Abstract
The breast cancer-associated gene 1 (BRCA1) is the most frequently mutated tumor suppressor gene in familial breast cancers. Mutations in BRCA1 also predispose to other types of cancers, pointing to a fundamental role of this pathway in tumor suppression and emphasizing the need for effective chemoprevention in these high-risk patients. Because the methyl ester of the synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO-Me) is a potent chemopreventive agent, we tested its efficacy in a highly relevant mouse model of BRCA1-mutated breast cancer. Beginning at 12 weeks of age, Brca1Co/Co;MMTV-Cre;p53+/- mice were fed powdered control diet or diet containing CDDO-Me (50 mg/kg diet). CDDO-Me significantly (P < 0.05) delayed tumor development in the BRCA1-mutated mice by an average of 5.2 weeks. We also observed that levels of ErbB2, pErbB2, and cyclin D1 increased in a time-dependent manner in the mammary glands in BRCA1-deficient mice, and CDDO-Me inhibited the constitutive phosphorylation of ErbB2 in tumor tissues from these mice. In BRCA1-deficient cell lines, the triterpenoids directly interacted with ErbB2, decreased constitutive phosphorylation of ErbB2, inhibited proliferation, and induced G0/G1 arrest. These results suggest that CDDO-Me has the potential to prevent BRCA1-mutated breast cancer.
Introduction
The need to find better ways to prevent breast cancer, especially in pre-menopausal women is self-evident. Therapy of “triple negative” (estrogen receptor, progesterone receptor, and Her2 expression) cancers, which are often associated with a mutation in the BRCA genes, has been particularly disappointing (1, 2). Women who are newly diagnosed with BRCA mutations have no realistic options, other than the personally unsatisfactory possibility of bilateral prophylactic mastectomy or “watchful waiting” with all of its attendant anxieties (3-5). There is thus a major need for new approaches to prevention, especially the development of new drugs that would be safe, free of undesirable side effects, and effective for chemoprevention when given to women over prolonged periods of time (2, 6, 7).
Over the past 30 years, many drugs have been developed for chemoprevention of ER-positive breast cancer, and two of these, tamoxifen and raloxifene, are approved by the FDA for clinical use as preventive agents (8, 9). The problem of chemoprevention of ER-negative breast cancer has been more difficult since drugs that block estrogen synthesis or estrogen action have limited or no use for ER-negative malignancies. However, some success has been achieved in experimental models, especially with rexinoids (selective ligands for the nuclear receptors known as RXR's) using the MMTV-Her2 neu transgenic mouse model (10-13). The newer development of mice with mutations in both BRCA1 as well as p53 (14-16) has now made it possible to study chemoprevention (17-19) that is relevant to the disease caused by a BRCA mutation in women. For the past 10 years, our laboratory has been developing new synthetic triterpenoids as agents for both chemoprevention and chemotherapy of cancer (20-22), and in the present article we now report, for the first time, the successful use of a triterpenoid, namely CDDO methyl ester (CDDO-Me), for inhibition of breast carcinogenesis induced by mutations of both BRCA1 and p53. Moreover, we also show mechanistic interactions between CDDO-Me and phospho-ErbB2, a known marker for ER-negative mammary tumorigenesis.
Materials and Methods
Reagents and in vitro assay
CDDO-Me and biotinylated triterpenoid (Bt-CDDO) were synthesized as described (23-25). For cell culture studies, compounds were dissolved in DMSO, and controls containing equal concentrations of DMSO (V < 0.1%) were included in all experiments. Sources of reagents and antibodies were as follows: antibodies against p21Waf1/Cip1 and Cdk4 from Santa Cruz Biotechnologies, (Santa Cruz, CA); ErbB2 from Lab Vision (Fremont, CA); pErbB2 and γH2AX from R&D systems (Minneapolis, MN); cyclin D1 from Cell Signaling Technology (Beverly, MA). The Brca1 mutant cell line W780 was derived from a mammary tumor in a Brca1Co/Co;MMTV-Cre;p53+/- mouse containing a targeted deletion of full-length Brca1 (15). W780 cells were cultured in DMEM with 5% FBS (Invitrogen, Carlsbad, CA) and were treated with CDDO-Me at the concentrations indicated in the text and in the figure legends. No additional cell testing was performed by the authors. For the immunoprecipitation experiments, W780 cells were treated with 3 μmol/L biotinylated triterpenoid for 1 h and lysed in 100 mmol/L Tris-HCl (pH 7.4), 1% Triton X-100. Total protein (1 mg) was incubated with 50 μL DynaBeads MyOne Streptavidin T1 (Invitrogen) for 1 h, pelleted, and washed four times with Tris-HCl-1% Triton X-100 buffer. Samples were resuspended in 40 μL Laemmli loading buffer, boiled for 5 min to remove the bound proteins from the beads, and analyzed by Western blotting (26).
Cell cycle analysis
Cells were treated with CDDO-Me or DMSO. After trypsinization, cells were fixed in 70% ethanol for 30 min at 4°C. The cells were washed twice with PBS, and then incubated for 30 min in the dark at 37°C in 1 ml of PBS containing 100 μg propidium iodide and 100 μg RNase A. After flow cytometry, histograms were generated using Cell Quest and Mod-Fit software.
In vivo experiments
All animal studies were done in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Dartmouth Medical School. For the serial sacrifice study, mammary glands from Brca1Co/Co;MMTV-Cre;p53+/-mice (15) at 12, 16, 20, 24, and 28 weeks of age were harvested. For the prevention studies, 12 wk old female Brca1Co/Co;MMTV-Cre;p5+/- mice were fed powdered 5002 rodent chow (PMI Feeds) or powdered diet containing CDDO-Me (50 mg/kg diet) and were palpated weekly for tumors. Mammary glands and tumor samples were fixed in neutral buffered formalin and embedded in paraffin blocks according to standard procedures, and sections were stained with hematoxylin and eosin (H&E). After deparaffinization and rehydration, antigen retrieval was performed by boiling in citrate buffer (pH 6.0). After cooling, slides were incubated for 60 min at room temperature with the following mouse monoclonal antibodies: ErbB2 (1:100; Neomarkers, Lab Vision Corporation, Fremont, CA), pErbB2 (1:300; R&D Systems, Minneapolis, MN), cyclin D1 (1:100; Cell Signaling Technology, Beverly, MA), or γH2AX (1:100; Immunotech, R&D Systems). All slides were developed with diaminobenzidine followed by hematoxylin counterstaining. Scoring was performed by the first author, who was blinded as to the primary antibodies and the treatment groups. For ErbB2, pErbB2, cyclin D1, and γH2AX the percentage of positively stained cells was estimated. Cases with 5% or fewer positively-stained cells were scored as 1, 2 for 5-20%, 3 for 20-50%, 4 for 50-80%, and 80% or more stained cells were denoted as 5.
Tissue levels
Six female Brca1Co/Co;MMTV-Cre;p53+/- mice were fed CDDO-Me in diet (50 mg/kg diet). After four days on diet, mammary glands were harvested and whole blood was collected into heparinized tubes. Mammary glands were homogenized in PBS and then all samples were extracted in acetonitrile, separated by reverse phase liquid chromatography, and detected by mass spectrometry as previously described (27). Standard curves were generated by serially diluting known concentrations of CDDO-Me in control blood or tissue homogenate. All samples were within the linear range of the standard curve.
Statistical analysis
When appropriate, data were expressed as means ± SD of at least three independent experiments. Statistical analysis for single parametric comparisons was performed using the Student's t test; the non-parametric data in Figure 3 was analyzed by the Kruskal-Wallis one-way ANOVA on ranks followed by Dunn's method. Figure 4A was analyzed using a Chi-square test and the Wilcoxon signed rank test, and percentages were analyzed using a Z-test. The criterion for statistical significance was p < 0.05.
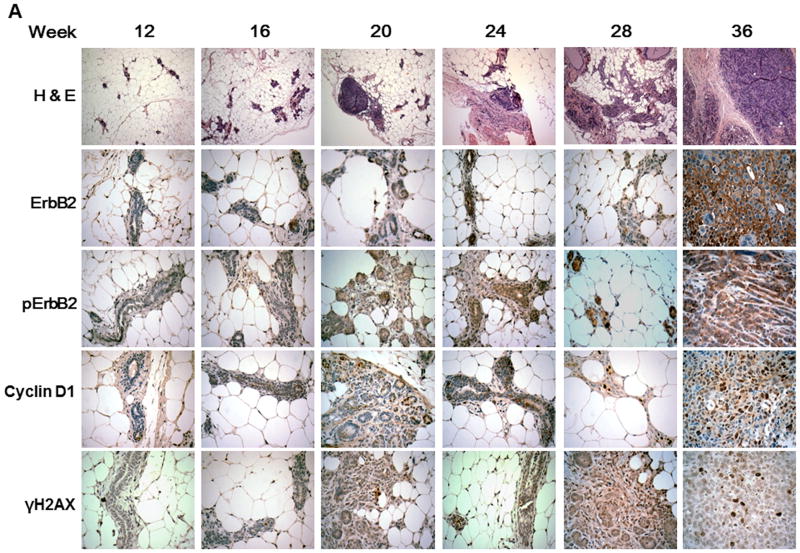
Figure 3. pERBb2 is overexpressed in BRCA-1 deficient mice.
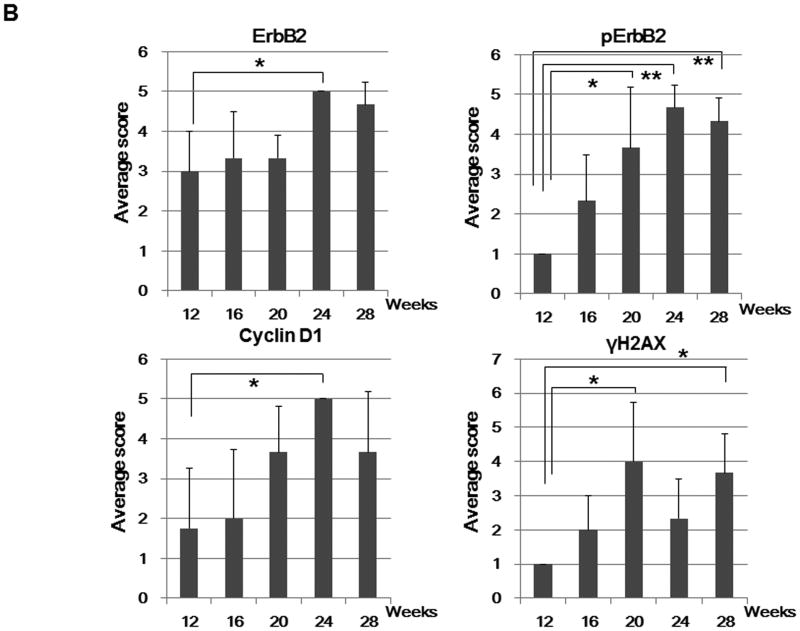
A, Histological and immunohistochemical analysis of mammary glands and tumors over time in Brca1 Co/Co;MMTV-Cre;p53+/- mice. Paraffin sections of the mammary glands or tumors from 12, 16, 20, 24, 28, and 36 week-old Brca1Co/Co;MMTV-Cre;p53+/- mice were stained with H & E (top) and the indicated antibodies (lower, ErbB2, pErbB2, cyclin D1, and γH2AX). Original magnifications: X 100 (H & E); X 400 (immunohistochemistry). B, The percentage of cells positive for ErbB2, pErbB2, cyclin D1, and γH2AX staining was scored in a blinded manner: 1, < 5% positive cells; 2, 5-20% positive; 3, 20-50% positive; 4, 50-80% positive; 5, > 80% positive. n = 3 per group. *, p < 0.05; **, p < 0.001. C, Western blot analysis of pErbB2, ErbB2, cyclin D1, and γH2AX levels in the mammary glands from mice 12, 16 and 20 weeks of age and in mammary tumors from 35 week-old BRCA1-deficient mice.
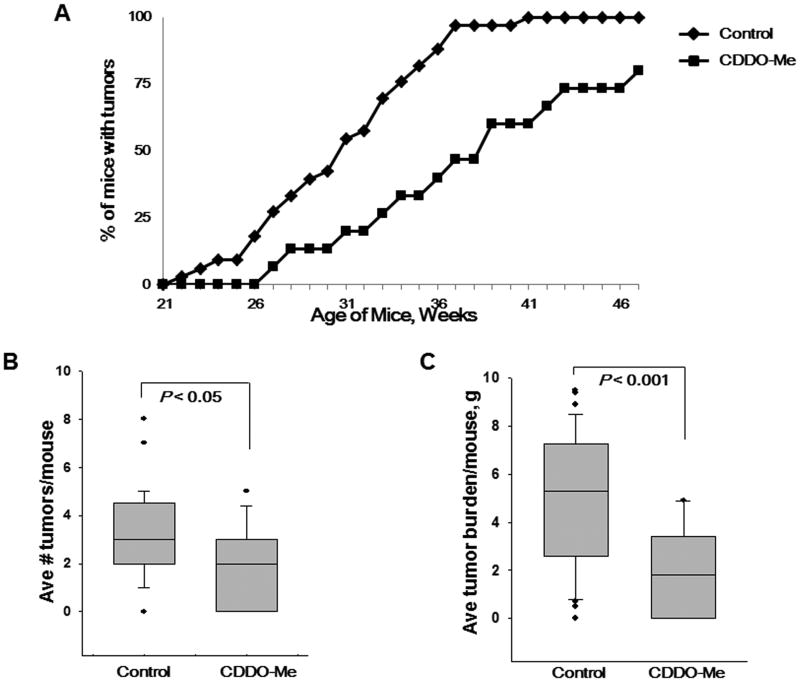
Figure 4. CDDO-Me delays tumor development in BRCA1-deficient mice.
A, Beginning at 12 wks of age, Brca1Co/Co;MMTV-Cre;p53+/- mice were fed powdered control diet or CDDO-Me (50 mg/kg diet). Mice were palpated weekly, and no tumors were found before the mice were 20 wks old. The CDDO-Me treated groups was significantly (P < 0.05) different than the control group for weeks 26-47. n = 33 mice in the control group and n = 15 in the CDDO-Me group. *, P < 0.05 versus control; Me, CDDO-methyl ester. Mean ± SE of the average number of tumors per mouse (B) and the average tumor burden per mouse (C).
Results
CDDO-Me inhibits the phosphorylation of ErbB2 and reduces the expression of cyclin D1 in BRCA1-deficient cells
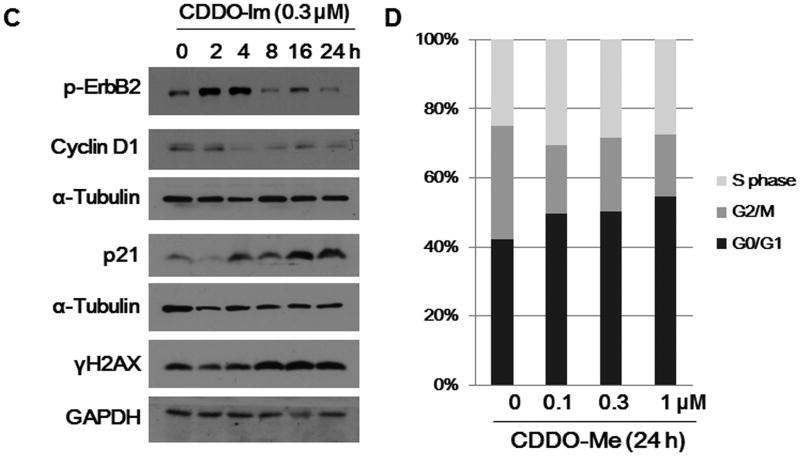
Deng and colleagues have previously shown that the ErbB2 (Her-2/neu) and cyclin D1 cell cycle regulatory proteins are overexpressed in the mammary tumors of Brca1 conditional knockout mice (15), and we have shown that synthetic triterpenoids inhibit proliferation and induce apoptosis in cell lines from these knockout mice (22). To determine whether the triterpenoids alter cellular and molecular proteins that regulate proliferation, we treated W780 breast cancer cells with CDDO-Me for 24 h. CDDO-Me inhibited the expression of p-ErbB2, cyclin D1 and CDK4, an important complex for cell cycle G1 phase progression, and markedly induced the expression of p21, a well known cell cycle inhibitor at the G1 phase, in a concentration- and time-dependent manner (Fig. 1A and B). This reduction is ErbB2 phosphorylation is not specific to CDDO-Me, as the synthetic triterpenoid CDDO-Imidazolide (CDDO-Im), which inhibits proliferation in BRCA1-deficient cell lines (22), also inhibited the expression of p-ErbB2 and induced the expression of p21 in the same cell line (Fig. 1C). Although CDDO-Im is equipotent to CDDO-Me, the stability and pharmacokinetic profile of CDDO-Me make it more suitable for in vivo studies. CDDO-Me showed similar effects on the same cell cycle regulators in E18-14C-27 breast cancer cells (data not shown), which express wild type BRCA1 and constitutively overexpress ErbB2 (10). CDDO-Me also induced G0/G1 arrest in the BRCA1-deficient cell lines, thereby reducing the percentage of cells in G2/M (Fig. 1D).
Figure 1. CDDO-Me inhibits the phosphorylation of ErbB2 and expression of cyclin D1 in BRCA1-deficient cells.
W780 cells were treated with different concentrations (A) of CDDO-Me (A, B) or CDDO-Im (C) for the indicated time periods (B, C) and soluble protein extracts were subjected to SDS-PAGE and Western blotting with the indicated antibodies. GAPDH and α-tubulin were used as loading controls. Cell cycle analysis (D) of W780 cells treated with CDDO-Me for 24 h and analyzed by flow cytometry.
CDDO-Me directly interacts with ErbB2
On the basis of a binding model, cysteine-805, located within the catalytic cleft of ErbB2, is ideally positioned for covalent interaction with irreversible inhibitors of ErbB2 that dock in the ATP binding pocket (28, 29). Because CDDO-Me could potentially form covalent bonds with sulfhydryl groups through Michael addition at carbon 1 or 9 (20, 30, 31), we determined whether a biotinylated analogue of CDDO (Bt-CDDO) could directly interact with ErbB2 (26). W780 cells were treated with Bt-CDDO, and lysates were precipitated with immobilized NeutrAvidin to isolate Bt-CDDO-protein complexes prior to Western blotting. As shown in Fig. 2A, Bt-CDDO directly interacts with ErbB2 but not with cyclin D1, which was used as a negative control. To further investigate the role of sulfhydryl groups in the interaction of CDDO-Me with ErbB2, we pretreated with the thiol modifying agents, N-acetylcysteine (NAC) and dithiothreitol (DTT) before treatment with CDDO-Me. As expected, pretreatment of W780 cells with the reducing agents NAC or DTT blocked Bt-CDDO binding to the ErbB2 protein (Fig. 2B), suggesting that CDDO-Me can form covalent adducts with cysteine thiols in ErbB2. The effects of CDDO-Me on the expression of pErbB2 and p21 were also significantly reversed when cells were pretreated with DTT (data not shown), indicating that CDDO-Me targets reactive cysteine residues in the ErbB2 protein.
Figure 2. CDDO-Me directly interacts with ErbB2.
A, W780 cells were treated with 3 μM of biotinylated triterpenoid (Bt-CDDO) for 1 h, triterpenoid-protein complexes were precipitated from cell lysates with strepavidin DynaBeads, and the levels of ErbB2 were assessed by Western blot analysis. Cyclin D1 was used as a negative control. B, The thiol modifying agents NAC and DTT abrogated the interaction between ErbB2 and Bt-CDDO-Me. W780 cells were treated with NAC or DTT for 1 h and/or Bt-CDDO-Me for an additional 1 h. NeutrAvidin precipitations were performed as described, and Bt-CDDO-ErbB2 complexes were detected by Western blot.
pErbB2 is overexpressed in BRCA-1 deficient mice
Our in vitro data indicate that CDDO-Me inhibits progression through the cell cycle and reduces phosphorylation of ErbB2, a receptor tyrosine kinase encoding the Her2/neu proto-oncogene that stimulates cell growth and differentiation. We next examined the expression of each of these proteins in the mammary glands of 12-28 week-old Brca1Co/Co;MMTV-Cre;p53+/- mice and found increased expression of these molecules with age (Fig. 3 A). Blinded analysis revealed that the levels of pErbB2, as detected by immunohistochemistry, were significantly increased in the mammary glands from the BRCA1-deficient mice after 20 weeks of age (Fig. 3B), suggesting that this molecule could be a potential biomarker in breast cancer induced by mutations in BRCA1. Phosphorylation of histone H2AX (γH2AX), an early marker of DNA damage, was also increased at 20 and 28 weeks. Although highly variable, Western analysis confirmed that these markers were up-regulated in the mammary glands from BRCA1-deficient mice in a time-dependent manner (Fig. 3C) and that pErbB2 is expressed at very high levels in tumors from these mice.
CDDO-Me delays tumor development in the mammary glands and extends survival of BRCA1-deficient mice
Because CDDO-Me is a potent chemopreventive agent in a variety of experimental models (20, 31), we investigated the ability of CDDO-Me to prevent mammary tumorigenesis in BRCA1-deficient mice. Mice were fed control diet or a diet containing CDDO-Me (50 mg/kg diet), beginning at 12 weeks of age. Mammary tumors were first detected at an average of 30.8 weeks of age in the control group, whereas the average first detection of tumors was significantly (P < 0.005) delayed to an average of 36 weeks in the mice fed CDDO-Me (Fig 4A). The average number of tumors per mice was also reduced (P < 0.05) in the mice fed CDDO-Me, with an average of 2.5 tumors per mouse compared to an average of 3.2 tumors per mouse in the control group (Fig. 4B). The average tumor burden per mouse (Fig. 4C) was only 2.9 g in the mice fed CDDO-Me vs. 5.1 g in the control mice (P < 0.001), and the average lifespan (Table 1) was significantly (P < 0.005) higher in the mice fed CDDO-Me compared to the controls (34.2 weeks and 39.3 weeks, respectively). In a pilot feeding study, an average of only 60 ± 30 nM CDDO-Me was detected in whole blood but 1.3 ± 0.6 μM CDDO-Me was detected in the mammary gland.
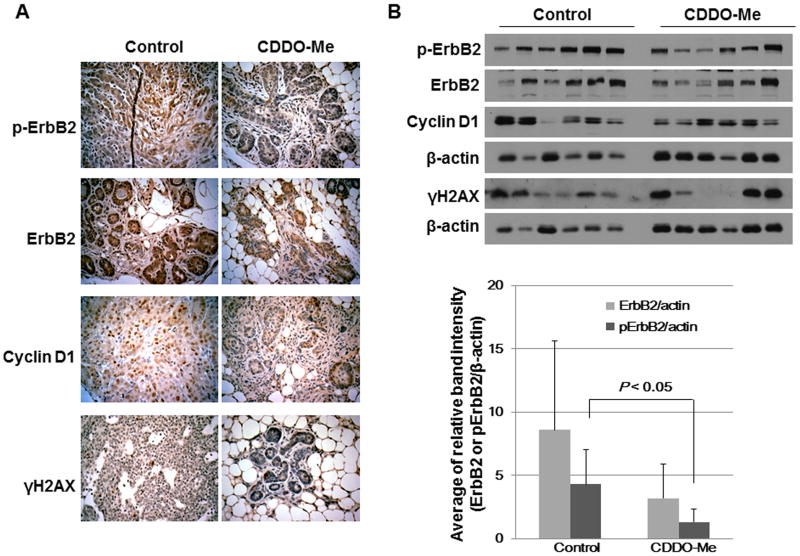
CDDO-Me attenuates the expression of pErbB2 in tumors from BRCA1-deficient mice
To test the ability of CDDO-Me to inhibit the expression of pErbB2 and cyclin D1 in vivo, BRCA1-mutated mice were fed control diet or CDDO-Me (50 mg/kg diet) for 18-24 weeks. Figure 5A shows that the levels of ErbB2, pErbB2, cyclin D1 and γH2AX were reduced by almost 50% in the mice fed CDDO-Me; the expression of pErbB2 was significantly (P < 0.05) decreased as characterized by H&E staining and Western blotting (Fig. 5 A and B).
Figure 5. CDDO-Me attenuates the expression of pErbB2 in tumors from BRCA1-deficient mice.
A, Immunohistochemical detection of protein expression in mice fed control diet or CDDO-Me in diet (50 mg/kg diet). Tumors were resected, fixed, and embedded in paraffin. Tissue sections were prepared for immunohistochemical staining and probed with appropriate antibodies (n = 4 per group; representative images are shown). B, Tumor tissue lysates from each group were immunoblotted with antibodies against pErbB2, ErbB2, cyclin D1, γH2AX and β-actin and quantified by densitometry. Bar represents mean ± SD of 6 tumors per group.
Discussion
We have shown the utility of a synthetic triterpenoid, CDDO-Me for inhibition of the process of carcinogenesis in a mouse model that is highly relevant to the development of invasive malignancy in women with a mutated BRCA1 gene. The suppression of malignancy in the mouse model (Fig. 4), although only partial, is nevertheless significant, and if it could be translated into clinical practice, would provide meaningful benefit to women who presently have no desirable therapeutic options. The dosage of triterpenoid that has been used in these experiments is apparently free of undesirable side effects in the mice, which continued to gain weight during the course of an almost year-long experiment.
Notably, a recent study has found that the EGFR inhibitor erlotinib delays tumor development in BRCA1-mutant mice (17), but erlotinib may be too toxic for clinical chemoprevention studies. CDDO-Me is not a conventional cytotoxic drug and has an excellent safety profile in humans (32). The well-tolerated dose used in these studies was well below the maximum-tolerated dose or the doses successfully used previously for treatment of experimental lung or breast cancer (21, 33). All mice continued to gain weight throughout the study, but the mice fed CDDO-Me did not gain as much weight as the control mice. Although we have shown that the combination of CDDO-Me and LG268 delays tumor development in MMTV-neu mice (21), this combination was not effective in this model, even though the weights of these mice were the same as mice fed CDDO-Me alone (data not shown). These data suggest that the delay in tumorigenesis in this model was not the result of toxicity or slower weight gain. Moreover, triterpenoids downregulate the fatty acid synthase pathway (34, 35), which contributes to weight gain and may contribute to the development of breast cancer driven by overexpression of ErbB2 (36, 37).
Mechanistically, we have shown important interactions between CDDO-Me and the protein target, ErbB2. ErbB2 is a validated target for drugs that are used in cancer chemotherapy, such as Herceptin. Many of the advances in chemotherapy of breast cancer have relied on the ability of drugs to modulate the activity of ErbB2, which undergoes phosphorylation to become biologically active. The biological relevance to carcinogenesis induced by BRCA mutation is shown here in Figure 3A which indicates that increased phosphorylation of ErbB2 occurs in mice as early as sixteen weeks of age. From a pharmacological perspective, we have shown that CDDO-Me inhibits phosphorylation of ErbB2 in cell cultures of breast cancer cells having a BRCA1 mutation; doses between 300-1000 nanoMolar are effective in vitro, and concentrations of 1.5 μM can be obtained in the mammary glands in vivo. Furthermore, CDDO-Me was able to suppress the expression of phospho-ErbB2 in actual tumors in mice that received this drug by chronic administration in the diet; levels of p-ErbB2 were reduced almost 50% in tumors obtained from treated mice (Fig. 5A and 5B). Finally, in experiments with a biotinylated analog of CDDO-Me, we have also shown that the triterpenoid directly interacts with ErbB2, presumably by Michael addition with a reactive cysteine at the catalytically active ATP binding pocket of this protein. It is well established that CDDO-Me and related triterpenoids are potent agents for Michael addition, although this is not a random process. Rather, the pentacyclic scaffold of CDDO-Me, together with the exocyclic methyl groups of this triterpenoid, provide a highly stereospecific platform for interaction with unique cysteine residues on target proteins (20, 30, 31). This is undoubtedly an important consideration in the relative safety of the use of these triterpenoids for chemoprevention.
In addition to the above mechanism, there are undoubtedly other mechanisms that contribute to the useful chemopreventive activity of CDDO-Me. It has previously been shown in both cell culture and in vivo experiments that CDDO-Me is a potent anti-angiogenic agent (38). Furthermore, recent proteomic studies have shown that the same biotinylated analog of CDDO-Me used here has multiple protein targets, most notably the mTOR complex and several of the nuclear receptors in the steroid receptor superfamily (39). The separate and individual contributions of each of these targets are difficult to determine in an in vivo context. Moreover, we cannot be certain how much of the chemopreventive activity of CDDO-Me is due to effects on stromal cells that comprise a particularly large fraction of the total cells in a carcinoma of the mammary gland (40-42).
The ultimate application of the results we have obtained here to prevention of breast cancer in women at exceptionally high risk still requires further development. It is most likely that CDDO-Me will be most effective if used in combination with other chemopreventive agents (6, 21). Whether such agents will turn out to be PARP inhibitors, rexinoids, or other anti-inflammatory agents remains to be determined. The practical use of CDDO-Me (generically known as bardoxolone methyl) for successful treatment of advanced diabetic nephropathy in a Phase 2 clinical trial (32, 43) indicates that synthetic oleanane triterpenoids can be given safely to patients in a useful manner. Considering the clinical problems facing young women who are newly diagnosed with a BRCA mutation, there is now a compelling need to push this area of research to the point that it becomes clinically practical.
Acknowledgments
These studies were supported by the Breast Cancer Research Foundation and the NIH (RO1 CA78814).
Footnotes
Disclosure of Potential Conflicts of Interest: M.B. Sporn: commercial research grant, Reata Pharmaceuticals, Inc.; M.B. Sporn and K. Liby: patent interest in synthetic triterpenoids. The other authors disclose no potential conflicts of interest.
References
- 1.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 2.Uray IP, Brown PH. Chemoprevention of hormone receptor-negative breast cancer: new approaches needed. Recent Results Cancer Res. 2011;188:147–62. doi: 10.1007/978-3-642-10858-7_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fackenthal JD, Olopade OI. Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nat Rev Cancer. 2007;7:937–48. doi: 10.1038/nrc2054. [DOI] [PubMed] [Google Scholar]
- 4.Trainer AH, Lewis CR, Tucker K, Meiser B, Friedlander M, Ward RL. The role of BRCA mutation testing in determining breast cancer therapy. Nat Rev Clin Oncol. 2010;7:708–17. doi: 10.1038/nrclinonc.2010.175. [DOI] [PubMed] [Google Scholar]
- 5.Salhab M, Bismohun S, Mokbel K. Risk-reducing strategies for women carrying BRCA1/2 mutations with a focus on prophylactic surgery. BMC Womens Health. 2010;10:28. doi: 10.1186/1472-6874-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sporn MB. Perspective: The big C - for Chemoprevention. Nature. 2011;471:S10–1. doi: 10.1038/471S10a. [DOI] [PubMed] [Google Scholar]
- 7.Sporn MB, Liby KT. Cancer chemoprevention: scientific promise, clinical uncertainty. Nat Clin Pract Oncol. 2005;2:518–25. doi: 10.1038/ncponc0319. [DOI] [PubMed] [Google Scholar]
- 8.Cuzick J, Decensi A, Arun B, Brown PH, Castiglione M, Dunn B, et al. Preventive therapy for breast cancer: a consensus statement. Lancet Oncol. 2011;12:496–503. doi: 10.1016/S1470-2045(11)70030-4. [DOI] [PubMed] [Google Scholar]
- 9.Davidson NE, Kensler TW. “MAPping” the course of chemoprevention in breast cancer. N Engl J Med. 2011;364:2463–4. doi: 10.1056/NEJMe1106052. [DOI] [PubMed] [Google Scholar]
- 10.Wu K, Zhang Y, Xu XC, Hill J, Celestino J, Kim HT, et al. The retinoid X receptor-selective retinoid, LGD1069, prevents the development of estrogen receptor-negative mammary tumors in transgenic mice. Cancer Res. 2002;62:6376–80. [PubMed] [Google Scholar]
- 11.Liby K, Rendi M, Suh N, Royce DB, Risingsong R, Williams CR, et al. The combination of the rexinoid, LG100268, and a selective estrogen receptor modulator, either arzoxifene or acolbifene, synergizes in the prevention and treatment of mammary tumors in an estrogen receptor-negative model of breast cancer. Clin Cancer Res. 2006;12:5902–9. doi: 10.1158/1078-0432.CCR-06-1119. [DOI] [PubMed] [Google Scholar]
- 12.Liby K, Royce DB, Risingsong R, Williams CR, Wood MD, Chandraratna RA, et al. A new rexinoid, NRX194204, prevents carcinogenesis in both the lung and mammary gland. Clin Cancer Res. 2007;13:6237–43. doi: 10.1158/1078-0432.CCR-07-1342. [DOI] [PubMed] [Google Scholar]
- 13.Brown PH, Subbaramaiah K, Salmon AP, Baker R, Newman RA, Yang P, et al. Combination chemoprevention of HER2/neu-induced breast cancer using a cyclooxygenase- 2 inhibitor and a retinoid X receptor-selective retinoid. Cancer Prev Res (Phila) 2008;1:208–14. doi: 10.1158/1940-6207.CAPR-08-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T, et al. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet. 1999;22:37–43. doi: 10.1038/8743. [DOI] [PubMed] [Google Scholar]
- 15.Brodie SG, Xu X, Qiao W, Li WM, Cao L, Deng CX. Multiple genetic changes are associated with mammary tumorigenesis in Brca1 conditional knockout mice. Oncogene. 2001;20:7514–23. doi: 10.1038/sj.onc.1204929. [DOI] [PubMed] [Google Scholar]
- 16.Bouwman P, Jonkers J. Mouse models for BRCA1 associated tumorigenesis: from fundamental insights to preclinical utility. Cell Cycle. 2008;7:2647–53. doi: 10.4161/cc.7.17.6266. [DOI] [PubMed] [Google Scholar]
- 17.Burga LN, Hu H, Juvekar A, Tung NM, Troyan SL, Hofstatter EW, et al. Loss of BRCA1 leads to an increase in epidermal growth factor receptor expression in mammary epithelial cells, and epidermal growth factor receptor inhibition prevents estrogen receptor-negative cancers in BRCA1-mutant mice. Breast Cancer Res. 2011;13:R30. doi: 10.1186/bcr2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poole AJ, Li Y, Kim Y, Lin SC, Lee WH, Lee EY. Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science. 2006;314:1467–70. doi: 10.1126/science.1130471. [DOI] [PubMed] [Google Scholar]
- 19.Bachelier R, Xu X, Li C, Qiao W, Furth PA, Lubet RA, et al. Effect of bilateral oophorectomy on mammary tumor formation in BRCA1 mutant mice. Oncol Rep. 2005;14:1117–20. [PubMed] [Google Scholar]
- 20.Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7:357–69. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- 21.Liby K, Risingsong R, Royce DB, Williams CR, Yore MM, Honda T, et al. Prevention and treatment of experimental estrogen receptor-negative mammary carcinogenesis by the synthetic triterpenoid CDDO-methyl Ester and the rexinoid LG100268. Clin Cancer Res. 2008;14:4556–63. doi: 10.1158/1078-0432.CCR-08-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim EH, Deng CX, Sporn MB, Liby KT. CDDO-imidazolide induces DNA damage, G2/M arrest and apoptosis in BRCA1-mutated breast cancer cells. Cancer Prev Res (Phila) 2011;4:425–34. doi: 10.1158/1940-6207.CAPR-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honda T, Rounds BV, Gribble GW, Suh N, Wang Y, Sporn MB. Design and synthesis of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, a novel and highly active inhibitor of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett. 1998;8:2711–4. doi: 10.1016/s0960-894x(98)00479-x. [DOI] [PubMed] [Google Scholar]
- 24.Honda T, Honda Y, Favaloro FG, Jr, Gribble GW, Suh N, Place AE, et al. A novel dicyanotriterpenoid, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-onitrile, active at picomolar concentrations for inhibition of nitric oxide production. Bioorg Med Chem Lett. 2002;12:1027–30. doi: 10.1016/s0960-894x(02)00105-1. [DOI] [PubMed] [Google Scholar]
- 25.Honda T, Janosik T, Honda Y, Han J, Liby KT, Williams CR, et al. Design, synthesis, and biological evaluation of biotin conjugates of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid for the isolation of the protein targets. J Med Chem. 2004;47:4923–32. doi: 10.1021/jm049727e. [DOI] [PubMed] [Google Scholar]
- 26.Yore MM, Liby KT, Honda T, Gribble GW, Sporn MB. The synthetic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole blocks nuclear factor-kappaB activation through direct inhibition of IkappaB kinase beta. Mol Cancer Ther. 2006;5:3232–9. doi: 10.1158/1535-7163.MCT-06-0444. [DOI] [PubMed] [Google Scholar]
- 27.Liby K, Yore MM, Roebuck BD, Baumgartner KJ, Honda T, Sundararajan C, et al. A novel acetylenic tricyclic bis-(cyano enone) potently induces phase 2 cytoprotective pathways and blocks liver carcinogenesis induced by aflatoxin. Cancer Res. 2008;68:6727–33. doi: 10.1158/0008-5472.CAN-08-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wissner A, Overbeek E, Reich MF, Floyd MB, Johnson BD, Mamuya N, et al. Synthesis and structure-activity relationships of 6,7-disubstituted 4-anilinoquinoline-3-carbonitriles. The design of an orally active, irreversible inhibitor of the tyrosine kinase activity of the epidermal growth factor receptor (EGFR) and the human epidermal growth factor receptor-2 (HER-2) J Med Chem. 2003;46:49–63. doi: 10.1021/jm020241c. [DOI] [PubMed] [Google Scholar]
- 29.Rabindran SK, Discafani CM, Rosfjord EC, Baxter M, Floyd MB, Golas J, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–65. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- 30.Couch RD, Browning RG, Honda T, Gribble GW, Wright DL, Sporn MB, et al. Studies on the reactivity of CDDO, a promising new chemopreventive and chemotherapeutic agent: implications for a molecular mechanism of action. Bioorg Med Chem Lett. 2005;15:2215–9. doi: 10.1016/j.bmcl.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 31.Sporn MB, Liby KT, Yore MM, Fu L, Lopchuk JM, Gribble GW. New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J Nat Prod. 2011;74:537–45. doi: 10.1021/np100826q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pergola PE, Krauth M, Huff JW, Ferguson DA, Ruiz S, Meyer CJ, et al. Effect of Bardoxolone Methyl on Kidney Function in Patients with T2D and Stage 3b-4 CKD. Am J Nephrol. 2011;33:469–76. doi: 10.1159/000327599. [DOI] [PubMed] [Google Scholar]
- 33.Liby K, Risingsong R, Royce DB, Williams CR, Ma T, Yore MM, et al. Triterpenoids CDDO-methyl ester or CDDO-ethyl amide and rexinoids LG100268 or NRX194204 for prevention and treatment of lung cancer in mice. Cancer Prev Res (Phila) 2009;2:1050–8. doi: 10.1158/1940-6207.CAPR-09-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin S, Wakabayashi J, Yates MS, Wakabayashi N, Dolan PM, Aja S, et al. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur J Pharmacol. 2009;620:138–44. doi: 10.1016/j.ejphar.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes DT, Martel PM, Kinlaw WB, Eisenberg BL. The synthetic triterpenoid CDDO-Im inhibits fatty acid synthase expression and has antiproliferative and proapoptotic effects in human liposarcoma cells. Cancer Invest. 2008;26:118–27. doi: 10.1080/07357900701522612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lupu R, Menendez JA. Targeting fatty acid synthase in breast and endometrial cancer: An alternative to selective estrogen receptor modulators? Endocrinology. 2006;147:4056–66. doi: 10.1210/en.2006-0486. [DOI] [PubMed] [Google Scholar]
- 37.Menendez JA, Lupu R, Colomer R. Targeting fatty acid synthase: potential for therapeutic intervention in her-2/neu-overexpressing breast cancer. Drug News Perspect. 2005;18:375–85. doi: 10.1358/dnp.2005.18.6.927929. [DOI] [PubMed] [Google Scholar]
- 38.Vannini N, Lorusso G, Cammarota R, Barberis M, Noonan DM, Sporn MB, et al. The synthetic oleanane triterpenoid, CDDO-methyl ester, is a potent antiangiogenic agent. Mol Cancer Ther. 2007;6:3139–46. doi: 10.1158/1535-7163.MCT-07-0451. [DOI] [PubMed] [Google Scholar]
- 39.Yore MM, Kettenbach AN, Sporn MB, Gerber SA, Liby KT. Proteomic analysis shows synthetic oleanane triterpenoid binds to mTOR. PLoS One. 2011;6:e22862. doi: 10.1371/journal.pone.0022862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–70. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polyak K, Kalluri R. The role of the microenvironment in mammary gland development and cancer. Cold Spring Harb Perspect Biol. 2010;2:a003244. doi: 10.1101/cshperspect.a003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 43.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011 doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]