Abstract
Background: In patients who have experienced a myocardial infarction (MI), n−3 (omega-3) PUFA status is low, whereas the risk of depression is increased.
Objective: The objective was to assess whether the plant-derived α-linolenic acid (ALA) and the fish fatty acids EPA and DHA would improve affective states.
Design: In a secondary analysis of the randomized, double-blind, placebo-controlled Alpha Omega Trial, 4116 of 4837 (85.1%) patients (aged 60–80 y; 79.2% men) who had experienced an MI were included. Margarine spreads were used to deliver 400 mg EPA-DHA/d, 2 g ALA/d, both EPA-DHA and ALA, or a placebo for 40 mo. At 40 mo, the endpoints of depressive symptoms (15-item Geriatric Depression Scale) and dispositional optimism (a 4-item questionnaire and the Life Orientation Test–Revised) were analyzed by using a posttest-only design.
Results: The 4 randomly assigned groups did not differ in baseline characteristics. ALA supplementation significantly increased plasma cholesteryl ester concentrations of ALA by 69%, and EPA-DHA supplementation increased plasma cholesteryl ester concentrations of EPA and DHA by 61% and 30%, respectively. Depressive symptoms or dispositional optimism did not differ between groups with the use of n−3 fatty acids compared with placebo at the 40-mo follow-up. The standardized mean (±SE) differences in depressive symptoms were as follows: for EPA-DHA plus ALA (n = 1009) compared with placebo (n = 1030), −0.025 ± 0.044 (P = 0.57); for EPA-DHA (n = 1007) compared with placebo, −0.048 ± 0.044 (P = 0.28); and for ALA (n = 1022) compared with placebo, −0.047 ± 0.044 (P = 0.29).
Conclusions: In patients who had experienced an MI, low-dose EPA-DHA supplementation, ALA supplementation, or a combination of both did not affect depressive symptoms and dispositional optimism. These findings are in accord with those from previous trials in individuals without psychopathology or without severe depressive symptoms. This trial was registered at clinicaltrials.gov as NCT00127452.
INTRODUCTION
The (very-) long-chain essential n−3 polyunsaturated fatty acids have been associated with several health benefits. EPA (20:5n−3) and DHA (22:6n−3) are essential fatty acids found primarily in fatty seafood (eg, salmon, sardines, herring, and mackerel). Populations with a low fish intake had higher rates of depression (1). Most (2–4), but not all (5, 6), cross-sectional and prospective studies showed that fish and n−3 fatty acid intake was inversely associated with depression and other poor affective states. In addition, several studies showed that depressed patients had low blood concentrations of n−3 fatty acids, particularly of DHA, compared with healthy controls (7). Clinical trials (8–13) and an updated meta-analysis of 35 clinical trials (14), generally of short duration (<6 mo), indicated that EPA-DHA administration had a small antidepressant effect in patients with major depression but not in participants with no or only mild depressive symptoms. For the parent compound of n−3 fatty acids found in vegetable oils, ALA4 (18:3n−3), there is only circumstantial evidence for beneficial effects on depression and dispositional optimism in adults (15, 16). In a large cohort study, the intake of ALA was inversely associated with incident depression during 10 y of follow-up (17). In a randomized trial in 51 children and adolescents with bipolar disorders, flaxseed oil rich in ALA did not significantly affect rating scale scores compared with placebo during the 16-wk follow-up (18).
Depressive symptoms and other negative affective states are common in patients with CHD, with ∼20% of post-MI patients suffering from major depression that is being undertreated (19, 20). Depression predicted new and recurrent adverse cardiovascular outcomes (21). With respect to positive affective states, dispositional optimism––defined in terms of generalized positive outcome expectancies and a future orientation––was related to a higher intake of n−3 fatty acids (22, 23) and a lower risk of CHD and cardiovascular mortality (24, 25). Because CHD was associated with lower intakes of fish and fish fatty acids (26, 27), and depressive symptoms in CHD patients were related to lower circulating DHA concentrations compared with CHD patients without depressive symptom (28–30), n−3 fatty acids may improve the affective state and prevent depression in patients with CHD.
We investigated in a randomized placebo-controlled trial (26, 27) whether long-term treatment with low doses of n−3 fatty acids are beneficial for the affective state in >4000 patients who had experienced an MI.
SUBJECTS AND METHODS
Study design and patients
For the present study, data were used from the Alpha Omega Trial, a secondary prevention study on the effect of n−3 fatty acids on cardiovascular diseases carried out in 4837 post-MI patients aged 60–80 y (26, 27). The flow diagram of the trial is shown in Figure 1. Patients who had experienced an MI were recruited by cardiologists from 32 hospitals from April 2002 through December 2006, with follow-up until December 2009. We aimed to assess whether low-dose n−3 fatty acids compared with placebo improved affective states in 4116 (of 4837; 85.1%) patients. The trial was conducted in accordance with the Declaration of Helsinki and approved by one central medical ethics committee (Haga Hospital, Leyenburg,The Hague, Netherlands, Office for Human Research Protections #IORG0004004) and by the committees of all participating hospitals. Written informed consent was obtained from all patients.
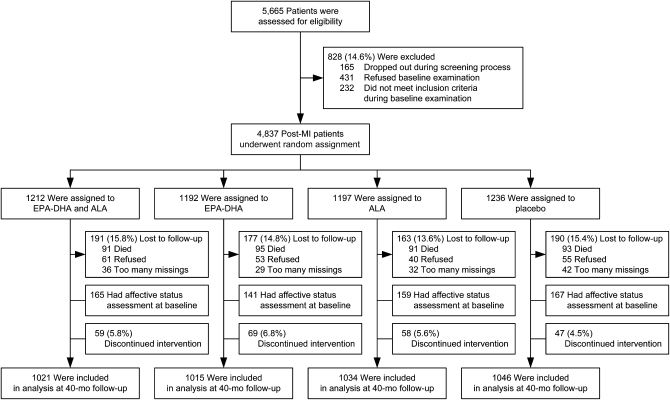
FIGURE 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram showing patient flow of men and women aged 60–80 y who had experienced an MI within 10 y before entering the study to receive 1 of 4 margarine spreads that were enriched with EPA-DHA, ALA, or both or placebo in a 2 × 2 factorial design. For the computation of the scores, the following missing items were allowed: total GDS-15 score, 1 or 2 out of 15; for the total LOT-R, 1 out of 6; and for the 4Q scores, 1 out of 4. ALA, α-linolenic acid; GDS-15, 15-item Geriatric Depression Scale; LOT-R, Life Orientation Test–Revised; MI, myocardial infarction; 4Q, 4-item optimism questionnaire.
Study protocol
Patients were randomly allocated according to a 2 × 2 factorial design to 1) 400 mg EPA-DHA plus 2 g ALA/d, 2) 400 mg EPA-DHA/d, 3) 2 g ALA/d, or 4) a placebo comparator margarine (in which EPA, DHA, or ALA was exchanged for oleic acid), with a presumed average margarine intake of 20 g/d. Margarines that the patients usually used were replaced with the study margarines, as opposed to supplementation with fish-oil capsules used in most previous trials (14). Randomization was performed with a 1:1:1:1 allocation ratio by using a computer random number generator. The margarines were similar to each other in taste, odor, texture, and color. Dosages were comparable to the Recommended Dietary Allowances for these n−3 fatty acids (26). The ratio of EPA to DHA in margarines that contained fish oil was, on average, 3:2. An objective measure of compliance was obtained by measurement of fatty acid composition in plasma cholesteryl esters from random samples of ∼750 patients at different time points (who all provided data on depression and optimism at 40 mo).
Depression and optimism endpoints
Questionnaires were completed by trained research nurses at home or in the hospital or self-completed when mailed to the patients’ homes. Depressive symptoms were measured by using the GDS-15 (31), a 15-item “yes or no” self-administered questionnaire to assess depressive symptoms during the past week in elderly subjects (Cronbach's α = 0.77). The full 30-item version of the GDS was found to have high reliability, specificity, and sensitivity for major depression in CHD patients (32). The total score ranges from 0 to 15, with higher scores indicating more depressive symptoms. For the computation of the GDS-15 score, 2 missing items were allowed and were subsequently imputed with the mean of the remaining items (n = 377 of 4068, 9.3%). The cutoff of >10 was used to identify patients with severe depressive symptoms. Patients were asked if they had ever been treated for depressive symptoms by a general practitioner, psychiatrist, psychotherapist, or social worker; and whether they had affected first-degree biological relatives.
Dispositional optimism was assessed by using 2 questionnaires: the LOT-R (23, 25) and a 4Q (23, 24, 33). The 4Q statements were as follows: “I still expect much from life,” “I do not look forward to the years to come,” “My days seem to pass by slowly,” and “I am still full of plans” (our translations). The 2 negatively worded items were reverse coded for computation of the 4Q sum score. Although the 4Q has not been validated in a psychometric sense, it did show predictive value for depressive symptoms (33) and cardiovascular mortality (24). One missing item per patient was allowed, which was imputed (n = 361 of 4084, 8.8%). The LOT-R is a 10-item questionnaire of which 4 items are filler items (23, 25). Three negatively worded items were reverse coded for computation of the LOT-R sum score. One missing item per patient was allowed, which was imputed (n = 183 of 4059, 4.5%). The total score range from 0 to 8 for the 4Q and from 0 to 24 for the LOT-R, with higher scores indicating greater optimism. Cronbach's α values were 0.58 and 0.55 for the 4Q and LOT-R, respectively.
Other variables
At baseline, data were collected on demographic factors (age, sex, educational level, marital status), lifestyle (smoking status, alcohol use), medical history, and self-rated health and medication use (26). Baseline data on medication use were coded according to the Anatomical Therapeutic Chemical Classification System and included benzodiazepines (Anatomical Therapeutic Chemical Classification codes: N05BA, N05CD, N03AE01, N05CF) and antidepressants (N06A) that were subclassified as selective serotonin reuptake inhibitors (N06AB), tricyclic antidepressant medications (N06AA), and other antidepressant medications (N06AF, N06AG, N06AX). Physical activity was assessed by the validated Physical Activity Scale for the Elderly (26). Patients were physically examined by trained research nurses at home or in the hospital, and measurement included weight and height (to calculate the BMI).
Statistical analysis
The power to detect an effect of EPA-DHA on depressive symptoms was calculated by using pooled standardized mean differences of 0.10 (14) and 0.45 (34) (as the overall effect was larger in trials that used supplements with ≥50% EPA) and a 2-sided α of 0.05. On the basis of the given numbers of ∼1000 experimental subjects and ∼1000 control subjects, the power ranged between 0.61 (14) and 1.00 (34) in 4-way analyses. On the basis of the given numbers of ∼2000 experimental subjects and ∼2000 control subjects, the power ranged between 0.89 (14) and 1.00 (34) in 2-way analyses.
Analyses in all 4116 patients were based on a predefined statistical analysis plan (see www.alphaomegatrial.com) by using a posttest-only design. An independent biostatistician performed unblinding of the treatment allocation after the primary analysis on affective state was completed. The groups who received n−3 fatty acid supplementation were compared with the placebo group in 4-way analyses (in which the each of the 3 n−3 groups was compared with the placebo group). In additional 2-way analyses, the 2 groups who received EPA-DHA were combined and compared with the 2 groups who did not receive EPA-DHA, and the 2 groups who received ALA were combined and compared with the 2 groups who did not receive ALA. Because of the positively skewed distributions of the GDS-15 and blood concentrations of ALA, EPA, and DHA, these variables were natural log-transformed before analyses. Because the distribution of 4Q scores was negatively skewed (due to the ceiling effect of the maximum score of 8), 9 minus the sum score was natural log-transformed. Back-transformed geometric means are presented in tables. To compare the groups’ demographic, medical, and psychiatric characteristics and to identify differences in fatty acid concentrations, protocol completion, depressive symptoms, and dispositional optimism, chi-square tests for categorical variables and 1-factor ANOVAs for continuous variables were used (Table 1). In multivariable general linear models we adjusted for relevant covariates (ie, age, sex, education, marital status, self-rated health, smoking status, alcohol use, physical activity, family history of depression, antidepressant use, and use of any psychotropic drug). Nonparametric Mann-Whitney tests were additionally used for group comparisons. ORs for the prevalence of moderate to severe depressive symptoms (GDS-15 ≥5) were calculated on the basis of logistic regression.
TABLE 1.
Baseline characteristics of the 4116 patients with coronary heart disease assigned to receive the n−3 fatty acids1
| Variables | EPA-DHA and ALA (n = 1021) | EPA-DHA (n = 1015) | ALA (n = 1034) | Placebo (n = 1046) | P value2 |
| Age (y) | 68.8 ± 5.53 | 68.8 ± 5.5 | 68.6 ± 5.5 | 68.7 ± 5.5 | 0.77 |
| Women [n (%)] | 214 (21.0) | 213 (21.0) | 222 (21.5) | 209 (20.0) | 0.86 |
| Time since MI, mean ± SD, y | 4.1 ± 3.0 | 4.2 ± 3.1 | 4.4 ± 3.3 | 4.3 ± 3.4 | 0.15 |
| Diabetes4 [n (%)] | 187 (18.3) | 206 (20.3) | 210 (20.3) | 189 (18.1) | 0.40 |
| Self-reported history of stroke [n (%)] | 72 (7.1) | 62 (6.1) | 70 (6.8) | 62 (5.9) | 0.69 |
| BMI5 (kg/m2) | 27.8 ± 3.8 | 27.7 ± 3.7 | 27.8 ± 3.7 | 27.7 ± 3.7 | 0.94 |
| Diastolic blood pressure (mm Hg) | 80.1 ± 11.2 | 81.1 ± 11.2 | 80.5 ± 11.0 | 80.3 ± 10.8 | 0.23 |
| Systolic blood pressure (mm Hg) | 141.5 ± 21.8 | 143.0 ± 21.2 | 141.9 ± 20.9 | 142.2 ± 21.7 | 0.42 |
| Higher education6 [n (%)] | 453 (44.6) | 428 (42.3) | 465 (45.4) | 469 (45.2) | 0.48 |
| Married [n (%)] | 825 (80.8) | 802 (79.0) | 837 (80.9) | 855 (81.7) | 0.46 |
| Current smoker [n (%)] | 137 (13.4) | 162 (16.0) | 160 (15.5) | 169 (16.2) | 0.29 |
| Alcohol use7 [n (%)] | 777 (76.3) | 762 (75.1) | 779 (75.4) | 778 (74.5) | 0.82 |
| No or light physical activity [n (%)] | 382 (37.7) | 401 (39.7) | 385 (37.5) | 428 (41.0) | 0.29 |
| Moderate to poor self-rated health [n (%)] | 227 (22.3) | 188 (18.6) | 224 (21.8) | 215 (20.7) | 0.16 |
| Family history of depression8 [n (%)] | 203 (20.0) | 195 (19.3) | 177 (17.3) | 207 (19.8) | 0.36 |
| Medication use [n (%)] | |||||
| Antidepressant | 35 (3.4) | 36 (3.5) | 29 (2.8) | 41 (3.9) | 0.56 |
| SSRI | 16 (1.6) | 16 (1.6) | 16 (1.5) | 29 (2.8) | 0.10 |
| TCA | 9 (0.9) | 13 (1.3) | 8 (0.8) | 9 (0.9) | 0.64 |
| Other antidepressant | 10 (1.0) | 7 (0.7) | 5 (0.5) | 3 (0.3) | 0.21 |
| Benzodiazepines | 94 (9.2) | 71 (7.0) | 79 (7.6) | 89 (8.5) | 0.27 |
| Any psychotropic drugs | 115 (11.3) | 103 (10.1) | 98 (9.5) | 119 (11.4) | 0.43 |
| Affective-state questionnaires9 | |||||
| Depressive symptoms score (GDS-15) | 1.43 (1.19, 1.69) | 1.23 (1.00, 1.49) | 1.35 (1.12, 1.61) | 1.36 (1.13, 1.61) | 0.74 |
| Dispositional optimism score (4Q) | 6.90 (6.70, 7.09) | 7.02 (6.81, 7.21) | 6.87 (6.67, 7.07) | 6.84 (6.63, 7.03) | 0.31 |
| Dispositional optimism score (LOT-R) | 14.8 (14.3, 15.2) | 14.5 (14.0, 15.0) | 14.6 (14.1, 15.1) | 14.7 (14.2, 15.2) | 0.87 |
Values may not total to the column total because of missing values for some variables. ALA, α-linolenic acid; GDS-15, Geriatric Depression Scale; LOT-R, Life Orientation Test–Revised; MI, myocardial infarction; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant; 4Q, 4-item optimism questionnaire.
Chi-square tests and ANOVA were used to determine significance.
Mean ± SD (all such values).
Diabetes was considered to be present if a patient reported having received the diagnosis from a physician, was taking antidiabetic drugs, or had an elevated plasma glucose concentration [≥7.8 mmol/L (40.5 mg/dL) in patients who had fasted >4 h or ≥11.1 mmol/L (200.0 mg/dL) in nonfasting patients].
BMI is calculated as weight in kilograms divided by height in meters squared.
Higher education was defined as higher vocational education, college, or university.
Alcohol use was defined as ≥1 glass/wk.
Family history of depression was assessed at 40 mo.
For depressive symptoms and dispositional optimism, data on 632 patients were available at baseline. Values are geometric means; 95% CIs in parentheses.
Regarding subgroup analyses, changes over time (40 mo compared with baseline) in depression and optimism scores could be analyzed in 632 patients with full data by using ANCOVA, with adjustment for the depression and optimism scores at baseline. The consistency of the effects of the study treatment on standardized scores (z scores) of depressive symptoms was further evaluated in prespecified subgroups on 10 characteristics. All tests were 2-tailed, with P < 0.05 denoting significance. The software used was SPSS version 17.0 (SPSS Inc).
RESULTS
Baseline characteristics and treatment
In the 4116 patients there were no differences in demographic, lifestyle, and biological factors or in the use of psychotropic medication between the 4 treatment groups (Table 1). In 632 patients who filled out the questionnaires on affective state at baseline, there were no differences in depressive symptoms and dispositional optimism between the 4 treatment groups. Of the 4837 original participants, there were 370 patients who died and 351 who did not complete the questionnaires (14.9%), and these were equally distributed among the randomly assigned groups (P = 0.97 and P = 0.14, respectively; Figure 1).
The 4116 patients included in the present analyses had a mean age of 68.7 y, and 20.8% of the patients were women. The mean period between the index MI and entry in the study was 4.2 y. Patients had a median intake of 15 g fish/d (IQR: 7–19 g/d) and 130 mg EPA-DHA/d (IQR: 0–210 mg/d) per day. At baseline, 3.4% were taking antidepressant medication and 8.2% were taking benzodiazepines.
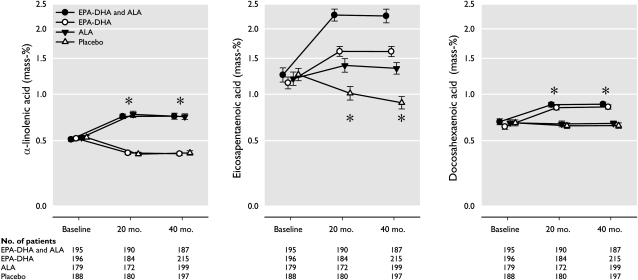
The median duration of follow-up was 40.9 mo (IQR: 39.4–41.6 mo), and the average (±SD) intake of the trial margarine was 19.4 ± 4.0 g/d, which was very close to the expected daily dose of 20 g/d and which implicates, on average, an additional 233 mg EPA/d, 155 mg DHA/d, and 1.9 g ALA/d in the appropriate groups. At the 40-mo visit, 94.3% of 4116 participants were still using their assigned margarine (which was equally distributed among the randomly assigned groups; Figure 1; P = 0.16). This high level of compliance was shown in increases in plasma cholesteryl esters at 40 mo (Figure 2): ALA supplementation increased serum ALA by 69.2% compared with placebo, and EPA-DHA supplementation increased serum EPA by 61.7% and serum DHA by 29.9% compared with placebo. Similar differences were observed at 20 mo. Blinding was successful, as shown by the fact that 74.8% of patients could not tell which margarine they were using, and the remaining patients were unable to do better than chance when guessing the identity of the 4 interventions.
FIGURE 2.
ALA, EPA, and DHA concentrations in plasma cholesteryl esters at baseline, 20 mo, and 40 mo in random samples of patients with coronary heart disease, according to n–3 fatty acid supplementation. Geometric mean (95% CI) values are presented on logarithmic scales. *P < 0.001 for group difference at that time point, as assessed by ANOVA. ALA, α-linolenic acid.
Outcomes
Because of some incomplete questionnaires among the 4116 participants, 40 mo of data were available for 4068 (98.8%) subjects for the GDS-15, for 4084 (99.2%) for the 4Q, and for 4059 (98.6%) for the LOT-R (Table 2). There was no evidence of a biological interaction between EPA-DHA or ALA and their effects on either depressive symptoms or dispositional optimism (Table 2). There were no differences between the 4 groups in depressive symptoms or dispositional optimism at 40 mo (Table 2). The standardized mean (±SE) differences of depressive symptoms were as follows: for EPA-DHA plus ALA (n = 1009) compared with placebo (n = 1030), −0.025 ± 0.044 (P = 0.57); for EPA-DHA (n = 1007) compared with placebo, −0.048 ± 0.044 (P = 0.28), and for ALA (n = 1022) compared with placebo, −0.047 ± 0.044 (P = 0.29; Table 2 and Figure 3). Depressive symptoms and dispositional optimism were not affected by EPA-DHA, ALA, or a combination of both at 40 mo, except for a slight beneficial effect on the LOT-R score in the EPA-DHA group compared with placebo that approached significance (P = 0.051; Table 2).
TABLE 2.
Four-way analysis on depressives symptoms and optimism outcome scores at 40 mo of follow-up in patients with coronary heart disease assigned to receive EPA-DHA plus ALA compared with placebo, EPA-DHA compared with placebo, and ALA compared with placebo1
| EPA-DHA and ALA |
EPA-DHA |
ALA |
Placebo (reference) |
||||||||
| Assessment scale at 40 mo | n | Value | P2 | n | Value | P2 | n | Value | P2 | n | Value |
| Depressive symptoms score (GDS-15) | |||||||||||
| Median (IQR) | 1009 | 1 (0–3) | 0.49 | 1007 | 1 (0–3) | 0.24 | 1022 | 1 (0–3) | 0.26 | 1030 | 2 (0–3) |
| Mean3 | 1009 | 1.43 (1.33, 1.53) | 0.57 | 1007 | 1.39 (1.29, 1.49) | 0.28 | 1022 | 1.39 (1.29, 1.49) | 0.29 | 1030 | 1.47 (1.37, 1.58) |
| Adjusted mean34 | 984 | 1.41 (1.32, 1.50) | 0.51 | 991 | 1.40 (1.31, 1.49) | 0.41 | 993 | 1.40 (1.31, 1.49) | 0.40 | 1010 | 1.45 (1.36, 1.55) |
| Severe depressive symptoms5 | |||||||||||
| n (%) | 1009 | 14 (1.4) | 1007 | 15 (1.5) | 1022 | 17 (1.7) | 1030 | 13 (1.3) | |||
| Crude OR (95% CI) | 1009 | 1.10 (0.52, 2.35) | 0.80 | 1007 | 1.18 (0.56, 2.50) | 0.66 | 1022 | 1.32 (0.64, 2.74) | 0.45 | 1030 | 1.0 (ref) |
| Adjusted OR (95% CI)4 | 984 | 1.00 (0.45, 2.21) | 0.99 | 991 | 1.29 (0.60, 2.78) | 0.51 | 993 | 1.40 (0.66, 2.97) | 0.38 | 1010 | 1.0 (ref) |
| Dispositional optimism score (4Q) | |||||||||||
| Median (IQR) | 1014 | 7 (5–8) | 0.15 | 1006 | 7 (6–8) | 0.18 | 1030 | 7 (6–8) | 0.35 | 1034 | 7 (5–8) |
| Mean3 | 1014 | 6.80 (6.72, 6.88) | 0.15 | 1006 | 6.78 (6.70, 6.87) | 0.26 | 1030 | 6.77 (6.68, 6.85) | 0.41 | 1034 | 6.72 (6.63, 6.80) |
| Adjusted mean34 | 990 | 6.82 (6.74, 6.89) | 0.40 | 992 | 6.79 (6.70, 6.86) | 0.07 | 999 | 6.76 (6.68, 6.84) | 0.21 | 1014 | 6.71 (6.63, 6.79) |
| Dispositional optimism score (LOT-R) | |||||||||||
| Median (IQR) | 1007 | 14 (12–16) | 0.50 | 1001 | 14 (12–17) | 0.050 | 1023 | 14 (12–16) | 0.68 | 1028 | 14 (12–16) |
| Mean3 | 1007 | 14.4 (14.2, 14.6) | 0.48 | 1001 | 14.6 (14.4, 14.7) | 0.09 | 1023 | 14.4 (14.2, 14.5) | 0.76 | 1028 | 14.3 (14.1, 14.5) |
| Adjusted mean34 | 983 | 14.4 (14.2, 14.6) | 0.46 | 988 | 14.6 (14.4, 14.8) | 0.051 | 993 | 14.4 (14.2, 14.6) | 0.70 | 1009 | 14.3 (14.1, 14.5) |
Because of some incomplete questionnaires among the 4116 participants, data were available for 4068 (98.8%) subjects for the GDS-15, for 4084 (99.2%) for the 4Q, and for 4059 (98.6%) for the LOT-R in unadjusted analyses. ALA, α-linolenic acid; GDS-15, Geriatric Depression Scale; LOT-R, Life Orientation Test–Revised; ref, reference; 4Q, 4-item optimism questionnaire.
Nonparametric Mann-Whitney test, ANOVA for 2 independent samples, or logistic regression analysis was used to determine significance as compared with the placebo group (ie, reference group).
Values are back-transformed geometric means; 95% CIs of the mean in parentheses.
Adjusted for age, sex, education (4 categories), marital status, self-rated health (3 categories), smoking status (3 categories), alcohol use, physical activity (3 categories), family history of depression, antidepressant use, and use of any psychotropic drug (by ANCOVA or logistic regression analysis, when appropriate).
Severe depressive symptoms was defined as a GDS-15 score >10 at 40 mo.
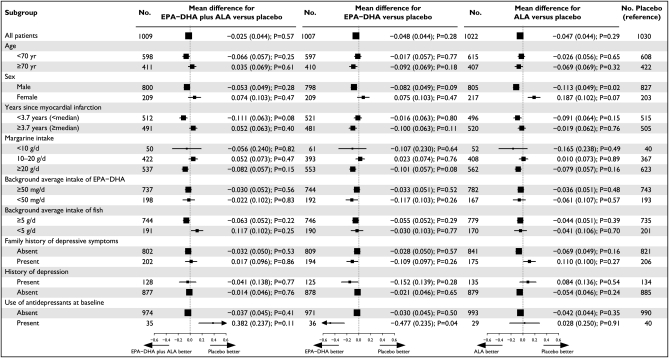
FIGURE 3.
Mean differences in depressive symptoms between prespecified subgroups, according to treatment group, in patients assigned to n−3 fatty acid supplementation groups compared with placebo. The horizontal bars represent SEs, and the vertical gray dotted lines indicate no mean difference. The size of the boxes is proportional to the number of patients. P values for the difference between the groups assigned to n−3 fatty acids compared with placebo are shown. GDS-15 scores were log-transformed because of their positively skewed distribution, and standardized scores were used in the analysis. The mean difference indicates the mean number of SDs in the GDS-15 score (ie, standardized z scores) by which the n–3 fatty acid–treated group was lower or higher than the placebo group at 40-mo follow-up. ALA, α-linolenic acid; GDS-15, 15-item Geriatric Depression Scale.
In addition, in 2-way analyses no significant differences were found. The standardized mean (±SE) differences of depressive symptoms were –0.013 ± 0.031 (P = 0.67) and –0.012 ± 0.031 (P = 0.69) for EPA-DHA (n = 2016) compared with placebo (n = 2052) and for ALA (n = 2031) compared with placebo (n = 2037), respectively.
At 40 mo, there were 59 (1.4%) patients with severe depressive symptoms (GDS-15 >10). The adjusted ORs for severe depressive symptoms compared with placebo were 1.00 in the EPA-DHA plus ALA group, 1.29 in the EPA-DHA group, and 1.40 in the ALA group (all P >0.3; Table 2). Similar results were found when using a cutoff for the GDS-15 of >7 (data not shown).
In the randomly assigned groups no significant differences in side effects such as gastrointestinal problems (eg, stomach upset) and other health problems were found (27).
Analyses of subgroups
In 632 subjects with both baseline and 40-mo values of depression and optimism scores, there were no differences between the changes over time between the n−3 fatty acid supplementation groups compared with the placebo group (Table 3). The risk of a decline in GDS-15 of 2 points over 40 mo was similar in those treated with n−3 fatty acids and placebo.
TABLE 3.
Changes in depressive symptoms and optimism outcome scores at baseline and at 40-mo follow-up in 632 patients with coronary heart disease assigned to receive EPA-DHA plus ALA compared with placebo, EPA-DHA compared with placebo, and ALA compared with placebo1
| EPA-DHA plus ALA (n = 165) |
EPA-DHA (n = 141) |
ALA (n = 159) |
Placebo (reference) (n = 167) |
||||
| Assessment scale | Estimate | P2 | Estimate | P2 | Estimate | P2 | Estimate |
| Depressive symptoms score (GDS-15) | |||||||
| Baseline | 1 (0–3)3 | 1 (0–2) | 1 (0–3) | 1 (0–3) | |||
| 40 mo | 1 (0–3) | 1 (0–3) | 1 (0–3) | 1 (0–3) | |||
| Change | 0.19 (–0.13, 0.50)4 | 0.37 | 0.32 (0.02, 0.61) | 0.23 | 0.12 (–0.23, 0.48) | 0.59 | 0.04 (–0.24, 0.31) |
| Dispositional optimism score (4Q) | |||||||
| Baseline | 7 (6–8) | 7 (6–8) | 7 (6–8) | 7 (6–8) | |||
| 40 mo | 7 (6–8) | 7 (6–8) | 7 (6–8) | 7 (6–8) | |||
| Change | −0.04 (–0.26, 0.19) | 0.94 | −0.33 (–0.58, –0.07) | 0.12 | −0.10 (–0.34, 0.14) | 0.75 | −0.03 (–0.23, 0.18) |
| Dispositional optimism score (LOT-R) | |||||||
| Baseline | 15 (13–7) | 15 (13–17) | 15 (13–17) | 15 (12–17) | |||
| 40 mo | 14 (12–16) | 14 (12–16) | 15 (12–16) | 14 (12–16) | |||
| Change | −0.53 (–0.98, –0.07) | 0.12 | 0.04 (–0.45, 0.53) | 0.92 | −0.21 (–0.66, 0.25) | 0.49 | −0.08 (–0.49, 0.34) |
The 632 patients with complete data on all 3 questionnaires at both time points were included in the analyses. Scores of the GDS-15 ranged from 0 to 15, with ≥5 consistent with moderate to severe levels of depressive symptoms and a diagnosis of depression. Scores of the 4Q ranged from 0 to 8 and of the LOT-R ranged from 0 to 24, with higher scores indicating higher levels of optimism. ALA, α-linolenic acid; GDS-15, Geriatric Depression Scale; LOT-R, Life Orientation Test–Revised; 4Q, 4-item optimism questionnaire; 95% CI, 95% CI of the mean.
ANCOVA was used to determine significance compared with the placebo group, with adjustment for baseline covariates.
Median; IQR in parentheses (all such values).
Mean; 95% CIs in parentheses (all such values).
Prespecified subgroup analyses did not show significant differences in effects of EPA-DHA and/or ALA compared with placebo on depressive symptoms between subgroups (Figure 3). There was no evidence for an effect of n−3 fatty acids compared with placebo in patients at increased risk of depression (ie, with a history of depression, with a family history of depression, or taking antidepressant medications at baseline). There was, however, a tendency in the small group who used antidepressant medications at baseline for a beneficial effect of EPA-DHA (P = 0.04; Figure 3).
DISCUSSION
In this multicenter, randomized, double-blind trial, low-doses of supplementation with either EPA-DHA or ALA were compared with placebo (ie, oleic acid) in >4000 patients who had experienced an MI. These interventions did not affect depressive symptoms and dispositional optimism in post-MI patients, most of whom had no psychiatric diagnosis and no depressed mood. Similar results were obtained in subgroups of patients taking antidepressants or with a (family) history of depression. However, we found that the small group of patients (n = 36) who were prescribed antidepressant medications at baseline showed a somewhat greater improvement in depressive symptoms when taking EPA-DHA compared with placebo (n = 40).
Our findings are in accord with trials carried out in cardiovascular patients. In a trial carried out in 122 CHD patients with major depression––many of whom also had experienced an MI––1680 mg EPA-DHA added to 50 mg sertraline/d did not result in better depression outcomes after 10 wk than did placebo oil added to sertraline (10). In a trial in which 102 stroke patients were randomly assigned, no effect on depressive symptoms was observed of an additional dose of 1000 mg EPA-DHA (12). In this secondary analysis of the Alpha Omega Trial, no formal diagnostic assessment of depression was carried out and post-MI patients were included who, in majority, did not suffer from psychopathology. Therefore, our trial could not answer the research question about the antidepressant effects of (high-dose) n−3 fatty acids in patients with major depression. However, our results have meaningful applicability to the context of the CHD patients who are willing to consume extra fish meals, n−3 fatty acid supplements, or functional foods rich in n−3 fatty acids if it would improve their affective status. Many of these persons already add extra n−3 fatty acids to their diets, and the outcome of depressive symptoms and optimism was therefore chosen to be relevant to the participants included in this trial. Consequently, the results may help MI patients to decide whether they will begin low-dose n−3 fatty acid supplementation to improve their level of mental well-being. Our findings suggest that this may be ineffective in the absence of severe depressive symptoms.
Other trials were carried out in healthy persons and patients with major depression. A recently published meta-analysis showed that EPA-DHA did not affect depressive symptoms in trials carried out in healthy subjects without psychiatric diagnoses and no depressed mood but had therapeutic effect in depressed patients (14). In these trials, higher doses of EPA-DHA (1–9 g/d) were used than those in our trial. The largest trial to date in 432 patients with major depression reported that 8 wk of 1.2 g EPA-DHA/d resulted in a small benefit over placebo that approached significance (13). Although high-dose EPA-DHA may reduce depressive symptoms in patients with major depression (13, 14), larger trials are needed to underpin this hypothesis further because there was evidence of publication bias and substantial heterogeneity among trials (14).
We did not find an effect of a low dose of EPA-DHA on dispositional optimism in patients who had experienced an MI. However, we found in observational studies that in populations characterized by low fish intakes, EPA-DHA intake was positively associated with optimism (22, 23). Yet, favorable associations in observational studies could be due to unmeasured or residual confounding, or reverse causation.
We are not aware of previous trials in adults investigating the effect ALA––the precursor of EPA and DHA––on affective states. In large cohort studies, a protective effect of ALA intake on incident depression was found in one (17) but not another (5) study. We found no overall effect of 2 g ALA supplementation/d, which increased daily intake of our patients by >100%, being consistent with the intended dose. ALA is present in green leafy vegetables and certain nuts and vegetable oils and would provide a more abundant plant source of n−3 fatty acids than would EPA and DHA. ALA supplementation was found to increase EPA (by 41.7%) but not DHA (Figure 2), because bioconversion through desaturation and elongation into DHA is rather inefficient in humans. Some studies found evidence for the idea that EPA is more beneficial for affective states than DHA (8, 9, 13). In a meta-analysis of randomized trials, n−3 fatty acid supplements containing ≥50% EPA showed an overall beneficial effect on depressive symptoms (34) with daily dose regimens generally above 1 g EPA+DHA. Our trial had null findings and used margarines that contained 60% EPA (and 40% DHA) but in a lower daily dose of 0.39 g EPA-DHA.
This study has several limitations. First, this is an ancillary study of the Alpha Omega Trial, which was designed to examine the effect of n−3 fatty acids on cardiovascular diseases and for which we reported that EPA-DHA or ALA did not significantly reduce the rate of major cardiovascular events or total mortality (27). This population comprised MI patients, most of whom had no depression at baseline. Therefore, we were not able to evaluate the treatment efficacy for reducing significant depressive symptoms in the large majority of our post-MI patients, and our findings cannot be generalized to depressed patients. Second, the dose of n−3 fatty acids used was relatively low for the active intervention conditions and may have been insufficient, although blood concentrations markedly increased. Pharmacologic rather than food-based doses may be needed to influence affective states. Third, change scores in the affective state could be calculated only in a subgroup, but these yielded similar findings. Fourth, we did not examine incident depression at regular intervals across the entire project period. Fifth, some patients (14.9%) did not complete the questionnaires at 40 mo for various reasons.
Our trial also has strengths. Randomization was successful, as evidenced by similar distributions of baseline demographic variables and risk factors for depression, including the affective state in a subgroup of 632 patients. Moreover, there was no differential mortality or other dropout among the randomly assigned groups, strengthening internal validity of this posttest-only trial. Other strengths are the study's largest sample size to date investigating the effects of n−3 fatty acids, assessments of both negative and positive affective states, effective blinding, the well-tolerated margarines, and the long-term follow-up. Moreover, this is the first study of ALA supplementation on depressive symptoms in adults; an earlier study was conducted in children and adolescents (18).
In summary, we conducted a randomized, double-blind, clinical trial in patients who had experienced an MI and who had a low habitual intake of fish. Our results do not provide evidence of a beneficial effect of low doses of dietary EPA-DHA or ALA on depression and optimism in MI patients without a depressive disorder. Further well-designed randomized controlled trials are required in patients with a major depressive disorder with the use of high doses of EPA-DHA and further studies with the use of ALA supplementation.
Acknowledgments
We express our gratitude to the following members of the Executive Committee for their invaluable contribution to the Alpha Omega Trial: Janette de Goede, Linda M Oude Griep, Eveline Waterham, and Annemarie M Teitsma-Jansen. Lucy Okma and Lisa Verberne are gratefully acknowledged for follow-up data collection and data processing. We thank all of the patients who participated in the Alpha Omega Trial. We also thank all general practitioners and cardiologists who provided information on clinical events and our team of research nurses for physical examination of trial participants. A list of members of the Alpha Omega Trial Group has been published elsewhere (26, 27).
The authors’ responsibilities were as follows—EJG, JMG and DK: designed and conducted the research; and EJG: analyzed the data, wrote the manuscript in close collaboration with JMG and DK, and has primary responsibility for final content. The sponsors had no role in the study design and no role in data collection, data analysis, data interpretation, or writing of the manuscript. The authors had final responsibility for the decision to submit the manuscript for publication after funding. The authors reported no conflicts of interest.
Footnotes
Abbreviations used: ALA, α-linolenic acid; CHD, coronary heart disease; 4Q, 4-item optimism questionnaire; GDS-15, 15-item Geriatric Depression Scale; LOT-R, Life Orientation Test–Revised; MI, myocardial infarction.
REFERENCES
- 1.Hibbeln JR. Fish consumption and major depression. Lancet 1998;351:1213. [DOI] [PubMed] [Google Scholar]
- 2.Tanskanen A, Hibbeln JR, Tuomilehto J, Uutela A, Haukkala A, Viinamäki H, Lehtonen J, Vartiainen E. Fish consumption and depressive symptoms in the general population in Finland. Psychiatr Serv 2001;52:529–31 [DOI] [PubMed] [Google Scholar]
- 3.Kamphuis MH, Geerlings MI, Tijhuis MA, Kalmijn S, Grobbee DE, Kromhout D. Depression and cardiovascular mortality: a role for n−3 fatty acids? Am J Clin Nutr 2006;84:1513–7 [DOI] [PubMed] [Google Scholar]
- 4.Colangelo LA, He K, Whooley MA, Daviglus ML, Liu K. Higher dietary intake of long-chain omega-3 polyunsaturated fatty acids is inversely associated with depressive symptoms in women. Nutrition 2009;25:1011–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hakkarainen R, Partonen T, Haukka J, Virtamo J, Albanes D, Lonnqvist J. Is low dietary intake of omega-3 fatty acids associated with depression? Am J Psychiatry 2004;161:567–9 [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Villegas A, Henriquez P, Figueiras A, Ortuno F, Lahortiga F, Martinez-Gonzalez MA. Long chain omega-3 fatty acids intake, fish consumption and mental disorders in the SUN cohort study. Eur J Nutr 2007;46:337–46 [DOI] [PubMed] [Google Scholar]
- 7.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry 2010;68:140–7 [DOI] [PubMed] [Google Scholar]
- 8.Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry 2002;59:913–9 [DOI] [PubMed] [Google Scholar]
- 9.Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002;159:477–9 [DOI] [PubMed] [Google Scholar]
- 10.Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer BC, Harris WS. Omega-3 augmentation of sertraline in treatment of depression in patients with coronary heart disease: a randomized controlled trial. JAMA 2009;302:1651–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Rest O, Geleijnse JM, Kok FJ, van Staveren WA, Olderikkert MG, Beekman AT, de Groot LC. Effect of fish oil supplementation on quality of life in a general population of older Dutch subjects: a randomized, double-blind, placebo-controlled trial. J Am Geriatr Soc 2009;57:1481–6 [DOI] [PubMed] [Google Scholar]
- 12.Poppitt SD, Howe CA, Lithander FE, Silvers KM, Lin RB, Croft J, Ratnasabapathy Y, Gibson RA, Anderson CS. Effects of moderate-dose omega-3 fish oil on cardiovascular risk factors and mood after ischemic stroke: a randomized, controlled trial. Stroke 2009;40:3485–92 [DOI] [PubMed] [Google Scholar]
- 13.Lespérance F, Frasure-Smith N, St-André E, Turecki G, Lespérance P, Wisniewski SR. The efficacy of omega-3 supplementation for major depression: a randomized controlled trial. J Clin Psychiatry 2011;72:1054–62 [DOI] [PubMed] [Google Scholar]
- 14.Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n−3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr 2010;91:757–70 [DOI] [PubMed] [Google Scholar]
- 15.Conklin SM, Harris JI, Manuck SB, Yao JK, Hibbeln JR, Muldoon MF. Serum omega-3 fatty acids are associated with variation in mood, personality and behavior in hypercholesterolemic community volunteers. Psychiatry Res 2007;152:1–10 [DOI] [PubMed] [Google Scholar]
- 16.Blondeau N, Nguemeni C, Debruyne DN, Piens M, Wu X, Pan H, Hu X, Gandin C, Lipsky RH, Plumier JC, et al. Subchronic alpha-linolenic acid treatment enhances brain plasticity and exerts an antidepressant effect: a versatile potential therapy for stroke. Neuropsychopharmacology 2009;34:2548–59 [DOI] [PubMed] [Google Scholar]
- 17.Lucas M, Mirzaei F, O'Reilly EJ, Pan A, Willett WC, Kawachi I, Koenen K, Ascherio A. Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: a 10-y prospective follow-up study. Am J Clin Nutr 2011;93:1337–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gracious BL, Chirieac MC, Costescu S, Finucane TL, Youngstrom EA, Hibbeln JR. Randomized, placebo-controlled trial of flax oil in pediatric bipolar disorder. Bipolar Disord 2010;12:142–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Melle JP, de Jonge P, Spijkerman TA, Tijssen JG, Ormel J, van Veldhuisen DJ, van den Brink RH, van den Berg MP. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004;66:814–22 [DOI] [PubMed] [Google Scholar]
- 20.Thombs BD, de Jonge P, Coyne JC, Whooley MA, Frasure-Smith N, Mitchell AJ, Zuidersma M, Eze-Nliam C, Lima BB, Smith CG, et al. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA 2008;300:2161–71 [DOI] [PubMed] [Google Scholar]
- 21.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004;66:802–13 [DOI] [PubMed] [Google Scholar]
- 22.Giltay EJ, Geleijnse JM, Zitman FG, Buijsse B, Kromhout D. Lifestyle and dietary correlates of dispositional optimism in men: The Zutphen Elderly Study. J Psychosom Res 2007;63:483–90 [DOI] [PubMed] [Google Scholar]
- 23.van de Rest O, de Goede J, Sytsma F, Oude Griep LM, Geleijnse JM, Kromhout D, Giltay EJ. Association of n-3 long-chain PUFA and fish intake with depressive symptoms and low dispositional optimism in older subjects with a history of myocardial infarction. Br J Nutr 2010;103:1381–7 [DOI] [PubMed] [Google Scholar]
- 24.Giltay EJ, Kamphuis MH, Kalmijn S, Zitman FG, Kromhout D. Dispositional optimism lowers the risk of cardiovascular death: the Zutphen Elderly Study. Arch Intern Med 2006;166:431–6 [DOI] [PubMed] [Google Scholar]
- 25.Tindle HA, Chang YF, Kuller LH, Manson JE, Robinson JG, Rosal MC, Siegle GJ, Matthews KA. Optimism, cynical hostility, and incident coronary heart disease and mortality in the Women's Health Initiative. Circulation 2009;120:656–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geleijnse JM, Giltay EJ, Schouten EG, de Goede J, Oude Griep LM, Teitsma-Jansen AM, Katan MB, Kromhout D; Alpha Omega Trial Group Effect of low doses of n-3 fatty acids on cardiovascular diseases in 4,837 post-myocardial infarction patients: design and baseline characteristics of the Alpha Omega Trial. Am Heart J 2010;159:539–46 [DOI] [PubMed] [Google Scholar]
- 27.Kromhout D, Giltay EJ, Geleijnse JM. n–3 Fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 2010;363:2015–26 [DOI] [PubMed] [Google Scholar]
- 28.Frasure-Smith N, Lesperance F, Julien P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol Psychiatry 2004;55:891–6 [DOI] [PubMed] [Google Scholar]
- 29.Parker GB, Heruc GA, Hilton TM, Olley A, Brotchie H, Hadzi-Pavlovic D, Friend C, Walsh WF, Stocker R. Low levels of docosahexaenoic acid identified in acute coronary syndrome patients with depression. Psychiatry Res 2006;141:279–86 [DOI] [PubMed] [Google Scholar]
- 30.Amin AA, Menon RA, Reid KJ, Harris WS, Spertus JA. Acute coronary syndrome patients with depression have low blood cell membrane omega-3 fatty acid levels. Psychosom Med 2008;70:856–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Craen AJ, Heeren TJ, Gussekloo J. Accuracy of the 15-item geriatric depression scale (GDS-15) in a community sample of the oldest old. Int J Geriatr Psychiatry 2003;18:63–6 [DOI] [PubMed] [Google Scholar]
- 32.Low GD, Hubley AM. Screening for depression after cardiac events using the Beck Depression Inventory-II and the Geriatric Depression Scale. Soc Indic Res 2007;82:527–43 [Google Scholar]
- 33.Giltay EJ, Zitman FG, Kromhout D. Dispositional optimism and the risk of depressive symptoms during 15 years of follow-up: the Zutphen Elderly Study. J Affect Disord 2006;91:45–52 [DOI] [PubMed] [Google Scholar]
- 34.Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr 2009;28:525–42 [DOI] [PubMed] [Google Scholar]