Abstract
Peer feedback affects adolescents’ behaviors, cognitions and emotions. We examined neural circuitry underlying adolescents’ emotional response to peer feedback using a functional neuroimaging paradigm whereby, 36 adolescents (aged 9–17 years) believed they would interact with unknown peers postscan. Neural activity was expected to vary based on adolescents’ perceptions of peers and feedback type. Ventrolateral prefrontal cortex (vlPFC) activity was found when adolescents indicated how they felt following feedback (acceptance or rejection) from peers of low vs high interest. Greater activation in both cortical (e.g. superior temporal gyrus, insula, anterior cingulate) and subcortical (e.g. striatum, thalamus) regions emerged in response to acceptance vs rejection feedback. Response to acceptance also varied by age and gender in similar regions (e.g. superior temporal gyrus, fusiform, insula), with greater age-related increases in activation to acceptance vs rejection for females than males. Affective response to rejection vs acceptance did not yield significantly greater neural activity in any region. vlPFC response suggests cognitive flexibility in reappraising initial perceptions of peers following feedback. Striatal response suggests that acceptance is a potent social reward for adolescents, an interpretation supported by more positive self-reported affective response to acceptance than rejection from high- but not low-interest peers.
Keywords: adolescence, cognitive flexibility, peer feedback, social reward
INTRODUCTION
Acceptance by social groups is fundamental to survival and reproduction across species (Baumeister and Leary, 1995; Insel and Fernald, 2004; Lieberman and Eisenberger, 2009). Social acceptance is particularly salient during adolescence when navigating one’s standing among peers, profoundly influences affect, behavior and cognition and provides a foundation for adult intimate relationships. The salience of peer acceptance powerfully motivates adolescents to conform to desirable peers’ patterns of behavior and thought (Allen et al., 2005). Indeed, many adolescents are concerned with peers’ views of them to the point where being accepted or rejected by peers strongly shapes social interactions and adjustment (Silverman et al., 1995; La Greca and Lopez, 1998; Muris et al., 1998). When accepted by their peers, adolescents tend to have social competence, high self-esteem, intimate friendships and popularity; when rejected, adolescents tend to exhibit social avoidance, anxiety, depression, suicidality, excessive risk-taking and substance use (Rubin et al., 2006).
While affective responses to peer feedback have been well-characterized in behavioral research, less is known about how such responses manifest in the adolescent brain. Extending from the neurobiology of affiliative behavior in maternal–infant attachment and pair bonding contexts (Nelson and Panksepp, 1998; Insel and Fernald, 2004; Depue and Morrone-Strupinsky, 2005), recent perspectives on adolescent neurodevelopment suggest that maturation of dissociable neural circuits supports social–emotional development (Nelson et al., 2005; Nelson and Guyer, 2011). These neural circuits are thought to underlie adolescent changes in affective and cognitive responses to social experiences precisely when the appeal of peers increases and the salience of family wanes (Nelson et al., 2005). The shift in what is socially salient to adolescents presumably reflects changes in three distinct neural networks devoted to detection of social–emotional stimuli, affective reactions to such stimuli and regulation of these reactions through higher order cognitive processes. The networks primarily involved in affective responses to and cognitive regulatory processing of social stimuli, and basic detection circuits to a lesser degree, undergo structural and functional maturation across adolescence and early adulthood (Nelson et al., 2005; Casey et al., 2008).
A network of regions along the ventral visual stream mediates detection and perceptual coding of salient social stimuli. These regions include the fusiform face area, superior temporal sulcus (STS) plus surrounding superior and medial temporal gyri and temporal parietal junction (TPJ), which encompasses the posterior region of the STS and inferior parietal lobule (Haxby et al., 2000; Adolphs, 2001; Decety et al., 2004; Saxe, 2006; Blakemore, 2008).
Upon detection of social stimuli, a core affective circuitry mediates attribution of meaning and salience to social information, including both approach and avoidance responses. Affective circuitry implicated in social feedback information processing includes the amygdala, striatum, hypothalamus, ventral anterior cingulate and anterior insula (Somerville et al., 2006; Guyer et al., 2009; Masten et al., 2009; Gunther-Moor et al., 2010).
Finally, successful social navigation requires individuals to rapidly and flexibly adjust to socially evaluative outcomes and to use current information to integrate and update goals and expectations (Nelson and Guyer, 2011). Such flexibility engages several prefrontal cortex (PFC) regions, including orbital, medial, ventral and ventrolateral areas, during a range of affective, cognitive and social processes (Anderson et al., 1999; Blair and Cipolotti, 2000; Amodio and Frith, 2006; Choudhury et al., 2006). Although implicated in the detection node, the STS and TPJ also serve critical social–cognitive and regulatory roles given, their relationship to higher order cognitive processes (e.g. perspective taking, mentalizing, theory of mind) (Saxe et al., 2004; Blakemore et al., 2007; Pfeifer et al., 2009).
Functional magnetic resonance imaging (fMRI) studies of adolescents’ neural responses in socially evaluative contexts demonstrate engagement of the so-called ‘affective’ and ‘cognitive regulatory’ nodes. For example, one study found increased activation in the striatum, hypothalamus, hippocampus and insula among older female adolescents while anticipating evaluation from appealing peers (Guyer et al., 2009). Related work showed that, relative to healthy adolescents, socially anxious adolescents demonstrated amygdala and vlPFC hyperactivation while anticipating evaluation from unappealing peers (Guyer et al., 2008) and persistent amygdala hyperactivation following peer rejection (Lau et al., in press). Other research has focused on adolescents’ responses to social exclusion and social feedback. In response to social exclusion, adolescents reported lower mood states than adults (Sebastian et al., 2010) and exhibited neural sensitivity in accordance with exclusion-related distress, with heightened insula activity correlated with high exclusion-related distress and vlPFC and ventral striatum activity associated with low exclusion-related distress (Masten et al., 2009). In response to social feedback, adolescents and young adults showed heightened ventromedial PFC (vmPFC) and striatal activity following acceptance when acceptance was expected and striatal and widespread frontal activity to rejection when rejection was expected (Gunther-Moor et al., 2010). Together, these results suggest that adolescents’ degree of interest in interacting with peers, expectations about being liked by peers and type of feedback received or social experience encountered modulates differential neural responses in regions supporting social affect and cognitive regulation.
With continued maturation of social information-processing regions during adolescence, age-related differences in activation are expected as adolescents refine the ability to self-regulate emotional responses and adjust goal-directed behavior during peer interactions (Nelson et al., 2005; Blakemore, 2008; Nelson and Guyer, 2011). Indeed, greater activation in PFC regions was found in adolescents vs adults when accessing self-knowledge and when thinking about themselves based on others’ perceptions of them (Pfeifer et al., 2007, 2009). When anticipating social feedback, increased age was associated with increased activity in affect-related regions when adolescents made positive vs negative appraisals of peers (Guyer et al., 2009). Likewise, age-related differences have emerged in activation of affective and cognitive-related regions following social feedback, such that greater striatal and PFC activity was found when adults, but not younger participants, were rejected after expecting to be rejected (Gunther-Moor et al., 2010).
The current study investigated neural responses to peer acceptance and rejection feedback in typically developing adolescents with methods that incorporate the complexity and dynamic nature of adolescent social interactions. We paired fMRI with an ecologically valid paradigm that delivers peer feedback within a simulated social context highly relevant to adolescents’ daily cognitions and emotions. Drawing on the social re-orientation framework (Nelson et al., 2005), we hypothesized that the three core neural circuits would be recruited when adolescents affectively responded to peer feedback. Because positive feedback from peers is a potent and salient social event for adolescents, we expected that peer acceptance vs rejection would generate activity in regions of the affective node (e.g. striatum) that have been implicated previously in reward processing within other socially appetitive contexts, e.g. mother–infant attachment, pair bonding and social acceptance (Bartels and Zeki, 2004; Leibenluft et al., 2004; Nitschke et al., 2004; Young and Wang, 2004; Gunther-Moor et al., 2010). Furthermore, we hypothesized that emotional responding to feedback would engage the cognitive regulatory node, including PFC, as these responses are integrated within a larger context of cognitive regulation and goal pursuit. Based on past work, we expected that adolescents’ degree of interest in the peers evaluating them would moderate response to feedback (Guyer et al., 2008, 2009), in support of the context dependency of social feedback effects (Gunther-Moor et al., 2010). Specifically, responding to acceptance from low-interest peers would involve regions mediating cognitive flexibility, whereas acceptance from high-interest peers would invoke reward processing and perspective-taking circuitry. Finally, given past age-related differences in responsivity of face processing regions during social evaluation (Guyer et al., 2009; Gunther-Moor et al., 2010), we expected that activation in such regions (e.g. fusiform) would vary by feedback type and age presumably via back projection of input from affective and cognitive regulatory nodes.
METHOD
Participants
Participants included 36 adolescents (16 females; aged 8.6–17.5 years, M = 13.54, s.d. = 2.5) recruited from the community with advertisements and financially compensated for participation. All participants were physically and psychiatrically healthy following a physical exam and interview with the Schedule for Affective Disorders–Present and Lifetime version (Kaufman et al., 1997). T-tests confirmed no differences between males and females in age, intelligent quotient scores (Wechsler, 1999), pubertal stage (Tanner, 1962), parent education and income (Table 1). Data on 34 participants engaging in a different set of distinct cognitive processes during a separate phase of this fMRI task have been reported previously (Guyer et al., 2009).
Table 1.
Mean (s.d.’s) for sample demographic characteristics
| Age in years | IQ | Pubertal stage | Parent education | Parent annual income | |
|---|---|---|---|---|---|
| Male (n = 20) | 13.44 (2.77) | 118.55 (9.09) | 3.21 (1.13) | 2.70 (0.57) | 4.28 (1.36) |
| Female (n = 16) | 13.66 (2.18) | 115.44 (10.46) | 3.33 (1.29) | 2.87 (1.06) | 4.60 (1.18) |
| Total (N = 36) | 13.54 (2.50) | 117.17 (9.70) | 3.26 (1.19) | 2.77 (0.81) | 4.42 (1.28) |
Note. Parent education ranged from 1 (high school graduate) to 4 (graduate training). Parent annual income ranged from 1 ($15 000–24 999) to 6 (>$180 000).
Procedures
Study procedures were approved by the institutional review board at the National Institute of Mental Health. All participants provided written assent and parents/legal guardians gave written informed consent for participation. Participants and their parents were informed during the consent process that they would receive misinformation at some point during the course of their testing; all participants were debriefed extensively at the conclusion of the study. No adverse reactions occurred.
fMRI task
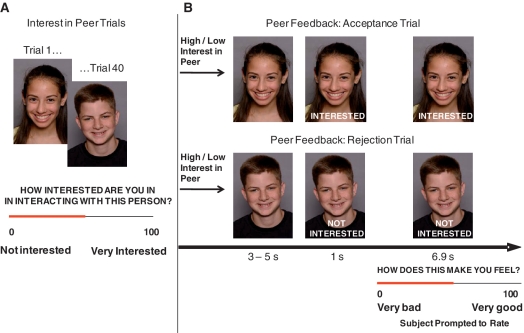
Participants completed the ‘Chatroom Task’, which simulated social interactions in two tasks (Figure 1). In Task 1, participants were led to believe they were participating in a nationwide investigation of Internet-based chat room communication among teenagers. They were told that, after an fMRI scan, they would chat online with another teenager from a collaborating institution. Participants then viewed on a laptop 40 photographs of peers (20 males) allegedly participating in the study and rated their interest in chatting with each peer (0 = not interested at all to 100 = very interested). Participants were also photographed, told that the ‘peers’ they had rated would similarly evaluate their pictures and view the ratings they had received, and that they would later chat with a mutually high-interest ‘peer’, based on their ratings, interests and hobbies. This deceptive approach was intended to increase task salience and followed Wendler’s (1996) recommendations for ethically permissible research using deception.
Fig. 1.
The Chatroom Task consisted of two phases. (A) In Phase 1, ∼2 weeks before the scan, participants rated how interested they were in interacting with peers based on photographs. A median split divided the ratings into low- and high-peer-interest groups. Participants were told that the same peers would learn how they had been rated and rate the participants’ photographs in a similar fashion. (B) In Phase 2, the participants were scanned. During the scan, participants viewed each received acceptance feedback that the peer was ‘Interested’ or rejection feedback that the peer was ‘Not Interested’ in chatting with them after the scan. After seeing the feedback, participants were asked to rate how the feedback made them feel (0 = very bad; 100 = very good).
The second task occurred 2 weeks later when participants underwent neuroimaging. This task used a rapid, event-related design which comprised 40 trials presented across 10.9–12.9 min. Participants viewed each photograph they had rated 2 weeks prior and were given ‘feedback’ that participants believed came from ratings made by the other ‘peers’. Feedback was actually determined randomly by computer algorithm. Each photograph was displayed for 3–5 s without feedback. Subsequently, the words ‘Interested’ or ‘Not Interested’ (indicating the peer’s desire to interact with the participant) was displayed at the bottom of the photograph. After 1 s, a rating scale was superimposed on the bottom of the photograph for the final 6.9 s that the photograph was displayed and participants rated their emotional response to the feedback (0 = makes me feel very bad to 100 = makes me feel very good). Half of the pictures depicting each gender were randomly assigned ‘Interested’ and half ‘Not interested’. Presentation order of feedback type was randomly determined. Participants’ peer interest ratings from Task 1 were used to bin neural responses on contrasts of acceptance vs rejection from peers of high vs low interest. Eight 3–5 s trials contained only a fixation cross, which were randomly intermixed throughout the experiment and served as a baseline. Interstimulus interval was 1 s.
Task stimuli were from the teen face emotion data set developed within our laboratory, which includes 40 digital headshots of actors aged 11–17 years (20 males) of varied ethnicities posing happy expressions with direct gaze (Nelson, 2004). Stimuli were well controlled on a variety of perceptual and cognitive demands. Actor attractiveness was not controlled in order to create a stimulus set of typical peers encountered by adolescents. E-prime software (Psychological Software Tools, Pittsburgh, PA, USA) presented the stimuli and recorded participants’ responses made using a hand-held device (Research Services Branch, NIMH, Bethesda, MD, USA).
Participants were debriefed postscan and told that no real interactions would occur. Only data from participants who believed the feedback was actually from the other ‘peers’ were included in analyses. Of the 50 participants recruited, 13 were excluded for head motion >3 mm and/or because they did not believe the feedback was from another participant. One participant was excluded due to technical problems.
fMRI data acquisition and preprocessing
Scanning occurred in a General Electric (Waukesha, WI, USA) Signa 3 Tesla magnet. Task stimuli were projected onto a screen at the foot of the scanner bed and viewed in a mirror mounted on the head coil. Head movement was constrained by foam padding. Functional scans were preceded by a localizer and a manual shim procedure. For functional image acquisition, each brain volume contained 29 contiguous 3.3-mm axial slices acquired parallel to the anterior commissure–posterior commissure line using a single-shot gradient echo with T2* weighting with the following parameters: 2300 ms repetition time (TR), 23 ms echo time (TE), 3.3 × 3.75 × 3.75 mm voxels, 64 × 64 matrix and 24-cm field of view (FOV). A high-resolution anatomical image was also acquired using a T1-weighted standardized magnetization prepared spoiled gradient recalled echo sequence to aid with spatial normalization with the following parameters: 124 1-mm axial slices, 8100 ms TR, 32 ms TE, 15° flip angle, number of excitations = 1, 256 × 256 matrix, 31.2 KHz bandwidth and 24 cm FOV.
Data analysis
Rating data collected before and during the scan were analyzed using SPSS (Chicago, IL, USA). FMRI data were preprocessed and analyzed using Analysis of Functional and Neural Images (AFNI) software version 2.56 b (Cox, 1996). Standard preprocessing of echo-planar imaging data included slice time correction, re-slicing to 1 mm isotropic voxels to place data in standard space, motion correction, spatial smoothing with a 6-mm full-width half-maximum Gaussian smoothing kernel, removal of signal deviations >2.5 s.d. from the mean using an AFNI de-spiking algorithm applied on a voxel-wise basis, a band-pass filtering algorithm to remove cyclical fluctuations in signal (either >0.011 Hz or <0.15 Hz) not temporally indicative of a hemodynamic response, and normalization of blood oxygen level-dependent (BOLD) signal intensity to percentage signal change using each subject’s voxel-wise time series mean as a baseline. Movement artifact was mitigated by using motion-correction parameters in the statistical model as nuisance covariates along with a covariate for mean intensity and linear drift. Ten initially recruited participants who moved >3 mm in any plane were excluded.
The statistical model was a gamma variate basis function convolved with the hemodynamic response function contained in AFNI and set to onset of each peer feedback event type, including picture presentation of the feedback and affective response rating period. ‘Peer feedback’ conditions were acceptance (‘Interested’) and rejection (‘Not Interested’) trials. Acceptance vs rejection was contrasted further by type of peer delivering feedback as determined by participants prescan ratings, i.e. ‘High-Interest’ and ‘Low-Interest’ peers. To maximize statistical power, these two ‘interest in peer’ conditions were determined using a median split of each participant’s ratings (M = 42.94, s.d. = 16.42, median = 44.53).
A general linear model determined the β-value and t-statistic per event type at each voxel. Whole-brain BOLD activation contrasts were created for each individual for each event type. This was followed by a second group-level, random-effects analysis of individual contrast values using the AFNI 3dANOVA3 program. This analysis of variance (ANOVA) tested the effects of interest in peers (high interest, low interest), feedback (acceptance, rejection) and the peer interest × feedback interaction. Standard criteria of P < 0.005 whole-brain uncorrected for multiple comparisons was used. The AFNI 3dclust program was used to create masks of significant clusters for main and interaction effects set at P < 0.005 and minimal cluster size of 100 voxels. The AFNI 3dmaskave program was used to compute average activation values of all voxels within functionally defined clusters for each participant. Mean activation values for functional clusters of interest were extracted per participant for the contrasts of significant main and interaction effects (e.g. acceptance-high-interest peers; acceptance-low-interest peers; rejection-high-interest peers; rejection-low-interest peers). Secondary analyses on extracted values were performed in SPSS to clarify effects in significant clusters.
Finally, to determine if participants’ age or gender influenced activation patterns, the key contrasts ‘acceptance–rejection feedback from high-interest peers’ and ‘acceptance–rejection feedback from low-interest peers’ were analyzed with the AFNI 3dRegAna procedure. Age, gender and the age × gender interaction were set as regressors for a voxel-wise whole-brain analysis with statistical threshold set to P < 0.005 and spatial extent set to 100 contiguous voxels.
RESULTS
Affective response to feedback
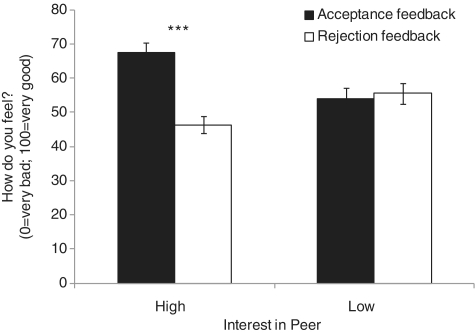
A 2 × 2 repeated measures ANOVA evaluated ratings of affective response to peer feedback, with peer interest (high, low) and feedback type (acceptance, rejection) as within-group factors. Significant main effects were found for peer interest [F(1, 35) = 4.49, P < 0.05) and feedback [F(1, 35) = 4.86, P < 0.05). These main effects were qualified by a significant peer interest × feedback interaction on adolescents’ affective response to feedback, F(1, 35) = 32.62, P < 0.001 (Figure 2). As expected, participants felt better having received acceptance (M = 67.66, s.e. = 2.68) vs rejection (M = 46.37, s.e. = 2.46) from peers of high interest (P < 0.001), but felt similarly in response to either feedback type from peers of low interest (n.s.; acceptance M = 53.97, s.e. = 3.25; rejection M = 55.56, s.e. = 2.98). Thus, participants’ ratings of peers made 2 weeks prior were related to their emotional responses to feedback during scanning.
Fig. 2.
A significant interest in peer-by-feedback-type interaction effect was found for adolescents’ affective response to evaluation, F(1, 35) = 32.62, P < 0.001. Higher levels of positive affect were reported when accepted vs rejected by peers of high interest (***P < 0.001).
A follow-up analysis using a 2 (peer interest) × 2 (feedback) × 2 (age group) × 2 (gender) repeated measures ANOVA tested potential moderating age effects (using median split, median age = 13.66) and/or gender. Main and interaction effects of age and gender on response to peer feedback were all non-significant; only the peer interest × feedback interaction was significant as reported above.
Neural response to feedback
Main effect of peer interest
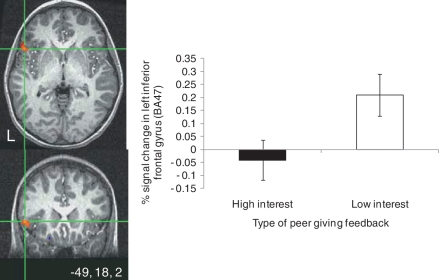
Whole-brain analyses indicated a significant main effect of participants’ prescan ratings of their interest in peers on IFG/Brodmann Area 47 (BA47) activity. Regardless of feedback (acceptance or rejection), greater activation was found in the left IFG/BA47 when feedback came from low- vs high-interest peers (Table 2; Figure 3). Comparisons of BOLD signal change parameter estimates extracted from the IFG/BA47 cluster during high- vs low-interest peer feedback trials revealed that IFG/BA47 response to low-interest peers reflected a significant increase in signal change above baseline [t(35) = 2.65, P = 0.01] whereas IFG/BA47 response to high-interest peers reflected a non-significant decrease in signal change below baseline.
Table 2.
Regions of activation for effects of interest in peer, feedback type and interest-in-peer by feedback-type interaction while adolescents rated how the feedback made them feel (N = 36)
| Region | x | y | Z | F | BA | Volume (vmul) |
|---|---|---|---|---|---|---|
| Interest in peer (High vs Low) | ||||||
| L inferior frontal gyrus | −49 | 18 | 2 | 10.87 | 47 | 289 |
| Feedback type (acceptance vs rejection) | ||||||
| Cortical | ||||||
| L superior frontal gyrus | −20 | 20 | 45 | 11.56 | 8 | 2498 |
| R superior frontal gyrus | 29 | 21 | 48 | 11.31 | 8 | 980 |
| L superior temporal gyrus | −52 | −56 | 25 | 14.47 | 39 | 208 |
| R superior temporal gyrus | 53 | −54 | 20 | 9.66 | 39 | 2390 |
| R middle temporal gyrus | 38 | −51 | 6 | 10.81 | 39 | 186 |
| R middle temporal gyrus | 36 | −59 | 27 | 10.08 | 39 | 146 |
| R cingulate gyrus; R precuneus | 13 | −48 | 29 | 9.62 | 31 | 269 |
| R cingulate gyrus | 19 | −37 | 22 | 9.10 | 31 | 768 |
| L angular gyrus; L precuneus | −40 | −73 | 32 | 11.68 | 39 | 299 |
| L precentral gyrus | −42 | −14 | 48 | 14.15 | 4 | 206 |
| R middle occipital gyrus | 36 | −70 | 9 | 10.26 | – | 141 |
| R anterior cingulate | 1 | 27 | 0 | 9.35 | 24 | 106 |
| R insula | 43 | 2 | 3 | 10.81 | 13 | 196 |
| R fusiform gyrus | 26 | −67 | −10 | 9.81 | 19 | 341 |
| L parahippocampal gyrus | −11 | −31 | 0 | 9.61 | – | 366 |
| Subcortical | ||||||
| R lentiform nucleus; R putamen | 30 | −14 | 3 | 15.72 | – | 684 |
| R caudate and caudate head | 8 | 16 | 0 | 12.45 | – | 236 |
| L thalamus | −1 | −29 | 7 | 9.04 | – | 253 |
| R hypothalamus | 4 | −4 | −16 | 9.61 | – | 116 |
| Interest in peer x feedback type | ||||||
| L posterior cingulate; L precuneus | −3 | −58 | 20 | 9.12 | 23 | 390 |
| R middle temporal gyrus | 56 | −24 | −3 | 9.32 | 21 | 182 |
| L uncus | −24 | 3 | −27 | 9.35 | 28 | 130 |
| R declive | 9 | −76 | −14 | 10.81 | – | 128 |
Threshold was set at P < 0.005 uncorrected. Cluster connectivity radius (rmm) was set at 0. Minimum cluster size (vmul) was set at 100. LPI coordinates are in Talairach space. F-value represents peak voxel in cluster. Broadmann area is based on peak voxel in cluster (– indicates Broadmann areas do not apply). L = left, R = right.
Fig. 3.
A significant main effect of interest in peer was found in the left inferior frontal gryus (IFG) when adolescents’ rated their affective response to peer feedback (thresholded at P < 0.005 for magnitude, uncorrected, 100 voxel minimum spatial extent). This effect emerged regardless of the type of feedback. Activation map shows cross-hairs centered on the maximum intensity value [Talairach coordinates (x, y, z): −49, 18, 21] for the cluster in the left IFG cluster (289 voxels). The bar graph shows parameter estimates extracted from the IFG cluster during high and low-peer interest feedback trials, relative to baseline.
Main effect of feedback
Acceptance vs rejection feedback (regardless of peer interest) elicited significant BOLD signal change in a number of regions previously implicated in social affiliation, social cognition, reward and social affect, including the superior frontal gyrus (SFG), superior temporal gyrus (STG), insula, fusiform gyrus, anterior cingulate and anterior and ventral striatum (caudate, putamen) (Table 2 shows all significant activations). No significant activations emerged to rejection.
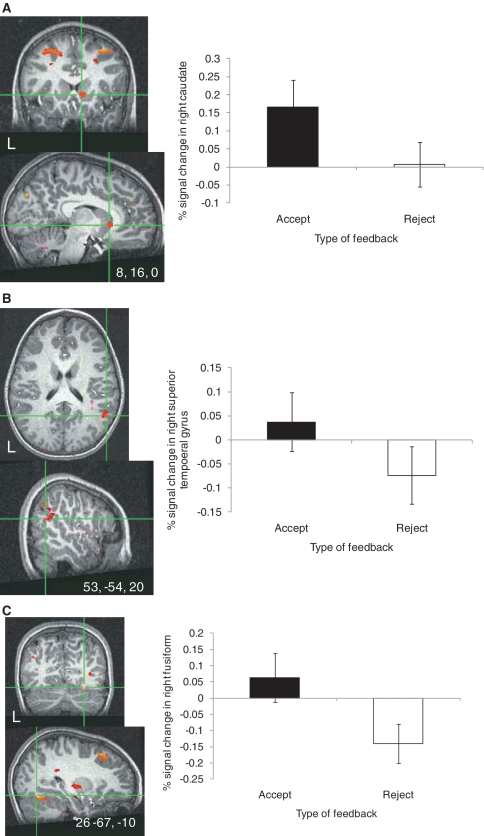
In support of expectations regarding the potentially rewarding nature of peer acceptance, acceptance vs rejection feedback elicited significantly greater activity in the right caudate (Figure 4A) and putamen. For display purposes, parameter estimates were extracted from the significant right caudate cluster during acceptance and rejection feedback relative to baseline. One-sample t-test results showed that in this region, acceptance significantly increased activation in the right caudate relative to baseline [t(35) = 2.22, P = 0.03), whereas rejection did not elicit significantly greater activation relative to baseline.
Fig. 4.
A significant main effect of feedback type was found in several cortical and subcortical regions when adolescents rated their affective response to peer feedback (thresholded at P < 0.005 for magnitude, uncorrected, 100 voxel minimum spatial extent). This effect emerged regardless of participants’ interest in peers. Activation maps show cross-hairs centered on the maximum intensity value for the activated cluster in the (A) right caudate [Talairach coordinates (x, y, z): 8, 16, 0; 236 voxels], (B) right superior temporal gyrus encompassing the temporal–parietal junction [Talairach coordinates (x, y, z): 53, −54, 20; 2390 voxels] and (C) right fusiform gyrus [Talairach coordinates (x, y, z): 26, −67, −10; 341 voxels]. Bar graphs show parameter estimates extracted from the right caudate, right superior temporal gyrus and right fusiform clusters during acceptance and rejection feedback trials, relative to baseline.
Another region that differed significantly in response to acceptance vs rejection feedback was the STG (Figure 4B). Extracted parameter estimates from the right STG cluster indicated that the difference between these events was due to less activation to rejection rather than greater activation to acceptance. Mean parameter estimates from each condition were not significantly different from zero.
A significant difference in response to acceptance vs rejection emerged in a third region of interest, the fusiform gyrus (Figure 4C). Parameter estimates extracted from the significant right fusiform cluster revealed a pattern similar to the STG. Specifically, the difference in response to these feedback types was due to less activation to rejection as opposed to greater activation to acceptance. Mean parameter estimates from each condition were not significantly different from zero.
Interaction of peer interest × feedback
Significant peer interest × feedback interactions were found (Table 2), such that participants’ prescan peer interest ratings (high, low) moderated subsequent neural response to acceptance vs rejection feedback in posterior cingulate/precuneus and middle temporal gyrus (MTG). Parameter estimates were extracted from and graphed for these two regions (Supplementary Figure S1). In the posterior cingulate/precuneus, acceptance from high-interest peers resulted in deactivation but rejection from low-interest peers induced a greater deactivation. A similar pattern emerged in the MTG.
Age and gender effects—feedback from high-interest peers
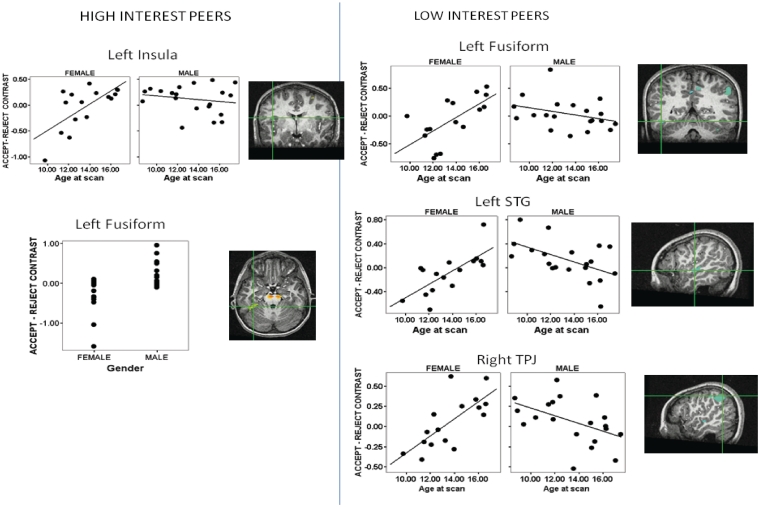
Acceptance vs rejection from peer participants rated high, elicited activations in some regions that varied by both age and gender. Main effects of age showed that activity in the postcentral gyrus, inferior parietal lobule (IPL), insula and cerebellum increased across age while activation in the middle frontal gyrus declined significantly across age. A main effect of gender showed that postcentral gyrus, fusiform gyrus and midbrain activity was significantly greater in males than females. An age × gender interaction was found for postcentral gyrus, IPL, insula and cerebellum activity (Table 3; Figure 5 shows scatterplots of insula and fusiform activity).
Table 3.
Age, gender and age × gender effects on neural response to acceptance vs rejection from high-interest peers
| Region | x | y | Z | t-value | BA | Volume (vmul) |
|---|---|---|---|---|---|---|
| Age | ||||||
| L postcentral gyrus | −56 | −22 | 47 | 4.28 | 2 | 703 |
| L inferior parietal lobule | −51 | −35 | 30 | 3.60 | 40 | 194 |
| R middle frontal gyrus | 33 | 0 | 53 | −3.43 | 6 | 190 |
| L insula | −40 | −4 | 16 | 4.00 | 13 | 169 |
| L cerebellum | −13 | −45 | −37 | 3.85 | – | 141 |
| R cerebellum | 25 | −45 | −36 | 3.98 | – | 177 |
| Gender | ||||||
| L postcentral gyrus | −41 | −19 | 32 | 3.86 | 3 | 753 |
| L fusiform gyrus | −34 | −35 | −22 | 3.77 | 20 | 352 |
| R postcentral gyrus | 44 | −25 | 31 | 3.32 | 3 | 340 |
| Pons | 1 | −20 | −34 | 3.77 | – | 127 |
| Midbrain | 12 | −18 | −20 | 4.09 | – | 610 |
| Age × gender | ||||||
| L postcentral gyrus | −56 | −19 | 46 | −4.30 | 2 | 660 |
| L cerebellum | −16 | −45 | −37 | −3.73 | – | 209 |
| L inferior parietal lobule | −54 | −34 | 33 | −3.30 | 40 | 140 |
| L insula | −40 | −3 | 15 | −3.74 | 13 | 101 |
Threshold was set at P < 0.005 uncorrected. Cluster connectivity radius (rmm) was set at 0. Minimum cluster size (vmul) was set at 100. LPI coordinates are in Talairach space. t-value represents peak voxel in cluster. Broadmann area is based on peak voxel in cluster (− indicates Broadmann areas do not apply). L = left, R = right.
Fig. 5.
A significant age × gender interaction effects on acceptance vs rejection feedback from (A) high-interest peers and from (B) low-interest peers. Thresholded at P < 0.005 for magnitude, uncorrected, 100 voxel minimum spatial extent. Activation maps show cross-hairs centered on the maximum intensity value for the activated cluster. Scatterplots show parameter estimates extracted from the acceptance vs rejection feedback from high-interest peers in the left insula [age × gender interaction; Talairach coordinates (x, y, z): −40, −3, 15; 101 voxels] and left fusiform [gender effect; Talairach coordinates (x, y, z): −34, −35, −22; 352 voxels] as well as from low-interest peers in the left fusiform [Talairach coordinates (x, y, z): −46, −45, −11; 190 voxels], left superior temporal gyrus [Talairach coordinates (x, y, z): −52, −18, −1; 1008 voxels] and right temporal parietal junction [Talairach coordinates (x, y, z): 52, −51, 45; 1494 voxels] (age × gender interactions).
Age and gender effects—feedback from low-interest peers
Acceptance vs rejection from peer participants rated as low, elicited activations that varied by age, gender and age × gender (Table 4). Fusiform, IPL, STG and midbrain activation all increased with increased age, while SFG and parahippocampal gyrus activation declined significantly with increased age. Males had greater fusiform, PHG and cerebellum activity whereas females had greater IFG activity. Finally, age × gender effects were found that primarily showed increasing activation across age among females and decreasing activation across age among males. Regions displaying this pattern included the left fusiform, left STG and right TPJ (Table 4 and Figure 5).
Table 4.
Age, gender, and age x gender effects on neural response to acceptance vs rejection from low-interest peers
| Region | x | y | Z | t-value | BA | Volume (vmul) |
|---|---|---|---|---|---|---|
| Age | ||||||
| L fusiform gyrus | −46 | −45 | −13 | 4.21 | 37 | 622 |
| R superior frontal gyrus | 21 | 34 | 52 | −4.42 | 8 | 318 |
| R inferior parietal lobule | 65 | −27 | 39 | 3.56 | 40 | 225 |
| R inferior parietal lobule | 56 | −31 | 44 | 3.33 | 40 | 137 |
| R inferior parietal lobule | 54 | −47 | 44 | 3.35 | 40 | 119 |
| R parahippocampal gyrus | 32 | −18 | −24 | −4.15 | 20 | 166 |
| L superior temporal gyrus | −52 | −18 | −1 | 3.77 | 21 | 150 |
| R midbrain | 13 | −18 | −22 | 4.23 | 37 | 179 |
| Gender | ||||||
| L cerebellum | −3 | −43 | −29 | 4.31 | – | 512 |
| R fusiform gyrus | 29 | −35 | −22 | 3.65 | 20 | 256 |
| R parahippocampal gyrus | 20 | −25 | −20 | 3.74 | 35 | 148 |
| L inferior frontal gyrus | −45 | 33 | 13 | −4.51 | 46 | 144 |
| Age × gender | ||||||
| R inferior parietal lobule | 52 | −51 | 45 | −4.18 | 40 | 1494 |
| L superior temporal gyrus | −52 | −18 | −1 | −4.55 | 22 | 1008 |
| R cerebellum | 12 | −55 | −37 | −4.04 | – | 428 |
| R precuneus | 10 | −66 | 49 | −3.84 | 7 | 391 |
| L fusiform gyrus | −46 | −45 | −11 | −3.82 | 37 | 190 |
| L midbrain | −10 | −27 | −11 | 4.76 | – | 189 |
| L fusiform gyrus | −28 | −36 | −17 | −3.74 | 20 | 180 |
| L posterior cingulate gyrus | −12 | −50 | 7 | −3.69 | 29 | 171 |
| L insula | −44 | 12 | 0 | −3.40 | 13 | 154 |
| R postcentral gyrus | 63 | −27 | 39 | −3.37 | 2 | 120 |
| R precuneus | 8 | −47 | 46 | −3.29 | 7 | 109 |
| R middle temporal gyrus | 53 | −19 | −4 | −3.63 | 21 | 100 |
Threshold was set at P < 0.005 uncorrected. Cluster connectivity radius (rmm) was set at 0. Minimum cluster size (vmul) was set at 100. LPI coordinates are in Talairach space. t-value represents peak voxel in cluster. Broadmann area is based on peak voxel in cluster (– indicates Broadmann areas do not apply). L = left, R = right.
DISCUSSION
In line with the social reorientation adolescent neurodevelopmental framework (Nelson et al., 2005), the current study generated expected regionally selective patterns of adolescents’ neural response to peer feedback. These patterns included a region showing differential activity based on adolescents’ initial interest in peers, regardless of feedback type; regions exhibiting increased response to positive vs negative feedback, regardless of interest in peers and regions whose response to feedback was moderated by interest in peers. Each of these findings will be discussed in turn, as well as results showing neural modulation by age and gender.
The vlPFC was the only region that varied significantly as a function of subject-reported peer interest, specifically in the left IFG, encompassing BA47. Specifically, greater IFG/BA47 activity emerged when adolescents reported how they felt receiving feedback specifically from peers with whom they were not interested in interacting. Previous work has shown a key role of the IFG/BA47 in response flexibility in other feedback-based contexts (Rolls, 2004; Remijnse et al., 2005; Ghahremani et al., 2010). For example, the IFG/BA47 is engaged during tasks that require cognitive processes such as response reversal learning, selecting stimuli of shifting value and updating expectancies following outcomes (Murray et al., 2007). This region has also codes changes in reward–punishment expectancies, not only for inanimate stimuli, but also for faces (Rolls, 2004). One interpretation of the current finding is that IFG/BA47, which continues to mature during adolescence, plays a role in reappraising social stimuli and updating expectancies following social feedback (Nelson and Guyer, 2011). Given the highly fluid and affective nature of adolescent peer affiliations, this would allow for updating and possibly adjusting an adolescent’s initial assessments of whether they liked certain peers after learning what peers thought of them. If this interpretation is correct, then it is noteworthy that the IFG/BA47 was activated more to a peer of little than of high interest. This suggests an adolescent’s low interest rating may be more amenable to change following positive or negative feedback than an adolescent’s high interest rating, and that reappraising and updating initial perceptions of peers are important processes to consider in future work. These results also resonate with past work indicating that adolescents’ initial impressions of peers modulates neural activity (Guyer et al., 2008, 2009) and support context dependency of neural response to social feedback (Gunther-Moor et al., 2010).
A second key finding revealed increased caudate and putamen activity following acceptance feedback. Striatal regions have been implicated in social reward processing and approach-related behavior (Aharon et al., 2001; Kampe et al., 2001; Aron et al., 2005; Depue and Morrone-Strupinsky, 2005). The striatal activity documented here likely reflects a positive emotional response to peer acceptance, consistent with the increased happiness that adolescents reported after being accepted by desirable peers. Unlike the emotional response ratings, striatal activity was not moderated by adolescents’ initial assessments of peers and may reflect a more basic response to social rewards not moderated by self-reported ratings. Acceptance vs rejection also elicited greater fusiform gyrus and STG activity. Both of these regions are implicated in basic detection and perception of social stimuli (Allison et al., 2000; Vaina et al., 2001; Haxby et al., 2002; Puce and Perrett, 2003; Jellema et al., 2004). Enhanced fusiform and STG activity could reflect more striatal and limbic input to these perceptual regions, which may, in turn, enhance perceptual encoding of peers after the receipt of positive feedback.
Notably, anterior cingulate cortex (ACC) activity increased during emotional responding to acceptance and insula activity decreased during emotional response to rejection. In contrast, other work in adolescents has found increased subgenual anterior cingulate, vPFC and insula activity in response to social exclusion (Masten et al., 2009). As the insula is implicated in interoception and subjective feelings of emotion (Craig, 2004), it may be involved in adolescents’ coding of the salience of socially evaluative events regardless of the event’s valence. The ACC result noted here is consistent with work in adults showing greater ventral ACC response to social acceptance and provides further validation of its sensitivity to affective processing of feedback among adolescents (Somerville et al., 2006).
Finally, posterior cingulate/precuneus and MTG response varied as a function of both interest in peer ratings and feedback type. As with the main effect of adolescents’ interest in peers, these interactions again suggest the influence of social context and expectations about others on neural response. The precuneus has been implicated in social- and self-knowledge and mentalizing tasks and may support deciphering the intentions of others (Pfeifer et al., 2007; Van Overwalle and Baetens, 2009). The MTG is likely involved in social perceptual processes in this context. Both of these regions displayed a relative increase in activation when feedback was inconsistent with whether adolescents thought they would like the peers. Similar patterns of social expectancy-related activations have been noted in adult and adolescent samples (Somerville et al., 2006; Gunther-Moor et al., 2010). The present results may indicate disengagement of these regions when feedback was consistent with prior expectations, but engagement when expectations were violated.
The present study also revealed activation patterns in many regions involved in social cognition that varied as a function of age, gender, or both in the contrasts of acceptance relative to rejection feedback for high and for low-interest peers. These regions included the fusiform, STG, insula and IPL/TPJ area. These effects generally indicated greater activation to acceptance than rejection for males than for females across all ages, greater activation to acceptance with increased age across gender, and greater age-related activation to acceptance for females than males. Activity in these areas suggest that making self-referential appraisals (‘Do I like this person?’) may rely more on integrating positive feedback about one’s self during emotional responding in later adolescence (Pfeifer et al., 2009).
Our age-related results should also be considered in light of recent work whereby participants viewed photographs of unfamiliar individuals and first had to rate their expectation of whether the depicted individual would like them or not and then received acceptance or rejection feedback (Gunther-Moor et al., 2010). Where positive feedback was expected, Gunther-Moor et al. (2010) reported results similar to the present findings. Specifically, acceptance induced greater activity than rejection in striatum, PFC and fusiform among other regions implicated in social cognition. We found similar patterns of increased activity to acceptance in these regions. As in past work on anticipating peer evaluation (Guyer et al., 2009), we found age-related linear increases in activation to acceptance within several regions involved in social affect and social reward, which suggests that the striatum is sensitive to positive types of social feedback as a function of age and gender. Work by Gunther-Moor et al. (2010) reported an opposing age-related shift of vmPFC and ventral striatum activity; specifically, greater activation to rejection than acceptance particularly in adults. This suggests that adults and adolescents may code negative social information differently. Additionally, our age-related findings and those of Gunther-Moor et al. (2010) emerged in different brain regions. Although regions in both studies play a role in social affect, age-related changes in the Gunther-Moor et al. (2010) study were concentrated more in the frontal regions, whereas our findings were more widespread. We also found that gender moderated many of the present study’s age-related differences in response to feedback and when anticipating peers’ evaluation (Guyer et al., 2009). These gender-related patterns suggest that older females may experience heightened affective responses to and cognitive regulation of peer feedback.
The neural response and self-report data described here demonstrate that, during the Chatroom task, psychiatrically healthy adolescents engage the social perceptual circuitry in combination with prefrontal systems that promote flexibility and limbic systems that denote reward value. One unexpected outcome from the current study is the general lack of response generated by rejection feedback. While other studies have indicated that adolescents show neural sensitivity to social exclusion (Masten et al., 2009; Sebastian et al., 2010; Lau et al., in press), the present data indicate increased response to acceptance and little to no response to rejection. This lack of response to rejection may reflect the task structure. If rejection came from peers that adolescents were more invested in (e.g. pictures of actual classmates) or if rejection was linked to tangible features of an adolescent’s personality, then the stimuli would likely be more salient and evocative. Alternatively, healthy adolescents may respond more to social signals conveying approach vs avoidance signals. This possibility should be addressed in future studies.
Some limitations apply to the present study. Complexities of real-life social interactions limit the degree to which specific cognitive and affective components can be represented naturalistically, while also manipulated experimentally. For example, why participants rated certain peers as socially desirable or undesirable may reflect processes not currently assessed with this task. However, participants’ initial ratings of each peer’s desirability and subsequent affective responses to feedback were strongly related. This suggests that participants’ initial impressions of peers relate to neural sensitivity in socially evaluative contexts such that not all peers are perceived equally; the meaning attributed to certain peers differentially influences neural responses to peer evaluation. Additional work should more precisely characterize the qualities of peers that differentially engage activity in these social perception, affective and cognitive regulatory circuits. Additional limitations relate to the use of deception. Some participants were excluded because they reported disbelief with the task. Although this approach may bias generalizability of the study’s results, analyses including participants who believed the manipulation are likely to represent adolescents’ typical reactions to encounters with peers. In addition, debriefing participants about the task deception limits longitudinal study of these processes. Nonetheless, longitudinal designs are needed to address how these patterns evolve across time within individuals.
The present study generated new knowledge of factors that modulate neural correlates of adolescents’ affective responses to socially evaluative feedback. Advances in clinical, developmental and social–cognitive fields of inquiry are anticipated from this interdisciplinary approach to the study of social influences on adolescent neurodevelopment. Future work is needed to test differences between adults and adolescents to determine which effects are specific to adolescents and examine these processes longitudinally. These results also highlight how observations based on direct measures of the brain can establish boundaries for developmental principles and frameworks, which in turn may generate more nuanced theories about how social cognition develops typically or goes awry in adolescent psychopathologies (Paus et al., 2008; Pine et al., 2008). Neural responsiveness to socially salient events may relate to the emergence or exacerbation of adolescent mood and anxiety disorders (Guyer et al., 2008; Lau et al., in press), the prevalence of which increases markedly during adolescence (Pine et al., 1998; Kessler et al., 2005). Finally, because adolescent neurodevelopment is intertwined with biological changes due to puberty, environment and context, neurophysiologic data can facilitate an understanding of how cognitive and affective responses to salient social events unfold and provide a benchmark for future clinical and developmental neuroscience studies.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Acknowledgments
The authors greatly appreciate the time given to us by the children and families involved in the study. In addition, the authors give special thanks to Harvey Iwamoto for task programming. The National Institute of Mental Health Intramural Research Program of the National Institutes of Health and National Institutes of Health Career Development (grant K99/R00 MH080076 to A.E.G.).
REFERENCES
- Adolphs R. The neurobiology of social cognition. Current Opinion in Neurobiology. 2001;11(2):231–9. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Aharon I, Etcoff N, Ariely D, Chabris CF, O'Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32(3):537–51. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Allen JP, Porter MR, McFarland FC, Marsh P, McElhaney KB. The two faces of adolescents' success with peers: adolescent popularity, social adaptation, and deviant behavior. Child Development. 2005;76(3):747–60. doi: 10.1111/j.1467-8624.2005.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends in Cognitive Sciences. 2000;4(7):267–78. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2(11):1032–7. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology. 2005;94(1):327–37. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21(3):1155–66. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117(3):497–29. [PubMed] [Google Scholar]
- Blair RJ, Cipolotti L. Impaired social response reversal: A case of ‘acquired sociopathy’. Brain. 2000;123(6):1122–41. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9(4):267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, den Ouden H, Choudhury S, Frith C. Adolescent development of the neural circuitry for thinking about intentions. Social Cognitive and Affective Neuroscience. 2007;2(2):130–9. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–26. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury S, Blakemore S-J, Charman T. Social cognitive development during adolescence. Social Cognitive and Affective Neuroscience. 2006;1(3):165–74. doi: 10.1093/scan/nsl024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. Human feelings: why are some more aware than others? Trends in Cognitive Sciences. 2004;8(6):239–41. doi: 10.1016/j.tics.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL, Sommerville JA, Chaminade T, Meltzoff AN. The neural bases of cooperation and competition: an fMRI investigation. Neuroimage. 2004;23(2):744–51. doi: 10.1016/j.neuroimage.2004.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: implications for conceptualizing a human trait of affiliation. Behavioral and Brain Sciences. 2005;28(3):313–50. doi: 10.1017/S0140525X05000063. discussion 350–95. [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, Monterosso J, Jentsch JD, Bilder RM, Poldrack RA. Neural components underlying behavioral flexibility in human reversal learning. Cerebral Cortex. 2010;20(8):1843–52. doi: 10.1093/cercor/bhp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther-Moor BG, van Leijenhorst L, Rombouts SA, Crone EA, Van der Molen MW. Do you like me? Neural correlates of social evaluation and developmental trajectories. Social Neuroscience. 2010;5:461–82. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of General Psychiatry. 2008;65(11):1303–12. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development. 2009;80(4):1000–15. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4(6):223–33. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biological Psychiatry. 2002;51(1):59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Annual Review of Neuroscience. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Jellema T, Maassen G, Perrett DI. Single cell integration of animate form, motion and location in the superior temporal cortex of the macaque monkey. Cerebral Cortex. 2004;14(7):781–90. doi: 10.1093/cercor/bhh038. [DOI] [PubMed] [Google Scholar]
- Kampe KK, Frith CD, Dolan RJ, Frith U. Reward value of attractiveness and gaze. Nature. 2001;413(6856):589. doi: 10.1038/35098149. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- La Greca AM, Lopez N. Social anxiety among adolescents: Linkages with peer relations and friendships. Journal of Abnormal Child Psychology. 1998;26(2):83–94. doi: 10.1023/a:1022684520514. [DOI] [PubMed] [Google Scholar]
- Lau JF, Guyer AE, Tone EB, et al. Neural responses to peer rejection in anxious adolescents: contributions from the amygdala-hippocampal complex. International Journal of Behavioral Development. in press doi: 10.1177/0165025411406854. [Epub 17 June 2011, doi:10.1177/0165025411406854] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers' neural activation in response to pictures of their children and other children. Biological Psychiatry. 2004;56(4):225–32. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI. Neuroscience. Pains and pleasures of social life. Science. 2009;323(5916):890–1. doi: 10.1126/science.1170008. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, et al. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4(2):143–57. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, Meesters C, Merckelbach H, Sermon A, Zwakhalen S. Worry in normal children. Journal of American Academy of Child Adolescent Psychiatry. 1998;37(7):703–10. doi: 10.1097/00004583-199807000-00009. [DOI] [PubMed] [Google Scholar]
- Murray EA, O'Doherty JP, Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. Journal of Neuroscience. 2007;27(31):8166–9. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE. Adolescent Facial Expressions Stimuli Database. 2004. Section on Development and Affective Neuroscience, National Institute of Mental Health. [Google Scholar]
- Nelson EE, Guyer AE. The development of the ventral prefrontal cortex and social flexibility. Developmental Cognitive Neuroscience. 2011;1:233–45. doi: 10.1016/j.dcn.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–74. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neuroscience and Biobehavioral Reviews. 1998;22(3):437–52. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage. 2004;21(2):583–92. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9(12):947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman MD, Dapretto M. "I know you are but what am I?!": neural bases of self- and social knowledge retrieval in children and adults. Journal of Cognitive Neuroscience. 2007;19(8):1323–37. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Borofsky LA, Dapretto M, Fuligni AJ, Lieberman MD. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Development. 2009;80(4):1016–38. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55(1):56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Pine DS, Guyer AE, Leibenluft E. Functional magnetic resonance imaging and pediatric anxiety. Journal of American Academy of Child and Adolescent Psychiatry. 2008;47(11):1217–21. doi: 10.1097/CHI.0b013e318185dad0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Perrett D. Electrophysiology and brain imaging of biological motion. Philosophical Transactions of Royal Society of London B: Biological Science. 2003;358(1431):435–45. doi: 10.1098/rstb.2002.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remijnse PL, Nielen MM, Uylings HB, Veltman DJ. Neural correlates of a reversal learning task with an affectively neutral baseline: an event-related fMRI study. Neuroimage. 2005;26(2):609–18. doi: 10.1016/j.neuroimage.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain and Cognition. 2004;55(1):11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Rubin KH, Bukowski WM, Parker JG. Peer interactions, relationships, and groups. In: Eisenberg N, William D, Richard ML, editors. Handbook of Child Psychology, Vol. 3, Emotional, and Personality Development. 6th edn. Hoboken, NJ: John Wiley & Son; 2006. pp. 571–645. [Google Scholar]
- Saxe R. Uniquely human social cognition. Current Opinion in Neurobiology. 2006;16(2):235–9. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Saxe R, Carey S, Kanwisher N. Understanding other minds: linking developmental psychology and functional neuroimaging. Annual Review of Psychology. 2004;55:87–124. doi: 10.1146/annurev.psych.55.090902.142044. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Viding E, Williams KD, Blakemore SJ. Social brain development and the affective consequences of ostracism in adolescence. Brain and Cognition. 2010;72(1):134–45. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Silverman WK, La Greca AM, Wasserstein S. What do children worry about? Worries and their relation to anxiety. Child Development. 1995;66(3):671–86. doi: 10.1111/j.1467-8624.1995.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9(8):1007–8. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Growth at Adolescence. Oxford: Blackwell Scientific Publications; 1962. [Google Scholar]
- Vaina LM, Solomon J, Chowdhury S, Sinha P, Belliveau JW. Functional neuroanatomy of biological motion perception in humans. Proceedings of the National Academy of Sciences USA. 2001;98(20):11656–61. doi: 10.1073/pnas.191374198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others' actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48(3):564–84. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Wendler D. Deception in medical and behavioral research: is it ever acceptable? Milbank Quarterly. 1996;74(1):87–114. [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nature Neuroscience. 2004;7(10):1048–54. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.