Abstract
Adolescent risk taking has been known to increase in the presence of peers. We hypothesized that peer interaction reduces the activation of the medial prefrontal cortex (mPFC) that is required for self-regulation of reward-driven behavior. We also expected that mPFC activity would be reduced more in those with greater surgency, a composite trait of behavioral approach, sensation seeking and positive affect. In our study, 20 15-year-old boys played a simulated driving video game alone and in the presence of peers who were encouraged to call out advice while we recorded the feedback-related negativity (FRN) event-related potential in response to an impending car crash. FRN amplitude was reduced both as a function of peer presence and increased surgency. More importantly, we also calculated intracerebral current source density at the time of the FRNs, and found that both greater surgency and peer presence are associated with reduced activity specifically in the mPFC. Riskier performance resulting in more car crashes resulted from the presence of peers only as an interaction with surgency, this interaction being related strongly to reduced activity in the ventromedial PFC.
Keywords: FRN, medial PFC, adolescence, risk taking, surgency
There is ample evidence that the dopamine reward system is highly reactive to gains and losses and that individuals vary in their responsivity of this system (Schultz, 1998; Kable and Glimcher, 2007; Joseph et al., 2009). Given that adolescence is a time of continuing brain changes in general (Gogtay and Thompson, 2010; Luciana, 2010; Schmithorst and Yuan, 2010; Segalowitz et al., 2010), and dramatic growth of the dopamine system in particular (Spear, 2000; Wahlstrom, et al., 2010b), it is to be expected that we should find large individual differences in the functioning of the dopamine-reward system during this period. In addition, it is not surprising to find somewhat different responses in the reward-system network in adolescents compared to adults when anticipating and receiving rewards vs losses (Bjork et al., 2004; Ernst and Fudge, 2009; Geier et al., 2010) and when faced with social stimulation and stressors (Doremus-Fitzwater et al., 2010; McCormick et al., 2010). While the direct evidence of dopamine system changes in adolescence is primarily from rodents and nonhuman primates, these changes are apparently widespread (Wahlstrom, et al., 2010a, b), with some evidence that the path in males is especially dramatic (Andersen and Teicher, 2000). This latter is exemplified in adolescent males’ penchant for risk taking, a focus of much current research and its relation to both the maturation of and individual differences in the reward system and the regulation of reward-related behaviors by the prefrontal cortex (PFC) (Steinberg et al., 2006; Romer et al., 2010).
The functional bases of such risk taking are partly summarized in the notion of self-regulation, which is a hallmark of healthy psychological functioning, especially with respect to emotional states (for reviews, see Gross, 2007). Although emotional states are mediated by limbic structures, most notably the ventral striatum for pleasure, approach and reward and the amygdala primarily for intense negative experiences, it is the human medial PFC (mPFC) and orbitofrontal cortex (OFC) that are associated with regulation of these emotional states (Ochsner and Gross, 2005; Wager et al., 2008; Ernst and Fudge, 2009). Disturbances in the ability to regulate especially negative emotions have been associated with altered anatomical (Whittle et al., 2008, 2009) and functional characteristics (Pizzagalli et al., 2001, 2006) of these mPFC structures.
Adolescent risk taking has long been associated with reduced behavioral self-regulation, but more recently the focus has shifted to risk-taking behavior moderated by the presence of peers (Arnett, 1992; Steinberg, 2007). Such peer presence combined with the increased emotional arousal induced by the excitement of risk taking is assumed to alter the functioning of the PFC, and thereby reduce the individual’s ability to self-regulate motivational states and hence behavior (Steinberg, 2007; Ernst and Fudge, 2009; Spear, 2010). In this article, we document for the first time that peer presence alters specifically mPFC activity at the time of negative performance feedback in human adolescents.
Despite immaturity of the frontal lobe associated with mechanisms of control and judgment in adolescence compared to adulthood (Ernst and Fudge, 2009; Steinberg et al., 2009; Steinberg, 2010), adolescents do not inherently have poor cognitive skills. When tested in the laboratory on indirect measures of risk taking, they reason and conclude as well as adults (Reyna and Farley, 2006). Rather, the issue is whether, in peer settings, adolescents’ cognitive judgment may be clouded by emotion and arousal (Steinberg et al., 2009) and/or adolescents may weigh costs and benefits differently.
Socialization factors may allow friends to influence an individual’s behavior by modeling, establishing group norms and encouraging risky behaviors (Perry and Jessor, 1985). The critical role of peer influence in adolescent risk taking is highlighted in Steinberg’s biobehavioral model, whereby peer interaction in adolescence becomes highly rewarding and may be processed similarly to other types of (nonsocial) rewards, i.e. via the nucleus accumbens (Steinberg, 2007). By peer interaction increasing reward motivation, the effectiveness of PFC control over risky behaviors diminishes. Thus, a maturing motivational system biases adolescents toward risky behavior in social contexts, while a relatively immature control system makes it difficult for adolescents to attenuate these behavioral drives. This control system is sometimes characterized as cognitive, centered on the dorsolateral PFC and its associated links to the dorsal anterior cingulate cortex (dACC) (Steinberg, 2007). However, emotional self-regulation is also dependent on ventromedial PFC (vmPFC) and rostral ACC (rACC), which directly link to limbic motivational structures (Spear, 2010) and these mPFC structures are associated with emotional self-regulatory capacity (Pizzagalli et al., 2001; Whittle et al., 2008).
In our study, we chose to include only males because of their greater propensity for risk taking. Participants played a video game whereby they could gain points by driving a car as far as possible without crashing into a brick wall that could suddenly appear. Thus, drivers could stop and keep the points accumulated at any time, but lost all their points on that trial if they risked driving too far and crashed. In a similar paradigm, Gardner and Steinberg (2005) found that only in the presence of peers, who were instructed to call out advice, were adolescents (13–16 years) more likely than youths (18–22 years) and adults (24 years and older) to crash into the wall. We used an event-related potential (ERP) source estimation approach to examine PFC activity in response to feedback signaling the impending car crash in adolescents with and without peers present. The feedback-related negativity (FRN), elicited in response to negative performance feedback, was used as a rough index of mPFC responsiveness. The FRN appears as a negative deflection in the ERP waveform around 250 ms after performance feedback and has been localized to the dACC (Gehring and Willoughby 2002) and the mPFC (Muller et al., 2005; Nieuwenhuis et al., 2005). According to one prominent theory, the FRN is thought to reflect the ACC being disinhibited by decreases in dopamine release from the mesencephalic dopamine system following unexpected or unpredicted error information (i.e. response errors negative environmental feedback) (Holroyd and Coles, 2002). Some evidence demonstrates that the amplitude of the FRN is sensitive to modulation in the dopamine system (Santesso et al., 2009) and individual differences, such as depressive symptoms (Santesso et al., 2008).
Similar to the FRN is the response-locked error-related negativity (ERN) that is elicited after response errors and is also localized to the ACC and mPFC (Van Veen and Carter, 2002; Debener et al., 2005) and can be modulated by dopamine (de Bruijn et al., 2004). Individual differences in the ERN have also been documented. For example, enhanced ERNs have been observed for individuals scoring high on negative affect (NA) (Luu et al., 2000) and obsessive-compulsiveness (Gehring et al., 2000), whereas reduced ERNs have been observed for individuals high on sensation seeking, sensitivity to reward and risk taking (Santesso and Segalowitz, 2009). Thus, these mPFC-produced negative ERP components appear to be increased in amplitude in individuals who experience greater NA in response to performance failures and to be diminished in individuals who respond to challenges and novelty with a positive approach and strong reward motivation.
In the present study, we expected that the amplitude of the FRN would be diminished when adolescents performed the driving task in the presence of peers as opposed to alone. In addition, we examined the extent to which the FRN was related to the adolescents’ level of what we refer to as surgency and inhibition. Surgency was defined here as a constellation of traits including behavioral activation/approach, sensation seeking and positive affect (PA). These traits have been demonstrated to be highly interrelated (Tellegen, 1985; Jorm, 1999; Smillie et al., 2006), similar to approach temperament (Tellegen, 1985; Elliot and Thrash, 2002) and are related to increased risk-taking behavior and positive outcome expectancies toward taking risks (Arkes et al., 1988; Newcomb and McGee, 1991; Horvath and Zuckerman, 1993; Zuckerman, 1994; Nygren et al., 1996). Inhibition was defined as a combination of behavioral inhibition and NA, also interrelated constructs (Tellegen, 1985; Jorm et al., 1999), and is similar to avoidance temperament (Elliot and Thrash, 2002). It was expected that surgency would be associated with diminished mPFC responses to negative feedback while inhibitory tendencies would be associated with increased mPFC responses. We expected that higher levels of surgency would augment the effect of peer presence on mPFC responses to losses.
Scalp FRN is one measure of mPFC activity during feedback, and it is a way to time the activity in mPFC related to receiving the feedback. However, it does not capture all activity in this large region and cannot differentiate variations within regional activity. In order to do this, we used Low Resolution Electromagnetic Tomography (LORETA) to estimate intracerebral current source density (CSD) at the time of the FRN in discrete regions of interest (ROIs) both to understand regional activity underlying the FRN and to test hypotheses concerning the effects of peer presence and of surgency on regional activation during negative feedback.
METHOD
Participants
Twenty 15–16-year-old adolescent males (mean age = 15.8 ± 0.76 years), were recruited from the community for EEG testing. Each of these ‘target’ males then recruited two same aged male friends although one group included a female friend. The target males were right-handed, without psychiatric or neurological conditions, without medication that affects consciousness, and had no history of head injury. Participants provided written consent and were given $25 each for their participation. All procedures received clearance from the Brock University Research Ethics Board in conformity with the Declaration of Helsinki, with signed consent for their participation obtained from both the participants and their parents or guardians. Due to a noisy EEG signal, one participant was excluded from the analyses.
Procedure
Driving simulation game
Risk taking was assessed using a computer driving simulation game consisting of 75 trials, similar to that in Gardner and Steinberg (2005). The target male was instructed to move a car as far as possible to earn points in order to beat a preset high score and win a prize. Participants started to earn points when the car was moving after a yellow light appeared and had between 1 and 20 s to safely stop the car and collect points before crashing into a wall. If they stopped safely and decided to move to the next trial, their points accumulated. If however, the wall appeared, pressing the button did nothing, the car crashed and points for that trial were lost. Participants could decide when to stop (by pressing a button) and restart the car (by pressing the same button again) as often as desired within each trial. Thus, they had to balance their desire to accumulate points against the possibility of crashing into the wall and losing the points for that trial. Participants could neither change the speed of the car, nor did they know when the wall would appear.
The target participant completed the task in a single visit to the lab in two conditions, counterbalanced across participants: alone and together in the presence of their two friends. During the together condition, the friends were instructed to verbally encourage game play and goad and/or provide verbal advice (but to not touch) the target participant. The target participant was told that he could either accept or decline his friends’ advice to maximize points in the game.
Personality measures
The Behavioral Inhibition and Behavioral Activation Scale developed by Carver and White (1994) is based on Gray’s theory that two general motivational systems underlie behavior and affect: a behavioral inhibition system (BIS) and a behavioral activation system (BAS) (Gray, 1989). Items included in the BIS scale focus on anxiety in response to a threatening situation, whereas the items included on the BAS scale emphasize activated approach toward a goal. Total (summed) scores were created separately for BIS and for BAS items.
The Sensation Seeking Scale Form-V (SSS-V, Zuckerman, 1994) is designed to measure four factors of sensation seeking: thrill and adventure seeking (attraction to physically risky activities), experience seeking (attraction to experience through mind and senses), disinhibition (desire to seek social stimulation in uninhibited social activities) and boredom susceptibility (aversion to monotony and preference for the unpredictable).
The Positive and Negative Affect Schedule (PANAS; Watson et al., 1988) was used to measure the participants’ positive and negative trait affect. Participants responded to 10 adjectives each for PA (e.g. interested, excited) and NA (e.g. irritated, nervous) to describe how they felt that day.
In order to reduce the number of variables, a composite measure for surgency was calculated by totaling standardized (Z-scored) values on the BAS, PA and sensation-seeking measures, and for inhibition by totaling standardized values on the BIS and NA measures.
Electroencephalogram recording and data collection
EEG was recorded continuously using a 128-channel Electrical Geodesics system (EGI) at 500 Hz with 0.1–100 Hz analog filtering referenced to the vertex. Impedance of all channels was kept below 50 kΩ. Data were processed using Brain Vision Analyzer (Brain Products GmbH). Data were first visually inspected for movement artifacts, filtered at 1–20 Hz and re-referenced off-line to an average reference. EEG segments were derived beginning 200 ms before and ending 600 ms after the appearance of the wall indicating the impending car crash on that trial. Ocular artifacts were corrected (Gratton et al., 1983) and trials with artifacts were automatically removed with a ±75-µV criterion. The FRN was scored as the most negative peak 200–400 ms after presentation of the wall feedback at the midline sites (FCz and Cz) relative to a pre-stimulus baseline between −200 and 0 ms.
Source localization of ERP data
LORETA (Pascual-Marqui et al., 1999) was used to estimate intracerebral CSD underlying the FRN within a 216–234-ms post-feedback time window, which captured the global field power peak of the FRN (228 ms) and the mean FRN peak latency at Cz (226 ms). CSD was derived from five ROIs which were based on previously defined Brodmann areas (Towle et al., 1993; Lancaster et al., 1997; Pizzagalli et al., 2006): rACC (BA 32/24), dACC (BA24’/B32’), vmPFC (medial BA 10/11), lateral PFC (lateral BA 10/11) and dorsolateral PFC (BA 46). CSD was computed as the linear weighted sum of the scalp electric potentials at each voxel in the ROI to yield power of current density (units are scaled to A/m2). For each subject, LORETA values were normalized to a total power of 1 and then log-transformed before statistical analyses.
RESULTS
Behavioral responses
The alone and together conditions did not differ in the number of points earned, t(18) = 0.59, P = 0.56 (alone = 542 005 ± 52 410, together = 556 172 ± 73 268), the number of crashes t(18) = 0.62, P = 0.54 (alone = 36.9 ± 6.8, together = 35.7 ± 8.8), the mean number of car restarts per trial t(18) = −1.78, P = 0.09 (alone = 0.73 ± 0.75, together = 1.24 ± 1.24) or the percentage of time the car was in motion t(18) = 1.99, P = 0.06 (alone = 0.91 ± 0.07, together = 0.87 ± 0.11), suggesting that the target participants, averaged over the personality variation, were able to adapt to the peer context enough to resist systematic behavioral dyscontrol.
However, there were differences between conditions when taking personality into account. Pearson correlations indicated that individuals scoring higher on the surgency measure made significantly more crashes in the together relative to the alone condition (semi-partial correlation between surgency and the difference in crash rate between conditions adjusting for inhibition scores: r = 0.53, P = 0.024) but inhibition was unrelated to the difference in crash rate (semi-partial correlation adjusting for surgency, r = −0.14, P = 0.51). The interaction between surgency and peer presence is captured by an analysis of variance comparing crash rate in the together and alone conditions as a function of high vs low scores on surgency (in a median split). The condition effect was not significant, F(1, 17) < 1, but the surgency × condition interaction was, F(1, 17) = 7.60, P = 0.013. Zero-order correlations for surgency and inhibition were not related to the crash rate in either condition (r’s were between 0.42 and −0.10, Ps > 0.074).
ERPs
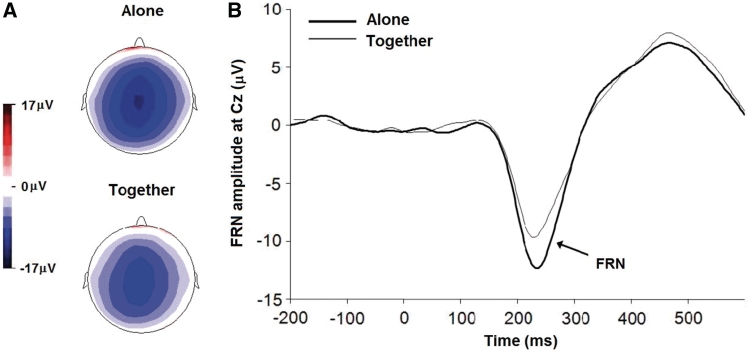
Grand-average ERP waveforms for the two conditions were calculated for the negative feedback. Topographical maps derived at the peak latency of the FRN show a negativity time locked to the appearance of the wall that is centered around the vertex and was slightly stronger for the alone compared to the together condition (Figure 1A). In a dipole source model, >99% of the variance in the topographies of the FRN in both conditions was accounted for by the same dipole sources located in BA32 (see Supplementary Data, Note 1).
Fig. 1.
Topographic maps and waveforms depicting the FRN. (A) Group average topographic maps of the FRN period during the alone (top) and together (bottom) condition; (B) group averaged ERP waveforms at Cz from 200 ms before to 600 ms after the presentation of wall feedback during the alone (heavy line) and together (light line) condition.
A mixed-model ANOVA was used to analyse the FRN with site (FCz, Cz) and condition (alone, together) as the within-subject factors. In all repeated measures analyses of variance, Greenhouse–Geisser correction was used where appropriate but original degrees of freedom are reported. There was a main effect for site, F(1, 18) = 7.72, P < 0.01, 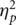 = 0.30, with the FRN maximal at site Cz, t(18) = 2.78, P = 0.01. A significant main effect for condition, F(1, 18) = 18.15, P < 0.001,
= 0.30, with the FRN maximal at site Cz, t(18) = 2.78, P = 0.01. A significant main effect for condition, F(1, 18) = 18.15, P < 0.001, 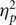 = 0.50, revealed that the FRN was 28% larger in the alone compared to the together condition (Figure 1B). The effect for condition was found at both FCz, t(18) = 3.48, P = 0.003 (alone = −12.3 ± 5.4, together = −9.6 ± 4.4) and at Cz, t(18) = 4.53, P < 0.001 (alone = −14.5 ± 4.4; together = −11.3 ± 4.3). The FRN amplitude at Cz was used for all subsequent analyses, as this is where the site and condition effects were maximal.
= 0.50, revealed that the FRN was 28% larger in the alone compared to the together condition (Figure 1B). The effect for condition was found at both FCz, t(18) = 3.48, P = 0.003 (alone = −12.3 ± 5.4, together = −9.6 ± 4.4) and at Cz, t(18) = 4.53, P < 0.001 (alone = −14.5 ± 4.4; together = −11.3 ± 4.3). The FRN amplitude at Cz was used for all subsequent analyses, as this is where the site and condition effects were maximal.
To eliminate the possibility that the presence of friends during the task simply distracted the participants causing attenuation of all ERP components due to increased latency jitter, we examined the N1 and P2 to positive feedback. The peak-to-peak N1-P2 amplitudes across conditions did not differ, F(1, 18) = 2.48, P = 0.13, 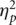 = 0.12, suggesting no greater jitter (variation in ERP latency) in the together condition. Further, the FRN to the negative feedback of the appearance of the wall was not spread out more in the together condition, as would have happened if the differences between conditions were due to latency jitter (see Figure 1B).
= 0.12, suggesting no greater jitter (variation in ERP latency) in the together condition. Further, the FRN to the negative feedback of the appearance of the wall was not spread out more in the together condition, as would have happened if the differences between conditions were due to latency jitter (see Figure 1B).
Correlations among performance, personality and the FRN
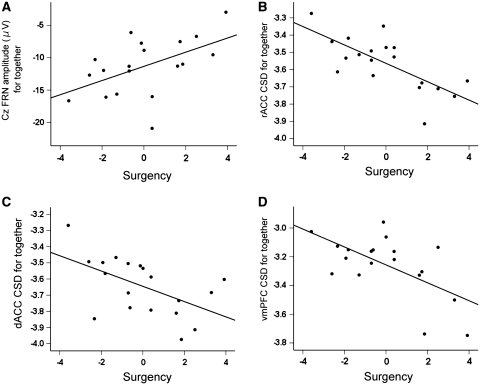
Higher surgency scores were associated with reduced FRN amplitudes during both the alone (r = 0.63, P < 0.01) and together condition (r = 0.52, P = 0.02; see Figure 2A and Table 1). The FRN was not related to the number of crashes in either condition (alone: r = 0.30, P = 0.22; together: r = 0.34, P = 0.16). The inhibition score did not relate significantly to the FRN amplitude or performance measures, except for a trend for greater inhibition to be related to reduced total points in the together condition (r = −0.42, P = 0.075).
Fig. 2.
Scatterplots between surgency scores and electrophysiological outcomes in the together condition: (A) the FRN amplitude at Cz (µV); (B) CSD in the rACC; (C) CSD in the dACC; (D) CSD in the vmPFC. CSD units are log amperes per square meter (A/m2).
Table 1.
Correlations between scores on surgency, inhibition and the FRN amplitude at Cz and CSD measures in the ACC and mPFC during each condition
| Surgency | Inhibition | |
|---|---|---|
| FRN amplitude | ||
| Alone | 0.63** | 0.38 |
| Together | 0.52* | 0.32 |
| CSD alone | ||
| rACC | −0.31 | −0.25 |
| dorsal ACC | 0.27 | −0.10 |
| ventral mPFC | −0.31 | −0.10 |
| lateral PFC | −0.31 | 0.01 |
| dorsal lateral PFC | −0.45 | −0.08 |
| CSD together | ||
| rACC | −0.72** | −0.31 |
| dorsal ACC | −0.55* | −0.08 |
| ventral mPFC | −0.61** | −0.17 |
| lateral PFC | −0.30 | 0.11 |
| dorsal lateral PFC | −0.20 | 0.24 |
*P < 0.05, **P < 0.01, two tailed.
LORETA current source estimation
Individual differences in the FRN for each condition were significantly related to activity in the rACC (Alone FRN: r = −0.55, P = 0.016; together FRN: r = −0.68, P = 0.001) but unrelated to activity in the dACC and vmPFC (P-values >0.06). However, condition significantly affected CSDs derived from the ROIs in medial prefrontal areas [rACC t(18) = 4.15, P = 0.001; dACC, t(18) = 2.78, P = 0.012; and vmPFC, t(18) = 3.92, P = 0.001], such that there was deactivation during the together condition, but there were no differences between conditions in lateral PFC or the dorsolateral PFC (P-values >0.25).
The CSD of each ROI was then correlated with performance and in more detail with respect to the personality characteristics (Table 1). The number of crashes in the together condition related highly to the activity in the vmPFC (r = −0.60, P = 0.006). None of the ROI activations correlated with the number of crashes in the alone condition. Surgency scores were highly related to activity in the together condition in the rACC (r = −0.72, P = 0.001), dACC (r = −0.56, P = 0.015) and vmPFC (r = −0.61, P = 0.006) (Figure 2B–D) but were unrelated to activity during the alone condition (P-values >0.05). Entering the activity levels simultaneously in a regression analysis accounting for surgency scores yielded similar results with only the together condition contributing significant unique variance. Neither the lateral PFC nor the dorsolateral PFC were related to surgency in either condition (P-values >0.06; see Supplementary Data, Note 2). No significant relations were found between the ROI activity and inhibition for either condition (P-values >0.19). When we separated participants scoring high vs low on surgency in a median split and performed a whole brain analysis corrected for multiple testing, the only regions that reliably separated the groups were the rACC and vmPFC (see Supplementary Data, Note 2).
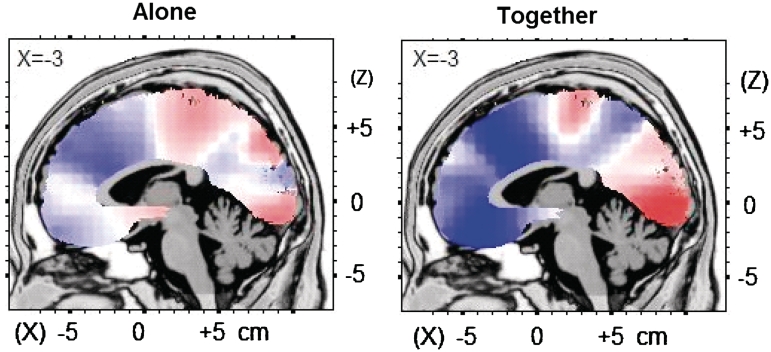
For illustrative purposes, a whole-brain correlation analysis between source activation and surgency indicated similar patterns for each condition but with differing strengths (Figure 3). In the together condition, higher surgency was associated specifically with deactivation in the dorsal and rACC and in the vmPFC; the maximum negative correlation was found at Talairach coordinates −3, 24, 36 (BA 32; r = −0.67, P = 0.001). This association of surgency with deactivation was considerably less for the alone condition, for which the maximum negative correlation was found at Talairach coordinates −3, 24, 22 (BA 32, r = −0.45, P = 0.05).
Fig. 3.
Regional activation during the FRN as related to surgency scores. Correlations of voxel-by-voxel activity during the FRN with surgency scores in the alone and together condition. Values unthresholded and displayed on an MNI template. Red, positive correlation; blue, negative correlation.
Using multiple regression, we found that activity from the rACC, dACC and vmPFC in the together condition accounted for 53% of the variance in predicting surgency, F(3, 15) = 5.72, P = 0.008, with none of the ROIs accounting for unique variance (rACC: sr = −0.25, P = 0.33; dACC: sr = −0.01, P = 0.96; vmPFC: sr = −0.16, P = 0.53). To more directly test differences in specific regional activity between conditions in predicting the surgency scores, activity levels from each ROI from the two conditions were entered simultaneously into regression analyses. Only the together condition provided unique variance in surgency for all three regions: rACC (together sr = −0.70, P = 0.001; alone sr = 0.24, P = 0.34), dACC (together sr = −0.55, P = 0.036; alone sr = 0.05, P = 0.84) and vmPFC (together sr = −0.61, P = 0.008; alone sr = −0.30, P = 0.23). No significant amount of variance in surgency could be accounted for by the lateral PFC (together sr = −0.13, P = 0.59; alone sr = −0.16, P = 0.53) or the dorsolateral PFC (together sr = −0.04, P = 0.88; alone sr = −0.41, P = 0.09). Similarly, adjusting for variance in lateral PFC regions did not appreciably reduce the prediction of surgency from mPFC regions, still accounting for 49% of the variance, F(3, 13) = 5.01, P = 0.016.
DISCUSSION
We recorded electrocortical responses of 15-year-old boys during a driving game designed to elicit negative feedback in response to risky performance. In the past using an analogous game, Gardner and Steinberg (2005) found more risks were taken when played in the presence of peers offering unsolicited advice, although there were wide individual differences within that study. We found wide individual differences as well in our task, some individuals showing an increase in crashes with peer presence and some showing a decrease, with the tendency to increase relating strongly to trait surgency. We show that activation of the mPFC in response to negative environmental feedback is attenuated in our participants when together with peers. Furthermore, the activity level of the mPFC, as reflected in CSD measures, correlates negatively with the individual’s surgency characteristic only when in the presence of peers. Such peer presence was interpreted as diminishing the likelihood of making cautious behavior choices, an effect presumably due to the activation of the socio-emotional neural system. According to this model, activation of the socio-emotional neural network would increase responses of the dopaminergic reward system (associated with the nucleus accumbens) and attenuate activation of PFC regions that normally act to inhibit behavioral impulses (Steinberg, 2007), presumably because of PFC inefficiencies arising from excessive dopamine levels (Spear, 2000; Wahlstrom et al., 2007). Because in our task this did not result in systematically lower performance, we can interpret the differing mPFC activation as reflecting a differing experience of the participant when with peers and not simply a disengagement from the task. Our results are the first to demonstrate that activity in the mPFC regions in adolescent boys is significantly reduced in the presence of peers.
An important neurobiological premise in this model is that social stimulation elicits a reward response and reduces attention to negative aspects of risk taking and performance failure. This in turn would reduce the likelihood of activating the PFC structures that regulate behavior in contexts in which greater attention is needed. This pattern would be exacerbated in situations with a too high or too low level of cathecholamine activity (Arnsten, 1997; Ernst and Fudge, 2009). Thus, we expected that individual differences in FRN and mPFC activity would relate to individual differences in the BAS, which is associated with the dopamine reward system (Gray, 1989). Indeed, higher surgency scores were associated with reduced FRN amplitudes and associated ACC and vmPFC activity during peer interaction. These results suggest that individuals with a greater positive drive to engage in risky activities have reduced emotional self-regulation when with peers as reflected in mPFC activity. Of course, we do not know the extent to which this effect is related to the nature of the chosen playmates. A synergy between the target participant and the peers may exacerbate the effect given the likelihood that high and low surgency individuals may have friends who are similarly high and low in trait surgency.
Furthermore, activation specifically in the vmPFC region during the peer-interaction condition predicted the riskiness of the behavior in terms of the number of car crashes. Of particular interest, surgency did not predict mPFC activation in the alone condition, when the boys did not have the challenge of dealing with interacting peers. Related research on neural networks associated with resistance to peer influence indicates a role for activation during negative emotional states for both lateral and mPFC and their links with other regions (Grosbras et al., 2007) in this trait. Our results support the notion that it is primarily when trying to handle the influence of peers in the context of negative feedback that the mPFC becomes critical.
These results suggest that the adolescent social neural response is moderated by one’s reward-seeking tendencies. Further research is needed, however, to determine which is the cause and which is the effect in this surgency–mPFC relation: increased reward seeking may put the adolescent in a state of low-mPFC reactivity, or relatively poorer cortical response may increase surgency.
These findings are consistent with the documented rise in activity of the mesocortical dopamine system during adolescence (Spear, 2000), which could put adolescents with relatively higher dopamine system activity at risk for disrupted mPFC function (Wahlstrom et al., 2007). If so, one would expect to find a weaker relation between surgency scores and mPFC activation in an adult peer-interactive situation. However, without data from other age groups, we cannot tell whether the surgency-mPFC relations in the present study are specific to adolescent boys and to the associated surge in dopamine.
The results of the present study are based on boys and should not be extrapolated to adolescent girls for several reasons. First, adolescent boys expect more benefits from risky behaviors than do girls, with this difference in expectation greater when among peers (Gardner and Steinberg, 2005). Second, adolescent girls (as well as women) are less susceptible to peer influence than are males (Steinberg and Monahan, 2007). Finally, stress has been shown to increase risk taking in men but reduce it in women (Lighthall et al., 2009). Although this latter has not been tested in adolescents, we might expect different moderating effects on PFC responses in a stress-related task for each gender.
Contrary to our predictions, inhibition was not associated with the FRN amplitude or activation of the mPFC ROIs. Although BIS has been related to enhanced ERNs and FRNs in adults (Boksem et al., 2006; De Pascalis et al., 2010), previous data from our laboratory failed to show an association in adolescents between ERN amplitude and sensitivity to punishment, a construct closely related to BIS (Santesso and Segalowitz, 2009), possibly suggesting a developmental change in the BIS.
The results of the present study are consistent with a general view that some personality traits potentially moderate the effect of peer influence. For example, individuals scoring higher on extroversion and sensation seeking are more influenced by peers to take risks (Slater, 2003). However, the possibility of an synergistic effect mentioned earlier is consistent with our data in that the overall group rate of risky performance (in terms of car crashes) did not change with peer presence, suggesting that peer presence reduced risk taking for some and increased it for others.
Our data provide support for a mPFC regulatory component in such a social network model for risk behaviors, although whether we can generalize to other domains of risk taking, such as drug use, sexual promiscuity and vandalism needs to be investigated. Of course, outside the laboratory peer influence may operate by mechanisms more subtle than overt goading or teasing, such as the desire to conform to group norms or an unconscious desire to change emotional states, such as anxiety and depression. It should not be surprising that being able to deal with such peer influences would relate to the individual’s neural processing of social information.
Interestingly, our data suggest that it is the mPFC regions that relate to the peer-presence effect and not the lateral (including dorsolateral) PFC regions as suggested by many researchers (Banks et al., 2007; Blair et al., 2007; Steinberg, 2007). We may have found this because the lateral PFC regions may act on affective and behavioral control only through the mPFC, especially the vmPFC, making the medial effects more proximal (Spear, 2010). Another possibility is that lateral PFC control signals may arise at time periods outside that of the FRN, which is the time of reaction to the negative feedback, possibly following the initial mPFC reaction. The two factors are, of course, not exclusive of each other.
Some important methodological conclusions can be drawn from our study. First, the FRN is well established in adolescents in this highly motivating game, just as the ERN is well established in adolescence using a standard flanker task. Of course, this does not preclude further maturation of the FRN, just as we found for the ERN (Santesso and Segalowitz, 2008). Second, we have shown that it is possible to record specific feedback-related ERP components during a highly arousing risk-taking task, something that is not possible with other vascular-based brain-imaging techniques.
Third, CSD measures may tap more directly than do scalp ERP measures into specific activation patterns that relate to personality and social context. We have shown that this activation has psychological importance even within the normative range of traits, just as has been shown for clinical studies (Pizzagalli et al., 2001). That is, whereas scalp ERP measures such as the FRN reflect the mixture of signals from various neural generating regions, stronger relations can be found by focusing on ROIs that have been shown to have relevant functional distinctions. In this case, although the dACC is often implicated in medial frontal negativities including the FRN (Gehring and Willoughby 2002; Van Veen and Carter, 2002), more rostral and ventral portions of the mPFC had the stronger relations with personality, mood, social context and performance (Pizzagalli et al., 2006). These more ventral regions have been associated with emotional regulation, having direct linkages to important motivation processes in the amygdala and ventral striatum regions (Bush et al., 2000).
In summary, we found that peer interaction reduces adolescent boys’ mPFC activation to negative feedback during a risk-taking task, and that this reduction is related to personality traits reflecting excitement, reward and sensation seeking. Adolescent boys, perhaps more than younger children or adults, are at risk for making dangerous decisions in contexts of high arousal, peer presence and real-life time pressures (Steinberg et al., 2009). Understanding the limitations of mPFC function within this context is critical to our understanding of psychological growth during this developmental period.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This study was supported by funds from the Natural Sciences and Engineering Research Council and the Social Sciences and Humanities Research Council of Canada. We would like to thank Allison Flynn and James Desjardins for their help in data collection, Michael Busseri and Louis Schmidt for initial discussions on the project and Cheryl McCormick for comments on the article.
REFERENCES
- Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neuroscience and Biobehavioral Reviews. 2000;24(1):137–41. doi: 10.1016/s0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Arkes HR, Herren LT, Isen AM. The role of potential loss in the influence of affect on risk-taking behavior. Organizational Behavior and Human Decision Processes. 1988;41(2):181–93. [Google Scholar]
- Arnett J. Reckless behavior in adolescence: a developmental perspective. Developmental Review. 1992;12(4):339–73. [Google Scholar]
- Arnsten AFT. Catecholamine regulation of the prefrontal cortex. Journal of Psychopharmacology. 1997;11(2):151–62. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala–frontal connectivity during emotion regulation. Social, Cognitive and Affective Neuroscience. 2007;2(4):303–12. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. The Journal of Neuroscience. 2004;24(8):1793–802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, et al. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35(1):430–40. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksem MA, Tops M, Wester AE, Meijman TF, Lorist MM. Error-related ERP components and individual differences in punishment and reward sensitivity. Brain Research. 2006;1101(1):92–101. doi: 10.1016/j.brainres.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;4(6):215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67(2):319–33. [Google Scholar]
- De Bruijn ER, Hulstijn W, Verkes RJ, Ruigt GS, Sabbe BG. Drug-induced stimulation and suppression of action monitoring in healthy volunteers. Psychopharmacology (Berl) 2004;177(1–2):151–60. doi: 10.1007/s00213-004-1915-6. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Varriale V, D’Antuono L. Event-related components of the punishment and reward sensitivity. Clinical Neurophysiology. 2010;121(1):60–76. doi: 10.1016/j.clinph.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. Journal of Neuroscience. 2005;25(50):11730–7. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain and Cognition. 2010;72(1):114–23. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot AJ, Thrash TM. Approach-avoidance motivation in personality: approach and avoidance temperaments and goals. Journal of Personality and Social Psychology. 2002;82(5):804–18. doi: 10.1037//0022-3514.82.5.804. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience and biobehavioral reviews. 2009;33(3):367–82. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Developmental psychology. 2005;41(4):625–35. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychological Science. 2000;11(1):1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295(5563):2279–82. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20(7):1613–29. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Thompson PM. Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain and Cognition. 2010;72(1):6–15. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55(4):468–84. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gray JR. Fundamental systems of emotion in the mammalian brain. In: Palermo DS, editor. Coping with Uncertainty: Behavioral and Developmental Perspectives. Hillsdale, NJ: Lawrence Erlbaum; 1989. pp. 173–95. [Google Scholar]
- Grosbras MH, Jansen M, Leonard G, et al. Neural mechanisms of resistance to peer influence in early adolescence. Journal of Neuroscience. 2007;27(30):8040–5. doi: 10.1523/JNEUROSCI.1360-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Handbook of Emotion Regulation. New York: Guilford; 2007. [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Horvath P, Zuckerman M. Sensation seeking, risk appraisal, and risky behavior. Personality and Individual Differences. 1993;14(1):41–52. [Google Scholar]
- Jorm AF, Christensen H, Henderson AS, Jacomb PA, Korten E, Rodgeres B. Using the BIS/BAS scales to measure behavioural inhibition and behavioural activation: factor structure, validity and norms in a large community sample. Personality and Individual Differences. 1999;26(1):49–58. [Google Scholar]
- Joseph JE, Liu X, Jiang Y, Lynam D, Kelly TH. Neural correlates of emotional reactivity in sensation seeking. Psychological Science. 2009;20(2):215–23. doi: 10.1111/j.1467-9280.2009.02283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10(12):1625–33. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, et al. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Human Brain Mapping. 1997;5(4):238–42. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthall NR, Mather M, Gorlick MA. Acute stress increases sex differences in risk seeking in the balloon analogue risk task. PLoS ONE. 2009;4(7):e6002. doi: 10.1371/journal.pone.0006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M. Adolescent brain development: current themes and future directions. Introduction to the special issue. Brain and Cognition. 2010;72(1):1–5. doi: 10.1016/j.bandc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Luu P, Collins P, Tucker DM. Mood, personality, and self-monitoring: negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. Journal of Experimental Psychology. General. 2000;129(1):43–60. doi: 10.1037//0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain and Cognition. 2010;72(1):73–85. doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Muller SV, Moller J, Rodriguez-Fornells A, Munte TF. Brain potentials related to self-generated and external information used for performance monitoring. Clinical Neurophysiology. 2005;116(1):63–74. doi: 10.1016/j.clinph.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Newcomb M, McGee L. Influence of sensation seeking on general deviance and specific problem behaviors from adolescence to young adulthood. Journal of Personality and Social Psychology. 1991;61(4):614–28. doi: 10.1037//0022-3514.61.4.614. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Slagter HA, von Geusau NJ, Heslenfeld DJ, Holroyd CB. Knowing good from bad: differential activation of human cortical areas by positive and negative outcomes. European Journal of Neuroscience. 2005;21(11):3161–8. doi: 10.1111/j.1460-9568.2005.04152.x. [DOI] [PubMed] [Google Scholar]
- Nygren TE, Isen AM, Taylor PJ, Dulin J. The influence of positive affect on the decision rule in risk situations: focus on outcome (and especially avoidance of loss) rather than probability. Organizational Behavior and Human Decision Processes. 1996;66(1):59–72. [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in cognitive sciences. 2005;9(5):242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Lehmann D, Koenig T, et al. Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiatry Research: Neuroimaging Section. 1999;90(3):169–79. doi: 10.1016/s0925-4927(99)00013-x. [DOI] [PubMed] [Google Scholar]
- Perry CL, Jessor R. The concept of health promotion and the prevention of adolescent drug abuse. Health Education Quarterly. 1985;12(2):169–84. doi: 10.1177/109019818501200204. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D, Pascual-Marqui RD, Nitschke JB, et al. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. American Journal of Psychiatry. 2001;158(3):405–15. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Peccoralo LA, Davidson RJ, Cohen JD. Resting anterior cingulate activity and abnormal responses to errors in subjects with elevated depressive symptoms: a 128-channel EEG study. Human Brain Mapping. 2006;27(3):185–201. doi: 10.1002/hbm.20172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyna VF, Farley F. Risk and rationality in adolescent decision making: Implications for theory, practice, and public policy. Psychological Science in the Public Interest. 2006;7(1):1–44. doi: 10.1111/j.1529-1006.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Romer D, Duckworth AL, Sznitman S, Park S. Can adolescents learn self-control? Delay of gratification in the development of control over risk taking. Prevention science: the official journal of the Society for Prevention Research. 2010;11(3):319–30. doi: 10.1007/s11121-010-0171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso DL, Evins AE, Frank MJ, Schetter EC, Bogdan R, Pizzagalli DA. Single dose of a dopamine agonist impairs reinforcement learning in humans: evidence from event-related potentials and computational modeling of striatal-cortical function. Human Brain Mapping. 2009;30(7):1963–76. doi: 10.1002/hbm.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ. Developmental differences in error-related ERPs in middle- to late-adolescent males. Developmental Psychology. 2008;44(1):205–17. doi: 10.1037/0012-1649.44.1.205. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ. The error-related negativity is related to risk taking and empathy in young men. Psychophysiology. 2009;46(1):143–52. doi: 10.1111/j.1469-8986.2008.00714.x. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Steele KT, Bogdan R, et al. Enhanced negative feedback responses in remitted depression. Neuroreport. 2008;19(10):1045–8. doi: 10.1097/WNR.0b013e3283036e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain and Cognition. 2010;72(1):16–25. doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80(1):1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Santesso DL, Jetha MK. Electrophysiological changes during adolescence: A review. Brain and Cognition. 2010;72(1):86–100. doi: 10.1016/j.bandc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Slater MD. Sensation-seeking as a moderator of the effects of peer influences, consistency with personal aspirations, and perceived harm on marijuana and cigarette use among younger adolescents. Substantial Use and Misuse. 2003;38(7):865–80. doi: 10.1081/ja-120017614. [DOI] [PubMed] [Google Scholar]
- Smillie LD, Jackson CJ, Dalgleish LI. Conceptual distinctions among Carver and White’s (1994) BAS scales: a reward-reactivity versus trait impulsivity perspective. Personality and Individual Differences. 2006;40(5):1039–50. [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The Behavioral Neuroscience of Adolescence. New York: Norton; 2010. [Google Scholar]
- Steinberg L. Risk Taking in Adolescence. Current Directions in Psychological Science. 2007;16(2):55–9. [Google Scholar]
- Steinberg L. A behavioral scientist looks at the science of adolescent brain development. Brain and Cognition. 2010;72(1):160–4. doi: 10.1016/j.bandc.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Cauffman E, Woolard J, Graham S, Banich M. Are adolescents less mature than adults?: minors’ access to abortion, the juvenile death penalty, and the alleged APA ‘flip-flop’. American Psychology. 2009;64(7):583–94. doi: 10.1037/a0014763. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Dahl R, Keating D, Kupfer D, Masten A, Pine DS. The study of developmental psychopathology in adolescence: Integrating affective neuroscience with the study of context. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology, Vol 2: Developmental neuroscience. 2nd edn. Hoboken, NJ: Wiley; 2006. pp. 710–41. [Google Scholar]
- Steinberg L, Monahan KC. Age differences in resistance to peer influence. Developmental Psychology. 2007;43(6):1531–43. doi: 10.1037/0012-1649.43.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellegen A. Structures of mood and personality and their relevance to assessing anxiety, with an emphasis on self-report. In: Tuma AH, Maser JD, editors. Anxiety and the Anxiety Disorders. Hillsdale, NJ: Erlbaum; 1985. pp. 681–706. [Google Scholar]
- Towle VL, Bolanos J, Suarez D, et al. The spatial location of EEG electrodes: locating the best-fitting sphere relative to cortical anatomy. Electroencephalogr Clinical Neurophysiology. 1993;86(1):1–6. doi: 10.1016/0013-4694(93)90061-y. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14(4):593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain and Cogniton. 2010a;72(1):146–59. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Hooper CJ, et al. Variations in the catechol O-methyltransferase polymorphism and prefrontally guided behaviors in adolescents. Biolgy of Psychiatry. 2007;61(5):626–32. doi: 10.1016/j.biopsych.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience and Biobehavioral Reviews. 2010b;34(5):631–48. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Whittle S, Allen NB, Fornito A, et al. Variations in cortical folding patterns are related to individual differences in temperament. Psychiatry Res. 2009;172(1):68–74. doi: 10.1016/j.pscychresns.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Whittle S, Yap MB, Yucel M, et al. Prefrontal and amygdala volumes are related to adolescents’ affective behaviors during parent-adolescent interactions. Proceedings of the National Acadamy of Sciences USA. 2008;105(9):3652–7. doi: 10.1073/pnas.0709815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Behavioral Expressions and Biosocial Bases of Sensation Seeking. New York, NY: Cambridge University Press; 1994. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.