClinicalTrials.gov NCT00005094) (5). The flow of patients through the study is summarized in the Figure. Beginning in November 1999, 2,457 potential participants at 91 clinical sites were entered into a 30-day placebo run-in period. After the run-in period, 2,035 patients with at least 80 percent adherence to medication use during the run-in period were subsequently randomly assigned to placebo, low-dose (200 mg, twice daily) or high-dose (400 mg, twice daily) celecoxib. Randomization was stratified on the basis of the use or non-use of low-dose aspirin (325 mg or less every other day or 162.5 mg or less every day) and clinical site. Aspirin users at baseline continued to take low-dose aspirin during the study; no other NSAID use was permitted. Patients were excluded if they had a history of familial adenomatous polyposis, hereditary nonpolyposis colon cancer, inflammatory bowel disease, or large-bowel resection other than appendectomy. Other exclusion criteria included a history of a renal or hepatic disorder, a clinically significant bleeding disorder, or treatment for a gastrointestinal ulcer before study entry. All patients provided written informed consent before enrollment and the study protocol was approved by the human subjects committee at each study site.
Figure. Flow of Patients Through the Study.
Patients who violated study entry criteria were those for whom the presence of an adenoma on colonoscopy at baseline could not be confirmed. Patients who withdrew consent for study participation included those who withdrew from the study for medical or nonmedical reasons, those who failed to complete a post-randomization colonoscopy for nonmedical reasons, or those who did not adhere to the protocol for other reasons. Adherence to the use of study medication was calculated as the duration of use in days, divided by 1095. Percentages do not always total 100 due to rounding.
Study drug treatment was initially planned for three years for all participants. However, at the recommendation of the APC trial Data Safety Monitoring Board (DSMB) treatment was terminated prematurely on December 17, 2004 based on the results of a safety analysis performed by an independent cardiovascular safety committee. At that time 1,762 patients (86.6 percent) had completed three years of treatment and 273 patients had 1 to 3 months of treatment remaining. In addition, 639 patients had begun participation in the extension study in which patients were planned to continue study medication in a blinded manner or continued in a surveillance only arm for an additional 2 years with a planned colonoscopy for identification and removal of colorectal polyps at 5 years after the initial randomization (Year 5 colonoscopy). Because all extension study patients were discontinued from study medication as of December 17, 2004, the median duration of treatment exposure in the extension study was 3.5 years and >94% of participants had been off study drug for at least 1 year at the time of the Year 5 colonoscopy (12).
Aspirin Use
Over the three year treatment period, patients who were taking low-dose aspirin (325 mg or less every other day or 162.5 mg or less every day) at study entry were supplied their usual dose of low-dose aspirin while they were on study medication. All patients were also supplied acetaminophen for treatment of minor pain and febrile illness. Investigators at the study site contacted patients every 2 months to counsel them to avoid nonprotocol use of aspirin or NSAIDs.
Outcome Ascertainment
A study investigator performed follow-up colonoscopies with endoscopic removal of polyps at one, three, and five years after randomization. A central study pathologist examined, in a blinded fashion, all polyps removed during these colonoscopies. Patients on treatment were contacted every 2 months by investigators at their study site to report any adverse events. Subjects in the extension study who discontinued study medication were contacted every 6 months to report adverse events. Adverse events were classified according to criteria from the Medical Dictionary for Regulatory Activities (MedDRA), version 8.1.
Genotyping
Among the 2,035 patients enrolled, 1,707 patients at the baseline exam provided a blood specimen. All patients who provided specimens provided separate, informed consent and a genotyping study protocol was approved by the Human Subjects Committee at Partners Healthcare. All genotyping was performed, in a blinded fashion, at the Dana-Farber Harvard Cancer Center High Throughput Polymorphism Detection Core on de-identified patient samples using previously described methods (13). In brief, we extracted genomic DNA from peripheral white blood cells by using the QiAmp 96 spin blood procedure (Qiagen, Chatsworth, CA) and performed genotyping using the Taqman Assay on the ABI Prism 7900HT Sequence Detection System (PE Applied Biosystems, Foster City, CA) in a 384-well format. We genotyped the T181A (rs2070959) and R184S (rs1105879) polymorphisms of UGT1A6. Among the 1707 patients, genotyping was unsuccessful for the T181A allele or R184S allele in 60 patients. These patients were subsequently excluded from the analysis. We inserted blinded quality control samples equal to 10% of the total number of samples to validate genotype identification procedures; concordance for these samples was 100%. Using the χ2 goodness-of-fit test, the genotype distribution was in Hardy-Weinberg equilibrium for the T181A allele (p=.42) and R184S allele (p=.85).
Statistical Analysis
Consistent with the intent-to-treat principle (5), we used the detection of an adenoma during the post-randomization colonoscopy at Year 1 or Year 3, regardless of whether the patient adhered to the treatment regimen, as the primary efficacy endpoint for the on-treatment analysis. There were 8 interval colorectal cancers that developed during treatment (3 on the placebo arm and 5 on the 400 mg-twice-daily arm). These were included as adenoma endpoints in the analyses. We used the Mantel-Cox test, which is a life-table extension of the Mantel-Haenszel statistic, with stratification for aspirin use or nonuse, sex, and age (<65 vs. ≥65 years). The Mantel-Cox procedure also provides a summary risk ratio, which is the weighted average of the relative risk over the two intervals and across strata of aspirin use, sex, and age (14, 15). Patients with no follow-up colonoscopy (n=163) were excluded from both follow-up intervals. A patient with a colonoscopy at Year 3 but with no colonoscopy at Year 1 was included in the analysis through Year 1, with the assumption that the patient had no adenoma at Year 1, and was then included in the analysis through Year 3 according to the findings of the colonoscopy at Year 3. The analyses at Year 3 excluded patients with an adenoma at Year 1 colonoscopy and patients with no adenoma at Year 1 and no colonoscopy at Year 3. We estimated the cumulative incidence of adenoma at 3 years within different subgroups using the Kaplan-Meier method.
To assess the impact of discontinuing celecoxib treatment on risk of recurrent adenoma, we calculated the percentage of patients with any adenoma among those who continued in the extension study at the Year 5 colonoscopy irrespective of the results of prior colonoscopies. Because celecoxib was discontinued for all participants on December 17, 2004, >94% of participants had been off study drug for at least 1 year at the time of the Year 5 colonoscopy. The summary risk ratio was also estimated by the Mantel-Cox method controlling for genotype and aspirin use.
For our analysis of adverse events, we included investigator-reported adverse events occurring after the first dose and up to 30 days after the last dose of study medication including events among patients who continued study medication in the 24 month extension study (12, 16). The risk ratio of an adverse event was estimated using Cox regression analysis stratified by different baseline hazards for low-dose aspirin use. The risk ratios among all patients were also adjusted for genotype.
Consistent with prior studies (10, 11, 17, 18), we defined patients with wild-type genotypes as having no variant R184S or T181A (wild-type/wild-type) and patients with variant genotypes as having ≥ one T181A+R184S, R184S, or T181A allele. The genotype groups were assessed for differences in baseline characteristics using analysis of variance for continuous variables and Chi-squared tests for categorical variables. To assess the heterogeneity of treatment between aspirin use and non-use, a test of the interaction between treatment and aspirin use from a logistic regression model was performed. We used the SAS version 9.2 (SAS institute, Cary, NC) for all analyses. All P values are two-sided.
Results
Among the 2,035 patients who were randomly assigned to treatment, 1,707 (84%) provided a suitable blood specimen for genotyping and 1647 (96%) of these patients were successfully genotyped (Figure). Colonoscopic endpoints were assessed in 1,484 (90%) of randomized subjects. Overall, 717 (44%) had wild-type genotypes and 930 (56%) had variant genotypes (≥ one T181A+R184S or R184S allele) (Supplementary Table). Baseline characteristics, including age, gender, and initial adenoma size and number, were largely similar according to genotype (Table 1).
Table 1.
Baseline Characteristics of the Patients According to UGT1A6 Genotype a
| Characteristics | All (n =1647) |
Wild-type (n = 717) |
Variant (n = 930) |
P value b |
|---|---|---|---|---|
| Age, median y, (range) | 59 (31, 88) | 59 (35, 87) | 59 (31, 88) | 0.38 |
| Women (%) | 32 | 32 | 32 | 0.97 |
| Race or ethnic group (%) c | ||||
| Non-hispanic White | 92 | 91 | 92 | 0.21 |
| Non-hispanic Black | 5 | 6 | 5 | |
| Hispanic | 2 | 1 | 2 | |
| Asian/Pacific Islander/Other | 1 | 1 | 1 | |
| Current cigarette smoker (%) | 17 | 18 | 16 | 0.59 |
| Body-mass index d | ||||
| Men | 28.7 (0.1) | 28.9 (0.2) | 28.6 (0.2) | 0.18 |
| Women | 29.0 (0.3) | 29.1 (0.4) | 29.0 (0.4) | 0.89 |
| Colorectal cancer in a parent (%) | 21 | 21 | 21 | 0.70 |
| Findings at baseline colonoscopy | ||||
| No. of adenomas | 2.1 (0.04) | 2.1 (0.06) | 2.1 (0.05) | 0.49 |
| At least one adenoma ≥ 1cm (%) | 43 | 42 | 44 | 0.56 |
| Multiple adenomas (%) | 56 | 56 | 55 | 0.60 |
| Adenoma burden, cm.e | 1.5 (0.03) | 1.5 (0.05) | 1.5 (0.04) | 0.96 |
| History of cardiovascular events (%) f | 14 | 14 | 14 | 0.82 |
| History of hypertension (%) | 40 | 40 | 41 | 0.64 |
| History of diabetes (%) | 9 | 9 | 10 | 0.34 |
| Use of low-dose aspirin (%) g | 32 | 33 | 31 | 0.35 |
| Randomized to placebo (%) | 34 | 34 | 34 | |
| Randomized to celecoxib, 200 mg twice daily (%) | 33 | 32 | 34 | |
| Randomized to celecoxib, 400 mg twice daily (%) | 33 | 34 | 32 |
Data are expressed as mean (SD) unless otherwise indicated. Wild-type UGT1A6 genotypes include individuals with no T181A or R184S alleles. Variant UGT1A6 genotypes include individuals with ≥ one variant (T181 or R184S) allele.
Test of difference between wild-type and variant genotype groups was calculated by analysis of variance for continuous variables, χ2 for categorical variables.
Race or ethnic group was determined by the investigator using predefined categories.
Body-mass index is the weight in kilograms divided by the square of the height in meters.
The adenoma burden was defined as the sum of the diameter of all adenomas reported during colonoscopy at baseline.
Cardiovascular events were defined as myocardial infarction, cerebrovascular disease, congestive heart failure, angina, and atherosclerotic heart disease.
Low-dose aspirin was defined as 325 mg or less every other day or 162.5 mg or less every day.
Because study medication was discontinued in December 2004, the colonoscopies conducted at 1 and 3 years after randomization represented maximal treatment exposure and were used to determine on-treatment efficacy (5, 12). We first examined the on-treatment effect of UGT1A6 genotype, aspirin use, and celecoxib on risk of adenoma through the 3-year study period. There was no overall association of the UGT1A6 genotype with adenoma risk (RR, 0.97; 95% CI, 0.86–1.10), which did not vary across all subgroups of aspirin use or celecoxib treatment. For patients of all genotypes, the estimated cumulative incidence of one or more adenomas by year 3 was 59.8% for those randomized to placebo, as compared with 43.3% for those randomized to low-dose (200 mg, twice daily) celecoxib (RR, 0.68; 95% CI, 0.59–0.79; P<.001), and 36.8% for those randomized to high-dose (400 mg, twice daily) celecoxib (RR, 0.54; 95% CI, 0.46–0.64; P<.001) (Table 2). There was no material alteration in these associations within subgroups defined by UGT1A6 genotype. We also did not observe a differential benefit of celecoxib treatment according to strata of concurrent aspirin use.
Table 2.
Risk of Adenoma According to Celecoxib Treatment, Stratified by Aspirin Use and UGT1A6 Genotype a
| Placebo | Celecoxib, 200 mg Twice Daily |
Celecoxib, 400 mg Twice Daily |
|
|---|---|---|---|
| All Genotypes | |||
| All patients, No. at risk b | 493 | 497 | 494 |
| Cumulative incidence, 3 yrs, % ± SE | 59.8 ± 2.3 | 43.3 ± 2.3 | 36.8 ± 2.3 |
| RR (95% CI) c | 1.0 | 0.68 (0.59–0.79) | 0.54 (0.46–0.64) |
| P value d | -- | <.001 | <.001 |
| Aspirin users, No. at risk b | 162 | 160 | 152 |
| Cumulative incidence, 3 yrs, % ± SE | 61.0 ± 4.0 | 46.0 ± 4.1 | 38.7 ± 4.1 |
| RR (95% CI) e | 1.0 | 0.69 (0.54–0.89) | 0.54 (0.41–0.73) |
| P value d | -- | .004 | <.001 |
| Non-aspirin users, No. at risk b | 331 | 337 | 342 |
| Cumulative incidence, 3 yrs, % ± SE | 59.2 ± 2.8 | 42.0 ± 2.8 | 36.0 ± 2.7 |
| RR (95% CI) e | 1.0 | 0.67 (0.56–0.81) | 0.54 (0.44–0.66) |
| P value d | -- | <.001 | <.001 |
| Patients with wild-type genotypes | |||
| All wild-type genotypes, No. at risk b | 212 | 213 | 221 |
| Cumulative incidence, 3 yrs, % ± SE | 62.3 ± 3.4 | 42.0 ± 3.5 | 36.3 ± 3.4 |
| RR (95% CI) f | 1.0 | 0.63 (0.50– 0.78) | 0.51 (0.40–0.65) |
| P value d | -- | <.001 | <.001 |
| Aspirin users, No. at risk b | 74 | 69 | 69 |
| Cumulative incidence, 3 yrs, % ± SE | 64.3 ± 3.4 | 44.0 ± 6.2 | 35.7 ± 6.1 |
| RR (95% CI) g | 1.0 | 0.64 (0.44– 0.92) | 0.51 (0.33–0.77) |
| P value d | -- | .013 | <.001 |
| Non-aspirin users, No. at risk b | 138 | 144 | 152 |
| Cumulative incidence, 3 yrs, % ± SE | 61.4 ± 4.2 | 41.0 ± 4.3 | 36.5 ± 4.0 |
| RR (95% CI) g | 1.0 | 0.62 (0.47– 0.82) | 0.51 (0.38–0.69) |
| P value d | -- | <.001 | <.001 |
| Patients with variant genotypes | |||
| All variant genotypes, No. at risk b | 281 | 284 | 273 |
| Cumulative incidence, 3 yrs, % ± SE | 57.9 ± 3.1 | 44.2 ± 3.1 | 37.1 ± 3.0 |
| RR (95% CI) f | 1.0 | 0.72 (0.59– 0.88) | 0.57 (0.45–0.71) |
| P value d | -- | .001 | <.001 |
| Aspirin Users, No. at risk b | 88 | 91 | 83 |
| Cumulative incidence, 3 yrs, % ± SE | 58.4 ± 5.4 | 47.4 ± 5.1 | 40.7 ± 5.6 |
| RR (95% CI) g | 1.0 | 0.74 (0.53– 1.04) | 0.57 (0.38–.86) |
| P value d | -- | .084 | .005 |
| Non-aspirin Users, No. at risk b | 193 | 193 | 190 |
| Cumulative incidence, 3 yrs, % ± SE | 57.6 ± 3.7 | 42.6 ± 3.7 | 35.6 ± 3.6 |
| RR (95% CI) g | 1.0 | 0.71 (0.56– 0.91) | 0.56 (0.44–.73) |
| P value d | -- | .006 | <.001 |
Cumulative risk of adenoma through follow-up colonoscopy at year 3. Patients were stratified at study entry according to the use or nonuse of low-dose aspirin (325 mg or less every other day or 162.5 mg or less every day). Patients not taking aspirin at baseline were required to abstain from taking it during the trial. Wild-type UGT1A6 genotypes include individuals with no T181A or R184S alleles. Variant UGT1A6 genotypes include individuals with ≥ one variant (T181 or R184S) allele.
No. at risk includes patients who underwent a follow-up colonoscopy at year 1 and/or year 3.
Risk ratio is estimated by the Mantel-Cox method, with stratification for genotype, aspirin use, time, age, and sex, with the placebo group as the referent group.
The p value is the Cochran-Mantel-Haenszel test of general association compared to the placebo group.
Risk ratio is estimated by the Mantel-Cox method, with stratification for genotype, time, age, and sex, with the placebo group as the referent group.
Risk ratio is estimated by the Mantel-Cox method, with stratification for aspirin use, time, age, and sex, with the placebo group as the referent group.
Risk ratio is estimated by the Mantel-Cox method, with stratification for time, age, and sex, with the placebo group as the referent group.
We specifically examined the influence of low-dose aspirin use on the on-treatment risk of adenoma through the colonoscopy at Year 3 (Table 3). Among all genotypes, low-dose aspirin was not associated with risk of adenoma (RR, 0.98; 95% CI, 0.86–1.15; P=0.79). This association was consistent across strata defined by genotype and randomized celecoxib treatment arm.
Table 3.
Risk of Adenoma According to Aspirin Use, Stratified by Celecoxib Treatment and UGT1A6 Genotype a
| Non-aspirin User | Aspirin User | |
|---|---|---|
| All Genotypes | ||
| All patients, No. at risk b | 1010 | 474 |
| Cumulative incidence, 3 yrs, % ± SE | 45.6 ± 1.6 | 48.8 ± 2.4 |
| RR (95% CI) c | 1.0 | 0.98 (0.86–.15) |
| P value d | -- | 0.79 |
| Randomized to placebo, No. at risk b | 331 | 162 |
| Cumulative incidence, 3 yrs, % ± SE | 59.2 ± 2.8 | 61.0 ± 4.0 |
| RR (95% CI) e | 1.0 | 0.96 (0.79–.16) |
| P value d | -- | .68 |
| Randomized to celecoxib 200 mg twice daily, No. at risk b | 337 | 160 |
| Cumulative incidence, 3 yrs, % ± SE | 42.0 ± 2.8 | 46.0 ± 4.1 |
| RR (95% CI) e | 1.0 | 1.02 (0.80–.31) |
| P value d | -- | 0.86 |
| Randomized to celecoxib 400 mg twice daily, No. at risk b | 342 | 152 |
| Cumulative incidence, 3 yrs, % ± SE | 36.0 ± 2.7 | 38.7 ± 4.1 |
| RR (95% CI) e | 1.0 | 0.97 (0.72–.30) |
| P value d | -- | 0.83 |
| Patients with wild-type genotypes | ||
| All wild-type genotypes, No. at risk b | 434 | 212 |
| Cumulative incidence, 3 yrs, % ± SE | 45.9 ± 2.5 | 48.3 ± 3.6 |
| RR (95% CI) f | 1.0 | 0.96 (0.79–.18) |
| P value d | -- | 0.73 |
| Randomized to placebo, No. at risk b | 138 | 74 |
| Cumulative incidence, 3 yrs, % ± SE | 61.4 ± 4.2 | 64.3 ± 6.0 |
| RR (95% CI) g | 1.0 | 0.96 (0.73–.26) |
| P value d | -- | .76 |
| Randomized to celecoxib 200 mg twice daily, No. at risk b | 144 | 69 |
| Cumulative incidence, 3 yrs, % ± SE | 41.0 ± 4.3 | 44.0 ± 6.2 |
| RR (95% CI) g | 1.0 | 0.97 (0.66–.43) |
| P value d | -- | 0.88 |
| Randomized to celecoxib 400 mg twice daily, No. at risk b | 152 | 69 |
| Cumulative incidence, 3 yrs, % ± SE | 36.5 ± 4.0 | 35.7 ± 6.1 |
| RR (95% CI) g | 1.0 | 0.97 (0.62–.50) |
| P value d | -- | 0.89 |
| Patients with variant genotypes | ||
| All variant genotypes, No. at risk b | 576 | 262 |
| Cumulative incidence, 3 yrs, % ± SE | 45.4 ± 2.2 | 49.0 ± 3.2 |
| RR (95% CI) f | 1.0 | 1.00 (0.83–.19) |
| P value d | -- | 0.96 |
| Randomized to placebo, No. at risk b | 193 | 88 |
| Cumulative incidence, 3 yrs, % ± SE | 57.6 ± 3.7 | 58.4 ± 5.4 |
| RR (95% CI) g | 1.0 | 0.96 (0.74–.25) |
| P value d | -- | .77 |
| Randomized to celecoxib 200 mg twice daily, No. at risk b | 193 | 91 |
| Cumulative incidence, 3 yrs, % ± SE | 42.6 ± 3.7 | 47.4 ± 5.1 |
| RR (95% CI) g | 1.0 | 1.06 (0.77–.47) |
| P value d | -- | 0.72 |
| Randomized to celecoxib 400 mg twice daily, No. at risk b | 190 | 83 |
| Cumulative incidence, 3 yrs, % ± SE | 35.6 ± 3.6 | 40.7 ± 5.6 |
| RR (95% CI) g | 1.0 | 0.97 (0.66–.43) |
| P value d | -- | 0.86 |
Cumulative risk of adenoma through follow-up colonoscopy at year 3. Patients were stratified at study entry according to the use or nonuse of low-dose aspirin (325 mg or less every other day or 162.5 mg or less every day). Patients not taking aspirin at baseline were required to abstain from taking it during the trial. Wild-type UGT1A6 genotypes include individuals with no T181A or R184S alleles. Variant UGT1A6 genotypes include individuals with ≥ one variant (T181 or R184S) allele.
No. at risk includes patients who underwent a follow-up colonoscopy at year 1 and/or year 3.
Risk ratio is estimated by the Mantel-Cox method, with stratification for treatment, genotype, time, age, and sex, with non-aspirin users as the referent group.
The p value is the Cochran-Mantel-Haenszel test of general association compared to the non-aspirin user group.
Risk ratio is estimated by the Mantel-Cox method, with stratification for genotype, time, age, and sex, with non-aspirin users as the referent group
Risk ratio is estimated by the Mantel-Cox method, with stratification for treatment, time, age, and sex, with non-aspirin users as the referent group
Risk ratio is estimated by the Mantel-Cox method, with stratification for time, age, and sex, with non-aspirin users as the referent group
Consistent with our previous analysis, we estimated the effect of celecoxib cessation on adenoma recurrence by examining the risk of any adenoma, irrespective of the results of prior colonoscopies, among patients undergoing a year 5 colonoscopy, when >94% of the cohort had discontinued celecoxib for at least 1 year (12). At year 5, among patients who used low-dose aspirin, there appeared to be a greater risk of recurrent adenoma among patients who were previously on low-dose celecoxib (RR, 1.45; 95% CI, 0.89–2.34) and high-dose celecoxib (RR, 1.53, 95% 1.00–2.53). In contrast, among patients who were not on low-dose aspirin, there did not appear to be an increased risk of recurrent adenoma after celecoxib withdrawal. Overall, this difference in adenoma recurrence according to aspirin use was not statistically significant (Pinteraction=.15). However, among patients with variant UGT1A6 genotypes who used low-dose aspirin, the risk of recurrent adenoma was especially high, with a RR of 1.60 (95% CI, 0.81–3.15) for those previously on low-dose and 1.98 (95% CI, 1.06–3.70) previously on high-dose celecoxib. In contrast, there was no increased risk associated with cessation of celecoxib among patients with variant genotype who did not use aspirin. A formal test of whether the risk of recurrence associated with withdrawal of celecoxib differed according to aspirin use approached statistical significance (Pinteraction=.06). There was also no increased risk among patients with wild-type genotype irrespective of aspirin use.
Although the number of events was limited, we conducted an exploratory analysis examining the influence of UGT1A6 genotype on investigator-reported adverse events associated with celecoxib treatment. We focused on cardiovascular and thrombotic events, renal and hypertensive disorders, and gastrointestinal ulceration and hemorrhage separately, consistent with the primary analysis of trial (Table 5). Among patients of any genotype, the cumulative incidence of cardiovascular and thrombotic events was 5.2% among those who received placebo, compared with 7.4% for low-dose celecoxib (RR, 1.41; 95% CI, 0.83–2.37) and 8.9% for high-dose celecoxib (RR, 1.66; 95% CI, 1.00–2.76). This substantially agreed with the primary analysis of the trial (5), as well as a separate, prespecified analysis of adjudicated cardiovascular events that was previously reported (7). There were no consistent differences in cardiovascular risk according to subgroups defined by UGT1A6 genotype. Among patients of any genotype, compared with placebo, there was an increased risk of renal and hypertensive disorders with low-dose (RR, 1.35; 95% CI, 1.03–1.76) but not high-dose celecoxib (RR, 1.01, 95% CI, 0.76–1.33). However, this risk appeared to be attenuated among those individuals with UGT1A6 variant genotypes (RR, 1.10; 95% CI, 0.78–1.54 for low-dose and RR, 0.76; 95% CI, 0.52–1.10) for high-dose). There did not appear to be an increased risk of gastrointestinal hemorrhage with either dose of celecoxib irrespective of genotype.
Table 5.
Incidence of Adverse Events after Randomization According to Celecoxib Dose, Stratified by UGT1A6 Genotype a
| Placebo | Celecoxib, 200 mg Twice Daily |
Celecoxib, 400 mg Twice Daily |
|
|---|---|---|---|
| All genotypes, No. at risk | 550 | 547 | 543 |
| Cardiovascular disorders, b No. events | 24 | 34 | 40 |
| Cumulative incidence, 3 yrs, % ±SE | 5.2 ± 1.1 | 7.4 ± 1.3 | 8.9 ± 1.4 |
| RR (95% CI) | 1.0 | 1.41 (0.83–.37) | 1.66 (1.00–.76) |
| Renal and hypertensive disorders, c No. events | 97 | 124 | 97 |
| Cumulative incidence, 3 yrs, % ± SE | 19.9 ± 1.9 | 25.8 ± 2.1 | 20.3 ± 1.9 |
| RR (95% CI) | 1.0 | 1.35 (1.03–.76) | 1.01 (0.76–.33) |
| Gastrointestinal ulceration/hemorrhage, d No. events | 57 | 57 | 54 |
| Cumulative incidence, 3 yrs, % ± SE | 12.0 ± 1.6 | 12.0 ± 1.6 | 11.5 ± 1.5 |
| RR (95% CI) | 1.0 | 0.98 (0.68–.42) | 0.94 (0.65–.36) |
| Patients with wild-type genotypes, No. at risk | 239 | 232 | 241 |
| Cardiovascular disorders, b No. events | 11 | 13 | 21 |
| Cumulative incidence, 3 yrs, % ± SE | 5.6 ± 1.6 | 6.1 ± 1.8 | 10.1 ± 2.2 |
| RR (95% CI) | 1.0 | 1.14 (0.51–.55) | 1.81 (0.87–.75) |
| Renal and hypertensive disorders, c No. events | 32 | 54 | 49 |
| Cumulative incidence, 3 yrs, % ± SE | 15.3 ± 2.6 | 26.8 ± 3.2 | 22.9 ± 3.1 |
| RR (95% CI) | 1.0 | 1.87 (1.21–.89) | 1.50 (0.96–.34) |
| Gastrointestinal ulceration/hemorrhage, d No. events | 23 | 26 | 17 |
| Cumulative incidence, 3 yrs, % ± SE | 12.4 ± 2.5 | 12.2 ± 2.4 | 8.3 ± 2.0 |
| RR (95% CI) | 1.0 | 1.14 (0.65–.00) | 0.68 (0.36–.27) |
| Patients with variant genotypes, No. at risk | 311 | 315 | 302 |
| Cardiovascular disorders, b No. events | 13 | 21 | 19 |
| Cumulative incidence, 3 yrs, % ± SE | 4.8 ± 1.4 | 8.4 ± 1.8 | 7.9 ± 1.8 |
| RR (95% CI) | 1.0 | 1.61 (0.81–3.22) | 1.52 (0.75–3.08) |
| Renal and hypertensive disorders, c No. events | 65 | 70 | 48 |
| Cumulative incidence, 3 yrs, % ± SE | 23.3 ± 2.7 | 25.1 ± 2.7 | 18.3 ± 2.5 |
| RR (95% CI) | 1.0 | 1.10 (0.79–1.54) | 0.76 (0.52–1.10) |
| Gastrointestinal ulceration/hemorrhage, d No. events | 34 | 31 | 37 |
| Cumulative incidence, 3 yrs, % ± SE | 11.7 ± 2.0 | 11.9 ± 2.1 | 14.1 ± 2.2 |
| RR (95% CI) | 1.0 | 0.88 (0.54–1.43) | 1.10 (0.69–1.76) |
Wild-type genotypes include individuals with no T181A or R184S alleles. Variant UGT1A6 genotypes include individuals with ≥ one variant (T181 or R184S) allele. Adverse events include those that were reported during the time after the first dose of the study drug until 30 days after the last dose of study drug. The analysis excludes 7 participants with genotype information that were randomized but never initiated treatment: 3 patients in the placebo group, 2 assigned to 200 mg of celecoxib twice daily, and 2 assigned to 400 mg of celecoxib twice daily. Data on adverse events include events reported among 639 patients who continued blinded treatment beyond the 36 month core phase of the study who were enrolled in the 24 month extension study.
Cardiovascular events include cardiovascular death or circulatory collapse, stroke, myocardial infarction, congestive heart failure, venous thrombosis or thromboembolism, cardiovascular therapeutic procedures, vascular therapeutic procedures, cerebrovascular disease, and vascular disease.
Renal and hypertensive events include elevated creatinine, fluid retention or edema, hypertension, proteinuria and renal failure.
Gastrointestinal ulceration and hemorrhage events include anemia, gastrointestinal bleeding, gastritis/duodenitis, upper or lower gastrointestinal ulceration, and other hemorrhage.
Discussion
In this large, randomized, placebo-controlled trial, celecoxib treatment was associated with a decrease in the three-year cumulative incidence of adenoma irrespective of concurrent low-dose aspirin use among patients with wild-type or variant UGT1A6 (≥ one T181A+R184S or R184S allele) genotypes, the principal enzyme responsible for aspirin metabolism. There also appeared to be no independent or additive effect of low-dose aspirin use on celecoxib efficacy. However, among individuals with variant UGT1A6 genotypes who used low-dose aspirin, there was an increased risk of recurrence associated with discontinuing treatment with celecoxib. Although statistical power was limited by the small number of events, it also appeared that among patients with variant genotypes, the increased incidence of renal and hypertensive events associated with celecoxib was attenuated.
In the Aspirin Polyp Prevention Study (APPS), low-dose, (81 mg/day) but not standard-dose (325 mg/day) aspirin treatment was associated with a lower risk of adenoma recurrence (1). Thus, in the APC trial, patients were stratified according to the use of low-dose aspirin at baseline and aspirin-users were required to remain on low-dose aspirin for the duration of the trial. In contrast with the APPS study, we did not find an effect of low-dose aspirin on adenoma recurrence, nor did it appear to enhance the chemopreventive effect of celecoxib. This is likely because patients who were aspirin users at study entry developed their baseline adenoma while using low-dose aspirin. Such patients may have been relatively resistant to the chemopreventative effect of aspirin, or may have had an exceptionally high risk of adenoma formation that was only partially suppressed by aspirin. Thus, continued exposure to aspirin may not appreciably influence adenoma risk when used concurrently with celecoxib. This is supported by our prior data which demonstrated that patients who developed their initial colorectal cancer in the setting of aspirin use did not benefit from continuing aspirin use after diagnosis of colorectal cancer. In contrast, patients who did not take aspirin prior to diagnosis of colorectal cancer but initiated aspirin use after diagnosis had a reduction in risk of colorectal-cancer specific mortality (19). It remains unclear if aspirin-naïve patients might benefit from use of both celecoxib and aspirin.
We also examined aspirin treatment in the APC trial in relation to UGT1A6 genotype since we and others, have found that the inverse association between aspirin use and adenoma risk was restricted to individuals with variant UGT1A6 genotypes who have a delayed aspirin metabolism (10, 11). However, we did not observe any significant modification of the effect of low-dose aspirin use in the on-treatment efficacy of celecoxib according to UGT1A6 genotype.
Because a large subset of patients underwent a colonoscopy at Year 5, when >94% of patients had been off-treatment for at least a year, the APC trial provided a unique opportunity to determine the risk of adenoma recurrence after discontinuing celecoxib. Overall, there did appear to be an increase in risk of adenoma associated with withdrawal of celecoxib among users of low-dose aspirin that was not apparent among non-users of aspirin. In particular, among patients with variant UGT1A6 genotypes who used aspirin, withdrawal of treatment with celecoxib was associated with a nearly two-fold higher risk of adenoma recurrence. In contrast, there was no increased risk of adenoma recurrence among non-users of aspirin with variant genotypes. Patients with variant UGT1A6 genotypes who use aspirin may have more sustained aspirin exposure resulting from their delayed metabolism. Thus, individuals with variant UGT1A6 genotype who developed a baseline adenoma despite aspirin use may represent a particularly aspirin resistant subgroup. Although addition of celecoxib to aspirin in this subset successfully suppressed adenoma formation, our results suggest that disease re-emerges with withdrawal of COX-2 inhibition, perhaps at a higher rate than among individuals who had not been exposed to celecoxib.
Our findings are supported by the five-year efficacy analysis of the Prevention of Colorectal Sporadic Adenomatous Polyps (PreSAP) trial, a similarly-designed, randomized, placebo-controlled, trial of 1,561 patients with recently removed colorectal adenomas, stratified by low-dose aspirin, which showed that treatment with celecoxib at 400 mg daily reduced risk of adenoma recurrence at a year 3 surveillance colonoscopy. As in our study, the effect of withdrawal of celecoxib was assessed in a subset of participants who agreed to a undergo colonoscopy 2 years after discontinuing treatment (year 5). Overall, compared to patients who discontinued placebo, patients who discontinued celecoxib had a 1.5-fold higher risk of recurrent adenoma with a more than 2-fold higher risk among low-dose aspirin users (20). These data also suggest that individuals who develop an adenoma despite exposure to aspirin may be particularly prone to recurrent neoplasia upon withdrawal of celecoxib.
Given the importance of the APC trial in identifying unexpected toxicity associated with celecoxib (7), we examined the influence of UGT1A6 genetic variation on risk of adverse events. There did appear to be attenuation of the risk of renal and hypertensive events among individuals with variant UGT1A6 genotypes. This may reflect a stronger counter-balancing effect of aspirin on the vascular effects of COX-2 selective inhibition among individuals with diminished aspirin metabolism. Nonetheless, because there were a limited number of adverse events associated with treatment, our findings are exploratory and require study in ongoing clinical trials examining the safety profile of celecoxib.
Our study had several strengths. First, celecoxib treatment was randomly assigned, dosing and treatment duration was strictly defined, and use of low-dose aspirin was carefully determined at baseline. Thus, it is unlikely that our findings are related to differential dosing, heterogeneity in treatment duration, or exposure to other NSAIDs. Second, as all patients were enrolled in a randomized trial, treatment and colonoscopic surveillance for outcomes, as well as reporting of adverse events, were uniform and standardized. Third, a large proportion of patients continued to be followed after discontinuation of treatment, permitting an analysis of the effect of withdrawal of COX-2 inhibition on recurrent disease.
Several limitations of this study deserve comment. First, aspirin use was not assigned. However, patients were initially stratified according to baseline use or non-use of low-dose aspirin (325 mg or less every other day or less than 162.5 mg/day), aspirin users were required to continue their usual pattern of aspirin intake during celecoxib treatment, and use of higher doses of aspirin and other NSAIDs was not permitted. Because the vast majority of aspirin users consumed less than 81 mg/day (>75%), we did not have sufficient numbers within other low-dose aspirin categories to examine potential heterogeneity in the effect of dose on outcomes. Second, we evaluated UGT1A6 genotype as a marker of aspirin sensitivity. Although two large studies demonstrated that variant UGT1A6 genotype modifies the effect of aspirin on adenoma risk (10, 11), another study did show that variant UGT1A6 genotype was associated with lower risk of adenoma recurrence independent of aspirin intake (17). Third, we had a limited number of adverse events for analysis, especially among patients with variant genotypes. Nonetheless, our study represents the largest cohort of patients who have been randomized to long-term treatment with celecoxib for whom toxicity data are available. Fourth, not all patients underwent a Year 5 colonoscopy to assess the effect of celecoxib withdrawal. However, there was no significant difference in baseline characteristics of individuals who underwent a Year 5 exam compared with those who did not (12). Last, we did not have a sufficient sample size to consider the joint effect of genetic variation in UGT1A6 with the cytochrome p450 enzyme isoform 2C9 (CYP2C9), the principal enzyme responsible for the metabolism of celecoxib (16).
Our study has specific clinical implications. First, our results do not suggest that concurrent use of low-dose aspirin influences the on-treatment effect of celecoxib on adenoma recurrence. Second, our data suggest that certain individuals may be relatively “resistant” to the chemopreventative effects of aspirin, with potential implications for chemopreventative responsiveness to other agents, including celecoxib. Such aspirin “resistance” has been well-described in the prevention of vascular disease and has also been associated with future risk of vascular events (21). Third, efforts to use genetic information to personalize and optimize chemoprevention have been identified as a high priority and may be warranted for specific patients. Our data suggest that with further study UGT1A6 genotypes could be used to determine the likelihood of disease recurrence after withdrawal of celecoxib or the risk of celecoxib-associated renal or hypertensive events.
In summary, we have shown that concurrent use of low-dose aspirin does not influence the efficacy of celecoxib in adenoma prevention. However, individuals who develop adenomas despite aspirin use within a genetic background associated with delayed aspirin metabolism may initially respond to celecoxib, but discontinuing celecoxib results in re-emergence of disease. Our data suggest that pharmacogenetic variability may be important in determining susceptibility to the benefits and hazards of aspirin and celecoxib and highlight the importance of developing alternative chemopreventative strategies for individuals who develop colorectal neoplasia despite use of aspirin.
Supplementary Material
Table 4.
Risk of Adenoma at 5-years According to Discontinuation of Celecoxib Treatment, Stratified by Aspirin Use and UGT1A6 Genotype a
| Prior Placebo |
Prior Celecoxib, 200 mg Twice Daily |
Prior Celecoxib, 400 mg Twice Daily |
|
|---|---|---|---|
| All Genotypes | |||
| All patients, No. at risk b | 183 | 166 | 189 |
| No. with adenoma, (%) c | 68 (37) | 64 (39) | 81 (43) |
| RR (95% CI) d | 1.0 | 1.04 (0.79–.36) | 1.14 (0.88–.47) |
| P value e | -- | 0.80 | 0.31 |
| Aspirin users, No. at risk b | 63 | 55 | 60 |
| No. with adenoma, (%) c | 19 (30) | 24 (44) | 29 (48) |
| RR (95% CI) f | 1.0 | 1.45 (0.89–.34) | 1.59 (1.00–.53) |
| P value e | -- | 0.13 | 0.05 |
| Non-aspirin users, No. at risk b | 120 | 111 | 129 |
| No. with adenoma, (%) c | 49 (41) | 40 (36) | 52 (40) |
| RR (95% CI) f | 1.0 | 0.88 (0.64–.23) | 0.98 (0.72–.32) |
| P value e | -- | 0.46 | 0.87 |
| Patients with wild-type genotypes | |||
| All wild-type genotypes, No. at risk b | 79 | 73 | 84 |
| No. with adenoma, (%) c | 30 (38) | 27 (37) | 38 (45) |
| RR (95% CI) g | 1.0 | 0.97 (0.64–.46) | 1.16 (0.81–.68) |
| P value e | -- | 0.89 | 0.42 |
| Aspirin users, No. at risk b | 32 | 27 | 27 |
| No. with adenoma, (%) c | 10 (31) | 11 (41) | 10 (37) |
| RR (95% CI) h | 1.0 | 1.30 (0.66– 2.59) | 1.19 (0.58–.41) |
| P value e | -- | 0.45 | 0.64 |
| Non-aspirin users, No. at risk b | 47 | 46 | 57 |
| No. with adenoma, (%) c | 20 (43) | 16 (35) | 28 (49) |
| RR (95% CI) h | 1.0 | 0.82 (0.49– 1.37) | 1.15 (0.76–.76) |
| P value e | -- | 0.44 | 0.51 |
| Patients with variant genotypes | |||
| All variant genotypes, No. at risk b | 104 | 93 | 105 |
| No. with adenoma, (%) c | 38 (37) | 37 (40) | 43 (41) |
| RR (95% CI) g | 1.0 | 1.09 (0.76–.56) | 1.12 (0.79–.58) |
| P value e | -- | 0.64 | 0.52 |
| Aspirin users, No. at risk b | 31 | 28 | 33 |
| No. with adenoma, (%) c | 9 (29) | 13 (46) | 19 (58) |
| RR (95% CI) h | 1.0 | 1.60 (0.81– 3.15) | 1.98 (1.06–.70) |
| P value e | -- | .17 | .02 |
| Non-aspirin users, No. at risk b | 73 | 65 | 72 |
| No. with adenoma, (%) c | 29 (40) | 24 (37) | 24 (33) |
| RR (95% CI) h | 1.0 | 0.93 (0.61– 1.42) | 0.84 (0.55–.29) |
| P value e | -- | .74 | .43 |
Risk of adenoma on the colonoscopy at Year 5 among 538 patients enrolled in the double-blinded extension of the APC trial. At the time of the Year 5 colonoscopy, 94% had been off study drug for at least 1 year. Patients were stratified at study entry according to the use or nonuse of low-dose aspirin (325 mg or less every other day or 162.5 mg or less every day). Patients not taking aspirin at baseline were required to abstain from taking it during the trial. Wild-type UGT1A6 genotypes include individuals with no T181A or R184S alleles. Variant UGT1A6 genotypes include individuals with ≥ one variant (T181 or R184S) allele.
No. at risk includes patients who underwent the Year 5 colonoscopy.
No. with adenoma includes the results of the colonoscopy at Year 5 without considering adenomas found at earlier timepoints.
Risk ratio controlling for genotype and aspirin use among patients who underwent Year 5 colonoscopy with placebo as the referent group
The p value is the Cochran-Mantel-Haenszel test of general association compared to the placebo group.
Risk ratio, controlling for genotype among patients who underwent Year 5 colonoscopy with placebo as the referent group
Risk ratio, controlling for aspirin use among patients who underwent Year 5 colonoscopy with placebo as the referent group
Risk ratio among patients who underwent Year 5 colonoscopy with placebo as the referent group
Acknowledgments
Funding: This work was supported by the National Cancer Institute at the National Institutes of Health (grant number R01 CA137178 to ATC and N01 CN95015 to MMB). Dr. Chan is a Damon Runyon Cancer Research Foundation Clinical Investigator.
Footnotes
Financial Disclosures: Dr. Chan has served as a consultant to Bayer HealthCare and Millennium Pharmaceuticals. Dr. Bertagnolli is the recipient of research funding from Pfizer Inc. Dr. Hawk has served as a consultant for Pozen Pharmaceutical Development Company. Ms. Hsu has no conflicts of interest. The statistical analysis of the entire data sets pertaining to efficacy and safety has been independently confirmed by Dr. Zauber, who is not employed by any corporate entity. The corresponding author had full access to all of the data and takes full responsibility for the veracity of the data and analysis.
References
- 1.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 2.Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348(10):883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 3.Benamouzig R, Deyra J, Martin A, Girard B, Jullian E, Piednoir B, et al. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology. 2003;125(2):328–336. doi: 10.1016/s0016-5085(03)00887-4. [DOI] [PubMed] [Google Scholar]
- 4.Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134(1):29–38. doi: 10.1053/j.gastro.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 6.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355(9):885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 7.Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, et al. Cardiovascular Risk Associated with Celecoxib in a Clinical Trial for Colorectal Adenoma Prevention. N Engl J Med. 2005;352(11):1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 8.Hutt AJ, Caldwell J, Smith RL. The metabolism of aspirin in man: a population study. Xenobiotica. 1986;16(3):239–249. doi: 10.3109/00498258609043527. [DOI] [PubMed] [Google Scholar]
- 9.Ciotti M, Marrone A, Potter C, Owens IS. Genetic polymorphism in the human UGT1A6 (planar phenol) UDP-glucuronosyltransferase: pharmacological implications. Pharmacogenetics. 1997;7(6):485–495. doi: 10.1097/00008571-199712000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Bigler J, Whitton J, Lampe JW, Fosdick L, Bostick RM, Potter JD. CYP2C9 and UGT1A6 genotypes modulate the protective effect of aspirin on colon adenoma risk. Cancer Res. 2001;61(9):3566–3569. [PubMed] [Google Scholar]
- 11.Chan AT, Tranah GJ, Giovannucci EL, Hunter DJ, Fuchs CS. Genetic variants in the UGT1A6 enzyme, aspirin use, the risk of colorectal adenoma. J Natl Cancer Inst. 2005;97(6):457–460. doi: 10.1093/jnci/dji066. [DOI] [PubMed] [Google Scholar]
- 12.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Breazna A, Kim K, et al. Five-year efficacy and safety analysis of the Adenoma Prevention with Celecoxib Trial. Cancer Prev Res (Phila Pa) 2009;2(4):310–321. doi: 10.1158/1940-6207.CAPR-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan AT, Tranah GJ, Giovannucci EL, Hunter DJ, Fuchs CS. A prospective study of genetic polymorphisms in the cytochrome P450 2C9 enzyme and the risk of distal colorectal adenoma. Clin Gastro Hepatol. 2004;2(8):704–712. doi: 10.1016/s1542-3565(04)00294-0. [DOI] [PubMed] [Google Scholar]
- 14.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 2nd ed. Hoboken, N.J.: J. Wiley; 2002. [Google Scholar]
- 15.Delong DM, Guirguis GH, So YC. Efficient computation of subset selection probabilities with application to Cox regression. Biometrika %R10.1093/biomet/81.3.607. 1994;81(3):607–611. [Google Scholar]
- 16.Chan AT, Zauber AG, Hsu M, Breazna A, Hunter DJ, Rosenstein RB, et al. Cytochrome P450 2C9 variants influence response to celecoxib for prevention of colorectal adenoma. Gastroenterology. 2009;136(7):2127–2136. doi: 10.1053/j.gastro.2009.02.045. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubner RA, Muir KR, Liu J-F, Logan RFA, Grainge M, Armitage N, et al. Genetic Variants of UGT1A6 Influence Risk of Colorectal Adenoma Recurrence. Clin Cancer Res. 2006;12(21):6585–6589. doi: 10.1158/1078-0432.CCR-06-0903. [DOI] [PubMed] [Google Scholar]
- 18.Samowitz WS, Wolff RK, Curtin K, Sweeney C, Ma KN, Andersen K, et al. Interactions between CYP2C9 and UGT1A6 polymorphisms and nonsteroidal anti-inflammatory drugs in colorectal cancer prevention. Clin Gastroenterol Hepatol. 2006;4(7):894–901. doi: 10.1016/j.cgh.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302(6):649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arber N, Spicak J, Racz I, Zavoral M, Breazna A, Gerletti P, et al. Five-Year Analysis of the Prevention of Colorectal Sporadic Adenomatous Polyps Trial. Am J Gastroenterol. 2011 doi: 10.1038/ajg.2011.116. [DOI] [PubMed] [Google Scholar]
- 21.Krasopoulos G, Brister SJ, Beattie WS, Buchanan MR. Aspirin "resistance" and risk of cardiovascular morbidity: systematic review and meta-analysis. BMJ. 2008;336(7637):195–198. doi: 10.1136/bmj.39430.529549.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.