Abstract
Capillary morphogenesis gene 2 (CMG2) is a type I membrane protein involved in the homeostasis of the extracellular matrix. While it shares interesting similarities with integrins, its exact molecular role is unknown. The interest and knowledge about CMG2 largely stems from the fact that it is involved in two diseases, one infectious and one genetic. CMG2 is the main receptor of the anthrax toxin, and knocking out this gene in mice renders them insensitive to infection with Bacillus anthracis spores. On the other hand, mutations in CMG2 lead to a rare but severe autosomal recessive disorder in humans called Hyaline Fibromatosis Syndrome (HFS). We will here review what is known about the structure of CMG2 and its ability to mediate anthrax toxin entry into cell. We will then describe the limited knowledge available concerning the physiological role of CMG2. Finally, we will describe HFS and the consequences of HFS-associated mutations in CMG2 at the molecular and cellular level.
Keywords: anthrax, CMG2, hyaline fibromatosis, TEM8
Introduction
The gene Cmg2 encodes a type I membrane protein that is expressed ubiquitously in the human body (Bell et al, 2001; Scobie et al, 2003), with the notable exception of the brain. While it was identified in 2001 as the second most upregulated gene upon formation of capillaries in 3D matrices (hence the name), its exact physiological roles remain poorly understood. Far more is, however, known about its ‘dark sides’—in disease. In 2003, Capillary morphogenesis gene 2 (CMG2) was identified as the second receptor for the anthrax toxin (Scobie et al, 2003), hence its official name of Anthrax Toxin Receptor 2 (ANTXR2). CMG2 shares 40% overall amino-acid identity with the first-identified anthrax receptor, Tumour Endothelial Marker 8 (TEM8 or ANTXR1) (Bradley et al, 2001), also a type I membrane protein. At the time, these findings received considerable attention from the scientific community and the broader media: a week after the attacks of 11 September 2001, letters containing anthrax spores were indeed sent to several news media offices, creating great terror. These events boosted anthrax-related research and interest therein and put CMG2 and TEM8 in the limelight. The same year, but less prominently, cmg2 was identified as the gene responsible for two rare autosomal recessive human genetic disorders, Infantile Hyaline Fibromatosis (ISH, (MIM 236490)) and Juvenile Hyaline Fibromatosis (JHF, (MIM 228600)), two variants of the same disease, now jointly called Hyaline Fibromatosis Syndrome (HFS) (Dowling et al, 2003; Hanks et al, 2003; El-Kamah et al, 2010).
We will here review the current knowledge about the structure of CMG2, its role as an anthrax receptor and its physiological function. We will also review findings regarding the related protein TEM8 that we feel are relevant to the understanding of CMG2. Finally, we will describe the knowledge gained from molecular and cellular studies on HFS-associated mutations.
Anthrax toxin
First, we will briefly describe the anthrax toxin—ironically the only well-characterized ligand for CMG2—since it has been widely used in studies to shed light on the molecular functions of CMG2 and TEM8. The anthrax toxin is composed of three independent polypeptide chains, of which two have enzymatic activities: the Lethal Factor (LF), which is a metalloprotease that cleaves MAP kinase kinases; and the Oedema Factor (EF), a calmodulin-dependent adenylate cyclase (Leppla, 1982; Duesbery et al, 1998; Vitale et al, 1998). The third subunit, called the Protective Antigen (PA), has the role of escorting EF and LF from the extracellular milieu to the cytosol of the target cell (for review, see Young and Collier, 2007). Only PA has the capacity to bind to target cells. In tissue culture systems, PA interacts with cells by binding to CMG2, TEM8 (for review, see van der Goot and Young, 2009) and, at least on RAW macrophages, also to heterodimeric complexes containing β1 integrin (Martchenko et al, 2010). In mice, however, CMG2 was found to be the main anthrax toxin receptor (Liu et al, 2009), and expression of CMG2 in myeloid cells in particular was shown to be essential for the establishment of an anthrax infection (Liu et al, 2010). Once bound to the cell surface, PA, which is initially an 83-kDa protein, is cleaved by the pro-protein convertase furin into a 63-kDa protein. PA63 has the capacity to oligomerize into heptameric or octameric rings, to which EF/LF can bind. The binding to CMG2/TEM8 and oligomerization of PA trigger the endocytosis of the multi-component receptor–PA–EF/LF complex. Upon arrival in endosomes, the acidic pH induces a conformational change in PA that leads to the formation of a transmembrane pore. Low pH also triggers unfolding of EF and LF, which can then be translocated through the PA channel to the other side of the endosomal membrane. Once in the cytosol, EF and LF exert their cytotoxic activity, the cellular and pathophysiological consequences of which have recently been reviewed (Moayeri and Leppla, 2009).
CMG2 structure
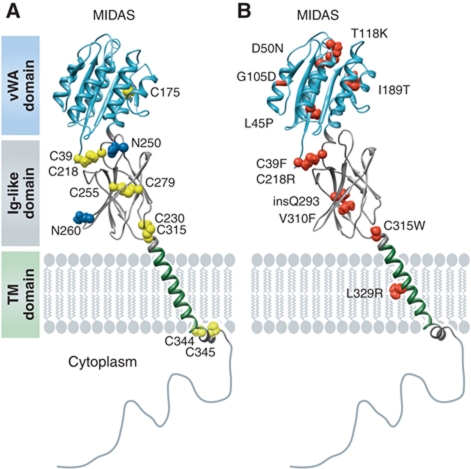
Four different CMG2 isoforms, generated by alternative splicing, have been predicted (NCBI's ACEMBLY) or identified (Scobie et al, 2003): CMG2489, CMG2488, CMG2386 and CMG2322 (UniProt database annotation). The relevance and tissue distribution of these isoforms has not been documented and most studies have dealt with the full-length variant CMG2489 (ANTR2_HUMAN: P58335). CMG2489 is a 489 amino-acid type I membrane glycoprotein which includes a signal peptide that targets it to the endoplasmic reticulum (ER) during synthesis, a von Willebrand factor type A (vWA) domain followed by an Ig-like domain in the extracellular portion of the molecule, a single 23 amino-acid transmembrane helix and a 148 residue cytoplasmic tail (Figure 1A). CMG2 is highly conserved among species that express it (84 and 62% human versus mouse or zebrafish, respectively), but is only found in vertebrates. The only protein in the database with which it shares significant similarity is TEM8 (see Figure 2 for an alignment of the cytosolic tails).
Figure 1.
CMG2 structure and HFS mutations. Schematic representation of the CMG2 structure. The vWA domain corresponds to the crystal structure (1tzn) and the Ig-like domain to our recently published model (Deuquet et al, 2011). (A) Residues in blue represent the N-glycosylation sites and cysteine residues are shown in yellow. (B) The position and identity of reported extracellular missense HFS mutations are mapped onto the CMG2 model. The figure was generated using UCSF Chimera© software (Pettersen et al, 2004).
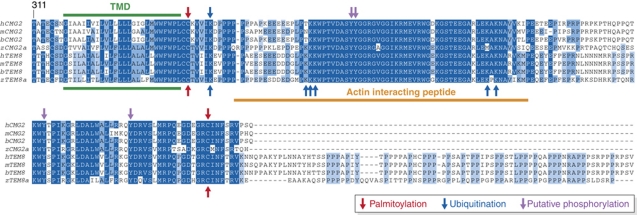
Figure 2.
The CMG2 cytoplasmic tail and post-translational modification sites. The cytosolic tails of human and zebrafish CMG2 and TEM8 were aligned to illustrate the high degree of identity between CMG2 and TEM8 and between species. The transmembrane domain is shown in green and the actin interacting TEM8 peptide in orange. Palmitoylation sites are indicated with red arrows, ubiquitination sites with blue arrows and potential phosphorylation sites in purple. While a requirement for CMG2 tyrosine phosphorylation has been demonstrated, the exact position of the tyrosine(s) has not been established. Modifications identified for CMG2 are shown above the alignment while modifications identified for TEM8 are shown at the bottom of the alignment. The alignment was generated with the Jalview software.
CMG2488 differs from CMG2489 only by their last 13 amino acids. CMG2386 is also a transmembrane protein with the same amino-acid composition as CMG2489, except that it lacks the Ig-like domain. As a consequence, it is retained in the ER (Bell et al, 2001; Deuquet et al, 2011). Finally, CMG2322 consists only of the signal peptide and the vWA domain, and is thus predicted to be secreted.
The CMG2 vWA domain and ligand binding
vWA domains are well-characterized protein–protein interaction domains found in some 500 human proteins, mostly involved in cell adhesion, such as extracellular matrix (ECM) proteins or integrins, in which this domain is called the inserted (I)-domain (Whittaker and Hynes, 2002). The CMG2 vWA domain harbours the typical features found in integrin I-domains (Figure 1A): a dinucleotide binding or Rossmann fold, with six α-helices surrounding a central six stranded β-sheet, creating a hydrophobic core between the closely packed sheet and helices (Shimaoka et al, 2002; Lacy et al, 2004). The structure of the TEM8 vWA domain is essentially the same (Fu et al, 2010). The top face of the vWA contains a metal ion-dependent adhesion site (MIDAS) motif (DXSXS…T…D, where X is any amino acid). The MIDASs of CMG2 and TEM8 are well conserved and can bind divalent cations such as Mg2+ or Ca2+ (Wigelsworth et al, 2004; Ramey et al, 2010). As in most I-domains (Springer, 2006), the C- and N-termini of the CMG2 vWA domain are in close proximity at the bottom face and are linked via a disulphide bond (Figure 1A).
So far, the only well-defined extracellular ligand for CMG2 and TEM8 is the anthrax PA. In-vitro PA binding studies in the presence of Mg2+ led to Kd values of 170 pM and 1.1 μM for CMG2 and TEM8 vWA domains, respectively (Wigelsworth et al, 2004; Scobie and Young, 2005). The affinity of PA for TEM8 is very similar to that of collagens for integrins (Shimaoka et al, 2002), in agreement with the fact that the toxin–receptor interaction to a large extent mimics the integrin-collagen binding, as shown by co-crystallization of PA with the CMG2 vWA domain (Lacy et al, 2004; Santelli et al, 2004).
The I-domains of integrins exist in two distinct conformations: open and closed, respectively, having high or low affinities for their ligand, due to structural changes in the MIDAS domain. To explain the differential affinity of PA for CMG2 and TEM8, and by analogy to integrins, it has been proposed that CMG2 is in an open state, while TEM8 is in equilibrium between open and closed states. The crystal structures of the isolated vWA domains of both CMG2 and TEM8, however, reveal an open conformation, with an acetate molecule mimicking the ligand (Lacy et al, 2004; Fu et al, 2010). The presence of non-conserved residues in the vWA–PA interface was in fact shown recently to be responsible for the difference in affinities of PA for TEM8 versus CMG2 (Fu et al, 2010). This does not exclude the fact that TEM8 can exist in different states at the surface of living cells, as recently shown using conformational antibodies (Yang et al, 2011). This might be equally true for CMG2 but has so far not been reported.
The immunoglobulin-like domain
The second extracellular domain of CMG2 is predicted to have an immunoglobulin (Ig) fold (Sun and Collier, 2010; Deuquet et al, 2011) and was modelled using fold recognition and manual modelling algorithms (Deuquet et al, 2011). As all Ig-like domains, it has a β-sandwich structure formed by two interacting antiparallel β-sheets with a Greek Key topology (Deuquet et al, 2011). This model suggested the existence of two disulphide bonds, the existence and identity of which were confirmed experimentally (Deuquet et al, 2011). The function of this domain is currently unknown, although the integrity of the disulphide bonds was shown to be necessary in assisting anthrax PA towards forming a transmembrane pore (Sun and Collier, 2010). The CMG2 Ig-like domain also contains two potential glycosylation sites that nicely localize to the surface of the domain (Figure 1A), at least one of which must be occupied as CMG2 was found to be glycosylated (Deuquet et al, 2009). Considering that a typical N-linked carbohydrate chain is almost as big as an Ig-like domain, this post-translational modification might well affect the folding or conformation of this domain.
The transmembrane domain and cytoplasmic tail of CMG2
Little if anything is known about the transmembrane domain (TMD) of CMG2, other than that it is two residues longer—23 versus 21—than the average single spanning membrane protein (Abrami et al, 2008b). Studies performed on the TMD of TEM8 suggest that it might self-associate into dimers or higher order structures (Go et al, 2006), a property that could well be shared by CMG2. We indeed found that mutations of cysteines in the CMG2 Ig-like domain lead to formation of disulphide bond formation between CMG2 molecules (Deuquet et al, 2011), supporting the notion that CMG2 might also self-assemble, or at least form clusters. The TMDs of both CMG2 and TEM8 are followed by two cysteine residues, which are sites of palmitoylation (Figures 1A and 2; Abrami et al, 2006) (see below).
The cytosolic tail of CMG2 has an unusual amino-acid composition with 40% charged residues, 15% proline residues and only 23% hydrophobic residues, compared with 19% charged, 3.5% proline and 33% hydrophobic for the extracellular domain. As such, the cytosolic tail of CMG2 is predicted as intrinsically disordered. Disordered domains, which constitute ≈30% of the human proteome, are thought to be important for regulatory processes (Dyson and Wright, 2005; Uversky and Dunker, 2010). Consistently, disordered regions are frequently associated with post-translational modifications, which allow them to interact with multiple partner proteins. Due to the low proportion of hydrophobic residues that generally trigger a hydrophobic collapse during protein folding, disordered domains are less compact than folded proteins (Marsh and Forman-Kay, 2010). For example, a domain the size of the CMG2 cytosolic tail (148 residues) would have an approximate dynamic radius of 20 Å if folded and of 30 Å if unstructured (Marsh and Forman-Kay, 2010). This allows the protein to sample an ≈2.4 × larger volume of cytoplasm (33 500 Å3 versus 113 100 Å3), thus increasing the probability of encounter with partner molecules. It has been shown for some disordered domains that they can undergo a disorder-to-order transition upon binding to specific interactors (Dyson and Wright, 2005; Uversky and Dunker, 2010). No structural studies are currently available for the cytosolic tail of CMG2. It has, however, been shown to interact with multiple partners during endocytosis (see below).
Similarities with integrins
CMG2 and TEM8 share some interesting properties with integrins, and could somehow be viewed as single chain integrins—integrins always being in the form of αβ dimers (Campbell and Humphries, 2011). Both proteins carry extracellular vWA domains involved in ligand binding that can potentially adopt an open or closed conformation, although this has not yet been shown for CMG2. The similarity is such that reproducing a mutation of a highly conserved phenylalanine residue in the αM integrin (Shimaoka et al, 2000) in TEM8 (F205W) led to the same effect: locking it into a high affinity state (Ramey et al, 2010). In contrast, mutation of T118A, in vicinity of the MIDAS, shifted the equilibrium to the inactive state (Ramey et al, 2010).
It has been extensively documented that the interaction of the integrin β-chain with actin governs the binding affinity of the α-chain for the ligand (Rose et al, 2007). Similarly, TEM8 was shown to interact with actin (Go et al, 2009; Abrami et al, 2010a; Yang et al, 2011) and the TEM8–actin interaction negatively correlate with the binding affinity of TEM8 for PA (Garlick and Mogridge, 2009; Go et al, 2009), that is, when binding of TEM8 to actin is reduced due to mutations in the cytosolic tail, the interaction with PA is enhanced. In reverse, binding of PA abolished the interaction of TEM8 with actin (Abrami et al, 2010a), again similarly to integrins (Campbell and Humphries, 2011). Thus, as integrins, TEM8 is able to mediate both outside-in (from PA to actin) and inside-out (from actin to the vWA domain) signalling. Whether the interaction with actin is direct or indirect is unclear. On one hand, a synthetic peptide corresponding to the actin binding segment of TEM8 (Figure 2) was found to bind and even bundle purified actin (Garlick and Mogridge, 2009)—suggesting a direct contact. On the other hand, not only actin, but also talin, vinculin and myosin II—components well known to be involved in the integrin–actin interaction—were detected in TEM8 pull-downs (Abrami et al, 2010a). The CMG2 cytosolic tail, although shorter by 73 residues than that of TEM8, contains the same actin interacting segment (Figure 2). Interaction with actin was, however, not readily detectable (Abrami et al, 2010a), suggesting that regulation of the CMG2–actin interaction (if such an interaction exists) differs from that of TEM8.
CMG2 and anthrax
As mentioned, both CMG2 and TEM8 were identified as receptors for anthrax PA, first using a gene recomplementation approach and then a similarity search (Bradley et al, 2001; Scobie et al, 2003). Recently, mice were generated lacking full-length CMG2, TEM8 or both (Liu et al, 2009). Experiments in which these mice were challenged with anthrax toxin or B. anthracis spores revealed that, while the lack of TEM8 had little effect on the sensitivity of the mice to intoxication/infection, the absence of CMG2 had a major effect—indicating that in mice, the main receptor is CMG2. This could be due either to the higher affinity of PA for CMG2 versus TEM8 (Liu et al, 2009), or to the fact that the cells important for infection and/or toxin induced death preferentially express CMG2.
In the anthrax context, the fundamental role of CMG2 and TEM8 is to bring the toxin to the appropriate location inside the cell. This requires a very precise timing of events since PA, once it has bound CMG2/TEM8 and has been processed by furin, must multimerize at the cell surface. Only then it can capture EF/LF from the extracellular medium and escort them to the interior of the cell. Internalization of monomeric PA in the absence of the enzymatic subunits is unproductive. CMG2 and TEM8 satisfy these timing requirements thanks to a series of well-coordinated post-translational modifications, such as palmitoylation and ubiquitination (see below).
In the absence of toxin, TEM8 is found in clusters at the plasma membrane, the organization of which requires the cortical actin cytoskeleton (Abrami et al, 2010a). Such clusters were not detected under the same conditions for CMG2 (Abrami et al, 2010a). This difference in behaviour was also apparent when analysing mobility of the two proteins in the membrane using fluorescence recovery after photobleaching (FRAP) (Abrami et al, 2010a). TEM8 showed a lower diffusion coefficient than CMG2 and a fraction of the protein was kept immobile in an actin-dependent manner (Abrami et al, 2010a).
Upon binding and oligomerization of PA, a conformational change is triggered in the TMDs and cytosolic tails of CMG2 and TEM8, which eventually leads to the activation of src-like kinases, which in turn phosphorylate CMG2/TEM8 (Abrami et al, 2010b; Figure 3). Phosphorylation of the receptor tail leads to the recruitment of the adaptor protein β-arrestin, which in turn allows binding of an E3 ubiquitin ligase that modifies the receptor on one or more cytosolic lysine residues. Mutations of five lysines were required to abrogate the ubiquitination signal of TEM8 (Figure 2), and the proto-oncogene cbl (Haglund and Dikic, 2005) was found to mediate the ubiquitination reaction (Abrami et al, 2008b, 2010a). In contrast, mutation of a single juxtamembranous lysine (Figure 2) was sufficient to prevent ubiquitination of CMG2 (Abrami et al, 2008b). The identity of the responsible E3 ligase has not been established for CMG2. Interestingly, the interaction of TEM8 with cbl was shown to require the redistribution of the receptor from the fluid plasma membrane regions to cholesterol-rich, lipid raft-like domains (Abrami et al, 2003, 2008b). The initial sequestration of TEM8 away from the cbl-containing raft-like domains to prevent premature ubiquitination is mediated by palmitoylation (Abrami et al, 2008b). This reversible post-translational modification consists in the addition of a C16 carbon acyl chain to one or more cytosolic cysteine residues, in particular juxtamembranous cysteines (Abrami et al, 2008b). Both CMG2 and TEM8 have been shown to undergo palmitoylation, possibly in the ER soon after synthesis but the responsible palmitoyltransferases have not been identified.
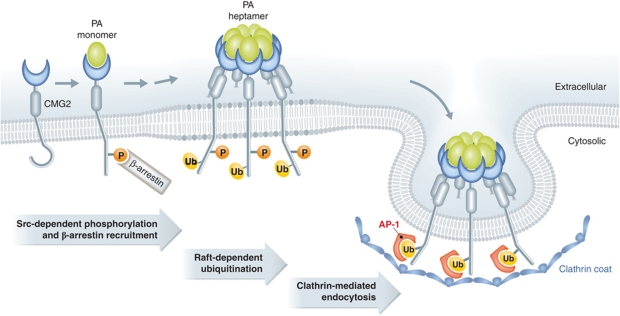
Figure 3.
Mechanisms of ligand-triggered CMG2 endocytosis. Anthrax protective antigen (PA) is the only well-characterized ligand of CMG2. Upon binding and oligomerization of PA to the CMG2 vWA domain, src-like kinases are activated on the cytosolic side leading to the phosphorylation of the CMG2 cytosolic tail. This allows the subsequent recruitment of the adaptor β-arresting, which in turns allow an E3 ubiquitin ligase, of so far unknown identity, to ubiquitinated CMG2 on a juxtamembranous lysine residue. Ubiquitination then triggers clathrin-dependent endocytosis of the CMG2–PA7mer complex in a manner that is dependent on the adaptor complex AP1. This clathrin-dependent endocytosis more over is dependent on the actin cytoskeleton.
Once ubiquitinated, the CMG2/TEM8 cytosolic tails trigger the formation of a clathrin coat in a manner dependent on the adaptor complex AP1 (Abrami et al, 2010a; Figure 3). This final clathrin-dependent endocytosis step critically depends on actin, irrespective of whether the receptor is CMG2 or TEM8 (Abrami et al, 2010a; Figure 3). The dependence of CMG2/TEM8-mediated uptake on AP1, instead of the more classical endocytic adaptor AP2 (Doherty and McMahon, 2009), on β-arrestin and on actin renders this ligand-triggered internalization route rather unconventional.
Upon arrival in endosomes, the PA multimers convert to their pore-forming conformation (Abrami et al, 2004). The pH threshold was shown to be lower for CMG2 than for TEM8 both in vivo (pH 5.0 for CMG2 versus pH 6.0 for TEM8) and in vitro (pH 5.7–5.8 for CMG2 versus pH 6.8–7.1 for TEM8) (Rainey et al, 2005; Scobie et al, 2007). Whether this difference in pH threshold actually affects the stage of the endocytic pathway where pore formation by PA occurs has not been directly addressed.
In HeLa cells, expressing almost exclusively TEM8 (Abrami et al, 2010a), pore formation was shown to occur in early endosomes. Interestingly, although membrane insertion of PA is known to lead to the translocation of EF/LF into the cytoplasm, the enzymatic subunits were found to remain associated with the early endosomes. Combined with the knowledge on receptor ubiquitination, these findings led to the proposal that upon arrival into early endosomes, the receptor–toxin complex is sorted into nascent intraluminal vesicles (Abrami et al, 2004), of this multivesicular organelle (Falguieres et al, 2009). The mechanisms of sorting were not further studied. However, it was shown that the toxin was subsequently transported to late endosomes from which release of LF occurs, presumably through the back fusion of intraluminal vesicles with the limiting membranes (Abrami et al, 2004; Sobo et al, 2007; Pons et al, 2008).
In the course of studies on anthrax toxin endocytosis, additional proteins have been found to interact with CMG2 and TEM8 and modulate toxin uptake, but their exact roles remain to be established. For one, in cells that express both receptors, CMG2 and TEM8 appear to interact with each other, either physically and/or genetically. RNAi against TEM8 was indeed shown to downregulate CMG2 at the protein, but not at the mRNA level (Abrami et al, 2008a). Then, a screen using expressed sequence tag (EST) technology to silence chromosomal genes and identify those that affect endocytosis of anthrax toxin led to the identification of two other interactors: the lipoprotein receptor-related 6 (LPR6) and ARAP3 (Lu et al, 2004; Wei et al, 2006).
LRP6 is a cell surface type I membrane protein that is well known for its role as a co-receptor during Wnt signalling. Upon binding of Wnt molecules to their Frizzled receptors, a ternary Wnt–Frizzled–LRP6 complex is formed leading to phosphorylation of the cytosolic tail of LRP6, an event required to transmit the Wnt signal to the nucleus. As a crucial component of Wnt signalling, LRP6 plays a role in many important cellular processes including embryogenesis, bone development and cell proliferation (Pinson et al, 2000; Tamai et al, 2000; Semenov et al, 2001). LRP6 can interact with both CMG2 and TEM8 receptors (Wei et al, 2006; Abrami et al, 2008a) and it appears that the cellular levels of CMG2/TEM8 regulate the levels of LRP6 at the protein level (Abrami et al, 2008a). Both silencing and overexpression of CMG2/TEM8 indeed lead to a post-translational downregulation of LRP6. Moreover, PA binding and oligomerization leads to tyrosine phosphorylation of LRP6 (in contrast to Wnt binding which leads to serine phosphorylation; Zeng et al, 2008), redistribution of LRP6 to raft-like domains and endocytosis (Abrami et al, 2008a). Thus, LRP6 appears to be part of the toxin–receptor complex upon endocytosis. Although LRP6 is not essential for anthrax toxin endocytosis (Young et al, 2007), silencing of LRP6 did delay the toxin uptake process, indicating that it might have a regulatory role (Abrami et al, 2008a).
ARAP3 is a multidomain cytosolic phosphoinositide-binding protein that also harbours two GTPase-activating protein (GAP) domains for the small GTPases Arf6 and RhoA, respectively (Krugmann et al, 2002). RhoA is a well-known regulator of actin dynamics, while Arf6 functions mainly in endocytic recycling (Takai et al, 2001). ARAP3 has been implicated in various processes such as cell adhesion and angiogenesis (Gambardella et al, 2010, 2011). Its role in endocytosis of CMG2 and TEM8 has however not been addressed.
Finally, not only do CMG2 and TEM8 share similarities with integrins, two recent studies suggest that they can be found together in a multicomponent complex (Jinnin et al, 2008; Martchenko et al, 2010). When studying RAW macrophages, which exclusively express CMG2, sensitivity towards anthrax toxin was found to be strongly affected by pre-incubation with anti-β1 integrin antibodies (Martchenko et al, 2010). Not only was α5β1 integrin found to be capable of binding PA in vitro with affinities similar to that of PA for TEM8, but β1 integrin was also found to co-internalize with PA. This study suggests that β1 integrin may modulate CMG2 function.
Evidence that TEM8 interacts with β1 integrin comes from a study on the genetic causes of infantile haemangioma (Jinnin et al, 2008). β1 integrin was found to interact with vascular endothelial growth factor receptor 2 (VEGFR2; Jinnin et al, 2008) and formation of this complex at the cell surface upon VEGF binding controls transcription of VEGFR1 (Roberts et al, 2004). Analysis of patient endothelial cells revealed a mutation in TMD of TEM8 (A326T) as well as mutations in VEGFR2, which were found to enhance the formation of a ternary complex β1 integrin–VEGFR2–TEM8, which in turn triggered a decrease in VEGFR1 expression (Jinnin et al, 2008).
Physiological role of CMG2
The physiological roles of CMG2 and TEM8 are still rather poorly understood. Knock-out mice have been generated. While these mice have been very interesting in terms of anthrax infection, they have provided little information regarding the functions of the proteins as no striking phenotypes could be observed (Liu et al, 2009), with the exception of an excess of ECM in various tissues of TEM8−/− mice (Cullen et al, 2009). Also, the double knock-out mutant was viable (Liu et al, 2009).
Several observations, however, clearly point towards a role in angiogenesis. CMG2 was identified in a screen for genes upregulated during angiogenesis and TEM8 is a tumour endothelial marker. As mentioned above, a mutation in TEM8 was associated with infantile haemangioma (Jinnin et al, 2008). A primary cause of HFS patient symptoms might be changes in the basal membrane of capillary vessels (Stucki et al, 2001). More recently, two studies have revealed a role of both CMG2 (Reeves et al, 2009) and TEM8 (Verma et al, 2011) in angiogenesis.
In mice, CMG2 was found to be expressed in endothelial cells of the lung, intestine, all layers of the skin, on smooth muscle cells, and in the vascular endothelium (Reeves et al, 2009). CMG2 expression was also found in both normal (quiescent) and malignant (angiogenic) vasculature in human tissue. In-vitro studies using Human Umbilical Vein Endothelial Cells (HUVECs) showed that while silencing cmg2 expression using an shRNA vector did not affect cell migration, it reduced cell proliferation. Moreover, cmg2 silencing decreased the ability of HUVECs to form a capillary network in 3D matrices (Reeves et al, 2009).
The role of TEM8 was addressed in the chicken choriallantoic membrane (CAM), since TEM8 but not CMG2 showed temporal expression during chick development (Verma et al, 2011). Consistent with the documented interaction of TEM8 with LRP6 (Wei et al, 2006; Abrami et al, 2008a), TEM8 was found to be a modulator of Wnt-dependent vascular morphogenesis in this system.
Despite these data, the exact functions of CMG2 and TEM8 at the cellular levels are still quite unclear. The identity of their preferential ligands needs to be firmly established. TEM8 has been found to bind collagens type VI (Nanda et al, 2004) and I (Hotchkiss et al, 2005), whereas CMG2 has been proposed to bind collagen type IV and laminin (Bell et al, 2001). The consequences of CMG2/TEM8–ligand interactions are unclear. Upon binding of cells to collagen I-coated plates, TEM8 was found to promote adhesion and spreading, in a manner dependent on its interaction with actin (Werner et al, 2006). On the other hand, the studies on anthrax toxin endocytosis, combined with the fact that pathogens are highly opportunistic organisms, suggest that CMG2 and TEM8 can signal upon ligand binding, and are able to rapidly traffic their ligand to degradation. Clearly, future studies are required to clarify the exact cellular roles of both proteins.
HFS and CMG2
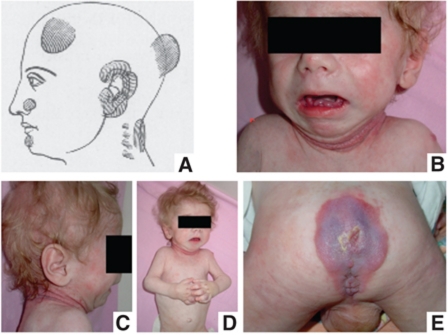
The first medical case of HFS was reported in 1903 (Whitfield and Robinson, 1903; Figure 4). The disease was first called Molluscum Fibrosum, then Juvenile Hyaline Fibromatosis and Infantile Systemic Hyalinosis (Drescher et al, 1967; Landing and Nadorra, 1986) and since it was realized that these were different manifestations of the same disease (Glover et al, 1991), the unifying term, Hyaline Fibromatosis Syndrome (El-Kamah, 2009), has been adopted. Some 150 medical cases have been reported showing no geographical or ethnic predisposition.
Figure 4.
Clinical presentation of HFS syndrome. (A) Whitfield's paper. (B–E) Images of a 10-month-old child with Infantile Hyalinosis show typical changes at a relatively early stage of the disease. Image (D) shows the flexed attitude (because of pain in the large joints) and the swollen interphalangeal joints. The skin over the interphalangeal joints is beginning to swell and will eventually develop into nodules. Erythema with pearly papules is developing in the skinfolds around the neck (BC). There is generalized erythema around the perineal and sacral region (the area of pressure) and in the perianal region the hallmark of the disease (E), the nodular tumours, are already developing. Similar tissue proliferations are developing over the gingiva (A).
HFS largely affects connective tissue, and its initial names refer to the accumulation of hyaline material in the skin and other organs (Landing and Nadorra, 1986; Fayad et al, 1987; Keser et al, 1999). HFS is a progressive, disfiguring and disabling disease, the onset of which can vary between birth and late childhood (Figure 4). ISH is the more severe form.
Affected newborns and infants do not show gross morphological defects. They may show thickening of the skin and of the large joints, flexural contractures of the large joints. A hypotonic, frog-legged position and poor development of the muscles are common features, due to reduced movements. Patients afflicted with ISH present a heightened susceptibility to bone fracture, chest infections and diarrhoea, which generally lead to death before the age of 2 years. Musculoskeletal imaging may show osteopaenia, multiple cystic osteolytic bone destruction and decalcification, with osteoporosis (Eich et al, 1998; Pirgon et al, 2007; Figure 4). Mental development, hearing and eyesight are described to be normal (Stucki et al, 2001). JHF has a later onset, does not show diarrhoea and patients may live well into adulthood.
Non-cancerous tissue proliferations and nodules are the most outstanding external features found for all patients (Figure 4) and are the hallmark of the disease. Interestingly, they are not present in the newborn but may develop over the first month of life. Nodules preferentially develop over points of mechanical stress or pressure, suggesting that HFS pathogenesis could be due to a disregulation of repair mechanisms after microtrauma (Urbina et al, 2004). The nodules were found to be rather cellular at the onset, containing elongated fibroblast-like cells embedded in an eosinophilic ECM and some thin-walled vessels, whereas older nodules contained mainly ECM (Mayer-da-Silva et al, 1988).
Only few studies have addressed the composition of patient nodules. An increase of two ECM components, which are crucial for the resistance of connective tissues to mechanical stress, have been found in nodules: collagens and glycosaminoglycans (GAGs). Collagens I (Tanaka et al, 2009) and VI were more abundant in nodules than in normal skin of the same patient (Murata et al, 1987; Glover et al, 1991; Katagiri et al, 1996; Tanaka et al, 2009). Changes in GAGs were also observed (Kitano et al, 1972; Breier et al, 1997; Iwata et al, 1980; Tzellos et al, 2009). In particular, a decrease in hyaluronic acid was found, likely due to a lower expression of hyaluronic acid synthases (Tzellos et al, 2009). These observations indicate that synthesis or degradation of ECM is abnormal in HFS patients.
Altogether, the clinical observations show that CMG2 is not essential during embryogenesis, and suggest that it might be important for tissue repair and integrity, possibly by controlling the homeostasis of the ECM.
Cmg2 mutations and their consequences
Due to the frequent occurrence in siblings from unaffected parents, HFS was thought to be an inherited recessive disorder. A genome-wide linkage search on four JHF affected individuals showed that the responsible gene localized at the chromosome position 4q21 (Rahman et al, 2002). Using positional cloning, cmg2 was subsequently found to be the affected gene (Dowling et al, 2003; Hanks et al, 2003). There is no evidence to support genetic heterogeneity in HFS, with a mutation on a second gene, since all analysed families so far showed linkage and/or mutation involving CMG2 as the causative agent.
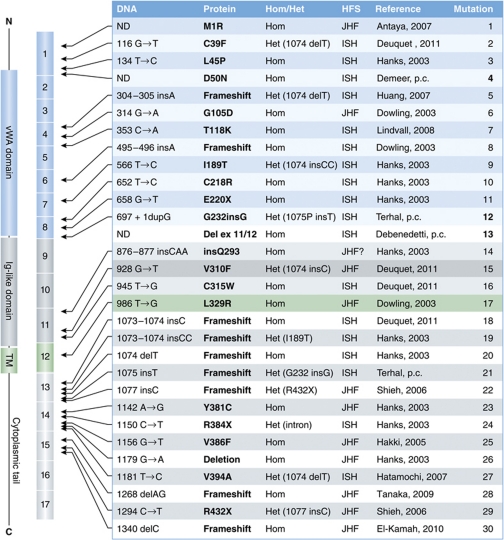
Some 34 different mutations have been identified, predominantly in exons and spread from exon 1 to exon 15 (Figure 5). Although most mutations have been identified only once, there is a mutational hotspot in exon 13 (positions 1074–1077) at which insertion of one or two bases or deletion of one base have been repeatedly reported (Figure 5, mutation/patient 18–22). Interestingly, some 40% of the reported patients are compound heterozygous, indicating that CMG2 mutations may not be extremely rare. These patients generally carry one allele with a modification at the exon 13 hotspot and one allele with a missense or non-sense mutation (Figure 5, mutation/patient 2, 5, 9, 12, 15, 27, 29).
Figure 5.
Hyaline Fibromatosis Syndrome mutations. The table summarizes the reported HFS mutations with the exception of the mutations found in introns. The protein structure is schematically shown on the far left, followed by the corresponding exons encoding these different protein domains. Hom=homozygous, Het=homozygous, ND=not determined, p.c.=personal communication.
So far, mutations can be classified into four major classes. Class I is composed of missense mutations in the vWA domain, such as D50N (own observation) and T118K (Lindvall et al, 2008; Figures 1B and 5), that do not affect plasma membrane targeting but impair ligand binding. Class II contains all other missense mutation in exons 1–11 (Figures 1B and 5, mutation/patient 2–16). With the exception probably of M1R where the initiating methionine is missing (Figure 5, mutation/patient 1), class II mutations affect the folding/stability of either the vWA domain or the Ig-like domain (Deuquet et al, 2009, 2011). In the case of Ig-like domain mutations, the induced folding defects prevent the formation of native intramolecular disulphide bonds leading to the formation of disulphide-linked high molecular weight complexes (Deuquet et al, 2011). As a consequence, the newly synthesized mutated CMG2 proteins are recognized by the ER quality control system and retained (Deuquet et al, 2009, 2011). For the rare cases where patient fibroblasts were available (Figure 5, patients 2 and 9; Deuquet et al, 2011), we found that ER retention was followed by rapid targeting to the ER degradation (ERAD) pathway. This, however, was not as efficient in a tissue culture transient expression system (Deuquet et al, 2009, 2011). Thus, it is unclear at present whether different ectodomain mutations are equally recognized by ER quality control. The efficiency of ERAD targeting might differ between class II mutations, an issue that is worth investigation in the context of personalized treatment (see below). This class of mutations also contains the G232insG mutation, corresponding to a three base insertion at position 697 on the gene. The resulting amino-acid insertion leads to an out-of-register β-strand in the Ig-like domain that therefore fails to fold properly (Deuquet et al, 2011). Finally, class II also includes the only reported mutation in the TMD L329R, corresponding to a missense mutation in exon 12 (Deuquet et al, 2009). While this domain does not appear to affect the folding of the ectodomain, it is recognized by the ER quality control as problematic, either because of the presence of a charged residue in the membrane or because this mutation affects quaternary assembly that would be required for ER exit.
Class III contains all frameshift mutations that lead to a premature stop codon (Figure 5, mutations 5, 8, 12, 18–22) as well as probably splicing mutations. These mutations are either predicted (mutations 5 and 8) or have been shown (all others) to lead to unstable mRNAs that are rapidly degraded (Tanaka et al, 2009; Deuquet et al, 2011, and own observations). As a consequence, the CMG2 protein was undetectable in cells from the few patients tested (Deuquet et al, 2011). This class may also contain mutations R384X and R432X, which lead to premature stops. This class also contains patient 13 (Figure 5) in which an unidentified mutation is a responsible for a splicing defect that leads to the deletion of exons 11 and 12. Finally, the three reported mutations that map to introns (IVS13+1G>A; IVS9+2T>C; IVS8+1G>A) are also likely to lead to premature stop codons (Dowling et al, 2003; Hanks et al, 2003).
The last class, class IV, contains the few reported missense mutations reported to map to the cytosolic tail of CMG2 (Y381C, V386F, V394A; Figure 5, mutations 23, 25, 27). These mutations are not expected to affect the mRNA level, nor the plasma membrane targeting of the protein. The prediction is therefore that these mutations will affect CMG2 function. For TEM8, the mutation corresponding to Y381C (Y383C) led to a diminished binding to actin and a concomitant increase in binding of anthrax PA (Go et al, 2009).
In terms of genotype–phenotype correlations, it appears that missense mutations in exons 1–12 generally, but not always, lead to the severe form of the disease as do mutations that lead to premature stop codons. In contrast, missense mutation in exons 13–17 correlates with the mild form of the disease, as exemplified by Patient 23 (Figure 5).
The diversity of the molecular consequences of cmg2 mutations clearly illustrates that the exact patient mutations need to be determined and analysed before any curative treatment can be envisioned. We found that the proteasome inhibitor, bortezomib also known as Velcade®, can rescue the class II CMG2 mutants tested, even in patients cells, leading to plasma membrane targeting of the protein (Deuquet et al, 2011). Rescued surface CMG2 was moreover capable of signalling in response to anthrax PA, indicating that it was functional, at least regarding this pathological role (Deuquet et al, 2011). These promising initial studies suggest that components of the ERAD machinery, not restricted to the proteasome itself, are interesting potential therapeutic targets for this class of mutations, as they are for a number of other conformational diseases such as cystic fibrosis. It will be of interesting to investigate whether HFS class III mutations, at least those where the transmembrane CMG2 protein is preserved, can be rescued using inhibitors of the non-sense mediated mRNA degradation pathway.
Concluding remarks
The cmg2 gene is ‘10-year old’ (Bell et al, 2001). During this period, tremendous progress has been made. It was found to be responsible for anthrax infection (Scobie et al, 2003; Liu et al, 2010) as well as for HFS (Dowling et al, 2003; Hanks et al, 2003). Some of the underlying molecular mechanisms have been described in detail: how CMG2 mediates entry of the anthrax toxin (van der Goot and Young, 2009), and how mutations in cmg2 affect the CMG2 protein (Deuquet et al, 2009, 2011). Still, years to come promise to be exciting since they should reveal the ‘good side’ of this protein, what it really does, during development (Verma et al, 2011), during angiogenesis (Reeves et al, 2009) and in normal adult life.
Acknowledgments
We are very grateful to the patients and their parents for supporting our efforts. We thank the Associazione ISI (http://www.associazioneisi.it) for linking patients as families suffering for HFS. A warm thank you to Mirko Bischofberger for generating Figure 3 and Thomas Lemmin for Figure 1. This work was supported by the Swiss National Science Foundation (GvdG), the Fondation Telethon Action Suisse, the Fondation SANTE-Vaduz/Aide au soutien des nouvelles therapies and the Gebert Rüf Stiftung.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abrami L, Bischofberger M, Kunz B, Groux R, van der Goot FG (2010a) Endocytosis of the anthrax toxin is mediated by clathrin, actin and unconventional adaptors. PLoS Pathog 6: e1000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrami L, Kunz B, Deuquet J, Bafico A, Davidson G, van der Goot FG (2008a) Functional interactions between anthrax toxin receptors and the WNT signalling protein LRP6. Cell Microbiol 10: 2509–2519 [DOI] [PubMed] [Google Scholar]
- Abrami L, Kunz B, Iacovache I, van der Goot FG (2008b) Palmitoylation and ubiquitination regulate exit of the Wnt signaling protein LRP6 from the endoplasmic reticulum. Proc Natl Acad Sci USA 105: 5384–5389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrami L, Kunz B, van der Goot FG (2010b) Anthrax toxin triggers the activation of src-like kinases to mediate its own uptake. Proc Natl Acad Sci USA 107: 1420–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrami L, Leppla SH, van der Goot FG (2006) Receptor palmitoylation and ubiquitination regulate anthrax toxin endocytosis. J Cell Biol 172: 309–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrami L, Lindsay M, Parton RG, Leppla SH, van der Goot FG (2004) Membrane insertion of anthrax protective antigen and cytoplasmic delivery of lethal factor occur at different stages of the endocytic pathway. J Cell Biol 166: 645–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrami L, Liu S, Cosson P, Leppla SH, van der Goot FG (2003) Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J Cell Biol 160: 321–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SE, Mavila A, Salazar R, Bayless KJ, Kanagala S, Maxwell SA, Davis GE (2001) Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci 114: 2755–2773 [DOI] [PubMed] [Google Scholar]
- Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA (2001) Identification of the cellular receptor for anthrax toxin. Nature 414: 225–229 [DOI] [PubMed] [Google Scholar]
- Breier F, Fang-Kircher S, Wolff K, Jurecka W (1997) Juvenile hyaline fibromatosis: impaired collagen metabolism in human skin fibroblasts. Arch Dis Child 77: 436–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell ID, Humphries MJ (2011) Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol 3: pii: a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen M, Seaman S, Chaudhary A, Yang MY, Hilton MB, Logsdon D, Haines DC, Tessarollo L, St Croix B (2009) Host-derived tumor endothelial marker 8 promotes the growth of melanoma. Cancer Res 69: 6021–6026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuquet J, Abrami L, Difeo A, Ramirez MC, Martignetti JA, van der Goot FG (2009) Systemic hyalinosis mutations in the CMG2 ectodomain leading to loss of function through retention in the endoplasmic reticulum. Hum Mutat 30: 583–589 [DOI] [PubMed] [Google Scholar]
- Deuquet J, Lausch E, Guex N, Abrami L, Salvi S, Lakkaraju A, Ramirez MC, Martignetti JA, Rokicki D, Bonafe L, Superti-Furga A, van der Goot FG (2011) Hyaline fibromatosis syndrome inducing mutations in the ectodomain of anthrax toxin receptor 2 can be rescued by proteasome inhibitors. EMBO Mol Med 3: 208–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT (2009) Mechanisms of endocytosis. Annu Rev Biochem 78: 857–902 [DOI] [PubMed] [Google Scholar]
- Dowling O, Difeo A, Ramirez MC, Tukel T, Narla G, Bonafe L, Kayserili H, Yuksel-Apak M, Paller AS, Norton K, Teebi AS, Grum-Tokars V, Martin GS, Davis GE, Glucksman MJ, Martignetti JA (2003) Mutations in capillary morphogenesis gene-2 result in the allelic disorders juvenile hyaline fibromatosis and infantile systemic hyalinosis. Am J Hum Genet 73: 957–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drescher E, Wyke S, Markiewicz C, Tegi S (1967) Juvenile fibromatosis in siblings (fibromatosis hyalinica multiplex juvenilis). J Pediatr Surg 2: 427–430 [Google Scholar]
- Duesbery NS, Webb CP, Leppla SH, Gordon VM, Klimpel KR, Copeland TD, Ahn NG, Oskarsson MK, Fukasawa K, Paull KD, Vande Woude GF (1998) Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280: 734–737 [DOI] [PubMed] [Google Scholar]
- Dyson HJ, Wright PE (2005) Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol 6: 197–208 [DOI] [PubMed] [Google Scholar]
- Eich GF, Hoeffel JC, Tschappeler H, Gassner I, Willi UV (1998) Fibrous tumours in children: imaging features of a heterogeneous group of disorders. Pediatr Radiol 28: 500–509 [DOI] [PubMed] [Google Scholar]
- El-Kamah GY, Fong K, El-Ruby M, Afifi HH, Clements SE, Lai-Cheong JE, Amr K, El-Darouti M, McGrath JA (2010) Spectrum of mutations in the ANTXR2 (CMG2) gene in infantile systemic hyalinosis and juvenile hyaline fibromatosis. Br J Dermatol 163: 213–215 [DOI] [PubMed] [Google Scholar]
- Falguieres T, Luyet PP, Gruenberg J (2009) Molecular assemblies and membrane domains in multivesicular endosome dynamics. Exp Cell Res 315: 1567–1573 [DOI] [PubMed] [Google Scholar]
- Fayad MN, Yacoub A, Salman S, Khudr A, Der Kaloustian VM (1987) Juvenile hyaline fibromatosis: two new patients and review of the literature. Am J Med Genet 26: 123–131 [DOI] [PubMed] [Google Scholar]
- Fu S, Tong X, Cai C, Zhao Y, Wu Y, Li Y, Xu J, Zhang XC, Xu L, Chen W, Rao Z (2010) The structure of tumor endothelial marker 8 (TEM8) extracellular domain and implications for its receptor function for recognizing anthrax toxin. PLoS One 5: e11203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella L, Anderson KE, Nussbaum C, Segonds-Pichon A, Margarido T, Norton L, Ludwig T, Sperandio M, Hawkins PT, Stephens L, Vermeren S (2011) The GTPase-activating protein ARAP3 regulates chemotaxis and adhesion-dependent processes in neutrophils. Blood 118: 1087–1098 [DOI] [PubMed] [Google Scholar]
- Gambardella L, Hemberger M, Hughes B, Zudaire E, Andrews S, Vermeren S (2010) PI3K signaling through the dual GTPase-activating protein ARAP3 is essential for developmental angiogenesis. Sci Signal 3: ra76. [DOI] [PubMed] [Google Scholar]
- Garlick KM, Mogridge J (2009) Direct interaction between anthrax toxin receptor 1 and the actin cytoskeleton. Biochemistry 48: 10577–10581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover MT, Lake BD, Atherton DJ (1991) Infantile systemic hyalinosis: newly recognized disorder of collagen? Pediatrics 87: 228–234 [PubMed] [Google Scholar]
- Go MY, Chow EM, Mogridge J (2009) The cytoplasmic domain of anthrax toxin receptor 1 affects binding of the protective antigen. Infect Immun 77: 52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go MY, Kim S, Partridge AW, Melnyk RA, Rath A, Deber CM, Mogridge J (2006) Self-association of the transmembrane domain of an anthrax toxin receptor. J Mol Biol 360: 145–156 [DOI] [PubMed] [Google Scholar]
- Haglund K, Dikic I (2005) Ubiquitylation and cell signaling. EMBO J 24: 3353–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S, Adams S, Douglas J, Arbour L, Atherton DJ, Balci S, Bode H, Campbell ME, Feingold M, Keser G, Kleijer W, Mancini G, McGrath JA, Muntoni F, Nanda A, Teare MD, Warman M, Pope FM, Superti-Furga A, Futreal PA et al. (2003) Mutations in the gene encoding capillary morphogenesis protein 2 cause juvenile hyaline fibromatosis and infantile systemic hyalinosis. Am J Hum Genet 73: 791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss KA, Basile CM, Spring SC, Bonuccelli G, Lisanti MP, Terman BI (2005) TEM8 expression stimulates endothelial cell adhesion and migration by regulating cell-matrix interactions on collagen. Exp Cell Res 305: 133–144 [DOI] [PubMed] [Google Scholar]
- Iwata S, Horiuchi R, Maeda H, Ishikawa H (1980) Systemic hyalinosis or juvenile hyaline fibromatosis. Ultrastructural and biochemical study of cultured skin fibroblasts. Arch Dermatol Res 267: 115–121 [DOI] [PubMed] [Google Scholar]
- Jinnin M, Medici D, Park L, Limaye N, Liu Y, Boscolo E, Bischoff J, Vikkula M, Boye E, Olsen BR (2008) Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med 14: 1236–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri K, Takasaki S, Fujiwara S, Kayashima K, Ono T, Shinkai H. (1996) Purification and structural analysis of extracellular matrix of a skin tumor from a patient with juvenile hyaline fibromatosis. J Dermatol Sci 13: 37–48 [DOI] [PubMed] [Google Scholar]
- Keser G, Karabulut B, Oksel F, Calli C, Ustun EE, Akalin T, Kocanaogullari H, Gumudis G, Doganavsargil E (1999) Two siblings with juvenile hyaline fibromatosis: case reports and review of the literature. Clin Rheumatol 18: 248–252 [DOI] [PubMed] [Google Scholar]
- Kitano Y, Horiki M, Aoki T, Sagami S (1972) Two cases of juvenile hyalin fibromatosis. Some histological, electron microscopic, and tissue culture observations. Arch Dermatol 106: 877–883 [PubMed] [Google Scholar]
- Krugmann S, Anderson KE, Ridley SH, Risso N, McGregor A, Coadwell J, Davidson K, Eguinoa A, Ellson CD, Lipp P, Manifava M, Ktistakis N, Painter G, Thuring JW, Cooper MA, Lim ZY, Holmes AB, Dove SK, Michell RH, Grewal A et al. (2002) Identification of ARAP3, a novel PI3K effector regulating both Arf and Rho GTPases, by selective capture on phosphoinositide affinity matrices. Mol Cell 9: 95–108 [DOI] [PubMed] [Google Scholar]
- Lacy DB, Wigelsworth DJ, Scobie HM, Young JA, Collier RJ (2004) Crystal structure of the von Willebrand factor A domain of human capillary morphogenesis protein 2: an anthrax toxin receptor. Proc Natl Acad Sci USA 101: 6367–6372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landing BH, Nadorra R (1986) Infantile systemic hyalinosis: report of four cases of a disease, fatal in infancy, apparently different from juvenile systemic hyalinosis. Pediatr Pathol 6: 55–79 [DOI] [PubMed] [Google Scholar]
- Leppla SH (1982) Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci USA 79: 3162–3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall LE, Kormeili T, Chen E, Ramirez MC, Grum-Tokars V, Glucksman MJ, Martignetti JA, Zaragoza MV, Dyson SW (2008) Infantile systemic hyalinosis: case report and review of the literature. J Am Acad Dermatol 58: 303–307 [DOI] [PubMed] [Google Scholar]
- Liu S, Crown D, Miller-Randolph S, Moayeri M, Wang H, Hu H, Morley T, Leppla SH (2009) Capillary morphogenesis protein-2 is the major receptor mediating lethality of anthrax toxin in vivo. Proc Natl Acad Sci USA 106: 12424–12429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Miller-Randolph S, Crown D, Moayeri M, Sastalla I, Okugawa S, Leppla SH (2010) Anthrax toxin targeting of myeloid cells through the CMG2 receptor is essential for establishment of Bacillus anthracis infections in mice. Cell Host Microbe 8: 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Wei W, Kowalski PE, Chang AC, Cohen SN (2004) EST-based genome-wide gene inactivation identifies ARAP3 as a host protein affecting cellular susceptibility to anthrax toxin. Proc Natl Acad Sci USA 101: 17246–17251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh JA, Forman-Kay JD (2010) Sequence determinants of compaction in intrinsically disordered proteins. Biophys J 98: 2383–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martchenko M, Jeong SY, Cohen SN (2010) Heterodimeric integrin complexes containing beta1-integrin promote internalization and lethality of anthrax toxin. Proc Natl Acad Sci USA 107: 15583–15588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-da-Silva A, Poiares-Baptista A, Guerra Rodrigo F, Teresa-Lopes M (1988) Juvenile hyaline fibromatosis. A histologic and histochemical study. Arch Pathol Lab Med 112: 928–931 [PubMed] [Google Scholar]
- Moayeri M, Leppla SH (2009) Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol Aspects Med 30: 439–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K, Motoyama T, Suka M, Ohno M, Kuboki Y (1987) High production of type VI collagen in multiple fibromatosis with multiple articular dysplasia. Biochem Biophys Res Commun 147: 275–281 [DOI] [PubMed] [Google Scholar]
- Nanda A, Carson-Walter EB, Seaman S, Barber TD, Stampfl J, Singh S, Vogelstein B, Kinzler KW, St Croix B (2004) TEM8 interacts with the cleaved C5 domain of collagen alpha 3(VI). Cancer Res 64: 817–820 [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25: 1605–1612 [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC (2000) An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407: 535–538 [DOI] [PubMed] [Google Scholar]
- Pirgon O, Atabek ME, Esen HH, Cangul H (2007) Infantile systemic hyalinosis with early thyroid dysfunction. J Pediatr Endocrinol Metab 20: 833–836 [DOI] [PubMed] [Google Scholar]
- Pons V, Luyet PP, Morel E, Abrami L, van der Goot FG, Parton RG, Gruenberg J (2008) Hrs and SNX3 functions in sorting and membrane invagination within multivesicular bodies. PLoS Biol 6: e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman N, Dunstan M, Teare MD, Hanks S, Edkins SJ, Hughes J, Bignell GR, Mancini G, Kleijer W, Campbell M, Keser G, Black C, Williams N, Arbour L, Warman M, Superti-Furga A, Futreal PA, Pope FM (2002) The gene for juvenile hyaline fibromatosis maps to chromosome 4q21. Am J Hum Genet 71: 975–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey GJ, Wigelsworth DJ, Ryan PL, Scobie HM, Collier RJ, Young JA (2005) Receptor-specific requirements for anthrax toxin delivery into cells. Proc Natl Acad Sci USA 102: 13278–13283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramey JD, Villareal VA, Ng C, Ward SC, Xiong JP, Clubb RT, Bradley KA (2010) Anthrax toxin receptor 1/tumor endothelial marker 8: mutation of conserved inserted domain residues overrides cytosolic control of protective antigen binding. Biochemistry 49: 7403–7410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves CV, Dufraine J, Young JA, Kitajewski J (2009) Anthrax toxin receptor 2 is expressed in murine and tumor vasculature and functions in endothelial proliferation and morphogenesis. Oncogene 29: 789–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DM, Kearney JB, Johnson JH, Rosenberg MP, Kumar R, Bautch VL (2004) The vascular endothelial growth factor (VEGF) receptor Flt-1 (VEGFR-1) modulates Flk-1 (VEGFR-2) signaling during blood vessel formation. Am J Pathol 164: 1531–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose DM, Alon R, Ginsberg MH (2007) Integrin modulation and signaling in leukocyte adhesion and migration. Immunol Rev 218: 126–134 [DOI] [PubMed] [Google Scholar]
- Santelli E, Bankston LA, Leppla SH, Liddington RC (2004) Crystal structure of a complex between anthrax toxin and its host cell receptor. Nature 430: 905–908 [DOI] [PubMed] [Google Scholar]
- Scobie HM, Marlett JM, Rainey GJ, Lacy DB, Collier RJ, Young JA (2007) Anthrax toxin receptor 2 determinants that dictate the pH threshold of toxin pore formation. PLoS One 2: e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scobie HM, Rainey GJ, Bradley KA, Young JA (2003) Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci USA 100: 5170–5174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scobie HM, Young JA (2005) Interactions between anthrax toxin receptors and protective antigen. Curr Opin Microbiol 8: 106–112 [DOI] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X (2001) Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol 11: 951–961 [DOI] [PubMed] [Google Scholar]
- Shimaoka M, Shifman JM, Jing H, Takagi J, Mayo SL, Springer TA (2000) Computational design of an integrin I domain stabilized in the open high affinity conformation. Nat Struct Biol 7: 674–678 [DOI] [PubMed] [Google Scholar]
- Shimaoka M, Takagi J, Springer TA (2002) Conformational regulation of integrin structure and function. Annu Rev Biophys Biomol Struct 31: 485–516 [DOI] [PubMed] [Google Scholar]
- Sobo K, Le Blanc I, Luyet PP, Fivaz M, Ferguson C, Parton RG, Gruenberg J, van der Goot FG (2007) Late endosomal cholesterol accumulation leads to impaired intra-endosomal trafficking. PLoS One 2: e851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA (2006) Complement and the multifaceted functions of VWA and integrin I domains. Structure 14: 1611–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucki U, Spycher MA, Eich G, Rossi A, Sacher P, Steinmann B, Superti-Furga A (2001) Infantile systemic hyalinosis in siblings: clinical report, biochemical and ultrastructural findings, and review of the literature. Am J Med Genet 100: 122–129 [DOI] [PubMed] [Google Scholar]
- Sun J, Collier RJ (2010) Disulfide bonds in the ectodomain of anthrax toxin receptor 2 are required for the receptor-bound protective-antigen pore to function. PLoS One 5: e10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Matozaki T (2001) Small GTP-binding proteins. Physiol Rev 81: 153–208 [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X (2000) LDL-receptor-related proteins in Wnt signal transduction. Nature 407: 530–535 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Ebihara T, Kusubata M, Adachi E, Arai M, Kawaguchi N, Utsunomiya J, Miki Y, Hiramoto M, Hattori S, Irie S (2009) Abnormal collagen deposition in fibromas from patient with juvenile hyaline fibromatosis. J Dermatol Sci 55: 197–200 [DOI] [PubMed] [Google Scholar]
- Tzellos TG, Dionyssopoulos A, Klagas I, Karakiulakis G, Lazaridis L, Papakonstantinou E (2009) Differential glycosaminoglycan expression and hyaluronan homeostasis in juvenile hyaline fibromatosis. J Am Acad Dermatol 61: 629–638 [DOI] [PubMed] [Google Scholar]
- Urbina F, Sazunic I, Murray G (2004) Infantile systemic hyalinosis or juvenile hyaline fibromatosis? Pediatr Dermatol 21: 154–159 [DOI] [PubMed] [Google Scholar]
- Uversky VN, Dunker AK (2010) Understanding protein non-folding. Biochim Biophys Acta 1804: 1231–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Goot G, Young JA (2009) Receptors of anthrax toxin and cell entry. Mol Aspects Med 30: 406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma K, Gu J, Werner E (2011) Tumor endothelial marker 8 amplifies canonical wnt signaling in blood vessels. PLoS One 6: e22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale G, Pellizzari R, Recchi C, Napolitani G, Mock M, Montecucco C (1998) Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem Biophys Res Commun 248: 706–711 [DOI] [PubMed] [Google Scholar]
- Wei W, Lu Q, Chaudry GJ, Leppla SH, Cohen SN (2006) The LDL receptor-related protein LRP6 mediates internalization and lethality of anthrax toxin. Cell 124: 1141–1154 [DOI] [PubMed] [Google Scholar]
- Werner E, Kowalczyk AP, Faundez V (2006) Anthrax toxin receptor 1/tumor endothelium marker 8 mediates cell spreading by coupling extracellular ligands to the actin cytoskeleton. J Biol Chem 281: 23227–23236 [DOI] [PubMed] [Google Scholar]
- Whitfield A, Robinson AH (1903) Remarkable series of cases of Molluscum Fibrosum in children. Trans R Med Chir Soc 1: 293–301 [PMC free article] [PubMed] [Google Scholar]
- Whittaker CA, Hynes RO (2002) Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol Biol Cell 13: 3369–3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigelsworth DJ, Krantz BA, Christensen KA, Lacy DB, Juris SJ, Collier RJ (2004) Binding stoichiometry and kinetics of the interaction of a human anthrax toxin receptor, CMG2, with protective antigen. J Biol Chem 279: 23349–23356 [DOI] [PubMed] [Google Scholar]
- Yang MY, Chaudhary A, Seaman S, Dunty J, Stevens J, Elzarrad MK, Frankel AE, St Croix B (2011) The cell surface structure of tumor endothelial marker 8 (TEM8) is regulated by the actin cytoskeleton. Biochim Biophys Acta 1813: 39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JA, Collier RJ (2007) Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem 76: 243–265 [DOI] [PubMed] [Google Scholar]
- Young JJ, Bromberg-White JL, Zylstra C, Church JT, Boguslawski E, Resau JH, Williams BO, Duesbery NS (2007) LRP5 and LRP6 are not required for protective antigen-mediated internalization or lethality of anthrax lethal toxin. PLoS Pathog 3: e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, Wang J, Doble B, Woodgett J, Wynshaw-Boris A, Hsieh JC, He X (2008) Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development 135: 367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]