Abstract
Modern medicine is expanding the possibilities of receiving “personalized” diagnosis and therapies, providing minimal invasiveness, technological solutions based on non-ionizing radiation, early detection of pathologies with the main objectives of being operator independent and with low cost to society. Our research activities aim to strongly contribute to these trends by improving the capabilities of current diagnostic imaging systems, which are of key importance in possibly providing both optimal diagnosis and therapies to patients. In medical diagnostics, cellular imaging aims to develop new methods and technologies for the detection of specific metabolic processes in living organisms, in order to accurately identify and discriminate normal from pathological tissues. In fact, most diseases have a “molecular basis” that detected through these new diagnostic methodologies can provide enormous benefits to medicine. Nowadays, this possibility is mainly related to the use of Positron Emission Tomography, with an exposure to ionizing radiation for patients and operators and with extremely high medical diagnostics costs. The future possible development of non-ionizing cellular imaging based on techniques such as Nuclear Magnetic Resonance or Ultrasound, would represent an important step towards modern and personalized therapies. During the last decade, the field of nanotechnology has made important progress and a wide range of organic and inorganic nanomaterials are now available with an incredible number of further combinations with other compounds for cellular targeting. The availability of these new advanced nanosystems allows new scenarios in diagnostic methodologies which are potentially capable of providing morphological and functional information together with metabolic and cellular indications.
Keywords: Intelligent nanosystems for cellular targeting, Magnetic resonance and ultrasound, Molecular imaging, Non-ionizing diagnostic techniques, Personalized medicine in the oncological and vascular field
INTRODUCTION AND EDUCATIONAL EXPERIENCE
Sergio Casciaro (Figure 1) was born in Lecce, Italy in 1971. He received his Laurea degree in Nuclear Engineering at Turin Polytechnic Engineering School (Politecnico di Torino) and his PhD in Bioengineering in 2003 at the University of Pisa.
Figure 1.

Sergio Casciaro, PhD, National Council of Research, Institute of Clinical Physiology, Campus Universitario Ecotekne, Via per Monteroni, 73100 Lecce, Italy.
From 1996, he was a research engineer at CERN - European Centre for Nuclear Research, Geneva, Switzerland, working on the design and tests of the Large Hadron Collider for high energy physics studies. Then, in 1998 he moved to EPFL - Swiss Federal Institute of Technology, Lausanne, Switzerland for a research program on thermo-fluid dynamic experiments working on an ASHRAE research investigation. Then he went to ISBEM - Istituto Scientifico Biomedico Euro Mediterraneo, Brindisi, Italy for advanced studies in the biomedical field with a special focus on image and signal processing of magnetic resonance imaging (MRI) and contrast-enhanced ultrasound (US) data. In 2000 and 2001 he was a visiting scientist at NIH - National Institute of Health, Bethesda, USA working with P.A. Bandettini in the functional imaging unit for advanced functional and morphological investigations of the brain. From 2002 he has been a research scientist at the National Council of Research, Institute of Clinical Physiology and leader of the Biomedical Engineering Science and Technology Division and director of the Nanoimaging Ultrasound LAB for non-ionizing cellular and molecular imaging using innovative nanosystems for diagnosis and therapy. He is and has been in charge of several national and European research projects. He is on the Expert Evaluator Panel of the European Commission for the 7th Framework Program (Health, NMP, etc.). He is a reviewer for the following international journals: Investigative Radiology, European Radiology, Radiology, Biomaterials, NeuroImage, Magnetic Resonance Imaging, IEEE TMI and others. He is an editorial board member of the World Journal of Radiology.
Dr. Casciaro is a member and chair of the scientific committee of several scientific associations (NESA Academy; ARISER Network; ICAR; MIMOS; CARS; LSMU- International Medical University, Berlin, Germany). He has been an invited lecturer at the Bioengineering Course at Lecce University and Invited Professor at 6 national and international master courses on advanced medical imaging methods and techniques. He is Editor in Chief of 3 international books and co-author of about 20 book chapters. He is the author of more than 70 scientific articles in peer-reviewed journals and international conference proceedings. He is the main inventor of 5 national and international patents. He has been appointed scientific director of the ARISER Annual Conference for 5 years. He received several awards and prizes as Young Investigator and in 2010 he was awarded the prestigious National Prize for Innovation by the PNICube National Association as well as the “Prize of Prize for Research” by the President of the Italian Republic at the Quirinale Palace in Rome, June 2011.
ACADEMIC STRATEGY AND GOALS
Sergio Casciaro has 15 years experience in multidisciplinary research at international level, as documented in detail in the next paragraphs. In particular, during the last decade, his research activities have been related to the development of novel methodologies for biomedical image and signal processing, and through the introduction of new contrast media, towards innovative and minimally invasive diagnoses and therapies. His experience is specifically related to three main research areas: (1) full experimental characterization and modeling of novel micro- and nano-scaled contrast media for non-ionizing imaging techniques, such as US and MRI; (2) automatic extraction of information from biomedical images and signals for anatomical and functional investigations; and (3) industrial research into the development of new biomedical systems with related patenting activities at a national and international level, bridging the gap between applied research and industries in this field.
Early diagnosis of cancer through a non-ionizing approach is one of the main research objectives. In fact, cancer is a huge and growing contributor to the burden of disease and premature death worldwide: every year, more than 12 000 000 new cancer cases are diagnosed (25% in Europe) and more than 7 500 000 people die due to cancer-related causes (23% in Europe)[1]; about 25% of all deaths in the EU are attributable to cancer, and in the age range 45-64 years the percentage increases to almost 50%[2]. In addition, population growth and ageing are expected to further increase these numbers.
The only possible way to significantly improve this situation is by the introduction of completely new diagnostic methods capable of identifying tumors in their very early stages of progression, ideally before the first cancer cell is generated, that is when, as a consequence of one or more risk factors, some cell nuclei start showing specific DNA damage that is likely to give rise to tumor degeneration. Therefore, the identification of distinctive DNA alterations is crucial to better understand the mechanisms of different cancers and to detect potential genomic markers for diagnosis and prognosis[3]. In this scenario, hepatocellular carcinoma in particular has recently been indicated as one of the tumor types where a more complete understanding of the underlying genetic alterations could have a major impact on the development of new treatment strategies[4]. A potentially promising strategy could be represented by the development of nanoparticle-based imaging agents, specifically designed to highlight possible genomic defects which are prone to subsequently develop cancer.
Non-invasive molecular imaging is an emerging field in the frame of Diagnostic Imaging which aims to develop new technologies which are able to detect processes at the molecular and cellular level in living organisms, in order to early and carefully identify and differentiate healthy tissue from pathological tissue for better diagnosis and therapy. The key feature of molecular imaging is the use of specific contrast agents that selectively identify chosen molecular targets or cellular processes, highlighting them on the corresponding image. The fundamental hypothesis of these new methods is that many diseases have a “molecular basis”, whose visualization may result in a number of benefits: early diagnosis, accurate staging, real-time monitoring of therapeutic treatment outcome (through the imaging of molecular markers), better prognosis on possible disease evolutions. This approach is particularly useful in the diagnosis of tumors, since in most cases the high mortality rate associated with these pathologies is due to a “late diagnosis”, done only after the tumor has reached an advanced stage.
Currently, the only diagnostic technique for which molecular imaging is already routinely used in clinics is positron emission tomography (PET), which is a highly expensive technique and, above all, involves the use of highly ionizing radiation with consequent risks for patients, operators and society. As a consequence, PET examinations can not be used for patient follow-up or for population screening purposes.
The idea of exploiting the properties of “molecular markers” has brought new perspectives for Diagnostic Imaging techniques, allowing the extension of molecular imaging applications to non-ionizing techniques, such as US and MRI, through the development and employment of innovative nano-sized “targeted” contrast agents.
ACADEMIC ACHIEVEMENTS
Sergio Casciaro started his research career in 1996 at the European Centre for Nuclear Research (CERN, Geneva, Switzerland) working on the design of the main superconducting components of the Large Hadron Collider, which nowadays represents the most important system for future fundamental research in the field of high energy physics, aimed at reproducing the “big bang” conditions at the origin of the universe. The theoretical design and experimental work conducted during his stay at CERN has been fully integrated in the final version of the system and the implemented guidelines have been instrumental for the system production in three European countries with a successful final test last year. This is undoubtedly one of the most important preliminary steps towards advancements in fundamental physics in the next century.
After research experience at the Swiss Federal Institute of Technology of Lausanne in 1999 working on fluid dynamic heat and mass transfer[5,6] and on starting up new experimental research activities, in 2000 he moved to the National Institute of Health (NIH, Bethesda, USA) in collaboration with ISBEM Institute of Brindisi and Pisa University, to work on the most advanced morphological and functional brain studies by means of MRI under the supervision of Dr. Peter A. Bandettini. He started the functional MRI brain activities in Pisa at the Institute of Clinical Physiology (IFC), the most important multidisciplinary biomedical institute of the Italian National Council of Research (CNR). From then on, all his energy and efforts have been devoted to the creation, from zero, of a multidisciplinary research group on biomedical engineering science and technology at the Lecce site of CNR-IFC. He developed an educational program on Biomedical Engineering at the Lecce University, where he recruited several young scientists for the PhD programs in Bioengineering; he implemented the research laboratories thanks to the regional, national and international grants he received in collaboration with many prestigious international scientific partners. Currently, he leads a multidisciplinary group of young researchers and PhD students, which in recent years has been actively involved in the creation and management of several research activities, and the annual organization of international scientific events.
The obtained research results, as described in detail in the following paragraphs, certify that the enormous personal and intellectual energy he invested has resulted in outstanding scientific outcomes.
During the last decade, the main research interests and activities of Sergio Casciaro have been related to the development of novel methodologies for biomedical image and signal processing, and through the introduction of new contrast media, towards innovative and minimally invasive diagnoses and therapies. Molecular, cellular, quantitative, automated and real time approaches have been of key significance in carrying out experimental and theoretical scientific investigations.
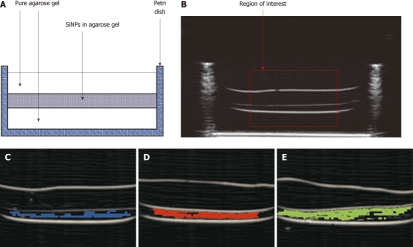
In particular, the experience gained is specifically related to the following main research areas: (1) theoretical simulations, design and full experimental characterization and modeling of novel micro- and nano-scaled contrast media for non-ionizing imaging techniques, like MRI and US[7-12] (Figure 2). These activities, whose final goal is the introduction of novel combined therapies by means of targeting, drug and gene delivery, have been performed, including custom designed phantoms for “in vitro” studies, as well as “ex vivo” experimentations and “in vivo” trials in animal models[13-15]; (2) automatic information extraction from biomedical images and signals for anatomical and functional investigations: automatic image segmentation and registration, tissue characterization (virtual biopsy), volume rendering for augmented and virtual reality applied to oncology radiotherapy and minimally invasive therapies (operation planning, intra-operative image guidance, training, etc.), functional MRI studies, employment of neural networks and expert systems for supporting medical decisions[16-30] (Figure 3); and (3) industrial research for the design and development of new systems and tools with the related intellectual property protection and patenting activities at a national and international level, bridging the gap between applied research and industries in this field.
Figure 2.
Example of automatic detection of accumulated Silica Nanoparticles. A: Scheme of the analyzed phantom; B: B-mode image of a control phantom with indication of the chosen ROI; C-E: Images of the analyzed ROIs with automatic detection results displayed in blue, red, and green for 150-nm, 320-nm, and 650-nm SiNPs, respectively. The sensitivity of the developed method for automatic nanoparticle detection had a maximum of 71% with 320-nm particles, whereas it was lower with both larger and smaller particles (sensitivity of 63% and 18%, respectively).
Figure 3.
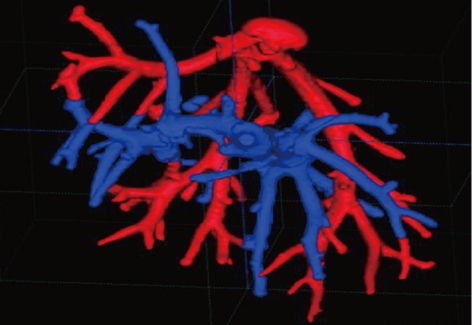
Example of a 3D automatic phantom image segmentation simulating liver vessel trees. The optimal algorithm configuration resulted in a vessel detection sensitivity of 100% for vessels of more than 1 mm in diameter, 50% in the range 0.5-1 mm and 14% in the 0.2-0.5 mm range. An average area overlap of 94.9% was obtained between automatically and manually segmented vessel sections, with an average difference of 0.06 mm2. The average values of corresponding false positive and false negative ratios were approximately 8% and 3%, respectively.
The guiding principles of the conducted research activity have always been the maximum exploitation and enhancement of non-ionizing imaging techniques (US and MRI) through innovative methodologies, based on novel image and signal processing algorithms and use of the most advanced contrast media, also nanotechnology-based.
Many scientific results contributed to the advancement of the research field and this can easily be verified thanks to the acceptance of publications in major international peer-reviewed multidisciplinary scientific journals, to the granted funding and to the winning of international prizes and awards.
In the MRI field, innovative scientific contributions have been made to the advancement of depiction of venous vessels into the brain by exploiting endogenous contrast mechanisms, revealing venous vessels smaller than 1 mm and enhancing many details undetectable in conventional venograms. This approach combines non-invasiveness with high resolution images. Thanks to these research outcomes, this methodology has been used to further characterize the most important and commonly used contrast mechanism for the detection and study of neuronal activations: the Blood Oxygen Level Dependent (BOLD) contrast. Sergio Casciaro’s work, has demonstrated the weakness of the BOLD mechanism in revealing neuronal electrical activity due to venous artefacts that are erroneously taken as actual brain activations, which can provide tremendous new inputs into surgical neuronal procedures. This work also received a Young Investigator Award in Sweden.
Important contributions have also been made in the field of US imaging, both with and without the use of contrast agents. In the literature, US contrast agents (UCAs) have been studied extensively, however, most of this research has focused on the single microbubble dynamic and not on contrast agent populations. For the first time, a new interpretation model was developed by Sergio Casciaro for studying the dynamic evolution of contrast agent populations over time and characterizing the main destruction mechanisms and their changes in terms of acoustic properties which can be exploited in the fields of targeting, gene and drug delivery. Experimental studies on the behavior of contrast microbubble populations have been selected twice as finalist papers in the Young Investigator Award Competition of the “European Symposium on Ultrasound Contrast Imaging”.
Another contribution is related to tissue characterization by means of spectral analysis through wavelets and independent component analysis, which is able to distinguish pathologic tissues from healthy tissues in a non-invasive and reliable way. Furthermore, all these tissue properties have been translated into artificial tissues reproducing with an excellent approximation not only the propagation of the main US signal component, but also its harmonic components normally essential for the accurate study of UCAs: “ad hoc” experimental set-ups for studying UCAs are available reducing the need for animal models and unnecessary sacrifice. This innovative technique for the manufacture of tissue mimicking phantoms for “in vitro” characterization of UCA behaviour was awarded the First Prize Poster Context in Chicago (IL, USA) during the “Annual Advances in Contrast Ultrasound” congress in 2005.
Finally, another important contribution was the automatic information extraction from medical images through innovative processing techniques in the case of abdominal images, without any need for user interaction, producing an important reduction in expensive manual operations. Furthermore, this method received the Best Paper Award at the ARISER Conference in 2007.
All previously summarized early scientific results received scientific recognition. First of all, by the acceptance of manuscripts in international peer-reviewed journals and conferences: from 2004 to 2009 more than sixty scientific articles were written and accepted in the field of biomedical imaging. Furthermore, four investigator awards were granted by international scientific committees and in two other cases Sergio Casciaro was a finalist in young investigator international competitions. Four patents have been also granted and several companies collaborate with Casciaro’s group on industrial exploitation. Additionally, several research funds have been granted to the afore-mentioned research activities at regional, national and international level. The following are the most significant and relevant research projects in which Sergio Casciaro had scientific leadership: a FIRB project on the development of new techniques and advanced methods for the employment of UCAs in diagnostic and therapeutic applications (about 600 k€; 2003-2006); the CERSUM project (European Center for Research and Development on applications of Ultrasound in Medicine; about 1.2 M€; 2005-2008); the ARISER project focused on the implementation of Augmented and Virtual Reality techniques for development of minimally invasive surgical procedures (“Marie Curie Actions” of the 6th FP, and Sergio Casciaro has been the Scientist in Charge of the “Image and Signal Processing” Workpackage; about 500 k€; 2005-2008; see also http://www.ariser.info); a Public-Private Laboratory for the development of innovative technologies for advanced medical diagnostics (850 k€ for the past 4 years). In the last 4 years Sergio Casciaro has been nominated as Scientific Director of the annual international conference and summer school on minimally invasive technologies (MIT) of the ARISER communities, together with several invitations to give talks at international events and institutions active in the biomedical-related research field. This last activity has been of key importance for triggering the inspiration and the challenge towards future MIT and related therapies.
PERSPECTIVE
Preliminary results available in the literature support the feasibility of nanoparticle contrast agents (NPCAs) for non-ionizing cellular imaging and concurrent therapy to treat specific pathologies. An absolutely new class of “theranostic” agents are under development in our laboratory, based on biocompatible nanoparticles consisting of a rigid multi-component core (superparamagnetic compound + silica, able to introduce variation of both magnetic susceptibility and acoustic impedance in the surrounding medium) and a softer polymeric shell, whose function will be to provide a pH- and/or thermo-sensitive encapsulation for loaded drug molecules and to act as a bridge for the conjugation of both fluorophores capable of emitting light in the infrared region (to be exploited in intraoperative fluorescence imaging) and aptamers for the selective detection of specific disease receptors. The possibility of adding a further component, like gold nanorods, to the nanoparticle core, in order to provide a strong optical absorption due to plasmon resonance effects, employable for both hyperthermia and optoacoustic imaging purposes, will also be evaluated.
The final goal is to develop and experimentally validate a minimally invasive nanotechnology-based solution to improve cancer diagnosis accuracy and subsequent disease management, through a multimodal imaging approach and a self-tailoring and self-monitoring therapeutic treatment. The undergoing research approach will try to satisfy the actual clinical needs for risk stratification, population screening and surgeon support during interventions, offering the possibility of combining elective repeatable and cheap diagnostic examinations (US) with highly specific clinical investigations (MRI) and also with intraoperative optical imaging modalities. The introduction of such diagnostic techniques involving nanoparticle “theranostic” agents represents a tremendous innovation compared to the state of the art of international literature. The entire systems and methods developed will then create an absolutely innovative diagnostic-therapeutic paradigm. Furthermore, it is reasonable to expect that the development of these new multimodal non-ionizing imaging modalities will allow significant improvements in the diagnostic performances of current imaging systems, and will have a strong influence on the advancements of the European technology and biomedical industry. Furthermore, the results of this research will create the basis to develop new advanced and integrated diagnostic systems, towards minimally invasive therapies of the future. Moreover, the targeting of NPCAs will allow local drug delivery with the combined use of hyperthermia systems for diagnosis and simultaneous cellular ablation of tumoral tissues towards a multi-therapy approach.
In conclusion, achievement of the ultimate goals of our main research will try to overcome PET limitations in the management of cancer pathologies by integrating diagnosis, therapy and treatment monitoring in a single non-ionizing procedure, and will open up new horizons in the field of early tumor diagnosis thanks to a revolutionary imaging approach.
ACKNOWLEDGMENTS
I thank all people, past and present colleagues, particularly Dr. Francesco Conversano, for their enthusiasm, help and contributions to our work and research goals. I am deeply grateful to several friends and investigators worldwide for their kind collaborations.
Footnotes
Supported by Italian Ministry of Research, Apulia Region, European Commission and National Council of Research
Peer reviewer: Frederik L Giesel, MD, PhD, MBA, National German Cancer Research Center (dkfz), Department of Radiology E010, 69120 Heidelberg, Germany
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Albreht T, McKee M, Alexe DM, Coleman MP, Martin-Moreno JM. Making progress against cancer in Europe in 2008. Eur J Cancer. 2008;44:1451–1456. doi: 10.1016/j.ejca.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Sakamoto JH, van de Ven AL, Godin B, Blanco E, Serda RE, Grattoni A, Ziemys A, Bouamrani A, Hu T, Ranganathan SI, et al. Enabling individualized therapy through nanotechnology. Pharmacol Res. 2010;62:57–89. doi: 10.1016/j.phrs.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zender L, Villanueva A, Tovar V, Sia D, Chiang DY, Llovet JM. Cancer gene discovery in hepatocellular carcinoma. J Hepatol. 2010;52:921–929. doi: 10.1016/j.jhep.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casciaro S, Thome JR. Thermal performance of flooded evaporators, part 1: review of boiling heat transfer studies. ASHRAE Trans. 2001;107:903–918. [Google Scholar]
- 6.Casciaro S, Thome JR. Thermal performance of flooded evaporators, part 2: review of void fraction, two-phase pressure drop, and flow pattern studies. ASHRAE Trans. 2001;107:919–930. [Google Scholar]
- 7.Conversano F, Franchini R, Lay-Ekuakille A, Casciaro S. In vitro evaluation and theoretical modelling of the dissolution behaviour of a microbubble contrast agent for ultrasound imaging. IEEE Sens J. 2011:Epub ahead of print. [Google Scholar]
- 8.Malvindi MA, Greco A, Conversano F, Figuerola A, Corti M, Bonora M, Lascialfari A, Doumari HA, Moscardini M, Cingolani R, et al. Magnetic/silica nanocomposites as dual-mode contrast agents for combined magnetic resonance imaging and ultrasonography. Adv Funct Mater. 2011;21:2548–2555. [Google Scholar]
- 9.Conversano F, Franchini R, Casciaro S. Characterization of microbubble contrast agents for echographic imaging through time-scheduled size distribution measurements. Sens Trans J. 2010;9:21–27. [Google Scholar]
- 10.Franchini R, Conversano F, Greco A, Verrienti R, Casciaro S. Ultrasound signal analysis applied to determine the optimal contrast dose for echographic examinations. Sens Trans J. 2010;9:48–55. [Google Scholar]
- 11.Casciaro S, Conversano F, Ragusa A, Malvindi MA, Franchini R, Greco A, Pellegrino T, Gigli G. Optimal enhancement configuration of silica nanoparticles for ultrasound imaging and automatic detection at conventional diagnostic frequencies. Invest Radiol. 2010;45:715–724. doi: 10.1097/RLI.0b013e3181e6f42f. [DOI] [PubMed] [Google Scholar]
- 12.Casciaro S, Palmizio Errico R, Conversano F, Demitri C, Distante A. Experimental investigations of nonlinearities and destruction mechanisms of an experimental phospholipid-based ultrasound contrast agent. Invest Radiol. 2007;42:95–104. doi: 10.1097/01.rli.0000251576.68097.d1. [DOI] [PubMed] [Google Scholar]
- 13.Casciaro S, Conversano F, Musio S, Casciaro E, Demitri C, Sannino A. Full experimental modelling of a liver tissue mimicking phantom for medical ultrasound studies employing different hydrogels. J Mater Sci Mater Med. 2009;20:983–989. doi: 10.1007/s10856-008-3644-6. [DOI] [PubMed] [Google Scholar]
- 14.Demitri C, Sannino A, Conversano F, Casciaro S, Distante A, Maffezzoli A. Hydrogel based tissue mimicking phantom for in-vitro ultrasound contrast agents studies. J Biomed Mater Res B Appl Biomater. 2008;87:338–345. doi: 10.1002/jbm.b.31108. [DOI] [PubMed] [Google Scholar]
- 15.Casciaro S, Demitri C, Conversano F, Casciaro E, Distante A. Experimental investigation and theoretical modelling of the nonlinear acoustical behaviour of a liver tissue and comparison with a tissue mimicking hydrogel. J Mater Sci Mater Med. 2008;19:899–906. doi: 10.1007/s10856-007-3007-8. [DOI] [PubMed] [Google Scholar]
- 16.Casciaro S, Franchini R, Massoptier L, Casciaro E, Conversano F, Malvasi A, Lay-Ekuakille A. Fully automatic segmentations of liver and hepatic tumors from 3-D computed tomography abdominal images: comparative evaluation of two automatic methods. IEEE Sens J. 2011:In press. [Google Scholar]
- 17.Conversano F, Franchini R, Demitri C, Massoptier L, Montagna F, Maffezzoli A, Malvasi A, Casciaro S. Hepatic vessel segmentation for 3D planning of liver surgery experimental evaluation of a new fully automatic algorithm. Acad Radiol. 2011;18:461–470. doi: 10.1016/j.acra.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Malvasi A, Tinelli A, Brizzi A, Guido M, Martino V, Casciaro S, Celleno D, Frigo MG, Stark M, Benhamou D. Intrapartum sonography for occiput posterior detection in early low dose combined spinal epidural analgesia by sufentanil and ropivacaine. Eur Rev Med Pharmacol Sci. 2010;14:799–806. [PubMed] [Google Scholar]
- 19.Lamata P, Lamata F, Sojar V, Makowski P, Massoptier L, Casciaro S, Ali W, Stüdeli T, Declerck J, Elle OJ, et al. Use of the Resection Map system as guidance during hepatectomy. Surg Endosc. 2010;24:2327–2337. doi: 10.1007/s00464-010-0915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casciaro S, Bianco R, Franchini R, Casciaro E, Conversano F. A new automatic phase mask filter for high-resolution brain venography at 3 T: theoretical background and experimental validation. Magn Reson Imaging. 2010;28:511–519. doi: 10.1016/j.mri.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Casciaro S, Bianco R, Distante A. Quantification of venous blood signal contribution to BOLD functional activation in the auditory cortex at 3 T. Magn Reson Imaging. 2008;26:1221–1231. doi: 10.1016/j.mri.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Massoptier L, Casciaro S. A new fully automatic and robust algorithm for fast segmentation of liver tissue and tumors from CT scans. Eur Radiol. 2008;18:1658–1665. doi: 10.1007/s00330-008-0924-y. [DOI] [PubMed] [Google Scholar]
- 23.Malvasi A, Tinelli A, Serio G, Tinelli R, Casciaro S, Cavallotti C. Comparison between the use of the Joel-Cohen incision and its modification during Stark’s cesarean section. J Matern Fetal Neonatal Med. 2007;20:757–761. doi: 10.1080/14767050701580531. [DOI] [PubMed] [Google Scholar]
- 24.Tinelli A, Malvasi A, Vergara D, Casciaro S. Emergency surgical procedure for failed methotrexate treatment of cervical pregnancy: a case report. Eur J Contracept Reprod Health Care. 2007;12:391–395. doi: 10.1080/13625180701502351. [DOI] [PubMed] [Google Scholar]
- 25.Tinelli A, Vergara D, Leo G, Malvasi A, Casciaro S, Leo E, Montinari MR, Maffia M, Marsigliante S, Lorusso V. Human papillomavirus genital infection in modern gynecology: genetic and genomic aspects. Eur Clin Obst Gyn. 2007;3:1–6. [Google Scholar]
- 26.Casciaro E, Silvano G, Assennato AC, Bambace S, Capomolla C, Carioggia V, Casciaro S, Fusco V, Lombardie R, Maiorana A, et al. Quality assurance in radiation oncology: Validation of methods and monitoring process to reduce errors and dysfunctions (quaraton) Radiother Oncol. 2006;78:S71–S72. [Google Scholar]
- 27.Malvasi A, Tinelli A, Brizzi A, Casciaro S. The dystocia in obstetrician. Minerva Anestesiol. 2006;72:54–58. [Google Scholar]
- 28.Tinelli A, Malvasi A, Schneider AJ, Keckstein J, Hudelist G, Barbic M, Casciaro S, Giorda G, Tinelli R, Perrone A, et al. [First abdominal access in gynecological laparoscopy: which method to utilize?] Minerva Ginecol. 2006;58:429–440. [PubMed] [Google Scholar]
- 29.Portaluri M, Casciaro S, Bambace S, Tramacere F, Casciaro E, Recchia V, Sanzo A, Pili G, Didonna V, Distante A. Quality assurance in radiotherapy. How to improve the effectiveness and completeness of an electronic patient’s chart. Ann Ist Super Sanita. 2005;41:493–499. [PubMed] [Google Scholar]
- 30.Portaluri M, Bambace S, Giuliano G, Di Paola L, Gianicolo ME, Distante S, Casciaro S. Fractionations in radiotherapy of brain metastases. Tumori. 2004;90:80–85. doi: 10.1177/030089160409000117. [DOI] [PubMed] [Google Scholar]