Abstract
Background
Several phase II trials in men with non-castrate PSA-recurrent prostate cancer have assessed the impact of novel non-hormonal agents on PSA kinetics. However, it is unknown whether changes in PSA kinetics influence metastasis-free survival (MFS).
Methods
We performed a retrospective post hoc analysis of 146 men treated in four phase II trials examining the investigational agents marimastat (a matrix metalloproteinase inhibitor; n=39), imatinib (a tyrosine kinase inhibitor; n=25), ATN-224 (a copper/zinc-superoxide dismutase inhibitor; n=22), and lenalidomide (an antiangiogenic/immunomodulatory drug; n=60). We investigated factors influencing MFS, including within-subject changes in PSA kinetics (PSA slope, doubling time, and velocity) before and after treatment initiation.
Results
After a median follow-up of 16.8 months, 70 patients (47.9%) developed metastases. In multivariable Cox regression models, factors that were independently predictive of MFS after adjusting for age and other clinical prognostic variables were baseline PSA doubling time (PSADT) (P=.05), baseline PSA slope (P=.01), on-study change in PSADT (P=.02), and on-study change in PSA slope (P=.03). In a landmark Kaplan-Meier analysis, median MFS was 63.5 months (95% CI 34.6–not reached) and 28.9 months (95% CI 13.5–68.0) for men with or without any decrease in PSA slope by 6 months after treatment, respectively.
Conclusions
This hypothesis-generating analysis suggests that within-subject changes in PSADT and PSA slope after initiation of experimental therapy may correlate with MFS in men with biochemically-recurrent prostate cancer. If validated in prospective trials, changes in PSA kinetics may represent a reasonable intermediate endpoint for screening new agents in these patients.
INTRODUCTION
In men with non-metastatic prostate-specific antigen (PSA)-recurrent prostate cancer following definitive local therapy, there is currently no consensus on optimal management.1,2 Treatment options include observation,3,4 continuous androgen deprivation therapy (ADT) initiated upon PSA recurrence,5,6 deferred ADT administered after the development of metastases or upon symptomatic progression,7,8 intermittent ADT,9,10 or enrollment in clinical trials.11 In addition, a subset of patients with PSA recurrence may benefit from salvage pelvic irradiation.12 In practice, early ADT is often employed in this patient population but this approach is associated with long-term cardiovascular and metabolic risks.13–15 Furthermore, a recent meta-analysis has shown that compared to deferred ADT, early ADT decreases prostate cancer-specific mortality but increases non-prostate cancer-specific mortality, and has no significant effect on overall survival.8 However, since no prospective randomized trial has specifically been conducted in men with non-metastatic PSA-recurrent disease to evaluate hormonal therapy initiated immediately or at the time of metastatic progression, there is still significant controversy surrounding the optimal timing of androgen suppression in these patients.16
In recent years, interest has emerged in identifying hormone-sparing therapies for the initial management of men with PSA-recurrent disease without detectable metastases. To date, a large number of biological and immunological agents have been evaluated in phase II clinical trials in these patients.17–25 However, progress in this area has been hampered by the use of suboptimal study endpoints, the absence of placebo-controlled trials, and the short duration of follow-up. Because of the inability to follow radiographic or clinical parameters in this setting, the majority of such studies have utilized changes in PSA kinetics (PSA doubling time, PSA slope, and PSA velocity) as their primary endpoint. Importantly, it is not known whether such PSA alterations are related to clinically meaningful outcomes such as metastasis-free survival (MFS) and overall survival.
We have previously conducted four phase II trials investigating experimental non-hormonal agents in men with non-metastatic PSA-recurrent prostate cancer after local therapy.19–22 All of these studies examined changes in PSA kinetics before and after study drug initiation as their primary endpoint, and none specifically evaluated MFS. The present study is a combined retrospective analysis of these four trials (n = 146), aiming to investigate the potential relationship between intra-subject changes in PSA kinetics and MFS. We hypothesized that improvements in PSA kinetics after initiation of such non-hormonal treatments would correlate with prolonged MFS in these patients.
PATIENTS AND METHODS
Patients
The designs of the four phase II studies included in this combined analysis have been described in detail previously.19–22 The first study19 was a randomized phase I/II trial evaluating 3 doses of an oral matrix metalloproteinase inhibitor, marimastat (5 mg, 20 mg, or 40 mg daily). A total of 39 patients were enrolled, and the primary efficacy endpoint was change in median PSA slope after 6 months of the study drug. The second trial20 was a single-arm phase II study of the oral tyrosine kinase inhibitor, imatinib (800 mg daily). A total of 25 men participated, and the primary endpoint was PSA response rate defined as a 50% decrease in PSA from baseline. The third study21 was a randomized phase II trial of 2 doses of an oral copper/zinc-superoxide dismutase inhibitor, ATN-224 (30 mg or 300 mg daily). A total of 47 patients were accrued, and the primary endpoint was change in mean PSA slope and mean PSA doubling time on study. The fourth trial22 was a randomized phase I/II study evaluating the oral antiangiogenic and immunomodulatory drug, lenalidomide (5 mg or 25 mg daily). A total of 60 men were enrolled, and the primary endpoint was change in median PSA slope after 6 months. None of these trials were designed a priori to capture data on MFS or overall survival.
All four studies were conducted at the Johns Hopkins Kimmel Cancer Center, Baltimore, MD. Three trials were single-center experiences while the ATN-224 study was performed through the Department of Defense/Prostate Cancer Foundation-sponsored consortium that also included 5 other centers. In all studies, eligible patients were required to have PSA-recurrent prostate cancer after local therapy (prostatectomy or radiotherapy), non-castrate levels of serum testosterone, non-metastatic disease as determined by CT and/or bone scan, and rising PSA levels. All trials used experimental agents that were not expected to mediate their effects through the endocrine axis. While on study, patients were required to have PSA assessments either every month (marimastat, ATN-224) or every 2 months (imatinib, lenalidomide). Patients were treated with study drug for either 6 months (marimastat, ATN-224, lenalidomide) or 12 months (imatinib), or less if unmanageable drug-related toxicities developed. In all trials, patients came off study upon PSA progression, clinical progression, metastatic progression, or death (whichever occurred first).
Study Design
The present study was a post hoc analysis of MFS using combined data from the four phase II studies outlined above. We retrospectively examined patient charts and/or electronic medical records for information on first metastatic occurrence and death. Metastatic disease was defined as the presence of osseous metastases visualized on bone scan (or MRI scan); and/or visceral (liver, lung, brain) or extra-pelvic nodal metastases visualized on CT scan. In the four studies, radiographic evaluations were performed either every 3 months (ATN-224) or every 6 months (marimastat, imatinib, lenalidomide), or sooner if clinically indicated. After trial completion, imaging studies were ordered at the discretion of the treating physicians (and scanning intervals ranged from 2 to 12 months). Radiologists were blinded to PSA results. Metastasis-free survival was defined as the time interval from study entry until initial metastasis or death. Patients were captured at the time of their first positive scan or censored at the time of their last confirmed negative scan. The data cut-off date was set as March 1st, 2010. This study was approved by the Johns Hopkins University institutional review board.
We used all available PSA values in the 12 months preceding study enrollment to calculate the baseline PSA kinetics parameters, and all available PSAs in the first 6 months after treatment initiation to calculate the on-study PSA kinetics. PSA slope was defined as the linear regression line of the natural log of PSA (in ng/mL) against time (in months).26 PSA doubling time (PSADT) was defined as the natural log of 2 divided by the slope of the linear regression line of the natural log of PSA against time (in months).26 PSA velocity was defined as the linear regression line of PSA (in the natural scale) against time (in months).27 Within-subject changes in PSA kinetics parameters before and after study enrollment were determined by comparing baseline values to on-study values. The aim of this study was to investigate the correlation between intra-subject changes in PSA kinetics and MFS, after adjusting for other clinical factors known to influence MFS.
The number of pre-treatment or on-study PSA values measured on any given patient ranged from a minimum of 3 to a maximum of 7. When calculating the (loge) PSA slope with a linear regression of the natural log of PSA against time, our assumption was that the PSA values on a logarithmic scale over time approximated a straight line, that the variance of residuals calculated from these regressions was constant over time, and that residuals were normally distributed. Given the small number of PSA values (3–7) per regression, assumptions of homoscedasticity and normality of the residuals from these regressions could not be evaluated for each individual patient. However, while not an assumption of the linear regressions, a test of normality of the distribution of the log of the PSA values was not significant (Kolmogorov-Smirnov test, P=.14).
Statistical Analysis
The primary objective of this study was to determine the independent contribution of changes in PSA kinetics on MFS. Time to metastasis was calculated from study entry to the date of metastasis or death. Event time distributions for this endpoint were estimated using the Kaplan-Meier method28 and 95% confidence intervals (CIs) were calculated by the method of Brookmeyer and Crowley.29 Landmark stratified Cox proportional hazards regressions were used to assess the effects of PSA kinetics on MFS. Models were stratified by study, and the landmark time was set at 6 months. This time point was chosen because all PSA values during the first 6 months after study entry were used to calculate on-study PSA kinetics. Such a landmark analysis prevents metastatic events that might occur during the first 6 months on study to be included in the analysis.
In the univariate analysis, factors that entered the model included age at study initiation (continuous variable), Gleason score (<7 vs. ≥7), tumor stage at diagnosis (T1/2 vs. T3/4), lymph node involvement at diagnosis (N0 vs. N1), use of ADT after PSA recurrence but before metastasis (yes vs. no), baseline PSADT (≥6 mo vs. <6 mo), baseline PSA velocity (below median vs. above median), baseline PSA slope (below median vs. above median), change in PSADT before and after study initiation (increase in PSADT vs. no increase), change in PSA velocity (decrease in velocity vs. no decrease), and change in PSA slope (decrease in slope vs. no decrease). In the multivariable analysis, only those variables with P-values from the univariate model of ≤0.10 were included. Notably, because all three PSA kinetic measures are a function of changes in PSA against time and are all strongly interrelated, three separate multivariable models each evaluating one kinetic measure at a time were created.
To account for the fact that on-study determinations of PSA kinetics are dependent upon the time point at which they are calculated, time-dependent covariate analyses of the relationship between log PSA or changes in log PSA and MFS were also conducted. This was performed using 3, 4 and 6 months of PSA data, respectively.
All P-values reported are two-sided, and the significance level was set at ≤0.05 for all analyses. Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, North Carolina) and R version 2.1 (National Cancer Institute, Bethesda, Maryland).
RESULTS
Patient Characteristics
Table 1 describes the clinical characteristics of men in each of the four trials. Twenty-five of 47 patients in the ATN-224 study (those enrolled at the other sites), did not have available data on metastasis and were excluded from the analysis. All 39 patients in the marimastat study, all 25 patients in the imatinib study, and all 60 patients in the lenalidomide study had full information available to determine metastasis-free survival (MFS). There were no statistically significant differences in any of the clinical variables listed in Table 1 between patients with (n=146) and without (n=25) metastasis information (data not shown). With a median follow-up in the combined evaluable cohort of 16.8 months, 70 patients (47.9%) developed metastases. The median MFS in the whole cohort was 38.1 months (95% CI, 22.9 to 61.7 months).
Table 1.
Patient Characteristics
| Characteristic | Trial
|
|||
|---|---|---|---|---|
| Marimastat (n=39) | Imatinib (n=25) | ATN-224 (n=22) | Lenalidomide (n=60) | |
| Minimum PSA requirement for trial entry | PSA ≥1.0 ng/mL | PSA ≥1.0 ng/mL | PSA ≥2.0 ng/mL | PSA ≥1.0 ng/mL |
| PSADT requirement for trial entry | Any PSADT | Any PSADT | PSADT ≤12 months | Any PSADT |
| Age, years | ||||
| Mean (Range) | 61 (48 to 77) | 65 (50 to 77) | 62 (53 to 75) | 63 (50 to 81) |
| Median | 58 | 67 | 63 | 64 |
| Local therapy | ||||
| Prostatectomy only | 19 (49%) | 5 (20%) | 10 (45%) | 24 (40%) |
| Radiotherapyonly | 4 (10%) | 9 (36%) | 3 (14%) | 11 (18%) |
| Both | 16 (41%) | 11 (44%) | 9 (41%) | 25 (42%) |
| Gleason score | ||||
| ≤ 6 | 0 (0%) | 8 (32%) | 5 (23%) | 13 (22%) |
| 7 | 24 (62%) | 12 (48%) | 7 (32%) | 31 (51%) |
| ≥ 8 | 15 (38%) | 5 (20%) | 10 (45%) | 16 (27%) |
| T stage | ||||
| T1 | 0 (0%) | 5 (20%) | 0 (0%) | 7 (12%) |
| T2 | 7 (18%) | 10 (40%) | 12 (55%) | 18 (30%) |
| T3 | 32 (82%) | 10 (40%) | 10 (45%) | 35 (58%) |
| N stage | ||||
| N0 | 34 (87%) | 25 (100%) | 18 (82%) | 53 (88%) |
| N1 | 5 (13%) | 0 (0%) | 4 (18%) | 7 (12%) |
| Use of ADT before metastases | ||||
| No | 27 (69%) | 13 (52%) | 17 (77%) | 53 (88%) |
| Yes | 12 (31%) | 12 (48%) | 5 (23%) | 7 (12%) |
| Baseline PSA, ng/mL | ||||
| Mean (Range) | 6.8 (0.7 to 36.5) | 15.0 (1.3 to 53.3) | 16.8 (2.1 to 89.3) | 13.3 (1.0 to 92.8) |
| Median | 3.9 | 11.0 | 7.1 | 7.0 |
| Baseline PSA doubling time, mo | ||||
| Mean (Range) | 4.9 (1.4 to 12.8) | 9.4 (1.9 to 26.2) | 4.7 (1.2 to 13.3) | 7.1 (0.8 to 32.2) |
| Median | 4.8 | 8.6 | 4.4 | 4.7 |
| Baseline PSA slope | ||||
| Mean (Range) | 0.18 (0.05 to 0.50) | 0.12 (0.03 to 0.37) | 0.22 (0.05 to 0.57) | 0.19 (0.02 to 0.88) |
| Median | 0.15 | 0.08 | 0.16 | 0.15 |
| Baseline PSA velocity, ng/mL/mo | ||||
| Mean (Range) | 0.6 (0.1 to 3.9) | 0.9 (0.1 to 3.2) | 2.6 (0.2 to 23.8) | 1.5 (0.1 to 16.6) |
| Median | 0.4 | 0.7 | 0.9 | 0.7 |
| Follow-up, mo | ||||
| Mean (Range) | 38.4 (4.7 to 121.4) | 43.5 (3.1 to 88.8) | 16.9 (1.2 to 34.3) | 15.7 (0.9 to 40.6) |
| Median | 21.8 | 39.7 | 18.1 | 15.3 |
Abbreviations: ADT, androgen deprivation therapy; PSA, prostate specific antigen; PSADT, prostate specific antigen doubling time.
Overall (n=146), median age at study entry was 63 years; 58 men (40%) had primary prostatectomy, 27 (18%) had primary radiotherapy, and 61 (42%) had prostatectomy and salvage radiotherapy; 26 men (18%) had Gleason score ≤6, 74 (51%) had Gleason score 7, and 46 (31%) had Gleason score ≥8; 12 men (8%) had T1 disease, 47 (32%) had T2 disease, and 87 (60%) had T3 disease; 130 men (89%) had node-negative disease, and 16 (11%) had node-positive disease; 110 men (75%) did not receive androgen deprivation therapy between developing PSA recurrence and metastatic disease, and 36 (25%) did receive ADT between PSA recurrence and metastasis (no patient received chemotherapy before metastasis); median PSA at baseline was 7.1 ng/mL; median PSA doubling time at baseline was 5.0 months; median PSA slope at baseline was 0.14; and median PSA velocity at baseline was 0.7 ng/mL/month.
Correlation of Changes in PSA Kinetics with Metastasis-Free Survival
In time-dependent covariate analysis, there was a statistically significant relationship between (log) PSA as well as changes in (log) PSA and MFS, using 3 months, 4 months, and 6 months of on-study PSA data (Table 2).
Table 2.
Stratified Time-Dependent Covariate Analysis of the Relationship Between (log) PSA or Changes in (log) PSA and Metastasis-Free Survival
| Analysis Type | (log) PSA
|
Change in (log) PSA
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| 3 Mo of on-study data | 1.47 | (1.16 – 1.88) | 0.0017 | 4.30 | (2.48 – 7.46) | <0.0001 |
| 4 Mo of on-study data | 1.62 | (1.26 – 2.07) | 0.0001 | 2.64 | (1.79 – 3.90) | <0.0001 |
| 6 Mo of on-study data | 1.84 | (1.44 – 2.36) | <0.0001 | 3.23 | (2.24 – 4.66) | <0.0001 |
Abbreviations: PSA, prostate-specific antigen; HR, hazard ratio; CI, confidence interval.
In univariate proportional hazards regression analyses (stratified by study), significant associations with MFS were observed for age, Gleason score, use of ADT before metastasis, baseline PSA doubling time (PSADT), baseline PSA velocity, baseline (log) PSA slope, change in PSADT, change in PSA velocity, and change in (log) PSA slope (Table 3). In these analyses, changes in the three measures of PSA kinetics were treated as dichotomous variables in an effort to facilitate clinical interpretation of the results. However, when treated as continuous variables, changes in the PSA kinetics parameters retained their association with MFS (change in PSADT: HR 0.99, 95% CI 0.98–1.00, P=.06; change in PSA velocity: HR 0.83, 95% CI 0.75–0.92, P=.01; change in PSA slope: HR 0.99, 95% CI 0.98–1.00, P=.06).
Table 3.
Stratified Univariate Cox Regression Analyses for Predicting Metastasis-Free Survival in Men with PSA-Recurrent Prostate Cancer Enrolled in All 4 Trials
| Variable | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|
| Age, years (Continuous) | 0.92 | (0.89 – 0.96) | 0.0001 |
| Local therapy | |||
| Surgery (± salvage radiotherapy) | 0.55 | (0.25 – 1.23) | 0.150 |
| Radiotherapy only | 1 [reference] | ||
| Gleason score | |||
| < 7 | 0.17 | (0.05 – 0.56) | 0.003 |
| ≥ 7 | 1 [reference] | ||
| T stage | |||
| T1–2 | 0.63 | (0.36 – 1.10) | 0.110 |
| T3 | 1 [reference] | ||
| N stage | |||
| N0 | 0.60 | (0.30 – 1.16) | 0.130 |
| N1 | 1 [reference] | ||
| Use of ADT before metastases | |||
| Yes | 0.08 | (0.03 – 0.21) | <0.0001 |
| No | 1 [reference] | ||
| Baseline PSA doubling time, mo | |||
| ≥ 6 mo | 0.26 | (0.13 – 0.49) | <0.0001 |
| < 6 mo | 1 [reference] | ||
| Baseline PSA velocity, ng/mL/mo | |||
| Below median (=0.7 ng/mL/mo) | 0.57 | (0.35 – 0.92) | 0.020 |
| Above median | 1 [reference] | ||
| Baseline (log) PSA slope | |||
| Below median (=0.14) | 0.23 | (0.13 – 0.42) | <0.0001 |
| Above median | 1 [reference] | ||
| Change in PSA doubling time*, mo | |||
| Increase | 0.30 | (0.14 – 0.65) | 0.002 |
| No increase | 1 [reference] | ||
| Change in PSA velocity*, ng/mL/mo | |||
| Decrease | 0.32 | (0.16 – 0.64) | 0.001 |
| No decrease | 1 [reference] | ||
| Change in (log) PSA slope* | |||
| Decrease | 0.30 | (0.14 – 0.65) | 0.002 |
| No decrease | 1 [reference] | ||
Because these variables are time-dependent covariates, a landmark univariate regression analysis was performed in these cases (with the landmark time set at 6 months).
Abbreviations: PSA, prostate-specific antigen; ADT, androgen deprivation therapy; CI, confidence interval.
Figure 1 demonstrates the effect of changes in PSADT (increase in PSADT after study entry vs. no increase), changes in PSA velocity (decrease in PSA velocity vs. no decrease), and changes in (log) PSA slope (decrease in PSA slope vs. no decrease) on MFS using Kaplan-Meier analysis.
Fig 1.
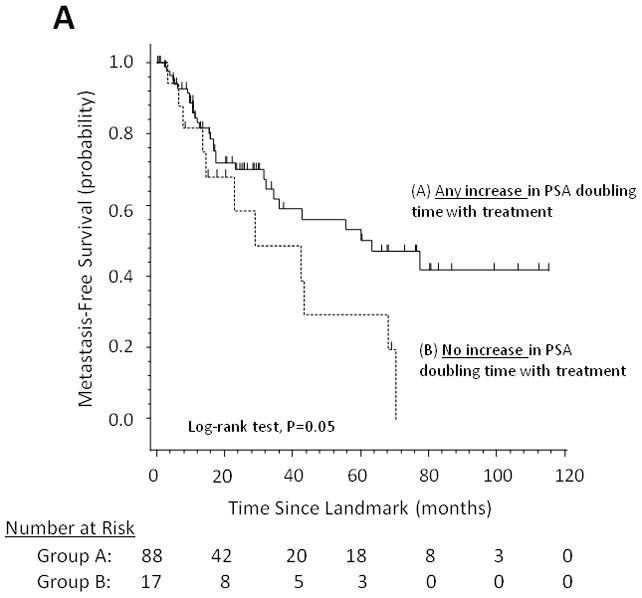
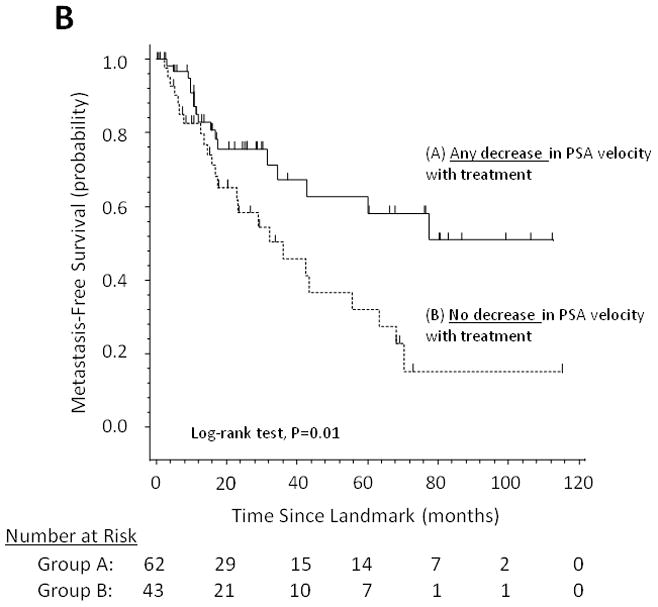
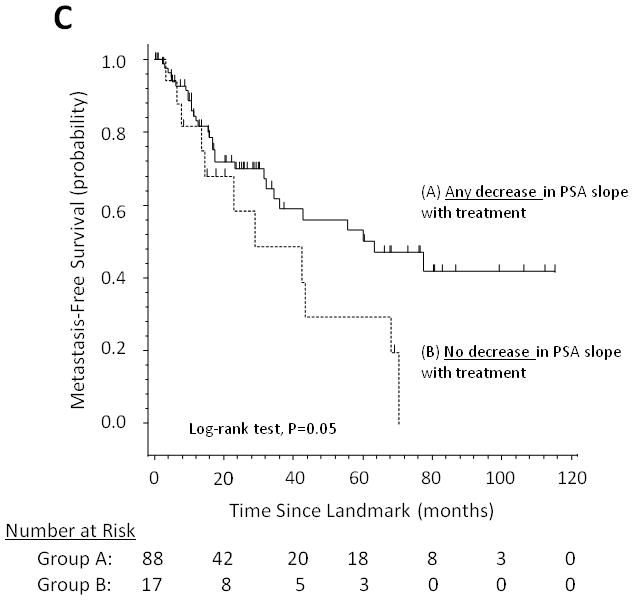
Metastasis-free survival stratified by (A) changes in PSA doubling time, (B) changes in PSA velocity, and (C) changes in log PSA slope.
In landmark multivariable analyses (Table 4), change in PSADT and change in (log) PSA slope emerged as significant independent predictors of MFS, while change in PSA velocity did not retain statistical significance. Our multivariable models were stratified by study to avoid assuming proportional hazards across the 4 different protocols. To test the discriminatory ability of the multivariable models, we calculated the concordance index (C) for Cox regressions. The c-statistics for the multivariable models using PSADT, velocity, and slope were 0.76, 0.76, and 0.78 respectively. These are within the range of acceptable discrimination (0.7 ≤ c-statistic < 0.8). The univariate kinetics models in Table 3 and the multivariable models in Table 4 were tested for proportional hazards by including time-by-covariate interaction terms in the models. To this end, none of these terms were significant (if a term had been significant, we would have adjusted the model by retaining the interaction term). In addition, plots of Schoenfeld residuals (with restricted cubic splines showing the smoothed relationship of the residuals with time) for each predictor in all models did not show any consistent trends with time. Global correlation with time tests of these Schoenfeld residuals for each multivariable model in Table 4 was not significant. This suggested that the proportional hazards assumption was satisfied by our data.
Table 4.
Stratified Landmark Multivariable Cox Regression Analyses for Predicting Metastasis-Free Survival, Considering Separately the Effect of (A) PSA Doubling Time Changes, (B) PSA Velocity Changes, and (C) PSA Slope Changes
| Variable | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|
| (A) Effect of PSA doubling time changes on metastasis-free survival | |||
| Age, years (Continuous) | 0.97 | (0.91 – 1.03) | 0.290 |
| Gleason score | |||
| < 7 | 0.23 | (0.05 – 1.16) | 0.080 |
| ≥ 7 | 1 [reference] | ||
| Use of ADT before metastases | |||
| Yes | 0.15 | (0.05 – 0.40) | 0.0002 |
| No | 1 [reference] | ||
| Baseline PSA doubling time, mo | |||
| ≥ 6 mo | 0.38 | (0.15 – 1.00) | 0.050 |
| < 6 mo | 1 [reference] | ||
| Change in PSA doubling time, mo | |||
| Increase | 0.35 | (0.15 – 0.83) | 0.020 |
| No increase | 1 [reference] | ||
| (B) Effect of PSA velocity changes on metastasis-free survival | |||
|---|---|---|---|
| Age, years (Continuous) | 0.95 | (0.90 – 1.01) | 0.080 |
| Gleason score | |||
| < 7 | 0.14 | (0.03 – 0.65) | 0.010 |
| ≥ 7 | 1 [reference] | ||
| Use of ADT before metastases | |||
| Yes | 0.12 | (0.04 – 0.37) | 0.0002 |
| No | 1 [reference] | ||
| Baseline PSA velocity, ng/mL/mo | |||
| Below median (=0.7 ng/mL/mo) | 0.49 | (0.22 – 1.10) | 0.080 |
| Above median | 1 [reference] | ||
| Change in PSA velocity, ng/mL/mo | |||
| Decrease | 0.60 | (0.27 – 1.30) | 0.190 |
| No decrease | 1 [reference] | ||
| (C) Effect of PSA slope changes on metastasis-free survival | |||
|---|---|---|---|
| Age, years (Continuous) | 0.98 | (0.92 – 1.04) | 0.430 |
| Gleason score | |||
| < 7 | 0.19 | (0.04 – 1.00) | 0.050 |
| ≥ 7 | 1 [reference] | ||
| Use of ADT before metastases | |||
| Yes | 0.12 | (0.04 – 0.35) | 0.0001 |
| No | 1 [reference] | ||
| Baseline (log) PSA slope | |||
| Below median (=0.14) | 0.35 | (0.15 – 0.80) | 0.010 |
| Above median | 1 [reference] | ||
| Change in (log) PSA slope | |||
| Decrease | 0.40 | (0.18 – 0.93) | 0.030 |
| No decrease | 1 [reference] | ||
Abbreviations: PSA, prostate-specific antigen; ADT, androgen deprivation therapy; CI, confidence interval.
Figure 2A considers on-study changes in (log) PSA slope as falling into one of 3 distinct clinical subgroups: those cases in which PSA slope decreases and becomes negative after study drug initiation (i.e. absolute PSA levels decline), those cases in which PSA slope decreases but remains positive (i.e. absolute PSA levels continue to increase but at a slower rate), and those cases in which PSA slope increases (i.e. absolute PSA levels rise at an accelerated rate). Figure 2B shows the stratification of MFS according to these 3 subgroups, demonstrating that post-landmark median MFS is 77.5 months (95% CI, 31.6 to not reached), 55.7 months (95% CI, 23.3 to not reached), and 28.9 months (95% CI, 13.5 to 68.0 months), respectively.
Fig 2.
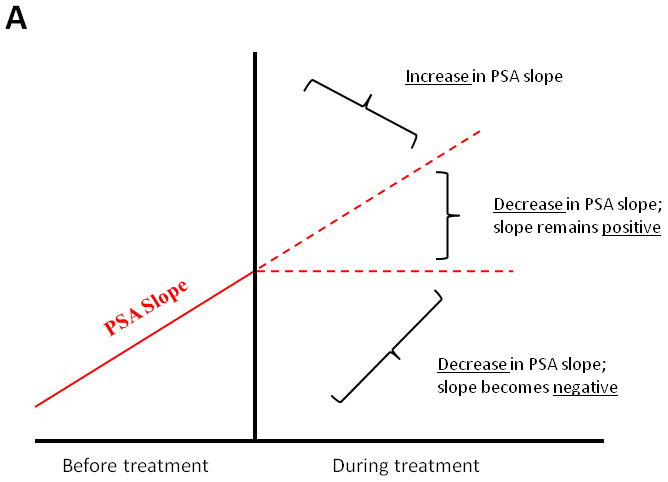
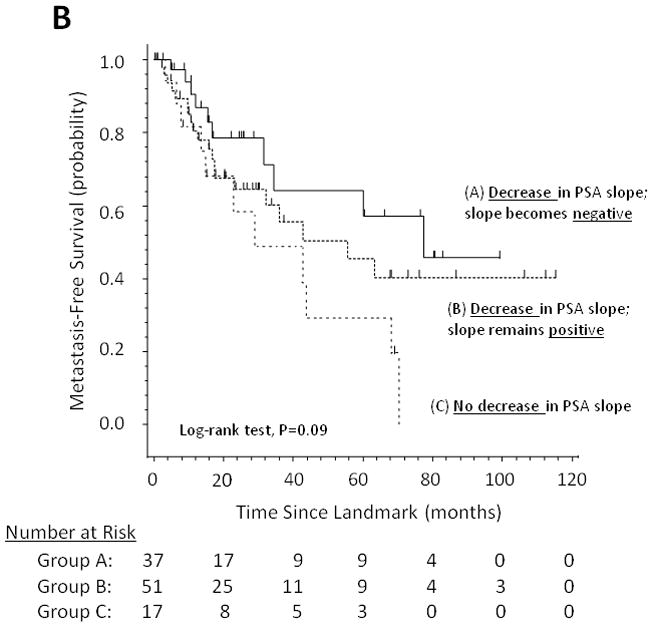
(A) Model showing theoretical changes in PSA slope after initiation of a non-hormonal experimental therapy. (B) Metastasis-free survival stratified by these three categories of PSA slope change
DISCUSSION
In men with non-castrate biochemically-recurrent prostate cancer after local therapy, the use of changes in PSA kinetics as an intermediate endpoint for evaluating the efficacy of non-hormonal experimental agents is attractive but unfounded. Exploring a potential association between changes in PSA kinetic measures and clinical outcomes would be important, as these PSA kinetics changes could serve as intermediate endpoints in therapeutic trials of such agents. This could offer the potential to derive reasonable conclusions about treatment efficacy while shortening the duration of follow-up required, overcoming a significant obstacle in testing novel non-hormonal agents in this patient population.
To our knowledge, this study is the first to document a correlation between changes in PSA kinetics and metastasis-free survival (MFS). Specifically, we have demonstrated that men whose PSADT increased after study entry (compared to pre-study PSADT) or whose PSA slope decreased (compared to pre-study PSA slope) had improved MFS. Importantly, the ability of changes in PSADT and PSA slope to predict MFS persisted after accounting for relevant clinical factors (e.g. age, Gleason score, and use of hormonal therapy before metastasis) as well as pre-treatment PSADT and PSA slope. Therefore, in addition to baseline PSA kinetic factors derived at study entry, post-treatment changes in these PSA kinetic measures were independently predictive of MFS.
For ease of clinical interpretation and applicability, we subdivided PSA kinetics changes into two clinical subgroups for use in the univariate and multivariable models. Interestingly, when patients were partitioned into 3 categories of PSA slope change, discreet MFS curves emerged in Kaplan-Meier analysis. To this end, MFS was longest for men whose PSA slopes decreased and became negative after study drug initiation, while MFS was shortest for men whose PSA slopes failed to decrease after study entry.
The results of this analysis can be compared to those of two related studies evaluating the correlation between PSA parameters and overall survival. In the first study involving patients with metastatic non-castrate prostate cancer receiving ADT, a PSA of ≤4 ng/mL after 7 months of ADT was a strong predictor of survival.30 In that same study, PSA progression (according to the Prostate Cancer Working Group 2 [PCWG2] definition31 within 7 months of ADT initiation also predicted for inferior survival.32 In the second study involving patients with castration-resistant prostate cancer receiving chemotherapy, a PSA decline of ≥30% was predictive of overall survival.33 In that same study, PSA progression (using the PCWG2 definition31) within 3 months of beginning chemotherapy also portended a worse survival.32 Although the present study was unable to evaluate overall survival due to an insufficient number of observed deaths at last follow-up, it adds to the body of literature suggesting that PSA parameters may correlate with meaningful clinical outcomes in men with prostate cancer.
This study has several limitations. First, this was a retrospective study and none of the four trials included in the combined analysis were designed to capture data on metastasis. In addition, because the majority of metastatic events occurred after patients had been taken off study, the frequency of subsequent bone scan and CT scan evaluations was dependent upon investigator practices and was not regulated. Second, there was no control over additional therapies (including hormonal therapy) administered to patients after they came off study in each of the four trials. Therefore, it was essential to adjust for the use of hormone therapy before metastasis in the multivariable models. Third, a minority of patients (14.6%) were excluded from analysis because of lack of available metastasis data. This may have introduced bias, although these patients did not differ statistically from those who did have available metastasis information with respect to any of the clinical variables. Fourth, the causal relationship between the study drugs and PSA kinetics changes cannot be proven in the absence of placebo control arms. A change in PSA kinetics after study entry, for example, may have been caused by more frequent PSA assessments on-study compared to pre-study PSA evaluations (which would not have been regulated). To this end, in a placebo-controlled trial evaluating the effect of celecoxib on PSADT in a similar patient population, 20% of 40 men in the placebo group had a post-treatment PSADT that was ≥200% of baseline.18 Finally, this study suffers from the known limitations of the landmark method,34 although this method was appropriately used here. For instance, this approach may result in loss of statistical power if a significant number of events (i.e. metastases, in this case) occur before the landmark time. In addition, it is generally difficult to determine whether the variable of interest (i.e. change in PSA kinetics, in this case) actually influences survival or if it simply acts as a marker of a more favorable prognosis.
In addition, because the studies included in this analysis used agents that are not particularly effective at altering PSA (with the exception of lenalidomide), this may have diminished our ability to detect a stronger association between PSA kinetics changes and MFS. Furthermore, the use of marginal drugs may potentially render the results of this analysis uninterpretable, because there is no direct evidence that on-study PSA kinetics changes were induced by the study drugs. Ultimately, the findings of this study will require confirmation in prospective trials using more effective agents as well as longer and more regimented follow-up.
In conclusion, this hypothesis-generating analysis suggests that within-subject changes in PSA kinetics (PSA doubling time, PSA slope) after initiation of non-hormonal experimental therapies may correlate with MFS in men with non-castrate PSA-recurrent prostate cancer. If these findings are validated in prospective trials using MFS as the primary endpoint, changes in PSA kinetics may represent a reasonable intermediate endpoint for screening new agents in this patient population. A prospective randomized trial aiming to validate these retrospective data is currently being designed.
Acknowledgments
We thank Gopal Bajaj, Tomasz Beer, Jennifer Callahan, Samuel Denmeade, Theodore DeWeese, Charles Drake, Renee Drew, Elizabeth Garrett-Mayer, Gilad Gordon, Susan Hudock, Menachem Laufer, Paul Mathew, Michael Morris, Roberto Pili, Steve Reich, Eli Rosenbaum, Charles Ryan, Victoria Sinibaldi, George Wilding, and Zhe Zhang.
This study was supported by DOD grant W81XWH-09-1-0149 (MAC), NIH/NCI grant P50 CA58236 (Prostate Cancer SPORE) (MAC, MAE), and an ASCO Cancer Foundation Young Investigator Award (ESA).
Footnotes
This work is original, and was presented as an abstract (poster discussion) at the 2010 ASCO Annual Meeting.
Authors’ disclosures: The authors indicate no potential conflicts of interest.
References
- 1.Boccon-Gibod L, Djavan WB, Hammerer P, et al. Management of prostate-specific antigen relapse in prostate cancer: a European Consensus. Int J Clin Pract. 2004;58:382–390. doi: 10.1111/j.1368-5031.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 2.Sandler HM, Eisenberger MA. Assessing and treating patients with increasing prostate-specific antigen following radical prostatectomy. J Urol. 2007;178:S20–S24. doi: 10.1016/j.juro.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 3.Antonarakis ES, Chen Y, Elsamanoudi SI, et al. Long-term overall survival and metastasis-free survival for men with prostate-specific antigen-recurrent prostate cancer after prostatectomy: analysis of the Center for Prostate Disease Research National Database. BJU Int. 2010 doi: 10.1111/j.1464-410X.2010.09878.x. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonarakis ES, Feng Z, Trock BJ, et al. The natural history of metastatic progression in men with PSA-recurrent prostate cancer after radical prostatectomy: long-term follow-up. BJU Int. 2011 doi: 10.1111/j.1464-410X.2011.10422.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–479. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 6.Tenenholz TC, Shields C, Ramesh VR, et al. Survival benefit for early hormone ablation in biochemically recurrent prostate cancer. Urol Oncol. 2007;25:101–109. doi: 10.1016/j.urolonc.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Moul JW, Wu H, Sun L, et al. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol. 2004;171:1141–1147. doi: 10.1097/01.ju.0000113794.34810.d0. [DOI] [PubMed] [Google Scholar]
- 8.Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 9.Opfermann KJ, Lai Z, Essenmacher L, et al. Intermittent hormone therapy in nonmetastatic prostate cancer. Clin Genitourin Cancer. 2006;5:138–143. doi: 10.3816/CGC.2006.n.030. [DOI] [PubMed] [Google Scholar]
- 10.Prapotnich D, Cathelineau X, Rozet F, et al. A 16-year clinical experience with intermittent androgen deprivation for prostate cancer: oncological results. World J Urol. 2009;27:627–635. doi: 10.1007/s00345-009-0393-1. [DOI] [PubMed] [Google Scholar]
- 11.Scher HI, Eisenberger M, D’Amico AV, et al. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 2004;22:537–556. doi: 10.1200/JCO.2004.07.099. [DOI] [PubMed] [Google Scholar]
- 12.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760–2769. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Hemelrijck M, Garmo H, Holmberg L, et al. Absolute and relative risk of cardiovascular disease in men with prostate cancer: results from the population-based PCBaSe Sweden. J Clin Oncol. 2010;28:3448–3456. doi: 10.1200/JCO.2010.29.1567. [DOI] [PubMed] [Google Scholar]
- 15.Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833–840. doi: 10.1161/CIRCULATIONAHA.109.192695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makarov DV, Humphreys EB, Mangold LA, et al. The natural history of men treated with deferred androgen deprivation therapy in whom metastatic prostate cancer developed following radical prostatectomy. J Urol. 2008;179:156–161. doi: 10.1016/j.juro.2007.08.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith MR, Manola J, Kaufman DS, et al. Rosiglitazone versus placebo for men with prostate carcinoma and a rising serum prostate-specific antigen level after radical prostatectomy and/or radiation therapy. Cancer. 2004;101:1569–1574. doi: 10.1002/cncr.20493. [DOI] [PubMed] [Google Scholar]
- 18.Smith MR, Manola J, Kaufman DS, et al. Celecoxib versus placebo for men with prostate cancer and a rising serum prostate-specific antigen after radical prostatectomy and/or radiation therapy. J Clin Oncol. 2006;24:2723–2728. doi: 10.1200/JCO.2005.03.7804. [DOI] [PubMed] [Google Scholar]
- 19.Rosenbaum E, Zahurak M, Sinibaldi V, et al. Marimastat in the treatment of patients with biochemically relapsed prostate cancer: a prospective randomized, double-blind, phase I/II trial. Clin Cancer Res. 2005;11:4437–4443. doi: 10.1158/1078-0432.CCR-04-2252. [DOI] [PubMed] [Google Scholar]
- 20.Bajaj GK, Zhang Z, Garrett-Mayer E, et al. Phase II study of imatinib mesylate in patients with prostate cancer with evidence of biochemical relapse after definitive radical retropubic prostatectomy or radiotherapy. Urology. 2007;69:526–531. doi: 10.1016/j.urology.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Lin J, Zahurak M, Beer TM, et al. A non-comparative randomized phase II study of two doses of ATN-224, a copper/zinc superoxide dismutase inhibitor, in patients with biochemically-recurrent hormone-naïve prostate cancer: a DOD/PCF study. Urol Oncol. 2011 doi: 10.1016/j.urolonc.2011.04.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keizman D, Zahurak M, Sinibaldi V, et al. Lenalidomide in nonmetastatic biochemically relapsed prostate cancer: results of a phase I/II double-blinded, randomized study. Clin Cancer Res. 2010;16:5269–5276. doi: 10.1158/1078-0432.CCR-10-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urba WJ, Nemunaitis J, Marshall F, et al. Treatment of biochemical recurrence of prostate cancer with granulocyte-macrophage colony-stimulating factor secreting, allogeneic, cellular immunotherapy. J Urol. 2008;180:2011–2017. doi: 10.1016/j.juro.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 24.McNeel DG, Dunphy EJ, Davies JG, et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol. 2009;27:4047–4054. doi: 10.1200/JCO.2008.19.9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiPaola RS, Chen Y, Bubley GJ, et al. A phase II study of PROSTVAC-V(vaccinia)/TRICOM and PROSTVAC-F(fowlpox)/TRICOM with GM-CSF in patients with PSA progression after local therapy for prostate cancer: results from ECOG 9802. ASCO Genitourinary Cancer Symposium [abstract 108]; Orlando. 2009. [Google Scholar]
- 26.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 27.D’Amico AV, Chen MH, Roehl KA, et al. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351:125–135. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–480. [Google Scholar]
- 29.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 30.Hussain M, Tangen CM, Higano C, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: Data from Southwest Oncology Group Trial 9346 (INT-0162) J Clin Oncol. 2006;24:3984–3990. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 31.Scher HI, Halabi S, Tannock I, et al. Design and endpoints of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussain M, Goldman B, Tangen C, et al. Prostate-specific antigen progression predicts overall survival in patients with metastatic prostate cancer: Data from Southwest Oncology Group Trials 9346 (Intergroup Study 0162) and 9916. J Clin Oncol. 2009;27:2450–2456. doi: 10.1200/JCO.2008.19.9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrylak DP, Ankerst DP, Jiang CS, et al. Evaluation of prostate-specific antigen declines for surrogacy in patients treated on SWOG 99-16. J Natl Cancer Inst. 2006;98:516–521. doi: 10.1093/jnci/djj129. [DOI] [PubMed] [Google Scholar]
- 34.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. J Clin Oncol. 2008;26:3913–3915. doi: 10.1200/JCO.2008.16.1000. [DOI] [PubMed] [Google Scholar]


