Abstract
Background: The circumstances under which the glycemic index (GI) and glycemic load (GL) are derived do not reflect real-world eating behavior. Thus, the ecologic validity of these constructs is incompletely known.
Objective: This study examined the relation of dietary intake to glycemic response when foods are consumed under free-living conditions.
Design: Participants were 26 overweight or obese adults with type 2 diabetes who participated in a randomized trial of lifestyle modification. The current study includes baseline data, before initiation of the intervention. Participants wore a continuous glucose monitor and simultaneously kept a food diary for 3 d. The dietary variables included GI, GL, and intakes of energy, fat, protein, carbohydrate, sugars, and fiber. The glycemic response variables included AUC, mean and SD of continuous glucose monitoring (CGM) values, percentage of CGM values in euglycemic and hyperglycemic ranges, and mean amplitude of glycemic excursions. Relations between daily dietary intake and glycemic outcomes were examined.
Results: Data were available from 41 d of monitoring. Partial correlations, controlled for energy intake, indicated that GI or GL was significantly associated with each glycemic response outcome. In multivariate analyses, dietary GI accounted for 10% to 18% of the variance in each glycemic variable, independent of energy and carbohydrate intakes (P < 0.01).
Conclusions: The data support the ecologic validity of the GI and GL constructs in free-living obese adults with type 2 diabetes. GI was the strongest and most consistent independent predictor of glycemic stability and variability.
INTRODUCTION
Dietary GI4 and GL are related constructs that refer to the rate at which available carbohydrate raises blood glucose during the postprandial period. GI is a food-classification system based on the rise in blood glucose after consumption of a test food, relative to a standard (eg, glucose or white bread) containing the same amount of carbohydrate (1). GL is calculated as the multiplicative product of GI and the amount of carbohydrate and thus is influenced by carbohydrate source and quantity (2). The postprandial AUC for glucose increases in a dose-response fashion with increasing carbohydrate portions of a food and varies by GI when carbohydrate load is held constant (3, 4).
The clinical utility of GI and GL has been the subject of considerable debate. Some researchers question evidence regarding the relevance of dietary GI and GL to health and raise several concerns about the methods used to determine GI (5, 6). Of particular relevance to the current study, the experimental constraints under which GI values are derived may not reflect real-life conditions. The test setting requires the subject to consume a specified portion (ie, containing 50 g available carbohydrate) of a single item, to be in a fasting state, and to consume the test food within a specified period of time. In addition, the optimal measure for assessing clinical significance (eg, incremental AUC above fasting, glycemic excursions) remains a topic of debate (5, 6). In the context of that debate, the current study was undertaken to examine the relations of dietary GI and GL (and other dietary variables) to glycemic response (as assessed with a CGM) when foods are consumed in self-selected amounts, at self-selected times, and in self-selected combinations by free-living overweight and obese individuals with type 2 diabetes.
SUBJECTS AND METHODS
Subjects
Participants were a subset of those who enrolled in a larger weight-loss trial (7) and were recruited between September 2006 and November 2007. Inclusion criteria for the parent study were a diagnosis of type 2 diabetes, an age of 18–65 y, and a BMI (kg/m2) of 27 to 45. Exclusion criteria were type 1 diabetes, uncontrolled hypertension or thyroid disease, unstable angina, malignant arrhythmias, myocardial infarction in the past year, cancer (active or in remission <5 y), clinically significant psychosocial impairment, pregnancy or lactation, or a history of cerebrovascular, renal, hepatic, or protein-wasting disease. Treatment with insulin, although not an exclusion criterion for the parent trial, was exclusionary in the ancillary study reported here.
Qualifying individuals were consecutively recruited from the parent trial. A total of 26 individuals (21 women, 5 men) volunteered to participate in the current study. Participants had a mean (±SD) age of 50.4 ± 9.3 y and BMI of 36.1 ± 5.3 and a glycated hemoglobin value of 6.7 ± 1.2%.
Procedures
Participants completed a 3-d period of CGM before the parent study intervention. At the start of each monitoring period, a registered nurse (who is also a Certified Diabetes Educator) inserted a sensor subcutaneously in the participant's abdomen and instructed the participant in the use of the CGM device (CGMS Gold; Medtronic). Participants were provided with a traditional glucometer (OneTouch Ultra; LifeScan) and compatible lancets and test strips for calibrating the CGM device at 12-h intervals. The device measures interstitial glucose (from which blood glucose is estimated) every 10 s and records the mean blood glucose value every 5 min. At the end of the 3-d monitoring period, participants returned to have the sensor withdrawn, and data were uploaded from the device to a computer for calculating variables by using software packaged with the CGM device. The reliability data for CGM in general and of the CGMS Gold system in particular are good (8–11).
During the CGM assessment period, participants also monitored their food and beverage intake. They were instructed to use food scales and dry and wet food measurement tools to assess portions and were told to record the amounts of all items consumed, along with a detailed description of each item, immediately after consumption. However, no specific nutrient targets or additional dietary instructions were given. At the end of each participant's assessment period, a research dietitian reviewed the intake records and queried the participants for additional data when records appeared incomplete or implausible.
Outcome measures
Glycemic response variables
We calculated glycemic response variables for each full 24-h period (midnight to midnight) for which complete glucose data were available. Glucose AUCs and glucose concentrations >0 mg/dL were calculated by using the trapezoidal method (12). Additional glycemic response variables included the SD of glucose values and the percentage of time spent in the euglycemic (71–180 mg/dL) and hyperglycemic (>180 mg/dL) ranges. The mean amplitude of glycemic excursions was calculated according to the methods described by Service et al (13).
Dietary variables
Dietary intake data were collected and analyzed by using Nutrition Data System for Research software version 2006, developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN (14). Dietary intake variables of interest included the following: total energy (kcal), GI (based on glucose as the reference food), GL, and intakes (g) of fat, protein, carbohydrate, sugar (total and added), and fiber (total, soluble, and insoluble). The reader is referred to page A11.12 of the Nutrition Data System for Research user manual (http://www.ncc.umn.edu/ndsrsupport/ndsrmanual2006.pdf) for a description of the methods used to calculate GI. Daily totals were calculated, corresponding to each complete 24-h (midnight to midnight) CGM period.
Statistical analysis
Bivariate Pearson's correlations were calculated to examine the relations between dietary intake and glycemic response variables observed in each 24-h period. These associations between dietary intake and glycemic response variables were examined in partial correlation analyses, with adjustment for total energy intake, to minimize confounding by differences in dietary intake related to body size. Dietary variables that were found to relate significantly to glycemic response variables in partial correlations were entered into multiple regression analyses, in a stepwise fashion, after forced entry of total energy intake. Data were analyzed by using SPSS version 16.0 (15); the α level was set at 0.05 for each test.
RESULTS
The 3-d assessment period yielded 50 full 24-h (midnight to midnight) glucose monitoring days. The AUC could not be computed for one of those days because of periodic malfunction of the sensor, which resulted in discontinuous data collection. On that day, however, other glycemic response indicators (eg, SD and MAGE) were computed. Full 24-h food records were unavailable for 9 d. Thus, complete glucose monitoring and dietary data were available for 41 d, with each participant contributing 1 or 2 d (mean = 1.6; SD = 0.5). Means and SDs for each day's dietary and glycemic response variables are shown in Table 1.
TABLE 1.
Daily dietary intake and glycemic response variables1
| Value | |
| Dietary intake (n = 41) | |
| Total energy (kcal) | 1912.0 ± 482.9 |
| Fat (g) | 75.0 ± 33.5 |
| Protein (g) | 83.3 ± 29.9 |
| Carbohydrate (g) | 231.9 ± 76.9 |
| Total sugar (g) | 83.8 ± 46.9 |
| Added sugar (g) | 52.3 ± 39.8 |
| Total fiber (g) | 18.2 ± 6.8 |
| Soluble fiber (g) | 4.7 ± 1.3 |
| Insoluble fiber (g) | 13.1 ± 5.4 |
| GI | 63.5 ± 6.1 |
| GL (g) | 134.4 ± 50.4 |
| Glycemic response variables (n = 50) | |
| AUC (mg · dL−1 · min−1)2 | 138.7 ± 41.4 |
| Mean (mg/dL) | 139.3 ± 41.43 |
| SD (mg/dL) | 27.3 ± 14.83 |
| Hyperglycemic values (%) | 30.1 ± 32.2 |
| Euglycemic values (%) | 68.7 ± 32.2 |
| MAGE (mg/dL) | 65.6 ± 34.9 |
All values are means ± SDs; n = 49. GI, glycemic index; GL, glycemic load; MAGE, mean amplitude of glycemic excursions.
Data not available on 1 d for one participant.
Values represent the sample mean ± SD for the 24-h glycemic response variables.
Dietary GI and glycemic response
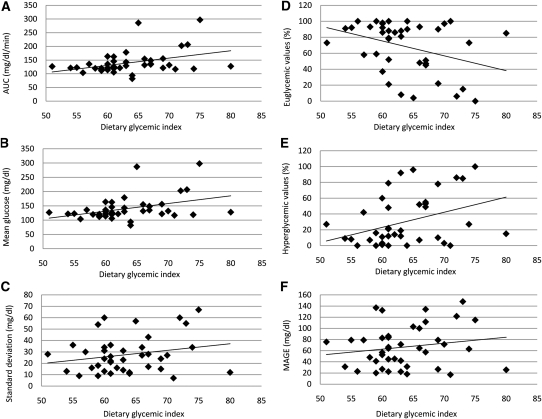
As shown in Table 2, bivariate correlation analyses showed that dietary GI was positively related to AUC (P = 0.01), mean glucose (P = 0.01), and the percentage of values in the hyperglycemic range (P = 0.02). GI was also negatively related to the percentage of values in the euglycemic range (P = 0.02). Control for energy intake in the partial correlations (Table 3) did not materially alter these relations. Scatter plots depicting the bivariate relations of GI to glycemic response outcomes are shown in Figure 1.
TABLE 2.
Bivariate correlations between dietary and glycemic response variables (n = 41)1
| Glycemic response variables | ||||||
| Dietary variables | AUC | Mean | SD | Hyperglycemic values | Euglycemic values | MAGE |
| mg · dL · min−1 | mg/dL | mg/dL | % | % | mg/dL | |
| Total energy (kcal) | 0.16 | 0.16 | 0.02 | 0.17 | −0.16 | −0.17 |
| Fat (g) | 0.11 | 0.11 | −0.10 | 0.15 | −0.13 | −0.23 |
| Protein (g) | −0.08 | −0.08 | −0.21 | −0.14 | 0.16 | −0.372 |
| Carbohydrate (g) | 0.20 | 0.21 | 0.22 | 0.22 | −0.23 | 0.09 |
| Total sugar (g) | −0.00 | −0.00 | 0.22 | 0.11 | −0.13 | 0.14 |
| Added sugar (g) | 0.09 | 0.09 | 0.22 | 0.17 | −0.17 | 0.14 |
| Total fiber (g) | 0.08 | 0.08 | 0.15 | 0.14 | −0.14 | 0.15 |
| Soluble fiber (g) | 0.16 | 0.16 | 0.03 | 0.18 | −0.17 | −0.05 |
| Insoluble fiber (g) | 0.04 | 0.04 | 0.17 | 0.11 | −0.11 | 0.19 |
| GI | 0.383 | 0.393 | 0.22 | 0.372 | −0.352 | 0.18 |
| GL (g) | 0.294 | 0.294 | 0.284 | 0.274 | −0.284 | 0.12 |
Cells contain zero-order correlation coefficients from Pearson's correlation analyses. GI, glycemic index; GL, glycemic load; MAGE, mean amplitude of glycemic excursions.
P ≤ 0.05.
P ≤ 0.01.
P ≤ 0.10.
TABLE 3.
Partial correlations between dietary and glycemic response variables, controlled for energy intake (n = 41)1
| Glycemic response variables | ||||||
| Dietary variables | AUC | Mean | SD | Hyperglycemic values | Euglycemic values | MAGE |
| mg · dL · min−1 | mg/dL | mg/dL | % | % | mg/dL | |
| Fat (g) | −0.04 | −0.04 | 0.22 | 0.02 | 0.01 | −0.17 |
| Protein (g) | −0.21 | −0.21 | −0.272 | −0.302 | 0.323 | −0.353 |
| Carbohydrate (g) | 0.13 | 0.14 | 0.313 | 0.14 | −0.18 | 0.313 |
| Total sugar (g) | −0.04 | −0.04 | 0.22 | 0.07 | −0.09 | 0.20 |
| Added sugar (g) | 0.05 | 0.05 | 0.21 | 0.13 | −0.14 | 0.19 |
| Total fiber (g) | 0.05 | 0.05 | 0.15 | 0.10 | −0.11 | 0.20 |
| Soluble fiber (g) | 0.10 | 0.10 | 0.03 | 0.11 | −0.11 | 0.03 |
| Insoluble fiber (g) | 0.02 | 0.02 | 0.17 | 0.09 | −0.09 | 0.22 |
| GI | 0.383 | 0.383 | 0.22 | 0.363 | −0.353 | 0.19 |
| GL (g) | 0.262 | 0.262 | 0.414 | 0.22 | −0.26 | 0.383 |
Cells contain partial correlation coefficients from Pearson's correlation analyses. GI, glycemic index; GL, glycemic load; MAGE, mean amplitude of glycemic excursions.
P ≤ 0.10.
P ≤ 0.05.
P ≤ 0.01.
FIGURE 1.
Scatter plots showing the relation between dietary glycemic index and glycemic response variables, including AUC (A), mean of glucose values (B), SD of glucose values (C), percentage of values in the euglycemic range (ie, 70–180 mg/dL; D), percentage of values in the hyperglycemic range (ie, >180 mg/dL; E), and MAGE (F). Lines of best fit were derived from bivariate Pearson's correlations. MAGE, mean amplitude of glycemic excursions.
Dietary GL and glycemic response
No significant relations between GL and glycemic outcomes were found in the uncontrolled bivariate correlations (Table 2). However, partial correlation analyses controlled for energy intake (Table 3) showed that GL was positively related to the SD of glucose values (P = 0.01) and MAGE (P = 0.02). The relations of GL to AUC and mean glucose were nearly significant (P = 0.10).
Other dietary variables and glycemic response
In the bivariate analysis (Table 2), protein intake was significantly and negatively related to MAGE (P = 0.02). This relation remained significant (P = 0.03) in the partial correlation analyses that controlled for energy intake (Table 3). Partial correlation analyses also found a significant positive relation between protein intake and percentage of CGM values in the euglycemic range (P = 0.05). Negative associations between protein intake and the SD of glucose values (P = 0.09) and the percentage of CGM values in the hyperglycemic range (P = 0.06) was nearly significant in partial correlation analyses.
Carbohydrate intake was unrelated to any glycemic response variable in bivariate correlations (Table 2), but was positively associated with the SD of glucose values (P = 0.05) and MAGE (P = 0.05) in the partial correlation analyses that controlled for energy intake (Table 3). None of the remaining dietary variables (intakes of fat, total sugar, added sugar, total fiber, soluble fiber, or insoluble fiber) were significantly correlated with any glycemic response outcome in bivariate or partial correlation analyses.
Multivariate analyses
Because carbohydrate intake, protein intake, and dietary GI were each related to more than one glycemic response variable, they were included in multivariate analyses. (GL was not included because of its high correlation with carbohydrate intake.) In each analysis, total energy intake was entered in the first step and carbohydrate, protein, and GI were entered in a stepwise fashion in the subsequent steps.
As shown in Table 4, only GI added significantly to the prediction of AUC (P < 0.01), mean glucose (P < 0.01), the SD of glucose values (P = 0.01), and the percentage of values in the hyperglycemic (P < 0.001) and euglycemic (P < 0.001) ranges. In contrast, both GI (P < 0.01) and carbohydrate intake (P = 0.04) were significant independent predictors of MAGE.
TABLE 4.
Multivariate analyses to determine independent contributions of GI and carbohydrate intake to glycemic response variables1
| AUC | Mean | SD | Hyperglycemic values | Euglycemic values | MAGE | |||||||
| β | R2 | β | R2 | β | R2 | β | R2 | β | R2 | β | R2 | |
| mg · dL · min−1 | mg/dL | mg/dL | % | % | mg/dL | |||||||
| Step 1 | ||||||||||||
| Energy | 0.06 | 0.00 | 0.06 | 0.00 | −0.06 | 0.00 | −0.02 | 0.00 | 0.01 | 0.00 | −0.11 | 0.01 |
| Step 2 | ||||||||||||
| Energy | 0.01 | 0.02 | −0.09 | −0.06 | 0.05 | −0.15 | ||||||
| GI | 0.36 | 0.132 | 0.38 | 0.143 | 0.32 | 0.102 | 0.41 | 0.173 | −0.42 | 0.183 | 0.32 | 0.102 |
| Step 3 | ||||||||||||
| Energy | −0.42 | |||||||||||
| GI | 0.33 | |||||||||||
| Carbohydrate | 0.36 | 0.054 | ||||||||||
Results from multiple regression analyses with stepwise entry of dietary GI and carbohydrate after forced entry of total energy intake. GI, glycemic index; MAGE, mean amplitude of glycemic excursions.
P ≤ 0.01.
P ≤ 0.001.
P ≤ 0.05.
DISCUSSION
The purpose of this study was to examine the relations of dietary variables—particularly GI and GL—to glycemic outcomes in a real-world setting. Participants in this study were obese individuals with type 2 diabetes who consumed their food and beverages without the restrictions imposed in laboratory tests of GI (ie, single items consumed in defined portions at a specified pace after a prolonged fast). In addition, the unit of analysis was a full day (rather than the usual 2- to 3-h postprandial period). Under these free-living conditions, dietary GI was significantly related to the AUC for glucose. This finding is consistent with that of Brynes et al (16), who reported a significant reduction in 24-h glucose AUC with a reduction in dietary GI among adults with type 2 diabetes. Furthermore, multivariate models in the current study found that dietary GI was significantly associated with all measured glycemic response indicators (AUC, mean and SD of glucose values, percentage of values in the hyperglycemic and euglycemic ranges, and MAGE) independently of energy and carbohydrate intakes. GI accounted for 10–18% of the unique variance in each outcome. These findings provide real-world evidence that consumption of a low-GI diet is beneficial for controlling blood glucose in individuals with type 2 diabetes.
MAGE, the average distance between glucose peaks and nadirs, is considered a “gold standard” measure of glycemic variability. Because MAGE is strongly related to oxidative stress (17), it may be an important variable in the onset and progression of diabetes-related complications. In the current study, MAGE was positively associated with dietary carbohydrate and GL, inversely associated with dietary protein, and not associated with dietary fat. After control for total energy intake in partial correlations, GL accounted for 14% to 17% of the variance in these glycemic outcomes. Co-ingestion of protein with carbohydrate augments insulin secretion and thereby attenuates the postprandial glycemic response (18), which provides a plausible explanation for the inverse association. Regarding dietary fat, Gentilcore et al (19) found that a preload of olive oil before a potato meal slowed gastric emptying and attenuated postprandial glycemia in patients with type 2 diabetes. However, consistent with the current study, co-ingestion of the oil with the meal had a relatively small effect on blood glucose (19).
According to the American Diabetes Association (20), monitoring carbohydrate intake is a “key strategy in achieving glycemic control,” and “the use of the glycemic index and glycemic load may provide a modest additional benefit for glycemic control over that observed when total carbohydrate is considered alone” (emphasis added). However, multivariate analyses conducted in the current study indicate that GI was a stronger independent predictor of glycemic stability and variability, as assessed with a CGM, than of total carbohydrate intake. Similarly, Bao et al (21) recently found that GI and GL were stronger predictors of the glycemic response to single foods than was carbohydrate content and that GL accounted for more variance than did carbohydrate in the glycemic response to a mixed meal.
Our study had some limitations, most notably the small sample size and the self-report nature of the dietary data. In addition, the calculation of dietary GI did not account for food processing or accompanying nutrients (eg, type and amount of protein and fat), and the participants did not reliably record the time of food intake; thus, the glycemic response could not accurately be linked to individual intake episodes. Nonetheless, the current findings suggest a greater role for low-GI (and, by extension, low-GL) diets in managing type 2 diabetes than is currently acknowledged. Standard indicators of glycemic control (eg, fasting blood glucose and glycated hemoglobin), may not be sufficiently sensitive to detect all clinically meaningful effects of a low-GI, low-GL diet on diabetes management. Given the apparent relevance of glycemic fluctuations to complications of diabetes, additional study is warranted.
Acknowledgments
The authors’ responsibilities were as follows—ANF: conducted the research, analyzed the data, and had primary responsibility for the final content; all authors wrote the paper. All authors read and approved the final manuscript and designed the research. ANF was employed full-time by the University of Pennsylvania when this study was designed and conducted and when the data were initially analyzed; however, he is currently employed by Nutrisystem Inc. The funding sources had no role in designing or implementing the study or in collecting, analyzing, or interpreting the data. CBE, TAW, and DSL reported no conflicts of interest.
Footnotes
Abbreviations used: CGM, continuous glucose monitoring; GI, glycemic index; GL glycemic load; MAGE, mean amplitude of glycemic excursions.
REFERENCES
- 1.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 1981;34:362–6 [DOI] [PubMed] [Google Scholar]
- 2.Salmerón J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, Stampfer MJ, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 1997;20:545–50 [DOI] [PubMed] [Google Scholar]
- 3.Brand-Miller JC, Thomas M, Swan V, Ahmad ZI, Petocz P, Colagiuri S. Physiological validation of the concept of glycemic load in lean young adults. J Nutr 2003;133:2728–32 [DOI] [PubMed] [Google Scholar]
- 4.Wolever TMS, Jenkins DJA. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr 1986;43:167–72 [DOI] [PubMed] [Google Scholar]
- 5.Gannon MC, Nuttall FQ. Factors affecting interpretation of postprandial glucose and insulin areas. Diabetes Care 1987;10:759–63 [DOI] [PubMed] [Google Scholar]
- 6.Pi-Sunyer FX. Glycemic index and disease. Am J Clin Nutr 2002;76(suppl):290S–8S [DOI] [PubMed] [Google Scholar]
- 7.Fabricatore AN, Wadden TA, Ebbeling CB, Thomas JG, Stallings VA, Schwartz S, Ludwig DS. Targeting dietary fat or glycemic load in the treatment of obesity and type 2 diabetes: a randomized controlled trial. Diabetes Res Clin Pract 2011;92:37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg S, Jovanovic L. Relationship of fasting and hourly blood glucose levels to HbA1c values: safety, accuracy, and improvements in glucose profiles obtained using a 7-day continuous glucose sensor. Diabetes Care 2006;29:2644–9 [DOI] [PubMed] [Google Scholar]
- 9.Mastrototaro J, Shin J, Marcus A, Sulur G. Star 1 Clinical Trial Investigators. The accuracy and efficacy of real-time continuous glucose monitoring sensor in patients with type 1 diabetes. Diabetes Technol Ther 2008;10:385–90 [DOI] [PubMed] [Google Scholar]
- 10.Chico A, Subirà M, Vidal-Ríos P, Novials A. The Continuous Glucose Monitoring System is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care 2003;26:1153–7 [DOI] [PubMed] [Google Scholar]
- 11.Diabetes Research in Children Network (DirecNet) Study Group, Buckingham BA, Kollman C, Beck R, Kalajian A, Fiallo-Scharer R, Tansey MJ, Fox LA, Wilson DM, Weinzimer SA, Ruedy KJ, Tamborlane WV. Evaluation of factors affecting CGMS calibration. Diabetes Technol Ther 2006;8:318–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purves RD. Optimum numerical integration methods for estimation of area-under-the-curve (AUC) and area-under-the-moment-curve (AUMC). J Pharmacokinet Biopharm 1992;20:211–26 [DOI] [PubMed] [Google Scholar]
- 13.Service JF, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 1970;19:644–55 [DOI] [PubMed] [Google Scholar]
- 14.Data N System for research. Minneapolis, MN: University of Minnesota Nutrition Coordinating Center, 2006. [computer software] [Google Scholar]
- 15.SPSS Inc Statistical package for the social sciences, version 16.0. Chicago, IL: SPSS Inc, 2007 [Google Scholar]
- 16.Brynes AE, Lee JL, Brighton RE, Leeds AR, Dornhorst A, Frost GS. A low glycemic diet significantly improves the 24-h blood glucose profile in people with type 2 diabetes, as assessed using the continuous glucose MiniMed monitor. Diabetes Care 2003;26:548–9 [DOI] [PubMed] [Google Scholar]
- 17.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006;295:1681–7 [DOI] [PubMed] [Google Scholar]
- 18.Ma J, Stevens JE, Cukier K, Maddox AF, Wishart JM, Jones KL, Clifton PM, Horowitz M, Rayner CK. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 2009;32:1600–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gentilcore D, Chaikomin R, Jones KL, Russo A, Feinle-Bisset C, Wishart JM, Rayner CK, Horowitz M. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J Clin Endocrinol Metab 2006;91:2062–7 [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association Standards of medical care in diabetes – 2011. Diabetes Care 2011;34(suppl 1):S11–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao J, Atkinson F, Petocz P, Willett WC, Brand-Miller JC. Prediction of postprandial glycemia and insulinemia in lean, young, healthy adults: glycemic load compared with carbohydrate content alone. Am J Clin Nutr 2011;93:984–96 [DOI] [PubMed] [Google Scholar]