Abstract
Background: Little is known about the joint association between glycemic index (GI), glycemic load (GL), and alcohol intake with type 2 diabetes (T2D).
Objective: The objective of this study was to examine whether alcohol intake alters the associations between carbohydrate quality (GI) or quality and quantity (GL) and T2D incidence in women.
Design: Participants from the Nurses’ Health Study who were free of T2D, cardiovascular disease, or cancer (n = 81,827) at baseline in 1980 were followed for 26 y. Cumulative averages of GI, GL, total carbohydrates, and alcohol intake were calculated every 2–4 y from validated food-frequency questionnaires. Cox proportional hazard models were used to adjust for covariates.
Results: We documented 6950 cases of T2D during follow-up. After adjustment for lifestyle and dietary factors, the positive association between GL and T2D risk was attenuated in subjects with higher alcohol intakes. RRs that compared the top and bottom quintiles of GL were 1.29 (95% CI: 1.11, 1.49; P-trend < 0.001) in women with alcohol intakes of 0 to <5 g/d, 1.34 (95% CI: 0.93, 1.92; P-trend = 0.05) in women with alcohol intakes of 5 to <15 g/d, and 0.99 (95% CI: 0.60, 1.65; P-trend = 0.82) in women with alcohol intakes ≥15 g/d (P-interaction = 0.02). However, a higher intake of alcohol did not modify the positive association between GI and T2D (P-interaction = 0.76).
Conclusion: Our findings suggest that a higher alcohol intake (≥15 g/d) attenuates the positive association between GL and T2D incidence.
INTRODUCTION
The prevalence of T2D4 is escalating with a major impact on morbidity and premature mortality worldwide (1). Alcohol intake was shown to be protective against T2D when consumed in moderate amounts compared with lifetime abstainers and became deleterious in excessive consumers (≥60 g/d in men and ≥50 g/d in women) according to a recent systematic review and meta-analysis of 20 cohort studies (2). A similar U-shaped relation has been shown in a previous meta-analysis of 15 prospective cohort studies with a 30% lower risk of T2D in moderate alcohol consumers (6–47 g/d) and no further risk reduction in heavier drinkers (≥48 g/d) or nondrinkers (3). In addition, positive associations between GI and GL and T2D risk have been shown in a recent meta-analysis of prospective cohorts (4). However, little is known about whether alcohol consumption modifies the association between GL or GI and T2D incidence. One trial conducted in 1975 showed that ethanol ingestion with food dampened the blood glucose rise and increased insulin sensitivity in both normal and diabetic subjects (5). Other trials were conducted to assess the effect of alcohol as part of a meal on different hormones in T2D patients. Ethanol was shown to suppress or delay the incretin (glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1, both of which are hormones in the gut that are known to enhance insulin secretion) response early postprandially when taken in a combination with a fat-rich mixed meal in T2D patients and eradicate the stimulation that carbohydrates would cause on the release of incretin and insulin (6). Although these effects were shown in T2D subjects (6), they were not consistently shown in healthy subjects (7–10). However, none of these studies have specifically examined whether alcohol intake could modify the known positive association between GI or GL and T2D in healthy women. Therefore, we prospectively examined whether alcohol intake altered associations between GI or GL and T2D incidence in the NHS cohort.
SUBJECTS AND METHODS
The NHS was established in 1976 and is a prospective cohort study of 121,700 registered female nurses (30–55 y of age at baseline) who reside in 11 states. Participants were mailed questionnaires at baseline and every second year to repeatedly assess lifestyle practices and chronic diseases occurrence. The study protocol was approved by the institutional review boards of Brigham and Women's Hospital and the Harvard School of Public Health.
Dietary assessment
Information on foods and beverages consumed in the previous year was first assessed in 1980. In 1984, dietary information was collected by using a 116-item FFQ. During 1986 through 2006, participants were asked to update their diet information every 4 y by using a similar but expanded 131-item semiquantitative FFQ that has been previously validated (11, 12). Participants were asked to select their usual intake of a standard portion of each food item. Nine responses were possible ranging from never or <1 time/mo to ≥6 times/d. The daily nutrient intake was calculated by multiplying the frequency of intake of each food by the nutrient content estimated by using food-composition tables from the Harvard University food-composition database, which was derived from the US Department of Agriculture sources (13) and summing across all items. To reflect both the quantity and quality of the carbohydrates consumed, GL was calculated by multiplying the carbohydrate content of each food by its GI, which is a measure of the relative postprandial blood glucose response per gram of carbohydrates, and then multiplying this value by the frequency of consumption and summing these values for all foods (14). Cereal fiber intake was defined and calculated as described previously (15). To reduce measurement error, the cumulative average values of 1980, 1984, 1986, 1990, 1994, 1998, and 2002 were computed for dietary variables including foods, nutrients, GI, and GL (12). The residual method was used to adjust intakes of total carbohydrates and cereal fiber for total energy (16). Total alcohol intake was calculated by using separate questions in the FFQ on the consumption of beer, wine, and spirits (17). The frequency of alcohol consumption, which ranged from none to 7 d/wk, was first asked in 1986. In a study to assess the validity and reproducibility of this alcohol-assessment method, self-reported alcohol consumption on questionnaires correlated highly (Spearman's r = 0.86) with independent measures of alcohol intake from 14 d of diet records and with serum HDL-cholesterol from fasting blood samples (r = 0.4), which is a nonspecific, and yet sensitive, marker of alcohol intake (18).
Measurement of nondietary factors
Age was calculated from the date of birth until the return date of the 1980 questionnaire. Height was assessed at the beginning of the study in 1976, and weight was self-reported on every questionnaire. Self-reported weights correlated highly with measured weights (r = 0.96) (19). BMI was calculated as weight (in kg) divided the square of height (in m). Current smoking status (yes/no) and quantity of cigarettes smoked per day were assessed on every questionnaire and used to assess an updated smoking status (never; past; current 1–14 cigarettes/d; current 15–24 cigarettes/d; current ≥25 cigarettes/d). In 1986, women were asked to complete an 8-item questionnaire regarding the average amount of time spent per week in different physical activities. Physical activity was expressed as hours per week and converted to metabolic equivalent task hours per week. Menopausal status and hormone use were assessed in 1976 and every 2 y thereafter. Women were classified as postmenopausal at the first report of natural menopause or surgery with bilateral oophorectomy.
Exclusion of participants at baseline
In the current analysis, participants with implausible energy intakes (<500 or >3500 kcal/d), who had a history of T2D, cardiovascular disease (ie, heart attack, stroke, angina, or coronary artery bypass graft), or cancer, who had missing values for the derived GL, or who had missed reporting their alcohol intake were excluded at baseline (1980). Thus, 81,827 participants remained for the current analysis.
Ascertainment of T2D cases
The outcome was T2D incidence between the return of the baseline questionnaire in 1980 and 30 June 2006. Women who reported a diagnosis of T2D in the biennial follow-up questionnaire were sent a supplementary questionnaire to confirm the diagnosis. The National Diabetes Data Group criteria were used to confirm a self-reported diagnosis of T2D (20). For T2D cases identified after 1998, the American Diabetes Association criteria were applied (21). Cases of type 1 diabetes were excluded. In a validation study of the supplementary questionnaire for diabetes diagnosis, a medical record review confirmed 98% of self-reported T2D cases (61 of 62 cases) (22).
Statistical analysis
Participants contributed follow-up time from the date they returned their baseline questionnaire to the date of diagnosis of T2D, death, loss to follow-up, or end of the study period, whichever came first. To examine the independent and joint associations of GL and alcohol intake with T2D, we estimated RRs and 95% CIs by using the Cox proportional hazards regression model with age in months as the time scale, the calendar year as a stratification variable, and time-varying covariates.
In the basic multivariate model 1, in addition to stratification by age and time period, we adjusted for known and suspected risk factors of T2D, including family history of T2D in 1986 (yes or no); BMI (<21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–32.9, 33–34.9, 35–39.9, or ≥40, or missing), energy intake (continuous; kcal/d), alcohol intake (0 to <5, 5 to <15, or ≥15 g/d), cereal fiber intake (quintiles; g/d), physical activity (1 to <3, 3 to <9, 9 to <18, 18 to <27, or ≥27 metabolic equivalent task hours/wk, or missing), smoking status (never, past, or currently 1–14, 15–24, or ≥25 cigarettes/d), menopausal status and hormone use (premenopausal, postmenopausal and never used hormones, postmenopausal and current hormone users, or postmenopausal and past hormone users). In an additional multivariate model 2, we further adjusted for coffee intakes (quintiles; cups/d; 1 cup = 237 mL), the ratio of polyunsaturated to saturated fatty acids (quintiles), trans fat intake (quintiles; g/d), and red-meat consumption (quintiles; servings/d). Cumulative averages of GI, GL, total carbohydrates, and dietary covariates (macronutrients and alcohol) were calculated at each time point to better represent the long-term diet and to minimize within-person variation. We stopped updating the diet (except for alcohol intake) when participants first reported a chronic disease diagnosis (eg, cancer, cardiovascular disease, or hypertension) because participants with any of these intermediate endpoints may have changed their diet. Because some people might have stopped drinking after self-reported disease diagnosis, we ran our main analysis where we updated alcohol intake and another sensitivity analysis where we stopped updating alcohol intake after self-reported disease diagnosis. All other covariates were updated at each time point. Tests for linear trends across quintiles (for GL, GI, or total carbohydrates) or categories (for alcohol intake) were performed by using Wald's test (1 df) of an ordinal term that represented median values of these quintiles or categories.
GI, GL, total carbohydrate intake, and alcohol intake were initially analyzed as main effects in quintiles in separate models. Stratified analyses and interaction tests were performed for GL (quintiles) and GI (quintiles) according to alcohol intake (0 to <5, 5 to <15, or ≥15 g/d) and to alcohol drinking frequency (none or 1–2 or 3–7 times/wk). Because the frequency of alcohol drinking was first assessed in 1986, we used 1986 as the baseline for the stratified analysis. A joint analysis was performed according to joint classifications of GL (quintiles) and alcohol intake (tertiles). The likelihood ratio test was used to compare the model including the cross-product terms [eg, median of tertiles of alcohol intake (ordinal) × median of quintiles of GL or GI (ordinal)] with a model that included only the main effects of alcohol intake and GL or GI. SAS software (version 9.1; SAS) was used for all analyses, and P < 0.05 was considered statistically significant.
RESULTS
In this cohort of 81,827 women, we documented 6950 incident T2D cases during 26 y of follow-up (1,961,881 person-years). Women with the highest GL diet at midpoint in 1990 had higher intakes of total carbohydrates, cereal fiber, total red meat, fruit, and vegetables, regular soda, and potatoes. These women were also less likely to smoke, drink alcohol, or drink coffee and more likely to be physically active that were women with a lower-GL diet (Table 1). Women with a higher-GI diet at midpoint in 1990 had higher intakes of total carbohydrate, cereal fiber, trans fat, total red meat, regular soda, and potatoes and lower intakes of alcohol, coffee, and fruit, and vegetables. They also smoked more and exercised less than did women with a lower-GI diet. Although GL correlated highly with carbohydrate intake (r = 0.90), GI was less strongly correlated with carbohydrate intake (r = 0.31). The correlation between GI and GL was 0.58. In our cohort, the mean baseline daily alcohol intake was 6.3 g (approximately one-half of a standard US drink), and the median alcohol intake was 0.8 g/d for the category of 0 to <5 g/d, 8.8 for the category of 5 to <15 g/d, and 23.9 for the category of ≥15 g/d. Only a small percentage of our sample [0.58% (n = 477)] had an average alcohol intake ≥50 g/d and was considered heavy drinkers. Furthermore, the top 5 contributors to baseline (1980) GL were orange juice (8.2%), white bread (8.1%), dark bread (7.5%), cola (6%), and fruit cocktails (5%), and the top 5 contributors for 1990 GL were mashed potatoes (7.6%), cold cereals (6.4%), dark bread (5.1%), bananas (4.4%), and orange juice (3.8%).
TABLE 1.
Age-standardized 1990 (midpoint) participant characteristics, by quintiles of glycemic load in US women from the NHS1
| Quintiles of glycemic load |
Quintiles of glycemic index |
|||||
| 1 | 3 | 5 | 1 | 3 | 5 | |
| n | 15,412 | 15,840 | 16,063 | 15,597 | 15,953 | 15,778 |
| Age (y) | 56.1 ± 7.02 | 56.0 ± 7.7 | 55.6 ± 7.4 | 56.7 ± 6.9 | 56.2 ± 7.1 | 54.9 ± 7.2 |
| Current postmenopausal hormone users [n (%)] | 5857 (38) | 6178 (39) | 5622 (35) | 6083 (39) | 6062 (38) | 5365 (34) |
| BMI (kg/m2) | 25.5 ± 4.7 | 25.5 ± 4.7 | 25.5 ± 4.9 | 25.6 ± 4.7 | 25.5 ± 4.7 | 25.3 ± 5.0 |
| Family history of T2D [n (%)] | 3391 (22) | 3960 (25) | 3855 (24) | 3587 (23) | 3829 (24) | 3787 (24) |
| Current smoking [n (%)] | 3237 (21) | 2376 (15) | 2249 (14) | 2496 (16) | 2339 (15) | 2998 (19) |
| Physical activity (MET-h/wk) | 14.9 ± 21.5 | 15.3 ± 21.0 | 16.3 ± 23.4 | 18.7 ± 24.4 | 15.5 ± 21.5 | 12.2 ± 19.0 |
| Alcohol intake (g/d) | 8.6 ± 11.6 | 6.1 ± 9.2 | 4.5 ± 7.5 | 8.5 ± 11.6 | 6.2 ± 9.0 | 4.5 ± 8.2 |
| Dietary glycemic load | 58.1 ± 11.5 | 98.6 ± 4.8 | 153 ± 22 | 79 ± 28 | 104 ± 30 | 121 ± 37 |
| Dietary glycemic index | 50 ± 3.8 | 52.5 ± 2.8 | 54.1 ± 2.6 | 47.6 ± 2.3 | 52.5 ± 0.4 | 56.5 ± 1.3 |
| Total carbohydrates (g/d)3 | 181 ± 35 | 200 ± 29 | 213 ± 29 | 192 ± 35 | 200 ± 31 | 206 ± 32 |
| Cereal fiber intake (g/d)3 | 3.1 ± 1.9 | 4.0 ± 1.8 | 4.0 ± 1.7 | 3.3 ± 2.0 | 4.0 ± 1.8 | 3.9 ± 1.7 |
| Coffee intake (cups/d) | 2.6 ± 1.7 | 2.4 ± 1.6 | 2.2 ± 1.7 | 2.9 ± 1.7 | 2.4 ± 1.6 | 1.9 ± 1.6 |
| Ratio of polyunsaturated to saturated fatty acids | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.5 ± 0.1 | 0.5 ± 0.1 |
| trans Fat intake (percentage of total energy) | 1.9 (0.6) | 1.9 (0.5) | 1.9 (0.5) | 1.7 (0.5) | 1.9 (0.5) | 2.1 (0.6) |
| Total red meat consumption (servings/d) | 0.9 ± 0.5 | 1.1 ± 0.5 | 1.3 ± 0.6 | 0.9 ± 0.6 | 1.1 ± 0.5 | 1.3 ± 0.6 |
| Total fruit and vegetables (servings/d) | 3.6 ± 1.5 | 4.9 ± 1.7 | 6.4 ± 2.4 | 5.3 ± 2.3 | 5.2 ± 2.0 | 4.2 ± 1.8 |
| Total regular carbonated beverages (servings/d) | 0.1 ± 0.2 | 0.2 ± 0.4 | 0.6 ± 1.0 | 0.1 ± 0.2 | 0.2 ± 0.4 | 0.6 ± 1.0 |
| Potatoes (servings/d) | 0.2 ± 0.2 | 0.3 ± 0.2 | 0.4 ± 0.2 | 0.2 ± 0.2 | 0.3 ± 0.2 | 0.4 ± 0.2 |
MET-h, metabolic equivalent task hours; NHS, Nurses’ Health Study; T2D, type 2 diabetes.
Mean ± SD (all such values).
Nutrient intakes were energy adjusted.
After adjustment for age, there was a positive dose-response relation between both GL and GI, and the incidence of T2D (all P-trend < 0.001; Table 2) and an inverse dose-response relation between total carbohydrate intake, alcohol intake, and T2D incidence (P-trend < 0.001). After further adjustment for lifestyle and dietary factors, trends remained significant for GL, GI, and alcohol intake, but total carbohydrate intake was no longer significant (P-trend = 0.39) (Table 2, model 5). Additional adjustment for intakes of coffee, trans fat, red meat, and the ratio of polyunsaturated to saturated fatty acids did not appreciably alter RRs. However, the total carbohydrate intake became positively associated with diabetes risk (P-trend = 0.01) (Table 2, model 6).
TABLE 2.
RRs of T2D for quintiles of GL, GI, and total carbohydrate intake1
| Values | P-trend | |||||
| GL (range) | 0 to <70 | 70–89 | >89–106 | >106–129 | >129 | |
| T2D cases (n) | 1239 | 1283 | 1390 | 1466 | 1572 | — |
| Person-years | 378,015 | 388,676 | 396,702 | 400,092 | 398396 | — |
| Age | 1.00 (reference)2 | 0.99 (0.91, 1.07) | 1.05 (0.97, 1.14) | 1.10 (1.02, 1.19) | 1.19 (1.10, 1.28) | <0.001 |
| MV model 13 | 1.00 (reference) | 1.02 (0.94, 1.11) | 1.12 (1.02, 1.22) | 1.21 (1.09, 1.34) | 1.30 (1.14, 1.47) | <0.001 |
| MV model 24 | 1.00 (reference) | 1.02 (0.94, 1.11) | 1.13 (1.03, 1.23) | 1.22 (1.10, 1.35) | 1.32 (1.16, 1.51) | <0.001 |
| GI (range) | 0.16–50 | >50–52 | >52–53 | >53–55 | >55 | |
| T2D cases | 1146 | 1322 | 1366 | 1445 | 1671 | |
| Person-years | 385,706 | 394,532 | 397,144 | 395,745 | 388,754 | |
| Age | 1.00 (reference) | 1.13 (1.04, 1.22) | 1.17 (1.08, 1.26) | 1.26 (1.16, 1.36) | 1.52 (1.41, 1.64) | <0.001 |
| MV model 33 | 1.00 (reference) | 1.17 (1.08, 1.26) | 1.24 (1.14, 1.34) | 1.31 (1.21, 1.42) | 1.55 (1.43, 1.67) | <0.001 |
| MV model 44 | 1.00 (reference) | 1.15 (1.06, 1.24) | 1.20 (1.11, 1.30) | 1.26 (1.16, 1.37) | 1.46 (1.34, 1.58) | <0.001 |
| Total carbohydrate intake (range, g/d) | 7–150 | >150–170 | >170–186 | >186–203 | >203 | |
| T2D cases | 1274 | 1450 | 1539 | 1381 | 1306 | |
| Person-years | 367,304 | 388,932 | 400,329 | 402,156 | 403,160 | |
| Age | 1.00 (reference) | 1.05 (0.97, 1.13) | 1.07 (0.99, 1.15) | 0.94 (0.87, 1.01) | 0.87 (0.80, 0.94) | <0.001 |
| MV model 53 | 1.00 (reference) | 0.99 (0.91, 1.07) | 1.05 (0.97, 1.13) | 0.99 (0.91, 1.08) | 1.04 (0.95, 1.13) | 0.39 |
| MV model 64 | 1.00 (reference) | 1.02 (0.94, 1.10) | 1.10 (1.01, 1.19) | 1.06 (0.97, 1.16) | 1.14 (1.03, 1.25) | 0.01 |
| Alcohol intake (g/d) | 0 to <5 | 5 to <15 | 15+ | |||
| T2D cases | 1993 | 4471 | 486 | |||
| Person-years | 436,339 | 1,277,265 | 24,8276 | |||
| Age | 1.00 (reference) | 0.53 (0.49, 0.56) | 0.46 (0.41, 0.50) | <0.001 | ||
| MV model 75 | 1.00 (reference) | 0.73 (0.68, 0.78) | 0.67 (0.61, 0.74) | <0.001 | ||
| MV model 86 | 1.00 (reference) | 0.74 (0.69, 0.79) | 0.68 (0.62, 0.76) | <0.001 | ||
GI, glycemic index; GL, glycemic load; MV, multivariate; T2D, type 2 diabetes.
RR (95% CI) from Cox proportional hazards models (all such values).
Adjusted for age (in mo), family history of T2D (yes or no), BMI (in kg/m2; <21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–32.9, 33–34.9, 35–39.9, or ≥40), energy intake (kcal; continuous), alcohol intake (0 to <5, 5 to <15, or ≥15 g/d), cereal fiber intake (quintiles; g/d), physical activity (1 to <3, 3 to <9, 9 to <18, 18 to <27, or ≥27 metabolic equivalent task hours/wk), smoking status (never, past, or currently 1–14, 15–24, or ≥25 cigarettes/d), and menopausal status and hormone use (premenopausal, postmenopausal and never used hormones, postmenopausal and current hormone users, or postmenopausal and past hormone users).
Same as in footnote 3 with additional adjustment for coffee intake (quintiles; cups/d; 1 cup = 237 mL), ratio of polyunsaturated to saturated fatty acids (quintiles), trans fat intake (quintiles; percentage of total energy), and red-meat consumption (quintiles; servings/d).
Same as in footnote 3 with additional adjustment for glycemic load (quintiles).
Same as in footnote 4 with additional adjustment for glycemic load (quintiles).
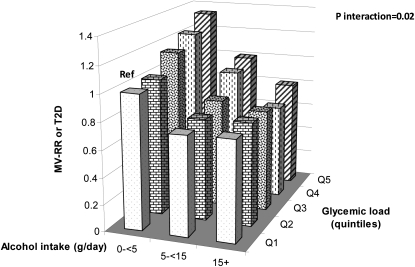
In the stratified analyses shown in Table 3, the positive association between increased GL and T2D incidence was evident in the group with no or low alcohol intake (0 to <5 g/d) (top quintile compared with reference category HR: 1.29; 95% CI: 1.11,1.49; P-trend < 0.001; model 1). This association remained positive, although it became borderline significant possibly because of the fewer number of cases in women with a moderate alcohol intake (5 to <15 g/d; HR for the same comparison: 1.34; 95% CI: 0.93, 1.92; P-trend = 0.05; model 2). However, the association became null in women with a higher alcohol intake (≥15 g/d; HR for the same comparison: 0.99; 95% CI: 0.60, 1.65; P-trend = 0.82; model 3) (P-interaction = 0.02) (Figure 1).
TABLE 3.
RRs of T2D for quintiles of GL within 3 strata of alcohol intake1
| Quintiles of GL within 3 strata of alcohol intake (g/d) |
||||||
| 0 to <70 | 70–89 | >89–106 | >106–129 | >129 | P-trend | |
| Alcohol intake of 0 to <5 g/d | ||||||
| Cases (n) | 862 | 971 | 1102 | 1188 | 1318 | — |
| Person-years | 206,999 | 236,881 | 257,668 | 270,934 | 290,632 | — |
| MV model 1 | 1.00 (reference)2 | 1.02 (0.93, 1.13) | 1.13 (1.02, 1.26) | 1.21 (1.07, 1.36) | 1.29 (1.11, 1.49) | <0.001 |
| Alcohol intake of 5 to <15 g/d | ||||||
| Cases (n) | 221 | 197 | 197 | 212 | 196 | — |
| Person-years | 98,448 | 95,797 | 91,094 | 88,194 | 76,957 | — |
| MV model 2 | 1.00 (reference) | 0.99 (0.80, 1.22) | 1.07 (0.84, 1.37) | 1.26 (0.95, 1.68) | 1.34 (0.93, 1.92) | 0.05 |
| Alcohol intake of ≥15 g/d | ||||||
| Cases (n) | 156 | 115 | 91 | 66 | 58 | — |
| Person-years | 72,568 | 55,998 | 47,939 | 40,964 | 30,807 | — |
| MV model 3 | 1.00 (reference) | 1.00 (0.76, 1.31) | 0.98 (0.71, 1.36) | 0.87 (0.58, 1.30) | 0.99 (0.60, 1.65) | 0.82 |
All MV models were adjusted for age (in mo), family history of T2D (yes or no), BMI (in kg/m2; <21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–32.9, 33–34.9, 35–39.9, or ≥40), energy intake (kcal; continuous), alcohol intake (g/d; continuous), cereal fiber intake (quintiles; g/d), physical activity (1 to <3, 3 to <9, 9 to <18, 18 to <27, or ≥27 metabolic equivalent task hours/wk), smoking status (never, past, or currently 1–14, 15–24, or ≥25 cigarettes/d), menopausal status and hormone use (premenopausal, postmenopausal and never used hormones, postmenopausal and current hormone users, or postmenopausal and past hormone users), coffee intake (quintiles; cups/d; 1 cup = 237 mL), ratio of polyunsaturated to saturated fatty acids (quintiles), trans fat intake (quintiles; percentage of total energy), and red-meat consumption (quintiles; servings/d). At baseline, the number of participants in each alcohol category was as follows: 53,102 for alcohol intake 0 to <5 g/d with the number of participants from quintiles 1 to 5 of GL being 9156, 9707, 11,111, 11,341, and 11,787, respectively; 18,681 participants for alcohol intake 5 to <15 g/d with the number of participants from quintiles 1 to 5 of GL being 4413, 3923, 3843, 3658, and 2844, respectively; and 10,044 participants for alcohol intake ≥15 g/d with the number of participants from quintiles 1 to 5 of GL being 2970, 2158, 1857, 1711, and 1348, respectively. P-interaction = 0.02. GL, glycemic load; MV, multivariate; T2D, type 2 diabetes.
RR (95% CI) from Cox proportional hazards models (all such values).
FIGURE 1.
Joint association (RRs from Cox proportional hazards models) of alcohol intake and glycemic load on T2D risk. All MV models were adjusted for age (in mo), family history of T2D (yes or no), BMI (in kg/m2; <21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–32.9, 33–34.9, 35–39.9, or ≥40), energy intake (kcal; continuous), cereal fiber intake (quintiles; g/d), physical activity (1 to <3, 3 to <9, 9 to <18, 18 to <27, or ≥27 metabolic equivalent task hours/wk), smoking status (never, past, or currently 1–14, 15–24, or ≥25 cigarettes/d), menopausal status and hormone use (premenopausal, postmenopausal and never used hormones, postmenopausal and current hormone users, or postmenopausal and past hormone users), coffee intake (quintiles; cups/d; 1 cup = 237 mL), ratio of polyunsaturated to saturated fatty acids (quintiles), trans fat intake (quintiles; percentage of total energy), and red-meat consumption (quintiles; servings/d). At baseline, the number of participants in each alcohol category was as follows: 53,102 for alcohol intake 0 to <5 g/d with the number of participants from quintiles 1 to 5 of glycemic load being 9156, 9707, 11,111, 11,341, and 11,787, respectively; 18,681 participants for alcohol intake 5 to <15 g/d with the number of participants from quintiles 1 to 5 of glycemic load being 4413, 3923, 3843, 3658, and 2844, respectively; and 10,044 participants for alcohol intake ≥15 g/d with the number of participants from quintiles 1 to 5 of glycemic load being 2970, 2158, 1857, 1711, and 1348, respectively. MV, multivariate; Ref, reference; T2D, type 2 diabetes.
A significant positive dose-response relation between GI and T2D incidence in each group of alcohol intake (all P-trend < 0.05) is shown in Table 4, and there was no evidence of the interaction between GI and alcohol intake (P-interaction = 0.76).
TABLE 4.
RRs of T2D for quintiles of GI within 3 strata of alcohol intake1
| Quintiles of GI within 3 strata of alcohol intake (g/d) |
||||||
| 0.16–50 | >50 to 52 | >52 to 53 | >53 to 55 | >55 | P-trend | |
| Alcohol intake of 0 to <5 g/d | ||||||
| Cases (n) | 807 | 981 | 1045 | 1185 | 1423 | — |
| Person-years | 213,168 | 234,826 | 252,356 | 271,235 | 291,529 | — |
| MV model 1 | 1.00 (reference)2 | 1.16 (1.05, 1.27) | 1.20 (1.09, 1.32) | 1.28 (1.17, 1.41) | 1.45 (1.32, 1.59) | <0.001 |
| Alcohol intake of 5 to <15 g/d | ||||||
| Cases (n) | 221 | 223 | 224 | 184 | 171 | — |
| Person-years | 102,368 | 102,200 | 95,683 | 84,773 | 65,465 | — |
| MV model 2 | 1.00 (reference) | 1.07 (0.88, 1.29) | 1.17 (0.96, 1.42) | 1.09 (0.89, 1.35) | 1.37 (1.10, 1.70) | <0.01 |
| Alcohol intake of ≥15 g/d | ||||||
| Cases (n) | 118 | 118 | 97 | 76 | 77 | — |
| Person-years | 70,169 | 57,505 | 49,105 | 39,737 | 31,760 | — |
| MV model 3 | 1.00 (reference) | 1.21 (0.93, 1.57) | 1.21 (0.91, 1.60) | 1.21 (0.89, 1.64) | 1.40 (1.03, 1.91) | 0.04 |
All MV models were adjusted for the same covariates as in Table 3. At baseline, the number of participants in each alcohol category was as follows: 53,102 for alcohol intake 0 to <5 g/d with the number of participants from quintiles 1 to 5 of GI being 9383, 9954, 10,460, 11,187, and 12,118, respectively; 18, 681 for alcohol intake 5 to <15 g/d with the number of participants from quintiles 1 to 5 of GI being 4130, 4149, 3859, 3597, and 2946, respectively; and 10,044 for alcohol intake ≥15 g/d with the number of participants from quintiles 1 to 5 of GI being 2718, 2279, 2034, 1667, and 1346, respectively. P-interaction = 0.76. GI, glycemic index; MV, multivariate; T2D, type 2 diabetes.
RR (95% CI) from Cox proportional hazards models (all such values).
In a secondary analysis, a similar pattern was seen between GL and T2D incidence on stratification with alcohol drinking frequency. However, the interaction was not significant (P-interaction = 0.34), which could have been due to the reduced power when alcohol drinking frequency was assessed. In sensitivity analyses, which was additionally controlled for low-fat dairy products, nuts, eggs, tea, and the MUFA:saturated fatty acid ratio (instead of the PUFA:saturated fatty acid ratio) in the models, did not significantly alter our results. Also, the exclusion of women who were heavy drinkers [consumed ≥50 g alcohol/d; 477 subjects (58%), including 11 T2D cases] did not materially change the results for the main effects and the interaction tests. All of the above-mentioned results did not materially change when we stopped updating alcohol intake after self-reported hypertension (data not shown).
DISCUSSION
In this large prospective study, a higher alcohol intake (≥15 g/d) appeared to attenuate the positive association between GL and T2D incidence. To our knowledge, this was the first prospective cohort to examine the effect modification of the association between carbohydrate quality and quantity and diabetes risk by alcohol intake.
According to a recent systematic review and meta-analysis of 20 cohort studies (2) and a previous meta-analysis of 15 prospective cohort studies (3) on alcohol intake and T2D risk, a U-shaped relation has been shown whereby moderate drinkers had a lower risk of T2D than did abstainers or heavy drinkers. These results were persistent in subjects with low or high BMI. Our current results, which were similar to our previous results in 1988 (23), were consistent with these findings; however, our study population included only a small number of heavy drinkers, which did not give us enough power to investigate any potential U-shape association with heavy drinking.
Moderate alcohol consumption has been shown to improve insulin sensitivity in healthy men (24) and nondiabetic postmenopausal women (25). One study showed that the habitual consumption of moderate alcohol in the form of wine (mean intake: 18 g alcohol/d for 30 d) lowered fasting serum insulin in T2D patients (11 women and 7 men) with a mean (range) age of 64 y (45–82 y) (26). Other randomized controlled trials of 3–12 mo in T2D patients concluded that moderate alcohol consumption reduced fasting plasma glucose (27) and hemoglobin A1c (28) but not postprandial plasma glucose (27) concentrations. Alcohol intake was also seen to be associated with decreased serum insulin and improved insulin sensitivity (29) potentially via increased concentrations of systemic adiponectin (30–34), which is an adipocyte-derived hormone known to enhance insulin sensitivity and to have antiinflammatory properties (35).
Little is known about whether the effect of GL on T2D risk is modified by alcohol consumption. One trial in normal and diabetic subjects showed that ethanol blunted the rise in blood glucose and increased the insulin response to co-ingested food (5). Another study showed that, in diabetic patients, alcoholic beverages that contained carbohydrates [eg, beer (3.7% alcohol and 3.1% glucose), sake (12.3% alcohol and 4.9% glucose), and shochu used as a control (20.5% alcohol and 0% glucose)] induced a blood glucose elevation that was proportionally attenuated by the ethanol content of these beverages, such that the elevation of blood glucose at 60 min was higher for beer than for sake and was not significant for shochu (36). Our results suggested an attenuation of the positive association between GL and T2D by alcohol intakes of ≥15 g/d (median intake: 23.9 d/d).
Both the quantity and the quality of the carbohydrate consumed affect the development of insulin resistance and T2D (4). Although GI quantifies the glycemic response to carbohydrates in specific foods (37), GL, which is the product of GI of a certain food by its carbohydrate content, represents the interaction between the quantity and the quality of carbohydrate. Alcohol metabolizes differently from carbohydrates, and its consumption might attenuate the adverse effects of high-GL foods on T2D risk by delaying the insulin-glucose responses. This lower postprandial blood glucose release requires less insulin quantity to clear blood glucose, which results in more insulin receptors and better insulin sensitivity. Lower fasting insulin concentrations and increased insulin sensitivity with chronic moderate alcohol consumption have been reported in international studies (38–40) and in subgroups of US young adults (41) and postmenopausal women (25). Moderate alcohol has also been suggested to inhibit the undesirable hepatic gluconeogeneis (42) by increasing the ratio of NADH:NAD+ within the liver cell (ie, by reducing NAD+ to NADH) because of the oxidation of alcohol to acetate, which would inhibit the citric acid cycle activity and the β-oxidation of fatty acids (43). Moreover, moderate alcohol has been suggested to affect carbohydrate metabolism in a variety of ways, including improved insulin sensitivity (44), reduced glycogenolysis (43), and fat oxidation (45), as well as altering the hormonal response (eg, glucagon and growth hormones) to hypoglycemia (46). Both reduced gluconeogenesis and glycogenolysis could lead to reactive hypoglycemia in rare cases dependent on the carbohydrate nature, the individual characteristics, and whether alcohol was consumed without food while glycogen stores were depleted (43). Although this effect suggests some stimulation of the pancreatic β cells by alcohol, other studies did not observe any increase in insulin or C-peptide after alcohol consumption in both normal and T2D subjects, which suggested that the β cell function was unaffected (47, 48). The association between GI and diabetes was not significantly modified by alcohol intake. Although GI is a marker of carbohydrate quality, GL represents both the quality (GI) and quantity (weight) of carbohydrates; thus, it is a better predictor of postprandial blood glucose response and insulin demand (49) and a better reflection of the glycemic burden of the overall diet. Indeed, as previously considered, neither GI (carbohydrate quality) nor total carbohydrate amount in isolation provides an accurate measure of insulin demand (14, 50). Nonetheless, both dietary GI and GL are significantly associated with increased risk of diabetes, independent of alcohol intake and other dietary factors.
Our study had notable strengths, including a large sample size, prospective design, high follow-up rates, repeated assessment of dietary and lifestyle information, and ability to control for the potential confounders. Further, to minimize the recall bias, we not only excluded, at baseline, participants with a major history of chronic diseases but also stopped updating dietary intakes after participants reported being diagnosed with certain chronic diseases that might have influenced their subsequent report of diet.
Several limitations warrant consideration. First, we did not have detailed data on alcohol drinking patterns to assess whether alcohol was always consumed with a meal in which it would be most effective at blunting GL. However, if anything, this would only add random error to our results and bias the interaction toward the null. Second, residual confounding is always a concern in observational studies; however, we controlled for known and suspected risk factors of T2D in our multivariate models. Third, because our study population predominantly consisted of female nurses with European ancestry, our results may not be generalizable to men or other ethnic groups. Fourth, in our analysis, BMI was updated every 2 y, and height was assessed at baseline, although it is likely to be reduced at older age and lead to an underestimate of BMI. However, given that repeated measures of BMI were adjusted in the analysis, this would not have a major impact on our results. Fifth, although our FFQ was not initially designed to determine differences in GI of foods, it was designed to explain the variance in the quantity and quality of carbohydrates consumed (11). However, measurement error in the assessment of dietary GI and GL is inevitable, which may have attenuated the observed associations. Sixth, although we had information on the different alcoholic types consumed (ie, beer, wine, and spirits), we did not have enough power to stratify the analysis by alcohol types. Last, we did not confirm our diabetes cases with the standard oral glucose tolerance test because this is unfeasible in large cohorts. However, the self-reported diabetes cases were confirmed via supplementary questionnaires that have been shown to be highly accurate on the basis of medical record review (51, 52).
In conclusion, our findings suggest that a higher alcohol intake (≥15 g/d) attenuates the effect of GL on T2D incidence. Additional studies are needed to confirm our findings in other populations and elucidate potential mechanisms.
Acknowledgments
The authors’ responsibilities were as follows—RAM: designed and conducted the analysis and wrote the manuscript; EBR, EG, MJS, and WCW: obtained funding, managed and conducted the cohort, and edited the manuscript; DSL: conceived the idea for the analysis, helped interpret the data, and edited the manuscript; and FBH: designed the study, helped interpret the data, edited the manuscript, obtained funding, and supervised the study. None of the authors declared a conflict of interest.
Footnotes
Abbreviations used: FFQ, food-frequency questionnaire; GI, glycemic index; GL, glycemic load; NHS, Nurses’ Health Study; T2D, type 2 diabetes.
REFERENCES
- 1.Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care 2003;26:917–32 [DOI] [PubMed] [Google Scholar]
- 2.Baliunas DO, Taylor BJ, Irving H, Roerecke M, Patra J, Mohapatra S, Rehm J. Alcohol as a risk factor for type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2009;32:2123–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care 2005;28:719–25 [DOI] [PubMed] [Google Scholar]
- 4.Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC. Glycemic index, glycemic load, and chronic disease risk–a meta-analysis of observational studies. Am J Clin Nutr 2008;87:627–37 [DOI] [PubMed] [Google Scholar]
- 5.McMonagle J, Felig P. Effects of ethanol ingestion on glucose tolerance and insulin secretion in normal and diabetic subjects. Metabolism 1975;24:625–32 [DOI] [PubMed] [Google Scholar]
- 6.Dalgaard M, Thomsen C, Rasmussen BM, Holst JJ, Hermansen K. Ethanol with a mixed meal decreases the incretin levels early postprandially and increases postprandial lipemia in type 2 diabetic patients. Metabolism 2004;53:77–83 [DOI] [PubMed] [Google Scholar]
- 7.Svartberg J, Holst JJ, Gutniak M, Adner N. The ethanol augmentation of glucose-induced insulin secretion is abolished by calcium antagonism with nifedipine: no evidence for a role of glucagon-like peptide-1 (GLP-1). Pancreas 1998;16:66–71 [DOI] [PubMed] [Google Scholar]
- 8.Fielding BA, Reid G, Grady M, Humphreys SM, Evans K, Frayn KN. Ethanol with a mixed meal increases postprandial triacylglycerol but decreases postprandial non-esterified fatty acid concentrations. Br J Nutr 2000;83:597–604 [DOI] [PubMed] [Google Scholar]
- 9.Suter PM, Gerritsen-Zehnder M, Hasler E, Gurtler M, Vetter W, Hanseler E. Effect of alcohol on postprandial lipemia with and without preprandial exercise. J Am Coll Nutr 2001;20:58–64 [DOI] [PubMed] [Google Scholar]
- 10.Raben A, Agerholm-Larsen L, Flint A, Holst JJ, Astrup A. Meals with similar energy densities but rich in protein, fat, carbohydrate, or alcohol have different effects on energy expenditure and substrate metabolism but not on appetite and energy intake. Am J Clin Nutr 2003;77:91–100 [DOI] [PubMed] [Google Scholar]
- 11.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 12.Willett WC. Nutritional epidemiology. New York, NY: Oxford University Press, 1998 [Google Scholar]
- 13.US Department of Agriculture Composition of foods - raw, processed, and prepared, 1963-1992. Agricultural Handbook No. 8 Series. Washington, DC: Department of Agriculture, Government Printing Office, 1993 [Google Scholar]
- 14.Salmerón J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 1997;277:472–7 [DOI] [PubMed] [Google Scholar]
- 15.de Munter JS, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med 2007;4:e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(suppl):1220S–8S [DOI] [PubMed] [Google Scholar]
- 17.Fuchs CS, Stampfer MJ, Colditz GA, Giovannucci EL, Manson JE, Kawachi I, Hunter DJ, Hankinson SE, Hennekens CH, Rosner B, et al. Alcohol consumption and mortality among women. N Engl J Med 1995;332:1245–50 [DOI] [PubMed] [Google Scholar]
- 18.Giovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, Willett WC. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol 1991;133:810–7 [DOI] [PubMed] [Google Scholar]
- 19.Willett WC, Hennekens CH, Bain C, Rosner B, Speizer FE. Cigarette smoking and non-fatal myocardial infarction in women. Am J Epidemiol 1981;113:575–82 [DOI] [PubMed] [Google Scholar]
- 20.Williamson DA, Rejeski J, Lang W, Van Dorsten B, Fabricatore AN, Toledo K. Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med 2009;169:163–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagberg LA, Lindholm L. Cost-effectiveness of healthcare-based interventions aimed at improving physical activity. Scand J Public Health 2006;34:641–53 [DOI] [PubMed] [Google Scholar]
- 22.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 2001;161:1542–8 [DOI] [PubMed] [Google Scholar]
- 23.Stampfer MJ, Colditz GA, Willett WC, Manson JE, Arky RA, Hennekens CH, Speizer FE. A prospective study of moderate alcohol drinking and risk of diabetes in women. Am J Epidemiol 1988;128:549–58 [DOI] [PubMed] [Google Scholar]
- 24.Goude D, Fagerberg B, Hulthe J. Alcohol consumption, the metabolic syndrome and insulin resistance in 58-year-old clinically healthy men (AIR study). Clin Sci (Lond) 2002;102:345–52 [DOI] [PubMed] [Google Scholar]
- 25.Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women:a randomized controlled trial. JAMA 2002;287:2559–62 [DOI] [PubMed] [Google Scholar]
- 26.Bantle AE, Thomas W, Bantle JP. Metabolic effects of alcohol in the form of wine in persons with type 2 diabetes mellitus. Metabolism 2008;57:241–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shai I, Wainstein J, Harman-Boehm I, Raz I, Fraser D, Rudich A, Stampfer MJ. Glycemic effects of moderate alcohol intake among patients with type 2 diabetes: a multicenter, randomized, clinical intervention trial. Diabetes Care 2007;30:3011–6 [DOI] [PubMed] [Google Scholar]
- 28.Marfella R, Cacciapuoti F, Siniscalchi M, Sasso FC, Marchese F, Cinone F, Musacchio E, Marfella MA, Ruggiero L, Chiorazzo G, et al. Effect of moderate red wine intake on cardiac prognosis after recent acute myocardial infarction of subjects with Type 2 diabetes mellitus. Diabet Med 2006;23:974–81 [DOI] [PubMed] [Google Scholar]
- 29.Davies MJ. Insulin secretagogues. Curr Med Res Opin 2002;18(suppl 1):s22–30 [DOI] [PubMed] [Google Scholar]
- 30.Joosten MM, Beulens JW, Kersten S, Hendriks HF. Moderate alcohol consumption increases insulin sensitivity and ADIPOQ expression in postmenopausal women: a randomised, crossover trial. Diabetologia 2008;51:1375–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imhof A, Plamper I, Maier S, Trischler G, Koenig W. Effect of drinking on adiponectin in healthy men and women: a randomized intervention study of water, ethanol, red wine, and beer with or without alcohol. Diabetes Care 2009;32:1101–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beulens JW, van Loon LJ, Kok FJ, Pelsers M, Bobbert T, Spranger J, Helander A, Hendriks HF. The effect of moderate alcohol consumption on adiponectin oligomers and muscle oxidative capacity: a human intervention study. Diabetologia 2007;50:1388–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beulens JW, Rimm EB, Hu FB, Hendriks HF, Mukamal KJ. Alcohol consumption, mediating biomarkers, and risk of type 2 diabetes among middle-aged women. Diabetes Care 2008;31:2050–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sierksma A, Patel H, Ouchi N, Kihara S, Funahashi T, Heine RJ, Grobbee DE, Kluft C, Hendriks HF. Effect of moderate alcohol consumption on adiponectin, tumor necrosis factor-alpha, and insulin sensitivity. Diabetes Care 2004;27:184–9 [DOI] [PubMed] [Google Scholar]
- 35.Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, Okamoto Y, Ohashi K, Nagaretani H, Kishida K, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation 2003;107:671–4 [DOI] [PubMed] [Google Scholar]
- 36.Hosaka S, Miyashita M, Inoue J, Maruyama K. The short-term effect of alcoholic beverage-intake on blood glucose levels in type 2 diabetic patients. Diabetes Res Clin Pract 2008;79:183–4 [DOI] [PubMed] [Google Scholar]
- 37.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 1981;34:362–6 [DOI] [PubMed] [Google Scholar]
- 38.Razay G, Heaton KW. Moderate alcohol consumption has been shown previously to improve insulin sensitivity in men. Br Med J 1997;314:443–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiechl S, Willeit J, Poewe W, Egger G, Oberhollenzer F, Muggeo M, Bonora E. Insulin sensitivity and regular alcohol consumption: large, prospective, cross sectional population study (Bruneck study). BMJ 1996;313:1040–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konrat C, Mennen LI, Caces E, Lepinay P, Rakotozafy F, Forhan A, Balkau B. Alcohol intake and fasting insulin in French men and women. The D.E.S.I.R. Study. Diabetes Metab 2002;28:116–23 [PubMed] [Google Scholar]
- 41.Flanagan DE, Moore VM, Godsland IF, Cockington RA, Robinson JS, Phillips DI. Alcohol consumption and insulin resistance in young adults. Eur J Clin Invest 2000;30:297–301 [DOI] [PubMed] [Google Scholar]
- 42.Siler SQ, Neese RA, Christiansen MP, Hellerstein MK. The inhibition of gluconeogenesis following alcohol in humans. Am J Physiol 1998;275:E897–907 [DOI] [PubMed] [Google Scholar]
- 43.van de Wiel A. Diabetes mellitus and alcohol. Diabetes Metab Res Rev 2004;20:263–7 [DOI] [PubMed] [Google Scholar]
- 44.Greenfield JR, Samaras K, Jenkins AB, Kelly PJ, Spector TD, Campbell LV. Moderate alcohol consumption, estrogen replacement therapy, and physical activity are associated with increased insulin sensitivity: is abdominal adiposity the mediator? Diabetes Care 2003;26:2734–40 [DOI] [PubMed] [Google Scholar]
- 45.Avogaro A, Valerio A, Miola M, Crepaldi C, Pavan P, Tiengo A, del Prato S. Ethanol impairs insulin-mediated glucose uptake by an indirect mechanism. J Clin Endocrinol Metab 1996;81:2285–90 [DOI] [PubMed] [Google Scholar]
- 46.Flanagan D, Wood P, Sherwin R, Debrah K, Kerr D. Gin and tonic and reactive hypoglycemia: what is important-the gin, the tonic, or both? J Clin Endocrinol Metab 1998;83:796–800 [DOI] [PubMed] [Google Scholar]
- 47.Avogaro A, Watanabe RM, Gottardo L, de Kreutzenberg S, Tiengo A, Pacini G. Glucose tolerance during moderate alcohol intake: insights on insulin action from glucose/lactate dynamics. J Clin Endocrinol Metab 2002;87:1233–8 [DOI] [PubMed] [Google Scholar]
- 48.Christiansen C, Thomsen C, Rasmussen O, Hauerslev C, Balle M, Hansen C, Hermansen K. Effect of alcohol on glucose, insulin, free fatty acid and triacylglycerol responses to a light meal in non-insulin-dependent diabetic subjects. Br J Nutr 1994;71:449–54 [DOI] [PubMed] [Google Scholar]
- 49.Vaughan L. Dietary guidelines for the management of diabetes. Nurs Stand 2005;19:56–64, quiz 66 [DOI] [PubMed] [Google Scholar]
- 50.Salmerón J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, Stampfer MJ, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of NIDDM in Men. Diabetes Care 1997;20:545–50 [DOI] [PubMed] [Google Scholar]
- 51.Eriksson MK, Franks PW, Eliasson M. A 3-year randomized trial of lifestyle intervention for cardiovascular risk reduction in the primary care setting: the Swedish Bjorknas study. PLoS ONE 2009;4:e5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337–43 [DOI] [PubMed] [Google Scholar]