Abstract
Background: Colorectal cancer has a natural history of several decades; therefore, the diet consumed decades before diagnosis may aid in understanding this malignancy.
Objective: The objective was to investigate diet during adolescence and 10 y before baseline (ages 40–61 y) in relation to colorectal cancer.
Design: Participants in the NIH-AARP Diet and Health Study (n = 292,797) completed a 124-item food-frequency questionnaire (FFQ) about diet in the past 12 mo and two 37-item FFQs about diet at ages 12–13 y and 10 y previously. Cox regression was used to estimate multivariate HRs and 95% CIs for colon (n = 2794) and rectal (n = 979) cancers within quintiles of exposures.
Results: Colon cancer risk was lower in the highest than in the lowest quintile of vitamin A (HR: 0.82; 95% CI: 0.72, 0.92) and vegetable (HR: 0.81, 0.70, 0.92) intakes during adolescence. Those in the highest intake category 10 y previously for calcium (HR: 0.83; 95% CI: 0.73, 0.94), vitamin A (HR: 0.81; 95% CI: 0.71, 0.92), vitamin C (HR: 0.83; 95% CI: 0.72, 0.95), fruit (HR: 0.84; 95% CI: 0.73, 0.97), and milk (HR: 0.78; 95% CI: 0.67, 0.90) had a lower risk of colon cancer, but a higher risk was observed for total fat (HR: 1.15; 95% CI: 1.01, 1.30), red meat (HR: 1.31; 95% CI: 1.12, 1.53), and processed meat (HR: 1.24; 95% CI: 1.06, 1.45). For rectal cancer, milk was inversely associated (HR: 0.75; 95% CI: 0.58, 0.96) with risk.
Conclusion: Adolescent and midlife diet may play a role in colorectal carcinogenesis.
INTRODUCTION
Colorectal cancer is the third most common cancer worldwide; thus strategies for prevention are of critical public health importance (1). Despite the predominating belief that diet plays an important role in the development of colorectal cancer, the literature has been largely inconclusive (2). However, the vast majority of epidemiologic research has investigated diets consumed within ∼10 y of diagnosis, with little research referencing diet earlier in life. Colorectal cancer is a multistep process with a natural history of several decades (3, 4); therefore, it is reasonable to hypothesize that diet earlier in life, rather than recent adult diet, may be informative in understanding this malignancy. A growing body of literature has documented a potential role for adolescent diet in the development of breast cancer, independent of diet consumed in recent adult life (5–7). Limited work has investigated early life (eg, childhood or adolescence), or mid-life (∼40–60 y of age) exposures in relation to colorectal carcinogenesis, although the available evidence suggests that transient caloric restriction in adolescence is associated with a lower risk of colorectal cancer after age 55 y (8). Furthermore, caloric restriction in adolescence or early adulthood is associated with a lower risk of developing a colorectal tumor characterized by the CpG island methylator phenotype (9), which suggests that adolescence or early adulthood may be a period susceptible to epigenetic modification. Only 2 epidemiologic studies have investigated early-life dietary exposures in relation to colorectal cancer, both of which were restricted to milk or dairy consumption and had mixed results (10, 11). Intakes of nutrients and specific food groups potentially influence the natural history of carcinogenesis; however, unlike severe caloric restriction, they provide a potentially achievable avenue for preventive dietary behavior. In this investigation, we used data from the NIH-AARP Diet and Health Study—a large prospective cohort—to examine the association between intake of nutrients and food groups at ages 12–13 y, diet 10 y before baseline, and risk of adenocarcinoma in the colon and rectum.
SUBJECTS AND METHODS
Study population
The NIH-AARP Diet and Health Study is a prospective cohort of men and women aged 50–71 y at recruitment from 6 US states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and 2 metropolitan areas (Atlanta, GA, and Detroit, MI). The study was described in detail previously (12). A self-administered baseline questionnaire regarding demographic and lifestyle characteristics, including diet in the previous 12 mo, was completed in 1995–1996 by a total of 617,119 individuals. A second questionnaire, hereafter called the risk factor questionnaire, was mailed to participants ∼6 months after completion of the baseline questionnaire; this questionnaire included abbreviated assessments of usual dietary intake 10 y previously (when participants were ∼40–61 y of age) and diet at ages 12–13 y. The risk factor questionnaire was returned by 334,907 individuals. For our analyses, we excluded individuals for whom either the baseline (n = 6959) or the risk factor questionnaire (n = 3424) was completed by proxy respondents, those with prevalent cancer at the administration of the baseline (n = 14,565) or risk factor (n = 4297) questionnaire, those who had a death only report for any cancer (n = 983), those with 0 person-years of follow-up (n = 19), and those in the extremes of energy intake (defined as more than twice the interquartile range of the Box-Cox logarithmic-transformed scale) for either diet in the previous 12 mo (n = 2334) or during ages 12–13 y (n = 595). The resulting cohort for our analysis included 292,797 participants (171,171 men and 121,626 women). The NIH-AARP Diet and Health Study was approved by the Special Studies Institutional Review Board of the US National Cancer Institute.
Cohort follow-up and case ascertainment
Cohort members were followed for change of address by using the US Postal Service. Periodic linkage of the cohort to the US Social Security Administration Death Master File, follow-up searches of the National Death Index, cancer registry linkage, questionnaire responses, and responses to other mailings were used to obtain vital status. We identified cancer cases through probabilistic linkage with state cancer registries. Our cancer registry ascertainment area was expanded beyond the 8 original states from which the cohort was recruited to include Texas and Arizona, where participants most commonly relocated to during follow-up. Approximately 5% of participants were lost to follow-up as a result of moving out of the 10 states. Our case ascertainment method, described elsewhere, indicates that ∼90% of cancers were identified through the cancer registries (13).
Colorectal cancer endpoints were defined by anatomic site and histologic code of the International Classification of Diseases for Oncology (14) and included codes C180-C189, C199, C209, and C260. We further classified cases as those in the colon (C180-189, C260) or rectum (C199, C209). We only included first primary diagnoses of adenocarcinoma; we excluded cases with unspecified histologies (n = 25), neuroendocrine tumors/carcinoids (n = 82), lymphomas (n = 25), sarcomas (n = 8), squamous cell carcinomas (n = 8), large cell carcinoma with rhabdoid phenotype (n = 1), cloacogenic carcinoma (n = 1), gastrinoma (n = 1), cribriform carcinoma (n = 1), and pigmented nevus (n = 2). Follow-up for these analyses began on the date the risk factor questionnaire was received and continued until censoring at the end of 2006 or when the participant moved out of the 10 state cancer registry areas, had a cancer diagnosis, or died, whichever came first.
Dietary assessment
Approximately 6 mo after the baseline questionnaire was completed, participants were asked to complete the risk factor questionnaire. This questionnaire included two 37-item food-frequency questionnaires (FFQs): one referencing diet during 12–13 y of age and the other referencing diet 10 y previously. Both 37-item FFQs began with introductory questions to help respondents focus on the time period of interest (15, 16); for example, for diet 10 y previously, the introductory statement in the questionnaire read as follows: “To help you focus on that period of time, this was during President Reagan's second term in office, and the following landmark events took place: the space shuttle Challenger disaster, the Iran-Contra hearing, and the Wall Street stock market crash.” In addition, both 37-item FFQs asked questions to help participants focus on the time period of interest, such as “what year was it?,” “where were you living (city and street)?,” “with whom were you living?,” “what school grade were you in?” (FFQ for 12-13 y of age, only), and “were you working outside the home?” (only for FFQ for 10 y previously). The 37-food items included in the FFQ were selected because of their major contribution to sources of fat, vitamin A, and vitamin C, which at the time of FFQ development were the nutrients hypothesized to be of most interest in carcinogenesis; in addition to these nutrients, period-relevant databases allowed us to evaluate energy, carbohydrate, protein, and calcium. Participants were asked to select from 9 categories of frequency of consumption, ranging from “never” to “2 or more times per day.” Portion size was not ascertained in the 37-item FFQs, but was estimated by assigning the median sex-specific portion size from US Federal government food and nutrition surveys consistent with the time period being queried. For the FFQ referencing diet during 12–13 y of age, data from boys and girls aged 12–13 y who completed the US Department of Agriculture 1965–1966 Household Food Consumption Survey (HFCS)—the first nationwide food consumption survey of individuals—were used to assign portion size. The HFCS was also used to determine energy and nutrient values (with the exception of fiber) per 100-g serving. The HFCS did not include values for fiber. Fiber values from the NHANES 1999–2000 database were attributed to all 3 survey periods. The energy or nutrient values per 100-g serving were multiplied by the median sex-specific serving size and multiplied again by the midpoint value per day for each frequency category, with the exception of the open-ended highest category (“2 or more times per day”), for which we used median frequency of consumption among boys and girls in the 1965 HFCS who reported consuming that specific item ≥2 times/d. A similar method was used for the 37-item FFQ referencing diet 10 y before baseline; only portion sizes and energy/nutrient (carbohydrate, total fat protein, calcium, vitamins A and C) intakes were based on NHANES-III (17). In addition, participants completed a 124-item FFQ pertaining to diet in the previous 12 mo (referred to as recent adult diet) as part of the 1995–1996 baseline questionnaire. This FFQ was based on the National Cancer Institute's Diet History Questionnaire; participants were asked to select from 10 categories of frequency of consumption, ranging from “never” to “6+ times per day” and from 1 of 3 possible portion sizes. Data were linked with the 1994–1996 US Department of Agriculture's Continuing Survey of Food Intakes by Individuals to ascertain energy and nutrient intakes (18).
To investigate food groups from the 124-item FFQ of diet in the previous 12 mo, we calculated variables based on the US Department of Agriculture's MyPyramid Equivalents Database (19). Data for the 37-item FFQs were insufficient to calculate the MyPyramid Equivalents Database; thus, we defined food groups (grains, vegetables, fruit, milk, red meat, processed meat, solid fat, and sweet baked goods) and calculated food group values as the sum of the frequency of intake for each food item within the food group.
Statistical analysis
The HRs and 95% CIs were estimated by using Cox proportional hazards regression, with person-years as the underlying time metric; analyses that used age as the underlying time metric yielded nearly identical HRs. The proportional hazard assumption was verified by using a time interaction model. For the main analyses, participants were categorized into quintiles of intake (with one exception), and the lowest quintile of intake was used as the reference group. The exception to the formation of quintiles occurred in the analysis of milk consumption during ages 12–13 y. For this analysis, >50% of the study population consumed milk either “1 time per day” or “2 or more times per day.” Because of the large number of intake frequencies clustered at these values, it was impossible to create nearly evenly populated quintiles of intake. Thus, for milk consumption at 12–13 y of age, we created 5 categories of intake defined respectively as follows: “never” to “1–11 times per year” (7%), “1–3 times per month” to “3–4 times per week” (21%), “5–6 times per week” (15%), “1 time per day” (22%), and “2 or more times per day” (35%). Tests for trend were calculated by assigning a median value for each quintile of consumption or, in the case of adolescent milk consumption, an ordinal variable for the category of consumption. Spearman correlation coefficients between quintile/category of intake during 12–13 y of age and in the 12 mo before baseline as well as between diet 10 y before baseline and diet in the previous 12 mo were calculated to evaluate the similarity of dietary exposures over time.
To examine changes in consumption patterns over time, we divided participants into tertiles of nutrient or food group intake at 2 time points: adolescence and recent adulthood, defined as the 12 mo before baseline. Individuals who were in the lowest category of consumption at both time points were the referent group, and comparisons were made to individuals who were in the highest consumption group as adolescents but were in the lowest category as adults, individuals who were in the lowest category as adolescents but were in the highest category as adults, individuals who remained in the middle tertile for both time points or whose tertile placement changed by only one category from adolescence to adulthood, and individuals who were in the highest consumption group as adolescents and as adults. To maximize clarity, we present results only for individuals in the extreme tertiles of intake and do not present results from the group of individuals who remained in the middle tertile for both time points or whose tertile placement changed by only one category from adolescence to adulthood.
The final multivariate models contained only variables that changed the HR by ≥10% or were established risk factors for colorectal cancer. These variables included age, BMI, energy intake, smoking, physical activity, and use of nonsteroidal antiinflammatory drugs at the time of the risk factor questionnaire, alcohol consumption at study baseline, sex, race, education, and self-report of a first-degree relative with a history of colon cancer. Variables that were evaluated but did not alter the HR by ≥10% or more included personal history of diabetes or colorectal polyps, being 12–13 y of age during 1942–1946 (a period of food rationing in the United States), colorectal cancer screening practices, stage at diagnosis, age at menarche, or BMI at age 18 y. Data on father's occupation were available for 179,202 individuals, but inclusion of this variable in analyses of this subset of individuals did not materially alter the HRs.
To test for heterogeneity between the anatomic subsites (colon compared with rectum and proximal colon compared with distal colon), we calculated the weighted average of the 2 β coefficients from the Cox model, with weights being proportional to the inverse of the variances. We then calculated the following chi-square statistic with one df:
wherein 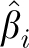 and
and 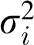 are the coefficient and its variance for each subsite and
are the coefficient and its variance for each subsite and 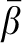 is the weighted average of the β coefficients. In addition, we conducted sensitivity analyses to examine the potential effects of preclinical disease on baseline diet by excluding the first 2 y of follow-up, and we stratified our analyses by sex. All statistical analyses were carried out by using Statistical Analytic Systems software version 9.1 (SAS Institute Inc).
is the weighted average of the β coefficients. In addition, we conducted sensitivity analyses to examine the potential effects of preclinical disease on baseline diet by excluding the first 2 y of follow-up, and we stratified our analyses by sex. All statistical analyses were carried out by using Statistical Analytic Systems software version 9.1 (SAS Institute Inc).
RESULTS
A total of 3773 cases of colorectal cancer (2480 in men and 1293 in women) were identified among the 292,797 eligible participants; of these, 2794 were located in the colon and 979 were in the rectum. Tests for heterogeneity did not indicate significant differences between proximal compared with distal colon tumors; therefore, results are presented only for colon and rectal cancer.
The study participants were predominately white (92.8%), and 58.5% were men. The mean age at administration of the risk factor questionnaire was 62.8 y. Those with cancer tended to be older, to consume more calories and alcohol, to be less educated, to be less physically active, and to not take nonsteroidal antiinflammatory drugs or hormone replacements as often as those without cancer (Table 1). Spearman correlation coefficients for adolescent and recent adult diet ranged from 0.09 for fat and protein to 0.30 for vegetables (see Supplemental Table 1 under “Supplemental data” in the online issue). Correlations among diet 10 y before baseline and recent adult diet were higher and ranged from 0.23 for protein to 0.54 for milk (see Supplemental Table 2 under “Supplemental data” in the online issue).
TABLE 1.
Distribution of covariates in the NIH-AARP Diet and Health Study (n = 292,797)
| Cohort(n = 292,797) | Colon cancer(n = 2794) | Rectal cancer(n = 979) | |
| Age (y) | 62.8 ± 5.31 | 64.7 ± 4.7 | 64.2 ± 4.9 |
| Sex, male [n (%)] | 171,171 (58.5) | 1799 (64.4) | 681 (70.0) |
| Married [n (%)] | 200,327 (68.4) | 1933 (69.2) | 700 (71.5) |
| First-degree relative with colon cancer [n (%)] | 26,367 (9.0) | 290 (10.4) | 72 (7.4) |
| BMI at baseline (kg/m2) | 26.9 (5.0) | 27.4 (5.0) | 27.3 (5.1) |
| Physically active at baseline, ≥5 times/wk [n (%)] | 58,862 (20.1) | 527 (18.9) | 185 (18.9) |
| Use of aspirin in previous 12 mo [n (%)] | 212,604 (73.3) | 1991 (72.3) | 690 (71.0) |
| Use of ibuprofen in previous 12 mo [n (%)] | 163,660 (56.6) | 1354 (49.1) | 486 (50.2) |
| Use of hormone replacement therapy, ever [n (%)]2 | 66,698 (54.8) | 426 (42.8) | 133 (44.6) |
| College graduate [n (%)] | 121,503 (41.5) | 1055 (37.8) | 325 (33.2) |
| Race [n (%)] | |||
| White | 271,813 (92.8) | 2602 (93.1) | 915 (93.5) |
| Black | 9194 (3.1) | 84 (3.0) | 20 (2.0) |
| Other3 | 11,790 (4.0) | 108 (3.9) | 44 (4.5) |
| Tobacco smoking [n (%)] | |||
| Never | 105,305 (36.0) | 884 (31.7) | 265 (27.1) |
| Former | 141,436 (48.3) | 1,489 (53.3) | 546 (55.8) |
| Current | 36,699 (12.5) | 334 (11.9) | 127 (13.0) |
| Total energy (kcal/d) | 1819 ± 771 | 1870 ± 819 | 1933 ± 842 |
| Alcohol intake at baseline (g/d) | 12.4 ± 30.9 | 14.8 ± 35.7 | 18.0 ± 42.0 |
Mean ± SD (all such values).
Among females only.
Includes Hispanic, Asian, Pacific-Islander, American Indian, Alaskan Native, and unknown.
Consumption of specific macronutrients (carbohydrate, total fat, or protein) during ages 12–13 y of age was not associated with either colon or rectal cancer, although a greater risk of rectal cancer was observed among individuals in the highest compared with the lowest quintile of fiber intake (Table 2). Individuals in the highest, compared with the lowest, quintile of calcium (HR: 0.85; 95% CI: 0.75, 0.95; P-trend = 0.03) and those in the highest quintile of vitamin A during ages 12–13 y (HR = 0.80; 95% CI: 0.71, 0.90; P-trend < 0.01) had a lower risk of colon cancer; although the association for calcium was attenuated after adjustment for adult intake (Table 2). No statistically significant associations were detected for calcium or vitamin A and rectal cancer or between vitamin C consumption and colon or rectal cancer. Tests for heterogeneity were significant only for the association of vitamin A and colon compared with rectal cancer (P-heterogeneity < 0.01).
TABLE 2.
Multivariate risks for the association between intakes of energy and nutrients at ages 12–13 y and colorectal cancer in the NIH-AARP Diet and Health Study (n = 292,797)
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-trend1 | |
| Carbohydrate | ||||||
| Median (g/1000 kcal) | 71 | 81 | 88 | 96 | 109 | – |
| Colon cancer (no. of cases) | 534 | 526 | 535 | 591 | 608 | – |
| Rectal cancer (no. of cases) | 188 | 205 | 209 | 180 | 197 | – |
| Colon2 | 1.00 | 0.98 (0.87, 1.11)3 | 0.99 (0.87, 1.12) | 1.06 (0.94, 1.19) | 1.04 (0.92, 1.17) | 0.30 |
| Colon + recent adult intake4 | 1.00 | 0.99 (0.88, 1.12) | 1.01 (0.89, 1.14) | 1.08 (0.96, 1.22) | 1.07 (0.95, 1.21) | 0.13 |
| Rectal2 | 1.00 | 1.10 (0.90, 1.35) | 1.13 (0.93, 1.38) | 0.98 (0.79, 1.20) | 1.03 (0.84, 1.27) | 0.84 |
| Rectal + recent adult intake4 | 1.00 | 1.11 (0.91, 1.36) | 1.15 (0.94, 1.40) | 1.00 (0.81, 1.23) | 1.07 (0.87, 1.32) | 0.82 |
| Total fat | ||||||
| Median (g/1000 kcal) | 46 | 51 | 54 | 57 | 61 | – |
| Colon cancer (no. of cases) | 596 | 587 | 527 | 540 | 544 | – |
| Rectal cancer (no. of cases) | 182 | 192 | 190 | 211 | 204 | – |
| Colon2 | 1.00 | 1.02 (0.91, 1.14) | 0.93 (0.82, 1.05) | 0.95 (0.84, 1.07) | 0.95 (0.84, 1.07) | 0.22 |
| Colon + recent adult intake4 | 1.00 | 1.01 (0.90, 1.14) | 0.92 (0.81, 1.03) | 0.94 (0.83, 1.06) | 0.93 (0.82, 1.05) | 0.12 |
| Rectal2 | 1.00 | 1.09 (0.88, 1.33) | 1.04 (0.84, 1.28) | 1.14 (0.93, 1.40) | 1.05 (0.85, 1.29) | 0.57 |
| Rectal + recent adult intake4 | 1.00 | 1.07 (0.87, 1.32) | 1.02 (0.83, 1.26) | 1.11 (0.91, 1.37) | 1.02 (0.83, 1.26) | 0.74 |
| Protein | ||||||
| Median (g/1000 kcal) | 35 | 39 | 41 | 43 | 47 | – |
| Colon cancer (no. of cases) | 577 | 582 | 565 | 540 | 530 | – |
| Rectal cancer (no. of cases) | 206 | 184 | 183 | 213 | 193 | – |
| Colon2 | 1.00 | 1.05 (0.93, 1.18) | 1.04 (0.93, 1.17) | 1.01 (0.90, 1.14) | 1.00 (0.89, 1.13) | 0.82 |
| Colon + recent adult intake4 | 1.00 | 1.06 (0.94, 1.19) | 1.05 (0.93, 1.18) | 1.02 (0.90, 1.15) | 1.01 (0.90, 1.14) | 0.93 |
| Rectal2 | 1.00 | 0.93 (0.76, 1.14) | 0.92 (0.75, 1.13) | 1.11 (0.92, 1.35) | 1.00 (0.82, 1.23) | 0.50 |
| Rectal + recent adult intake4 | 1.00 | 0.91 (0.74, 1.11) | 0.91 (0.74, 1.11) | 1.10 (0.91, 1.33) | 0.99 (0.81, 1.22) | 0.58 |
| Fiber | ||||||
| Median (g/1000 kcal) | 4.5 | 5.8 | 6.9 | 8.2 | 10.8 | – |
| Colon cancer (no. of cases) | 521 | 561 | 534 | 570 | 608 | – |
| Rectal cancer (no. of cases) | 154 | 182 | 223 | 207 | 213 | – |
| Colon2 | 1.00 | 1.05 (0.93, 1.18) | 0.95 (0.85, 1.08) | 0.99 (0.88, 1.12) | 1.02 (0.90, 1.15) | 0.99 |
| Colon + recent adult intake4 | 1.00 | 1.06 (0.94, 1.20) | 0.97 (0.86, 1.10) | 1.01 (0.90, 1.15) | 1.05 (0.93, 1.19) | 0.61 |
| Rectal2 | 1.00 | 1.16 (0.93, 1.44) | 1.38 (1.12, 1.70) | 1.24 (1.00, 1.53) | 1.27 (1.03, 1.57) | 0.06 |
| Rectal + recent adult intake4 | 1.00 | 1.16 (0.94, 1.44) | 1.39 (1.13, 1.72) | 1.25 (1.01, 1.55) | 1.29 (1.04, 1.60) | 0.04 |
| Calcium | ||||||
| Median (mg/1000 kcal) | 262 | 356 | 442 | 582 | 746 | – |
| Colon cancer (no. of cases) | 632 | 546 | 547 | 553 | 516 | – |
| Rectal cancer (no. of cases) | 217 | 197 | 200 | 179 | 186 | – |
| Colon2 | 1.00 | 0.87 (0.77, 0.98) | 0.87 (0.77, 0.97) | 0.88 (0.79, 0.99) | 0.85 (0.75, 0.95) | 0.03 |
| Colon + recent adult intake4 | 1.00 | 0.88 (0.79, 1.00) | 0.90 (0.80, 1.01) | 0.93 (0.83, 1.04) | 0.90 (0.80, 1.02) | 0.28 |
| Rectal2 | 1.00 | 0.90 (0.74, 1.10) | 0.92 (0.76, 1.12) | 0.86 (0.71, 1.05) | 0.92 (0.75, 1.12) | 0.37 |
| Rectal + recent adult intake4 | 1.00 | 0.92 (0.75, 1.11) | 0.94 (0.77, 1.15) | 0.88 (0.72, 1.08) | 0.95 (0.78, 1.16) | 0.59 |
| Vitamin A | ||||||
| Median (IU/1000 kcal) | 1556 | 2012 | 2404 | 2897 | 3992 | – |
| Colon cancer (no. of cases) | 606 | 569 | 515 | 578 | 526 | – |
| Rectal cancer (no. of cases) | 174 | 193 | 199 | 211 | 202 | – |
| Colon2 | 1.00 | 0.92 (0.82, 1.03) | 0.82 (0.73, 0.93) | 0.91 (0.81, 1.02) | 0.80 (0.71, 0.90) | <0.01 |
| Colon + recent adult intake4 | 1.00 | 0.93 (0.82, 1.04) | 0.83 (0.74, 0.94) | 0.93 (0.83, 1.04) | 0.82 (0.72, 0.92) | <0.01 |
| Rectal2 | 1.00 | 1.13 (0.92, 1.39) | 1.15 (0.94, 1.41) | 1.23 (1.01, 1.51) | 1.16 (0.95, 1.43) | 0.18 |
| Rectal + recent adult intake4 | 1.00 | 1.13 (0.92, 1.39) | 1.16 (0.94, 1.42) | 1.25 (1.01, 1.53) | 1.19 (0.96, 1.47) | 0.12 |
| Vitamin C | ||||||
| Median (mg/1000 kcal) | 17 | 25 | 36 | 54 | 84 | – |
| Colon cancer (no. of cases) | 616 | 564 | 551 | 531 | 532 | – |
| Rectal cancer (no. of cases) | 193 | 224 | 205 | 197 | 160 | – |
| Colon2 | 1.00 | 0.91 (0.82, 1.03) | 0.92 (0.82, 1.03) | 0.93 (0.83, 1.05) | 0.93 (0.83, 1.05) | 0.46 |
| Colon + recent adult intake4 | 1.00 | 0.92 (0.82, 1.04) | 0.94 (0.84, 1.06) | 0.95 (0.85, 1.07) | 0.96 (0.85, 1.08) | 0.85 |
| Rectal2 | 1.00 | 1.17 (0.96, 1.42) | 1.11 (0.91, 1.35) | 1.11 (0.90, 1.35) | 0.93 (0.75, 1.15) | 0.19 |
| Rectal + recent adult intake4 | 1.00 | 1.18 (0.97, 1.43) | 1.11 (0.91, 1.36) | 1.13 (0.92, 1.39) | 0.96 (0.77, 1.19) | 0.34 |
Linear test for trend derived by using the median value of each quintile.
Adjusted for energy at ages 12–13 y, age at completion of risk-factor questionnaire, sex, BMI, race, education, physical activity, alcohol consumption, smoking, use of nonsteroidal antiinflammatory drugs, use of hormone replacement therapy, and self-report of a first-degree relative with a history of colon cancer.
HR; 95% CI in parentheses (all such values); calculated by Cox proportional hazard regression models.
Adjusted for energy at ages 12–13 y, energy in recent adulthood, nutrient of interest in recent adulthood, age at completion of risk-factor questionnaire, sex, BMI, race, education, physical activity, alcohol consumption, smoking, use of nonsteroidal antiinflammatory drugs, use of hormone replacement therapy, and self-report of a first-degree relative with a history of colon cancer.
Examination of food groups consumed during ages 12–13 y showed a statistically significant reduction in colon cancer among individuals with high consumption of vegetables, and this association persisted after adjustment for recent adult vegetable intake (quintile 5 compared with quintile 1: HR = 0.81; 95% CI: 0.70, 0.92; P-trend = 0.01; Table 3). High milk consumption was also associated with a lower colon cancer risk, but this association was attenuated after adjustment for adult milk consumption. No associations were found between intake of any of the food groups examined at 12–13 y of age and rectal cancer (Table 3), and the test for heterogeneity of the association of vegetable intake with colon compared with rectal cancer was statistically significant (P-heterogeneity = 0.03).
TABLE 3.
Multivariate risks for the association between food group intake at ages 12–13 y and colorectal cancer in older adulthood in the NIH-AARP Diet and Health Study (n = 292,797)
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-trend1 | |
| Grains2 | ||||||
| Median (times consumed/d) | 0.30 | 0.79 | 1.00 | 1.07 | 2.02 | – |
| Colon cancer (no. of cases) | 565 | 536 | 478 | 513 | 702 | – |
| Rectal cancer (no. of cases) | 208 | 222 | 150 | 180 | 219 | – |
| Colon3 | 1.00 | 0.99 (0.86, 1.10)4 | 1.03 (0.91, 1.17) | 1.04 (0.91, 1.18) | 1.09 (0.96, 1.25) | 0.10 |
| Colon + recent adult intake5 | 1.00 | 0.99 (0.87, 1.12) | 1.05 (0.92, 1.19) | 1.05 (0.92, 1.20) | 1.11 (0.98, 1.27) | 0.07 |
| Rectal3 | 1.00 | 1.02 (0.84, 1.24) | 0.82 (0.66, 1.02) | 0.88 (0.70, 1.09) | 0.82 (0.66, 1.03) | 0.07 |
| Rectal + recent adult intake5 | 1.00 | 1.02 (0.84, 1.24) | 0.82 (0.66, 1.02) | 0.89 (0.71, 1.10) | 0.84 (0.67, 1.05) | 0.10 |
| Vegetables6 | ||||||
| Median (times consumed/d) | 0.39 | 0.82 | 1.20 | 1.66 | 2.57 | – |
| Colon cancer (no. of cases) | 662 | 529 | 548 | 563 | 492 | – |
| Rectal cancer (no. of cases) | 191 | 210 | 189 | 215 | 174 | – |
| Colon3 | 1.00 | 0.84 (0.75, 0.94) | 0.86 (0.76, 0.97) | 0.87 (0.77, 0.98) | 0.80 (0.70, 0.91) | 0.01 |
| Colon + recent adult intake5 | 1.00 | 0.85 (0.75, 0.95) | 0.87 (0.77, 0.98) | 0.88 (0.78, 1.00) | 0.81 (0.70, 0.92) | 0.01 |
| Rectal3 | 1.00 | 1.21 (0.99, 1.47) | 1.08 (0.88, 1.34) | 1.26 (1.02, 1.55) | 1.10 (0.87, 1.39) | 0.52 |
| Rectal + recent adult intake5 | 1.00 | 1.19 (0.98, 1.46) | 1.07 (0.86, 1.32) | 1.23 (1.00, 1.53) | 1.07 (0.84, 1.36) | 0.72 |
| Fruit7 | ||||||
| Median (times consumed/d) | 0.11 | 0.41 | 0.80 | 1.28 | 2.07 | – |
| Colon cancer (no. of cases) | 614 | 627 | 537 | 513 | 504 | – |
| Rectal cancer (no. of cases) | 212 | 229 | 180 | 197 | 161 | – |
| Colon3 | 1.00 | 0.99 (0.89, 1.11) | 0.99 (0.88, 1.11) | 0.99 (0.88, 1.12) | 0.94 (0.82, 1.08) | 0.36 |
| Colon + recent adult intake5 | 1.00 | 1.01 (0.90, 1.13) | 1.01 (0.89, 1.14) | 1.00 (0.88, 1.14) | 0.98 (0.85, 1.12) | 0.67 |
| Rectal3 | 1.00 | 1.05 (0.87, 1.28) | 0.97 (0.79, 1.19) | 1.11 (0.90, 1.38) | 0.91 (0.72, 1.15) | 0.49 |
| Rectal + recent adult intake5 | 1.00 | 1.07 (0.89, 1.30) | 1.00 (0.81, 1.23) | 1.16 (0.94, 1.43) | 0.97 (0.76, 1.23) | 0.90 |
| Milk8 | ||||||
| Median (times consumed/d) | 0 | 0.50 | 0.79 | 1.00 | 3.00 | – |
| Colon cancer (no. of cases) | 220 | 648 | 427 | 602 | 903 | – |
| Rectal cancer (no. of cases) | 68 | 219 | 139 | 237 | 316 | – |
| Colon3 | 1.00 | 1.00 (0.86, 1.15) | 0.89 (0.76, 1.06) | 0.86 (0.73, 1.01) | 0.84 (0.71, 0.99) | 0.04 |
| Colon + recent adult intake5 | 1.00 | 1.00 (0.86, 1.17) | 0.94 (0.79, 1.11) | 0.91 (0.77, 1.07) | 0.92 (0.78, 1.10) | 0.42 |
| Rectal3 | 1.00 | 1.03 (0.78, 1.36) | 0.91 (0.68, 1.23) | 1.05 (0.79, 1.40) | 0.94 (0.70, 1.26) | 0.43 |
| Rectal + recent adult intake5 | 1.00 | 1.07 (0.79, 1.38) | 0.93 (0.69, 1.26) | 1.08 (0.81, 1.43) | 0.99 (0.73, 1.34) | 0.72 |
| Red meat9 | ||||||
| Median (times consumed/d) | 0.31 | 0.68 | 1.01 | 1.36 | 1.99 | – |
| Colon cancer (no. of cases) | 596 | 552 | 550 | 559 | 537 | – |
| Rectal cancer (no. of cases) | 205 | 178 | 201 | 194 | 201 | – |
| Colon3 | 1.00 | 1.01 (0.89, 1.14) | 1.03 (0.91, 1.16) | 1.12 (0.98, 1.28) | 1.14 (0.97, 1.33) | 0.05 |
| Colon + recent adult intake5 | 1.00 | 0.99 (0.88, 1.12) | 1.00 (0.88, 1.14) | 1.08 (0.95, 1.24) | 1.09 (0.93, 1.28) | 0.14 |
| Rectal3 | 1.00 | 0.92 (0.74, 1.13) | 1.03 (0.83, 1.27) | 1.06 (0.84, 1.33) | 1.13 (0.87, 1.47) | 0.19 |
| Rectal + recent adult intake5 | 1.00 | 0.90 (0.73, 1.11) | 0.99 (0.80, 1.23) | 1.01 (0.81, 1.27) | 1.08 (0.83, 1.40) | 0.35 |
| Processed meat10 | ||||||
| Median (times consumed/d) | 0.1 | 0.35 | 0.58 | 0.93 | 1.35 | – |
| Colon cancer (no. of cases) | 570 | 451 | 606 | 647 | 520 | – |
| Rectal cancer (no. of cases) | 194 | 134 | 227 | 228 | 196 | – |
| Colon3 | 1.00 | 0.97 (0.85, 1.10) | 1.07 (0.94, 1.20) | 1.11 (0.98, 1.25) | 1.13 (0.98, 1.31) | 0.03 |
| Colon + recent adult intake5 | 1.00 | 0.96 (0.85, 1.09) | 1.05 (0.93, 1.19) | 1.08 (0.95, 1.23) | 1.11 (0.96, 1.28) | 0.06 |
| Rectal3 | 1.00 | 0.81 (0.65, 1.01) | 1.08 (0.88, 1.32) | 1.04 (0.84, 1.32) | 1.13 (0.89, 1.45) | 0.10 |
| Rectal + recent adult intake5 | 1.00 | 0.80 (0.64, 1.00) | 1.05 (0.86, 1.29) | 1.01 (0.82, 1.25) | 1.09 (0.85, 1.40) | 0.18 |
| Solid fat11 | ||||||
| Median (times consumed/d) | 0.21 | 0.57 | 1.00 | 1.07 | 2.00 | – |
| Colon cancer (no. of cases) | 522 | 769 | 647 | 232 | 624 | – |
| Rectal cancer (no. of cases) | 177 | 270 | 228 | 93 | 211 | – |
| Colon3 | 1.00 | 1.01 (0.90, 1.13) | 0.97 (0.85, 1.10) | 0.88 (0.74, 1.04) | 0.92 (0.80, 1.06) | 0.26 |
| Colon + recent adult intake5 | 1.00 | 1.01 (0.90, 1.13) | 0.96 (0.85, 1.09) | 0.87 (0.74, 1.03) | 0.92 (0.80, 1.05) | 0.31 |
| Rectal3 | 1.00 | 1.03 (0.85, 1.25) | 1.02 (0.83, 1.26) | 1.01 (0.77, 1.32) | 0.93 (0.77, 1.18) | 0.58 |
| Rectal + recent adult intake5 | 1.00 | 1.01 (0.83, 1.23) | 0.99 (0.80, 1.23) | 0.98 (0.74, 1.28) | 0.90 (0.71, 1.14) | 0.60 |
| Sweet baked goods12 | ||||||
| Median (times consumed/d) | 0.10 | 0.30 | 0.63 | 1.00 | 1.79 | – |
| Colon cancer (no. of cases) | 595 | 569 | 513 | 571 | 546 | – |
| Rectal cancer (no. of cases) | 220 | 193 | 171 | 214 | 181 | – |
| Colon3 | 1.00 | 0.92 (0.81, 1.03) | 0.95 (0.84, 1.08) | 0.94 (0.82, 1.07) | 1.02 (0.88, 1.18) | 0.41 |
| Colon + recent adult intake5 | 1.00 | 0.92 (0.82, 1.03) | 0.96 (0.85, 1.08) | 0.94 (0.83, 1.07) | 1.03 (0.89, 1.19) | 0.35 |
| Rectal3 | 1.00 | 0.80 (0.66, 0.98) | 0.81 (0.66, 1.00) | 0.90 (0.73, 1.10) | 0.82 (0.64, 1.06) | 0.48 |
| Rectal + recent adult intake5 | 1.00 | 0.81 (0.66, 0.99) | 0.82 (0.67, 1.01) | 0.91 (0.74, 1.12) | 0.84 (0.65, 1.07) | 0.55 |
Linear test for trend derived by using the median value of each quintile.
White bread or rolls, dark bread or rolls (rye, whole grain, whole wheat, and pumpernickel), and pizza.
Adjusted for energy at ages 12–13 y, age at completion of risk-factor questionnaire, sex, BMI, race, education, physical activity, alcohol consumption, smoking, use of aspirin and ibuprofen, use of hormone replacement therapy, and self-report of a first-degree relative with a history of colon cancer.
HR; 95% CI in parentheses (all such values); calculated by Cox proportional hazard regression models.
Adjusted for energy at ages 12–13 y, energy in recent adulthood, nutrient of interest in recent adulthood, age at completion of risk-factor questionnaire, sex, BMI, race, education, physical activity, alcohol consumption, smoking, use of aspirin and ibuprofen, use of hormone replacement therapy, and self-report of a first-degree relative with a history of colon cancer.
Broccoli, carrots, baked beans, lettuce salads, fresh tomato, tomato or vegetable soup, and other vegetables, including corn, peas, and green beans.
Fresh apples (not cooked), orange or grapefruit juice, oranges, grapefruit, tangerines, and canned fruit, such as peaches, pears, and applesauce.
Whole milk, including on cereals; categories of intake were assigned rather than quintiles because of clustering of intake frequency.
Ground beef, roast beef or steak, cold cuts, bacon or sausage, and hot dogs.
Bacon or sausage, cold cuts, and hot dogs.
Butter and margarine.
Cookies, cake, or donuts.
Investigation of diet 10 y before baseline, when participants were 40–61 y of age, showed a greater number of statistically significant associations with colon and rectal cancer than did diet at ages 12–13 y. After adjustment for recent adult diet, a statistically significant positive association between total fat consumption 10 y before baseline was detected for colon cancer (quintile 5 compared with quintile 1: HR= 1.15; 95% CI: 1.01, 1.30; P-trend = 0.02), but not for rectal cancer (Table 4), although the test for heterogeneity indicated that the risks were not statistically different (P-heterogeneity = 0.31). After adjustment for adult diet, no significant associations were detected for intake of carbohydrate or protein with colon or rectal cancer, although a borderline significant positive association between fiber and rectal cancer was observed (quintile 5 compared with quintile 1: HR: 1.26; 95% CI: 1.00, 1.58; P-trend = 0.06), but not for colon cancer (P-heterogeneity = 0.05). A lower risk of colon cancer was found for individuals in the highest quintile of calcium (HR: 0.83; 95% CI: 0.73; 0.94; P-trend < 0.01), vitamin A (HR: 0.81; 95% CI: 0.71, 0.92; P-trend = 0.03), and vitamin C (HR: 0.83; 95% CI: 0.72; 0.95, P-trend = 0.02) in the 10 y before baseline, and these associations remained after adjustment for recent adult diet; no associations were observed between these 3 nutrients and rectal cancer. A food group–based analysis of diet 10 y before baseline adjusted for recent adult consumption showed a statistically significant lower risk of colon cancer for those in the highest category of fruit (HR: 0.84; 95% CI: 0.73, 0.97; P-trend = 0.02) or milk (HR: 0.78; 95% CI: 0.67, 0.90; P-trend < 0.01), and a greater risk for those in the highest quintile of red meat intake (HR: 1.31; 95% CI: 1.12, 1.53; P-trend < 0.01) and processed meat (HR: 1.24; 95% CI: 1.06, 1.45; P-trend < 0.01) (Table 5). A reduced risk of rectal cancer was observed among individuals in the highest category of milk intake 10 y before baseline (HR: 0.75; 95% CI: 0.58, 0.96; P-trend = 0.05), whereas those in the highest quintile of grains had a borderline significantly greater rectal cancer risk, although the trend was not statistically significant (HR: 1.28; 95% CI: 1.00; 1.64, P-trend = 0.12). Tests for heterogeneity for the association between food groups and colon or rectal cancer were significant only for fruit (P-heterogeneity < 0.01).
TABLE 4.
Multivariate risks for the association between intake of energy and nutrients 10 y before baseline and colorectal cancer in the NIH-AARP Diet and Health Study (n = 295,845)1
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-trend2 | |
| Carbohydrate | ||||||
| Median (g/1000 kcal) | 85 | 98 | 106 | 115 | 129 | – |
| Colon cancer (no. of cases) | 575 | 545 | 543 | 588 | 568 | – |
| Rectal cancer (no. of cases) | 193 | 193 | 206 | 202 | 191 | – |
| Colon3 | 1.00 | 0.80 (0.80, 1.01)4 | 0.86 (0.77, 0.97) | 0.90 (0.80, 1.01) | 0.84 (0.74, 0.94) | <0.01 |
| Colon + recent adult intake5 | 1.00 | 0.92 (0.82, 1.04) | 0.90 (0.80, 1.02) | 0.95 (0.84, 1.07) | 0.89 (0.78, 1.01) | 0.13 |
| Rectal3 | 1.00 | 0.97 (0.80, 1.19) | 1.01 (0.83, 1.23) | 0.96 (0.79, 1.18) | 0.90 (0.73, 1.10) | 0.32 |
| Rectal + recent adult intake5 | 1.00 | 1.00 (0.81, 1.22) | 1.05 (0.86, 1.29) | 1.03 (0.84, 1.27) | 1.00 (0.81, 1.24) | 0.90 |
| Total fat | ||||||
| Median (g/1000 kcal) | 35 | 41 | 44 | 47 | 52 | – |
| Colon cancer (no. of cases) | 560 | 546 | 542 | 573 | 598 | – |
| Rectal cancer (no. of cases) | 179 | 205 | 215 | 196 | 192 | – |
| Colon3 | 1.00 | 0.99 (0.88, 1.12) | 1.02 (0.90, 1.15) | 1.10 (0.97, 1.24) | 1.20 (1.06, 1.35) | <0.01 |
| Colon + recent adult intake5 | 1.00 | 0.99 (0.88, 1.12) | 1.00 (0.88, 1.13) | 1.06 (0.94, 1.21) | 1.15 (1.01, 1.30) | 0.02 |
| Rectal3 | 1.00 | 1.13 (0.92, 1.38) | 1.21 (0.99, 1.48) | 1.11 (0.90, 1.36) | 1.09 (0.88, 1.34) | 0.47 |
| Rectal + recent adult intake5 | 1.00 | 1.09 (0.88, 1.34) | 1.15 (0.93, 1.41) | 1.03 (0.83, 1.28) | 1.00 (0.81, 1.25) | 0.89 |
| Protein | ||||||
| Median (g/1000 kcal) | 32 | 37 | 41 | 44 | 50 | – |
| Colon cancer (no. of cases) | 585 | 577 | 544 | 573 | 540 | – |
| Rectal cancer (no. of cases) | 192 | 189 | 195 | 197 | 212 | – |
| Colon3 | 1.00 | 1.00 (0.89, 1.12) | 0.95 (0.84, 1.07) | 1.01 (0.90, 1.13) | 0.94 (0.83, 1.06) | 0.34 |
| Colon + recent adult intake5 | 1.00 | 1.02 (0.90, 1.14) | 0.97 (0.86, 1.09) | 1.03 (0.92, 1.16) | 0.96 (0.85, 1.09) | 0.61 |
| Rectal3 | 1.00 | 0.99 (0.81, 1.22) | 1.03 (0.84, 1.26) | 1.02 (0.83, 1.25) | 1.08 (0.88, 1.32) | 0.43 |
| Rectal + recent adult intake5 | 1.00 | 0.98 (0.80, 1.21) | 1.02 (0.83, 1.25) | 1.01 (0.82, 1.24) | 1.06 (0.86, 1.30) | 0.56 |
| Fiber | ||||||
| Median (g/1000 kcal) | 5 | 7 | 8 | 10 | 13 | – |
| Colon cancer (no. of cases) | 593 | 562 | 556 | 569 | 539 | – |
| Rectal cancer (no. of cases) | 177 | 198 | 216 | 202 | 192 | – |
| Colon3 | 1.00 | 0.95 (0.84, 1.06) | 0.92 (0.82, 1.04) | 0.94 (0.83, 1.05) | 0.90 (0.80, 1.02) | 0.12 |
| Colon + recent adult intake5 | 1.00 | 0.97 (0.86, 1.09) | 0.97 (0.86, 1.09) | 0.99 (0.88, 1.12) | 0.97 (0.85, 1.10) | 0.75 |
| Rectal3 | 1.00 | 1.11 (0.91, 1.36) | 1.24 (1.01, 1.51) | 1.17 (0.96, 1.44) | 1.15 (0.93, 1.42) | 0.25 |
| Rectal + recent adult intake5 | 1.00 | 1.13 (0.92, 1.39) | 1.29 (1.05, 1.58) | 1.24 (1.01, 1.54) | 1.26 (1.00, 1.58) | 0.06 |
| Calcium | ||||||
| Median (mg/1000 kcal) | 259 | 325 | 393 | 483 | 610 | – |
| Colon cancer (no. of cases) | 661 | 575 | 578 | 483 | 522 | – |
| Rectal cancer (no. of cases) | 217 | 232 | 177 | 178 | 181 | – |
| Colon3 | 1.00 | 0.84 (0.75, 0.95) | 0.82 (0.73, 0.91) | 0.68 (0.61, 0.77) | 0.73 (0.65, 0.82) | <0.01 |
| Colon + recent adult intake5 | 1.00 | 0.88 (0.79, 0.99) | 0.87 (0.78, 0.98) | 0.75 (0.66, 0.85) | 0.83 (0.73, 0.94) | <0.01 |
| Rectal3 | 1.00 | 1.05 (0.87, 1.26) | 0.79 (0.64, 0.96) | 0.79 (0.65, 0.97) | 0.82 (0.67, 1.00) | <0.01 |
| Rectal + recent adult intake5 | 1.00 | 1.06 (0.88, 1.28) | 0.81 (0.66, 0.99) | 0.83 (0.67, 1.02) | 0.85 (0.69, 1.06) | 0.05 |
| Vitamin A | ||||||
| Median (IU/1000 kcal) | 1714 | 2492 | 3357 | 4683 | 8117 | – |
| Colon cancer (no. of cases) | 639 | 546 | 538 | 575 | 521 | – |
| Rectal cancer (no. of cases) | 203 | 224 | 189 | 172 | 197 | – |
| Colon3 | 1.00 | 0.83 (0.74, 0.93) | 0.82 (0.73, 0.93) | 0.88 (0.79, 0.99) | 0.79 (0.71, 0.92) | <0.01 |
| Colon + recent adult intake5 | 1.00 | 0.84 (0.75, 0.95) | 0.84 (0.75, 0.95) | 0.91 (0.81, 1.03) | 0.81 (0.71, 0.92) | 0.03 |
| Rectal3 | 1.00 | 1.10 (0.91, 1.33) | 0.96 (0.78, 1.17) | 0.87 (0.71, 1.08) | 1.02 (0.83, 1.24) | 0.70 |
| Rectal + recent adult intake5 | 1.00 | 1.09 (0.90, 1.32) | 0.96 (0.78, 1.17) | 0.88 (0.71, 1.09) | 1.05 (0.84, 1.30) | 0.96 |
| Vitamin C | ||||||
| Median (mg/1000 kcal) | 31 | 55 | 81 | 112 | 169 | – |
| Colon cancer (no. of cases) | 654 | 545 | 547 | 550 | 523 | – |
| Rectal cancer (no. of cases) | 214 | 223 | 183 | 186 | 179 | – |
| Colon3 | 1.00 | 0.85 (0.76, 0.95) | 0.84 (0.74, 0.94) | 0.83 (0.74, 0.93) | 0.78 (0.70, 0.88) | <0.01 |
| Colon + recent adult intake5 | 1.00 | 0.88 (0.78, 0.99) | 0.88 (0.78, 1.00) | 0.88 (0.77, 1.00) | 0.83 (0.72, 0.95) | 0.02 |
| Rectal3 | 1.00 | 1.08 (0.89, 1.30) | 0.99 (0.75, 1.11) | 0.91 (0.75, 1.11) | 0.90 (0.74, 1.11) | 0.13 |
| Rectal + recent adult intake5 | 1.00 | 1.09 (0.90, 1.33) | 0.92 (0.75, 1.14) | 0.95 (0.77, 1.18) | 0.96 (0.77, 1.21) | 0.48 |
A greater number of participants completed the food-frequency questionnaire about diet 10 y previously than about diet during ages 12–13 y. Thus, the n values for Tables 4 and 5 are greater than those for Tables 2 and 3.
Linear test for trend derived by using the median value of each quintile.
Adjusted for energy at ages 12–13 y, age at completion of risk-factor questionnaire, sex, BMI, race, education, physical activity, alcohol consumption, smoking, use of nonsteroidal antiinflammatory drugs, use of hormone replacement therapy, and self-report of a first-degree relative with a history of colon cancer.
HR; 95% CI in parentheses (all such values); calculated by Cox proportional hazard regression models.
Adjusted for energy at ages 12–13 y, energy in recent adulthood, nutrient of interest in recent adulthood, age at completion of risk-factor questionnaire, sex, BMI, race, education, physical activity, alcohol consumption, smoking, use of nonsteroidal antiinflammatory drugs, use of hormone replacement therapy, and self-report of a first-degree relative with a history of colon cancer.
TABLE 5.
Multivariate risks for the association between food group intake 10 y before baseline and colorectal cancer in older adulthood in the NIH-AARP Diet and Health Study (n = 295,845)1
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-trend2 | |
| Grains3 | ||||||
| Median (times consumed/d) | 0.25 | 0.58 | 0.87 | 1.08 | 2.07 | – |
| Colon cancer (no. of cases) | 566 | 518 | 614 | 524 | 597 | – |
| Rectal cancer (no. of cases) | 172 | 195 | 219 | 181 | 218 | – |
| Colon4 | 1.00 | 0.98 (0.87, 1.10)5 | 1.00 (0.88, 1.13) | 1.10 (0.96, 1.25) | 1.10 (0.96, 1.27) | 0.09 |
| Colon + recent adult intake6 | 1.00 | 0.99 (0.88, 1.12) | 1.02 (0.90, 1.15) | 1.13 (0.99, 1.29) | 1.14 (0.98, 1.31) | 0.05 |
| Rectal4 | 1.00 | 1.19 (0.97, 1.47) | 1.14 (0.92, 1.40) | 1.22 (0.95, 1.55) | 1.22 (0.95, 1.55) | 0.26 |
| Rectal + recent adult intake6 | 1.00 | 1.21 (0.98, 1.49) | 1.16 (0.94, 1.44) | 1.24 (0.98, 1.56) | 1.28 (1.00, 1.64) | 0.12 |
| Vegetables7 | ||||||
| Median (times consumed/d) | 0.53 | 0.99 | 1.42 | 1.98 | 2.85 | – |
| Colon cancer (no. of cases) | 641 | 561 | 518 | 546 | 553 | – |
| Rectal cancer (no. of cases) | 194 | 197 | 201 | 190 | 203 | – |
| Colon4 | 1.00 | 0.90 (0.80, 1.01) | 0.81 (0.72, 0.91) | 0.84 (0.75, 0.95) | 0.87 (0.77, 0.99) | 0.06 |
| Colon + recent adult intake6 | 1.00 | 0.91 (0.81, 1.02) | 0.82 (0.73, 0.93) | 0.86 (0.76, 0.98) | 0.88 (0.77, 1.01) | 0.13 |
| Rectal4 | 1.00 | 1.06 (0.86, 1.29) | 1.06 (0.87, 1.30) | 1.01 (0.82, 1.25) | 1.14 (0.92, 1.42) | 0.33 |
| Rectal + recent adult intake6 | 1.00 | 1.04 (0.85, 1.28) | 1.05 (0.86, 1.29) | 0.99 (0.80, 1.24) | 1.12 (0.88, 1.41) | 0.47 |
| Fruit8 | ||||||
| Median (times consumed/d) | 0.16 | 0.56 | 0.99 | 1.35 | 2.10 | – |
| Colon cancer (no. of cases) | 637 | 550 | 558 | 533 | 541 | – |
| Rectal cancer (no. of cases) | 209 | 208 | 200 | 183 | 185 | – |
| Colon4 | 1.00 | 0.88 (0.79, 0.99) | 0.86 (0.77, 0.97) | 0.80 (0.70, 0.90) | 0.82 (0.72, 0.92) | <0.01 |
| Colon + recent adult intake6 | 1.00 | 0.90 (0.80, 1.01) | 0.89 (0.78, 1.01) | 0.82 (0.72, 0.94) | 0.84 (0.73, 0.97) | 0.02 |
| Rectal4 | 1.00 | 1.03 (0.85, 1.26) | 0.98 (0.81, 1.20) | 0.88 (0.72, 1.08) | 0.91 (0.73, 1.12) | 0.17 |
| Rectal + recent adult intake6 | 1.00 | 1.07 (0.88, 1.31) | 1.05 (0.85, 1.29) | 0.97 (0.77, 1.21) | 1.05 (0.82, 1.34) | 0.96 |
| Milk9 | ||||||
| Median (times consumed/d) | 0.03 | 0.28 | 0.79 | 1.0 | 2.0 | – |
| Colon cancer (no. of cases) | 628 | 660 | 503 | 555 | 473 | – |
| Rectal cancer (no. of cases) | 218 | 227 | 186 | 182 | 172 | – |
| Colon4 | 1.00 | 0.85 (0.76, 0.95) | 0.81 (0.72, 0.92) | 0.73 (0.65, 0.82) | 0.70 (0.61, 0.79) | <0.01 |
| Colon + recent adult intake6 | 1.00 | 0.88 (0.79, 0.99) | 0.87 (0.76, 0.98) | 0.79 (0.69, 0.90) | 0.78 (0.67, 0.90) | <0.01 |
| Rectal4 | 1.00 | 0.83 (0.69, 1.00) | 0.87 (0.71, 1.06) | 0.69 (0.56, 0.85) | 0.74 (0.59, 0.92) | <0.01 |
| Rectal + recent adult intake6 | 1.00 | 0.81 (0.67, 0.99) | 0.85 (0.69, 1.06) | 0.68 (0.54, 0.85) | 0.75 (0.58, 0.96) | 0.05 |
| Red meat10 | ||||||
| Median (times consumed/d) | 0.18 | 0.43 | 0.66 | 0.96 | 1.49 | – |
| Colon cancer (no. of cases) | 516 | 556 | 571 | 552 | 624 | – |
| Rectal cancer (no. of cases) | 174 | 175 | 197 | 209 | 230 | – |
| Colon4 | 1.00 | 1.13 (1.00, 1.28) | 1.17 (1.03, 1.33) | 1.23 (1.08, 1.41) | 1.46 (1.26, 1.69) | <0.01 |
| Colon + recent adult intake6 | 1.00 | 1.09 (0.96, 1.24) | 1.10 (0.96, 1.25) | 1.14 (0.99, 1.31) | 1.31 (1.12, 1.53) | <0.01 |
| Rectal4 | 1.00 | 1.00 (0.81, 1.24) | 1.08 (0.88, 1.34) | 1.21 (0.97, 1.51) | 1.24 (0.97, 1.59) | 0.03 |
| Rectal + recent adult intake6 | 1.00 | 0.95 (0.76, 1.18) | 0.99 (0.79, 1.24) | 1.07 (0.85, 1.36) | 1.06 (0.81, 1.38) | 0.42 |
| Processed meat11 | ||||||
| Median (times consumed/d) | 0.05 | 0.15 | 0.30 | 0.53 | 1.02 | – |
| Colon cancer (no. of cases) | 411 | 576 | 571 | 617 | 644 | – |
| Rectal cancer (no. of cases) | 120 | 201 | 205 | 214 | 245 | – |
| Colon4 | 1.00 | 1.03 (0.90, 1.17) | 1.11 (0.98, 1.27) | 1.24 (1.09, 1.42) | 1.30 (1.13, 1.51) | <0.01 |
| Colon + recent adult intake6 | 1.00 | 1.02 (0.89, 1.16) | 1.09 (0.95, 1.25) | 1.20 (1.04, 1.39) | 1.24 (1.06, 1.45) | <0.01 |
| Rectal4 | 1.00 | 1.19 (0.95, 1.50) | 1.26 (1.00, 1.59) | 1.31 (1.03, 1.66) | 1.40 (1.09, 1.81) | 0.02 |
| Rectal + recent adult intake6 | 1.00 | 1.18 (0.93, 1.49) | 1.21 (0.95, 1.54) | 1.24 (0.96, 1.59) | 1.30 (0.99, 1.70) | 0.16 |
| Solid fat12 | ||||||
| Median (times consumed/d) | 0.08 | 0.50 | 0.79 | 1.00 | 2.00 | – |
| Colon cancer (no. of cases) | 563 | 631 | 521 | 545 | 559 | – |
| Rectal cancer (no. of cases) | 186 | 241 | 176 | 203 | 179 | – |
| Colon4 | 1.00 | 0.99 (0.88, 1.11) | 1.01 (0.89, 1.14) | 0.96 (0.85, 1.09) | 1.05 (0.92, 1.19) | 0.42 |
| Colon + recent adult intake6 | 1.00 | 0.98 (0.87, 1.10) | 0.99 (0.87, 1.12) | 0.94 (0.83, 1.07) | 1.01 (0.89, 1.15) | 0.80 |
| Rectal4 | 1.00 | 1.12 (0.92, 1.36) | 0.99 (0.80, 1.23) | 1.07 (0.87, 1.31) | 0.97 (0.78, 1.21) | 0.52 |
| Rectal + recent adult intake6 | 1.00 | 1.07 (0.88, 1.30) | 0.93 (0.75, 1.15) | 0.99 (0.80, 1.21) | 0.87 (0.69, 1.09) | 0.11 |
| Sweet baked goods13 | ||||||
| Median (times consumed/d) | 0.05 | 0.20 | 0.35 | 0.73 | 1.35 | – |
| Colon cancer (no. of cases) | 636 | 445 | 596 | 578 | 564 | – |
| Rectal cancer (no. of cases) | 228 | 167 | 190 | 203 | 197 | – |
| Colon4 | 1.00 | 0.89 (0.79, 1.01) | 1.04 (0.92, 1.17) | 0.99 (0.88, 1.11) | 1.02 (0.90, 1.15) | 0.47 |
| Colon + recent adult intake6 | 1.00 | 0.90 (0.79, 1.02) | 1.04 (0.93, 1.17) | 0.99 (0.88, 1.12) | 1.01 (0.89, 1.15) | 0.54 |
| Rectal4 | 1.00 | 0.93 (0.76, 1.13) | 0.89 (0.73, 1.07) | 0.92 (0.75, 1.10) | 0.92 (0.75, 1.10) | 0.61 |
| Rectal + recent adult intake6 | 1.00 | 0.94 (0.77, 1.16) | 0.91 (0.74, 1.11) | 0.94 (0.77, 1.15) | 0.93 (0.75, 1.15) | 0.66 |
A greater number of participants completed the food-frequency questionnaire about diet 10 y previously than about diet during ages 12–13 y. Thus, the n values for Tables 4 and 5 are greater than those for Tables 2 and 3.
Linear test for trend derived by using the median value of each quintile.
White bread or rolls, dark bread or rolls (rye, whole grain, whole wheat, and pumpernickel), and pizza.
Adjusted for energy at ages 12–13 y, age at completion of risk-factor questionnaire, sex, BMI, race, education, physical activity, alcohol consumption, smoking, use of aspirin and ibuprofen, use of hormone replacement therapy, and self-report of a first-degree relative with a history of colon cancer.
HR; 95% CI in parentheses (all such values); calculated by Cox proportional hazard regression models.
Adjusted for energy at ages 12–13 y, energy in recent adulthood, nutrient of interest in recent adulthood, age at completion of risk-factor questionnaire, sex, BMI, race, education, physical activity, alcohol consumption, smoking, use of aspirin and ibuprofen, use of hormone replacement therapy, and self-report of a first-degree relative with a history of colon cancer.
Broccoli, carrots, baked beans, lettuce salads, fresh tomato, tomato or vegetable soup, and other vegetables, including corn, peas, and green beans.
Fresh apples (not cooked), orange or grapefruit juice, oranges, grapefruit, tangerines, and canned fruit such as peaches, pears, and applesauce.
Whole milk, including on cereals; categories of intake were assigned rather than quintiles because of clustering of intake frequency.
Ground beef, roast beef or steak, cold cuts, bacon or sausage, and hot dogs.
Bacon or sausage, cold cuts, and hot dogs.
Butter and margarine.
Cookies, cake, or donuts.
Results excluding cases accrued in the first 2 y of follow-up were not materially different from the overall study population. We found no significant interactions by sex, except for dietary fiber and colon cancer for adolescent diet (P < 0.001) and diet 10 y before baseline (P < 0.001). Stratified analyses of fiber intake by sex showed a positive association among women in the highest quintile of fiber intake during adolescence (HR: 1.29; 95% CI: 1.05, 1.59), but a nonsignificant inverse association among men (HR: 0.95; 95% CI: 0.82, 1.11). However, for diet 10 y before baseline, this association was nonsignificantly greater in women (HR: 1.15; 95% CI: 0.92, 1.43) but significantly lower in men (HR: 0.78; 95% CI: 0.66, 0.93).
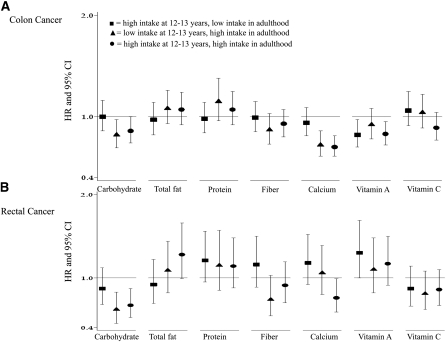
In an effort to determine how change in consumption patterns over time affect colon and rectal cancer risk, we examined the combinations of tertiles of intake at age 12–13 y and baseline in 1995 when the participants were 50–71 y of age. The percentage of individuals belonging to the various tertile combinations ranged from a low of 6.6% (for participants in the highest tertile of consumption of calories, fat, or vegetables in adolescence but in the lowest tertile of consumption for these dietary factors in recent adulthood) to 22.8% of individuals who were high milk consumers during both adolescence and recent adulthood). Relative to individuals who were in the lowest tertile of consumption at both time points, colon cancer risk was lower for those in the highest tertile of consumption at both time points for intakes of carbohydrate (HR: 0.86; 95% CI: 0.74, 1.00), calcium (HR: 0.70; 95% CI: 0.61, 0.81), and vitamin A (HR: 0.83; 95% CI: 0.72, 0.95) (Figure 1A). A significant inverse association with vitamin A consumption was also observed when high consumption occurred only in adolescence, and significant inverse associations between consumption of carbohydrate or calcium and colon cancer were observed when high consumption occurred in recent adult diet. Similar to the findings for colon cancer, a lower risk of rectal cancer was observed among individuals in the highest tertiles of consumption at both time points for carbohydrate (HR: 0.67; 95% CI: 0.52, 0.87) and calcium (HR: 0.76; 95% CI: 0.59, 0.99) (Figure 1B). Although a significant inverse relation with rectal cancer was observed among individuals who were high carbohydrate consumers (recent adult diet), a significant inverse association between high calcium intake and rectal cancer was observed only among individuals who were high consumers at both time points.
FIGURE 1.
HRs and 95% CIs for colon cancer (A) and rectal cancer (B) by change in tertiles of nutrient intakes from adolescence to recent adulthood relative to individuals in the lowest tertile of intake at both time points.
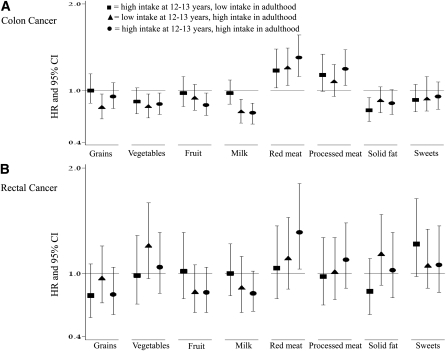
Analyses of the change in consumption of food groups (Figure 2) showed a lower risk of colon cancer among individuals in the highest tertile as both adolescents and adults relative to those in the lowest tertile at both time points for vegetables (HR: 0.84; 95% CI: 0.72, 0.97), fruit (HR: 0.83; 95% CI: 0.71, 0.97), and milk (HR: 0.74; 95% 0.64, 0.85) (Figure 2A). Furthermore, the reduction in colon cancer risk among high fruit consumers was observed only with high fruit consumption in both time periods, whereas the reduction in colon cancer with high milk consumption was observed when consumption was high during recent adult life, but not when consumption was high in adolescence and low in recent adulthood. A greater risk of colon cancer was noted among those with high consumption, at both time points, of the following food groups relative to those with low consumption at both time points: red meat (HR: 1.38; 95% CI: 1.16, 1.64) and processed meat (HR: 1.25; 95% CI: 1.06, 1.47); the red meat association was evident if the individuals were in the highest intake category at any point in time. Examination of rectal cancer showed a greater risk among individuals in the highest tertile of consumption at both time points for red meat (HR: 1.39; 95% CI: 1.04, 1.85) (Figure 2B). The association was not significant if high consumption occurred only in adolescence or only in recent adulthood.
FIGURE 2.
HRs and 95% CIs for colon cancer (A) and rectal cancer (B) by change in tertiles of food group intakes from adolescence to recent adulthood relative to individuals in the lowest tertile of intake at both time points.
DISCUSSION
In this large prospective study, we found evidence that diet in adolescence and mid-life, both of which are outside the time period routinely assessed in nutritional epidemiology studies, may modify the risk of colon and rectal cancer. Specifically, we found that individuals with a high consumption of vitamin A or vegetables during ages 12–13 y had a reduction in colon cancer risk after age 50 y. Furthermore, a high intake of calcium, vitamin A, or vitamin C during ages 40–61 y was inversely associated with colon cancer after age 50 y. Similar protective associations were observed among high consumers of fruit or milk during ages 40–61 y, and deleterious associations with colon cancer were observed among those with a high intake of fat, red meat, and processed meat during ages 40–61 y and for processed meat and rectal cancer. In addition, significant P-trend values indicating a dose-response relation were observed for all but one of the aforementioned dietary variables.
A novel aspect of our study was the evaluation of how dietary change over time affects colorectal cancer risk. We found multiple instances in which an effect was observed when consumption was high in both adolescence and recent adult life, but not when consumption was low in adolescents who became high consumers as adults or vice versa. This suggests that the pattern of exposure over the life course may play an important role in colorectal cancer risk.
Validation studies of diet recalled in the distant past are limited. Reproducibility among responses to an FFQ about diet during high school (15–35 y in the past) among Nurses’ Health Study II participants indicated moderate to strong reproducibility (20). Furthermore, correlation between FFQ responses by the nurses and their mothers regarding their child's diet were also moderate. However, validation of parental 7-d food records regarding the diet of their 13–18-y-old children from the Fels Longitudinal Study were generally not well correlated with the offspring's response to an FFQ administered roughly 48 y later, although there was variation ranging from −0.53 to 0.99 (21). Although the ideal study design would ascertain diet in youth and prospectively follow participants for decades until cancer incidence, the methods used in the current investigation are among the best available to address life course exposures in relation to cancer in the absence of this archetype, a topic that is designated as a “research priority” by the American Institute for Cancer Research (22).
The 37-item FFQs used in our study considered select nutrients and food groups, and the food-group assessment did not disaggregate complex mixtures of food. A comparison of nutrient values derived from our FFQ about diet during adolescence with those estimated for 12–13-y-olds in the HFCS indicated higher values for the HFCS. This finding is not surprising given that our FFQ consisted of only 37 items and the HFCS had an open-ended recall. Despite the lower estimates of intake, we expect that the ranking is accurate. In addition, it is possible that misclassification of self-reported lifestyle characteristics occurred; if misclassification was nondifferential, the results would most likely be biased toward the null (23). The strengths of our study included the large study population, which accrued 2794 colon and 979 rectal cancers, and the use of period-relevant nutrition surveys and databases to estimate serving size and nutrient information.
Evidence from the Netherlands Cohort Study suggests that adolescence and early adulthood are critical periods for epigenetic modification associated with future colorectal cancer risk (9). Recent work from the same cohort documented lower colon cancer risk among men who experienced severe caloric restriction during adolescence (8). Few studies have examined intakes of specific nutrients or food groups during adolescence and the risk of colorectal cancer. Prospective dietary data collected during adolescence showed a positive association between dairy product intake and colorectal cancer risk in the British-based Boyd-Orr cohort (11). This is somewhat surprising given that milk and calcium intake in adulthood are generally associated with a lower risk of colorectal cancer (2), although the small number of colorectal cancer cases (n = 76) is important to note. Contrary to the Boyd Orr findings, a recent study of participation in New Zealand government-sponsored school milk programs indicated a reduced colorectal cancer risk with program participation, including evidence of a dose-response effect with increased milk consumption (10). In our study, after control for recent adult intake, the associations for calcium and milk intakes during adolescent in relation to colon cancer were attenuated. Adult dietary consumption was not investigated in the New Zealand Study.
We found significant inverse relations between vegetable and vitamin A intakes during adolescence and colon cancer. Post hoc analyses indicated that the top 3 adolescent contributors to vitamin A intake (carrots, whole milk, and tomato or other vegetables soups) provided ∼54% of this nutrient; because 2 of the 3 leading contributors to vitamin A are vegetables, it is not surprising that both vitamin A and vegetables were inversely associated with colon cancer. Recent vegetable intake in adults was previously shown to be associated with a reduction in colorectal cancer among men in the NIH-AARP Diet and Health Study (24).
Although certain cohort studies have >20 y of follow-up data, data are lacking on the association of dietary exposures corresponding to mid-life with the outcome of colorectal cancer after control for more recent dietary exposure. Given the natural history of the adenoma-carcinoma sequence (25), it is reasonable to hypothesize that diet in mid-life could be an important exposure period for colorectal cancer—a malignancy that typically occurs after the sixth decade of life. Results from our study indicate that certain nutrients and food groups consumed during the ages 40–61 y were associated with risk of colorectal cancer 10–20 y in the future, independent of future dietary characteristics. This suggests that dietary intake earlier in the life course than previous explored (24, 26–29) may play a role in colorectal cancer.
Our finding that fiber consumption in the highest quintile during adolescence was positively associated with rectal cancer was unexpected, as were the results from sex-stratified analyses of fiber and colon cancer suggesting an greater risk of colon cancer among women with high fiber consumption during adolescence. Fiber was the only nutrient for which period-relevant nutrient data were not available; fiber values from NHANES 1999–2000 were imputed for all 3 time points and may have been a source of error. Furthermore, median adolescent fiber intake from the FFQ was 8.7 g/d, which is well below the amount (>30 g/d) reported in other studies reporting an inverse relation between fiber and colorectal cancer (30).
Our analyses of combinations of consumption levels during adolescence and recent adulthood attempted to address the relative importance of life stages on cancer risk. Our results indicate that, for certain dietary exposures, the protective or deleterious effect was present only when individuals were high consumers in both adolescence and recent adulthood. We found evidence that the pattern of exposure over the life course was particularly relevant for a protective effect of fruit against colon cancer and of calcium against rectal cancer and for the adverse relations between processed meat and colon cancer and between red meat and rectal cancer.
In summary, our results suggest that certain dietary exposures during ages 12–13 y are associated with colorectal cancer decades later, and certain dietary exposures during ages 40–61 y are associated with colorectal cancer 10–20 y in the future. Exposures during both time points are associated with colon or rectal cancer independent of dietary exposures occurring closer in time to the cancer diagnosis. Our results indicate that exposure over the life course likely plays a significant role in determining colorectal cancer risk. This is among the first investigations to determine a role of diet during adolescence and mid-life on colorectal carcinogenesis. Future studies are required to confirm these findings.
Supplementary Material
Acknowledgments
We are indebted to the participants in the NIH-AARP Diet and Health Study for their outstanding cooperation. We also thank Sigurd Hermansen and Kerry Grace Morrissey from Westat for study outcomes ascertainment and management and Leslie Carroll at Information Management Services for data support and analysis. We acknowledge the loss and memory of Arthur Schatzkin, who died 20 January 2011. He conceived and launched the NIH-AARP Diet and Health Study and had great personal warmth and humor, tremendous intellectual curiosity and honesty, a genuine interest in all, and a passion for improving public health through exemplary science. He will be dearly missed. Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, and Emory University. Cancer incidence data from California were collected by the California Department of Health Services and Cancer Surveillance Section. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, and the State of Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (FCDC) under contract with the Florida Department of Health (FDOH). Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry and Louisiana State University Medical Center in New Orleans. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey State Department of Health and Senior Services. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, PA. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services.
The authors’ responsibilities were as follows—EHR, ACMT, YP, and AJC: contributed to the analysis and drafting of the manuscript; FET, NP, and AFS: contributed to the development of the FFQ, the data analysis, and the drafting of the manuscript; BIG: contributed to the statistical analysis and drafting of the manuscript; and ARH: contributed to the study design and concept. None of the authors declared any conflicts of interest. The views expressed herein are solely those of the authors and do not necessarily reflect those of the FCDC or FDOH. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Footnotes
Abbreviations used: FFQ, food-frequency questionnaire; HFCS, household food consumption survey.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, Cancer incidence and mortality worldwide: IARC CancerBase no. 10. Available from: http://globocan.iarc.fr (cited 20 March 2010)
- 2.World Cancer Research Fund/American Institute for Cancer Research Food, nutrition, and physical activity, and the prevention of cancer: a global perspective. Washington, DC: AICR, 2007 [Google Scholar]
- 3.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell 1996;87:159–70 [DOI] [PubMed] [Google Scholar]
- 4.Winawer SJ. Natural history of colorectal cancer. Am J Med 1999;106(1A):3S–6S; discussion 50S–1S [DOI] [PubMed] [Google Scholar]
- 5.Linos E, Willett WC, Cho E, Frazier L. Adolescent diet in relation to breast cancer risk among premenopausal women. Cancer Epidemiol Biomarkers Prev 2010;19:689–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frazier AL, Li L, Cho E, Willett WC, Colditz GA. Adolescent diet and risk of breast cancer. Cancer Causes Control 2004;15:73–82 [DOI] [PubMed] [Google Scholar]
- 7.Shu XO, Jin F, Dai Q, Wen W, Potter JD, Kushi LH, Ruan Z, Gao YT, Zheng W. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers Prev 2001;10:483–8 [PubMed] [Google Scholar]
- 8.Hughes LA, van den Brandt PA, Goldbohm RA, de Goeij AF, de Bruine AP, van Engeland M, Weijenberg MP. Childhood and adolescent energy restriction and subsequent colorectal cancer risk: results from the Netherlands Cohort Study. Int J Epidemiol 2010;39:1333–44 [DOI] [PubMed] [Google Scholar]
- 9.Hughes LA, van den Brandt PA, de Bruine AP, Wouters KA, Hulsmans S, Spiertz A, Goldbohm RA, de Goeij AF, Herman JG, Weijenberg MP, et al. Early life exposure to famine and colorectal cancer risk: a role for epigenetic mechanisms. PLoS ONE 2009;4:e7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox B, Sneyd MJ. School milk and risk of colorectal cancer: a national case-control study. Am J Epidemiol 2011;173:394–403 [DOI] [PubMed] [Google Scholar]
- 11.van der Pols JC, Bain C, Gunnell D, Smith GD, Frobisher C, Martin RM. Childhood dairy intake and adult cancer risk: 65-y follow-up of the Boyd Orr cohort. Am J Clin Nutr 2007;86:1722–9 [DOI] [PubMed] [Google Scholar]
- 12.Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, Hurwitz PE, Coyle L, Schussler N, Michaud DS, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol 2001;154:1119–25 [DOI] [PubMed] [Google Scholar]
- 13.Michaud DS, Midthune D, Hermansen S, Leitzmann M, Harlan LC, Kipnis V, Schatzkin A. Comparison of cancer registry case ascertainment with SEER estimates and self-reporting in a subset of the NIH-AARP Diet and Health Study. J Registry Manage 2005;32:70–5 [Google Scholar]
- 14.WHO International classification of diseases for oncology. 3rd ed Geneva, Switzerland: WHO, 2000 [Google Scholar]
- 15.Loftus EF, Marburger W. Since the eruption of Mt. St. Helens, has anyone beaten you up? Improving the accuracy of retrospective reports with landmark events. Mem Cognit 1983;11:114–20 [DOI] [PubMed] [Google Scholar]
- 16.van der Vaart W, Glasner T. Personal landmarks as recall aids in survey interviews. Field Methods 2011;23:37–56 [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) Third National Health and Nutrition Examination Survey (NHANES III). 2009 Version current 30 March 2011. Available from: http://www.cdc.gov/nchs/nhanes/nh3data.htm (cited 15 April 2010)
- 18.Subar AF, Midthune D, Kulldorff M, Brown CC, Thompson FE, Kipnis V, Schatzkin A. Evaluation of alternative approaches to assign nutrient values to food groups in food frequency questionnaires. Am J Epidemiol 2000;152:279–86 [DOI] [PubMed] [Google Scholar]
- 19.Friday JE, Bowman SA. MyPyramid equivalents database for USDA Survey Food Codes, 1994-2002. Version 1.0. October 2006. Available from: http://www.barc.usda.gov/bhnrc/fsrg (cited 20 March 2011)
- 20.Maruti SS, Feskanich D, Rockett HR, Colditz GA, Sampson LA, Willett WC. Validation of adolescent diet recalled by adults. Epidemiology 2006;17:226–9 [DOI] [PubMed] [Google Scholar]
- 21.Chavarro JE, Rosner BA, Sampson L, Willey C, Tocco P, Willett WC, Chumlea WC, Michels KB. Validity of adolescent diet recall 48 years later. Am J Epidemiol 2009;170:1563–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Institute for Cancer Research Grant application package for 2010/2011. 2010. Available from: http://www.aicr.org/site/DocServer/2010_2011_Grant_Application_Package.pdf?docID=4341 (cited 15 March 2011)
- 23.Jurek AM, Greenland S, Maldonado G, Church TR. Proper interpretation of non-differential misclassification effects: expectations vs observations. Int J Epidemiol 2005;34:680–7 [DOI] [PubMed] [Google Scholar]
- 24.George SM, Park Y, Leitzmann MF, Freedman ND, Dowling EC, Reedy J, Schatzkin A, Hollenbeck A, Subar AF. Fruit and vegetable intake and risk of cancer: a prospective cohort study. Am J Clin Nutr 2009;89:347–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61:759–67 [DOI] [PubMed] [Google Scholar]
- 26.Cross AJ, Leitzmann MF, Gail MH, Hollenbeck AR, Schatzkin A, Sinha R. A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med 2007;4:e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park Y, Leitzmann MF, Subar AF, Hollenbeck A, Schatzkin A. Dairy food, calcium, and risk of cancer in the NIH-AARP Diet and Health Study. Arch Intern Med 2009;169:391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park Y, Subar AF, Kipnis V, et al. Fruit and vegetable intakes and risk of colorectal cancer in the NIH-AARP diet and health study. Am J Epidemiol 2007;166:170–80 [DOI] [PubMed] [Google Scholar]
- 29.Schatzkin A, Mouw T, Park Y, Subar AF, Kipnis V, Hollenbeck A, Leitzmann MF, Thompson FE. Dietary fiber and whole-grain consumption in relation to colorectal cancer in the NIH-AARP Diet and Health Study. Am J Clin Nutr 2007;85:1353–60 [DOI] [PubMed] [Google Scholar]
- 30.Bingham SA, Day NE, Luben R, Ferrari P, Slimani N, Norat T, Clavel-Chapelon F, Kesse E, Nieters A, Boeing H, et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet 2003;361:1496–501 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.