Abstract
We developed an ecologically valid virtual peer interaction paradigm—the Chatroom Interact Task in which 60 pre-adolescents and adolescents (ages 9–17 years) were led to believe that they were interacting with other youth in a simulated internet chatroom. Youth received rejection and acceptance feedback from virtual peers. Findings revealed increased pupil dilation, an index of increased activity in cognitive and affective processing regions of the brain, to rejection compared to acceptance trials, which was greater for older youth. Data from a cell-phone Ecological Momentary Assessment (EMA) protocol completed following the task indicated that increased pupillary reactivity to rejection trials was associated with lower feelings of social connectedness with peers in daily life. Eyetracking analyses revealed attentional biases toward acceptance feedback and away from rejection feedback. Biases toward acceptance feedback were stronger for older youth. Avoidance of rejection feedback was strongest among youth with increased pupillary reactivity to rejection, even in the seconds leading up to and following rejection feedback. These findings suggest that adolescents are sensitive to rejection feedback and seek to anticipate and avoid attending to rejection stimuli. Furthermore, the salience of social rejection and acceptance feedback appears to increase during adolescence.
Keywords: adolescence, social interaction, peer rejection, pupil dilation, eyetracking, ecological momentary assessment
A fundamental human characteristic is the need to preserve the social self, which includes vigilance to threats that may jeopardize social esteem, social status and relative self-worth (Bowlby, 1969; Maslow, 1987; Baumeister and Leary, 1995). These responses are particularly salient during the transition into and through adolescence. Adolescence is associated with enhanced sensitivity to social evaluation and enhanced desire for social affiliation (O'Brien and Bierman, 1988; Steinberg and Morris, 2000; Brown, 2004). Social comparison becomes entrenched in daily life (Brown and Lohr, 1987), potentially making adolescents reactive to cues of social acceptance and rejection. Yet, mechanisms of these processes have rarely been examined in adolescents due largely to difficulties in creating believable environments in which peer acceptance and rejection can take place during mechanistic assessments. Here we examine peer acceptance and rejection in the laboratory using a novel ecological task.
Sensitivity to social status may be linked, at least in part, to remodeling of regions of the adolescent brain involved in social information processing (Spear, 2000; Nelson et al., 2005; Blakemore, 2008). These changes coincide with changes in the social context that contribute to increased salience of the social sphere and perceived social evaluation in daily life. For example, parent–child conflict increases during early adolescence (Steinberg and Silk, 2002) and social affiliation with peers and romantic interests becomes increasingly important (Furman, 2002; Brown, 2004). Adolescent peer and romantic relationships are often intense, volatile, unstable and involve increasingly complex and salient social hierarchies such as in-groups and crowds (Connolly et al., 2000; Brown, 2004).
Although limited, mechanistic studies support increased sensitivity to social and emotional information in adolescence. For example, Silk et al. (2009) found that mid-to-late pubertal youth had a greater pupillary response to affective words than pre-to-early pubertal youth, controlling for participants’ age. Several neuroimaging studies show increased neural activity on emotional face processing tasks in adolescents compared to adults. For example, adolescents have been found to have greater amygdala reactivity relative to adults to sad and fearful faces (Killgore and Yurgelun-Todd, 2007; Guyer et al., 2008a) and greater activation in the amygdala, medial prefrontal cortex (MPFC) and anterior cingulate cortex (ACC) compared to adults during passive viewing of emotional faces (Monk et al., 2003).
Despite this growing body of research on emotional information processing in adolescence, little is known about how youth process social interactions associated with social acceptance and rejection. Most studies have utilized passive viewing or decision-making related to affective faces—in most cases the faces of adults—and have not investigated how youth interact with live peers or respond to real social feedback. Ecologically-valid social interaction paradigms could fill this gap, facilitating investigation of mechanisms of social rejection and acceptance during preadolescence and adolescence. Yet, practical and ethical constraints in the use of live peer confederates make the investigation of peer interaction in the laboratory challenging. One emerging research approach involves the development of virtual interaction and feedback paradigms (Guyer et al., 2008b; Masten et al., 2009). For example, experiments using Eisenberger et al. (2003) virtual ball tossing paradigm (‘Cyberball’) in adults have shown that brain regions associated with social information processing such as the dorsal anterior cingulate cortex (dACC), anterior insula and right ventral prefrontal cortex (RVPFC) are more active during periods of social exclusion than during periods of social inclusion. Masten et al. (2009) similarly found increased activity in the insula and the right ventral prefrontal cortex in adolescents during periods of social exclusion compared to inclusion on the cyber ball task, as well as increased activity in the subgenual ACC and ventral striatum that was unique to adolescents.
More explicit feedback has also been explored using tasks that provide participants with rigged feedback about how desirable or likable they are based on their photographs (Somerville et al., 2006; Guyer et al., 2008b; Moor et al., 2010). For example, Davey et al. (2010) investigated how young adults respond to ‘being liked’ by asking participants to view photos of other ‘virtual participants’ whom they were led to believe had rated them as ‘likable’ based on their own photographs. Guyer et al. (2008b, 2009) developed a Chatroom task to investigate youth's neural response to anticipated and rigged feedback from virtual peers they believed had evaluated them as potential interaction partners for an upcoming online chat session. Initial results showed that anxious adolescents expected peers to rate them as less desirable and had greater activation in the amygdala than healthy controls when judging how interested the virtual peers would be in chatting with them, especially for peers they did not want to interact with (Guyer et al., 2008b).
To better evaluate the dynamics of explicit social information processing, it would be useful to meld features of Cyberball, such as dynamic ecological inclusion/rejection, with the explicit judgments used in the Chatroom task. Towards that end, we developed a new version of the Chatroom task, Chatroom Interact, in which the subject interacts with virtual peers and experiences both rejection and acceptance from these peers during simulated online interaction. Unlike most other existing social feedback tasks (i.e. Davey, et al., 2010; Moor, et al., 2010), the Chatroom Interact Task utilizes photographs of other children and adolescents rather than using photographs of young adults. It differs from the original version of the Chatroom Task (Guyer et al., 2008b) in that it involves a period of live simulated interaction with the virtual peers. It also differs from the original version of the Chatroom task through additional features designed to increase the subjects’ sense of engagement with virtual peers so that they would presumably care more about receiving rejection and acceptance feedback from these peers. These included an expanded age range (9–17) that allowed us to more closely match photographs based on the subject's age and the addition of biographical profiles describing virtual peers. We designed this task to be used during neuroimaging and/or psychophysiological assessment. Here, we present pupillary, eyetracking and Ecological Momentary Assessment (EMA) data from the task development study to demonstrate that the task can provide valuable information on how adolescents process social acceptance and rejection.
We focus on pupillary reactivity to acceptance and rejection during the Chatroom-Interact task because pupillary responses coincide with activity in brain regions linked to emotional and cognitive processing including the dorsolateral prefrontal cortex (Siegle et al., 2003b) anterior cingulate cortex (Critchley et al., 2005) and amygdala (Koikegami and Yoshida, 1953). The pupil becomes more dilated in response to stimuli that require greater cognitive load or that have greater emotional intensity (Beatty, 1982; Beatty and Lucero Wagoner, 2000; Siegle et al., 2003a, 2004). This response occurs because the pupil is innervated by brain structures involved in both cognitive and emotional processing. Inhibition of the constrictor muscle occurs through parasympathetic innervation of the Edinger Whestphal nucleus which receives extensive inputs from cortical and limbic regions. Stimulation of the dilator muscle occurs through a hypothalamic pathway which also receives corticolimbic inputs. Thus, stimulation of limbic regions such as the amygdala increases pupil diameter (Koikegami and Yoshida, 1953), as does stimulation of the midbrain reticular formation (Beatty, 1986). The midbrain reticular formation receives afferent projections from the prefrontal cortex, which are implicated in emotion regulation (Szabadi and Bradshaw, 1996), and sends efferent projections to the ocular motor nuclei.
Pupil dilation also provides valuable information about the time-course of brain activation in response to a stimulus. The pupil remains dilated as long as the processing demand persists and, because pupillary responses can be sampled frequently (every 16 ms in the present study), provides a dynamic measure of changes in brain activity following exposure to the stimulus. For example, pupillometry can be used to differentiate early vs sustained reactions to emotional stimuli. Information about the intensity and time course of adolescents’ responses to peer acceptance and rejection obtained via pupillometry can provide valuable information to inform and complement future neuroimaging studies.
Recent concurrent pupillary/fMRI studies (Siegle et al., 2003; Siegle et al., 2003b; Critchley, et al., 2005; Urry et al., 2009) have indicated that pupillary responses on emotional information processing tasks appear most strongly associated with activity in several prefrontal regions associated with cognitive control and emotion regulation. For example, Siegle et al. 2003 found that decreased pupil dilation in response to negative emotional words was predictive of remission in cognitive therapy for adults with depression. Decreased pupil dilation on this task was associated with decreased activity in the dorso-lateral prefrontal cortex (DLPFC), an area linked to executive control and emotion regulation. Similarly, Siegle et al. (2003a) found that increased pupillary responses were associated with increased dorso-lateral prefrontal function on a digit-sorting task. Critchley et al. (2005) found associations of pupillary motility with the anterior cingulate on a Stroop task and Urry et al. (2009) found increased pupil dilation during conditions of increased cingulate and dorsomedial prefrontal activity.
In the present study, we compared pupillary reactivity to rejection and acceptance trials. Because of the marked effects of peer rejection on self-esteem and psychological adjustment in youth (Rudolph et al., 1997; Kistner et al., 1999; Dodge et al., 2003; Lopez and DuBois, 2005), as well as Masten et al.'s (2009) findings of greater activity in social information processing regions in response to social exclusion compared to inclusion among adolescents, we hypothesized that youth would have a stronger response to rejection than acceptance, resulting in greater pupil dilation in response to rejection compared to acceptance trials. We expected this response to increase with age. We also explored whether pupillary reactivity to rejection and acceptance would differ based on the gender of the virtual peer. Although there is little existing research to guide hypotheses, Davey et al. (2010) found greater activation in the caudal orbitofrontal cortex (OFC) and anterior insula to positive feedback from the opposite gender compared to the same gender. On the other hand, extensive research on the construct of homophily indicates that individuals tend to form the strongest associations with those most like themselves (McPherson et al., 2001), suggesting that youth may care more about social acceptance and rejection by peers of the same gender. For this reason, hypotheses regarding the effects of rater gender remained exploratory with no specific hypotheses.
A second goal of the study was to demonstrate ecological validity for the Chatroom Interact task by showing that physiological data obtained during the task were related to real world peer interaction. Eisenberger et al. (2007) used EMA to demonstrate that adults with greater dACC activity to social exclusion also felt more socially rejected in their everyday lives. We have also shown that pupillary reactivity on a word valence identification task in youth was related to the experience of positive and negative emotions in daily life (Silk et al., 2007). For the present study, we administered an EMA protocol to the youth in the study in which they were interviewed via cell-phone about social and emotional experiences in daily life. Following Eisenberger et al. (2007), we examined whether reactivity to social acceptance and rejection was related to feelings of closeness with peers during daily interactions. We hypothesized that youth who were more reactive to social acceptance and rejection on the Chatroom Interact Task would report feeling less close to their peers in daily life.
Finally, we examined whether youth exhibited differential attention to acceptance and rejection cues using eyetracking, and whether this pattern changed with age. One mechanism that individuals use to regulate emotions is to selectively attend to certain affective stimuli (Gross, 1998), often using gaze aversion to avert attention away from an undesirable stimulus (Rothbart et al., 1992). Eyetracking can be used to reveal patterns of visual attention, including focus of visual attention and gaze aversion (Isaacowitz et al., 2009). We hypothesized that youth would systematically alter gaze patterns following feedback. We further expected that gaze position and pupil diameter would be related, such that greater pupillary response to rejection would be associated with greater shifts in attentional focus.
METHODS
Participants
Participants were 60 typically developing children and adolescents. Participants (32 female) ranged in age from 9.3 to 17.6 (M = 13.2; SD = 2.5). The sample was 80% Caucasian, 13% African American and 7% Biracial. Exclusion criteria for the study included: (i) symptoms suggestive of an Axis I psychiatric disorder based on the Child or Adolescent Symptom Inventory-4 (Gadow and Sprafkin, 1998a, 1998b), (ii) the existence of a major systemic medical illness, (iii) a history of serious head injury, or (iv) having eye problems or difficulties in vision not corrected by the use of glasses or contact lenses.
Procedure
All participants were recruited from community advertisements and existing research projects. Participants completed an initial phone screen and two 2-h laboratory visits during which questionnaires and pupillary assessments were completed.
Psychiatric screen
Parents of children ≥12 years completed the Adolescent Symptom Inventory 4 (ASI-4; Gadow and Sprafkin, 1998a) and parents of children <12 years completed the Child Symptom Inventory 4 (CSI-4; Gadow and Sprafkin, 1998b). The ASI-4 and CSI-4 both inquire about child behavior over 17 categories related to DSM-IV (American Psychological Association, 1994) diagnoses. The ASI-4 and CSI-4 demonstrate convergent and discriminant validity with clinician diagnoses (Gadow et al., 2005; Gadow and Sprafkin, 1998a, 1998b).
Pupil/eyetracking assessment
Testing occurred in a moderately lit room. Participants sat ∼68 cm from the monitor. Data were collected using a table-mounted RK-464 eye-tracker. The eye-tracker consisted of a video camera and infrared light source that were pointed at a participant's eye, and a device that tracked the location and size of the pupil and corneal reflection using these tools. Data were recorded at 60 Hz (every 16.7 ms) and passed digitally from the eye-tracker to a computer that stored the acquired data along with signals marking the beginning of trials, the end of fixation and stimulus onset time. The resolution for a typical participant was better than 0.05 mm pupil diameter.
Pupil/eyetracking preprocessing
Data were cleaned using our standard procedures (e.g. Silk, et al., 2007). Linear interpolations replaced blinks throughout the data set. Trials comprised of >50% blinks were removed from consideration. Data were smoothed using a 10-point weighted average filter. Linear trends in pupil dilation calculated over blocks of 15 trials were removed to eliminate effects of slow drift in pupil diameter not related to trial characteristics. Pupillary responses were calculated by subtracting baseline pupil diameter from diameter during a trial. Baselines were calculated using the first 10 samples (167 ms) of each trial, during which time participants were viewing virtual peers’ photos, but not yet engaged in selection of targets or receiving feedback. We chose to leave the images on the screen during this period so that there would not be changes in pupil dilation driven by a light reflex in response to images moving on and off the screen between trials.
Eye position was calculated based on the x- and y-coordinates of the eye-gaze minus a corneal-reflection signal, which accounts for small head movements. To calibrate the eyetracker, at the beginning of the assessment session, participants were asked to fixate sequentially on nine points arranged at the top-left, top-middle, top-right, middle-left, middle, middle-right, bottom-left, bottom-middle and bottom-right of the screen. Traces for gaze position, computed as pupil centroid minus the centroid of the corneal reflection, were plotted on a grid with estimated screen-corner locations immediately following the calibration using gaze position row and column averages locked to calibration-point onset to estimate the calibration point locations. When the gaze–position–trace did not correspond closely to the estimated calibration points the calibration was redone until an adequate calibration could be obtained. Adequate calibrations were obtained for all participants. Gaze position for the chatroom task was scaled and offset based on the parameters obtained from the calibration task.
Chatroom Interact task
The Chatroom Interact Task was designed to investigate reactions to social acceptance and rejection from virtual peers in an on-line setting. The task consisted of two phases. On a first assessment day, participants were asked to view smiling photos and biographical profiles of other age-matched preadolescents or adolescents (virtual peers). The photos of virtual peers were of child actors and/or youth residing in a different state who consented to be photographed by a photographer for the task development phase of the study. Participants were told they would have the opportunity to interact online with several youth at remote sites at their next visit as part of an internet communication study. Based on photos and profiles, participants were asked to choose the top five males and top five females that they would be interested in interacting with at their next visit. Participants were asked to provide their own biographical profile based on a series of questions and their picture was taken.
The authors created an initial set of gender and age-specific profiles (age groups: 9 to 11, 12 to 14, 15 to 17) which were then presented to, evaluated and revised after feedback from multiple focus groups. Profiles were standardized so that three pieces of information were included in all profiles matched to information requested from participants for their own profiles: (i) Activities (i.e. ‘belongs to the art club,’ ‘plays soccer’), (ii) ‘Something you like’ (i.e. ‘likes playing with her two dogs,’ ‘likes dancing’, ‘likes Wii bowling’) and (iii) Someday I want to: (i.e. ‘wants to be a policeman’, ‘wants to be a skater,’ ‘wants to be a doctor’). Five unique profiles were created for each gender/age group. Information in each profile was designed to hang together to make a believable, age-appropriate child/adolescent, and to appeal to participants with a range of interests and social status. An example of a profile for a 9- to 11-year-old girl was ‘Cara: Belongs to the art club, likes making jewelry, wants to be a teacher’; an example 12- to 14-year-old boy profile was, ‘Bryan: JV tennis captain, Likes to play video games with friends, Wants to own and manage a hotel’; an example 15- to 17-year old girl profile was: ‘Kathy: Won poetry writing context, Likes reading, Wants to be a writer.’
Approximately 2 weeks following the initial assessment day, participants returned to the laboratory to complete the interaction phase. They were told that they had been matched with two of the males and two of the females selected from the first visit and that these peers were ready to participate in a ‘chat game’ via remote connection. They reviewed biographical profiles for selected peers. Pictures of the peers and participant were then projected on the screen two at a time, as the subject and the two virtual peers took turns selecting who they would rather talk to about a series of common teen interests (e.g. music, TV, friends; Figure 1). Fifteen discussion topics were selected and refined via focus groups.
Fig. 1.
Depiction of an example same gender trial on the Chatroom Interact Task.
The chat game proceeded in six blocks. Each block was comprised of 15 trials in which a person was chosen or not chosen as the preferred person to discuss each topic. Topics were presented randomly and repeated in each block. The first three blocks were played with the same gender virtual peers and the second three blocks were played with the opposite gender virtual peers. In blocks 1 and 6, the subject made choices among the two virtual peers. Analyses focus on blocks 2 through 5, in which the subject was chosen/not chosen by the virtual peers (first same gender, then opposite-gender). Trials were arranged in blocks so that participants experienced two accept blocks in which they were chosen two-third of the time (one same-gender and one opposite-gender) and two reject blocks in which they were rejected two-third of the time (one same-gender and one opposite-gender). The order of accept and reject blocks and trials were randomized within gender grouping.
Each block began with an instruction about who would be making choices for that block (agent). The photograph of the agent was shown at the bottom left corner of the screen and the photographs of the other two players were shown next to each other in the middle of the screen, as in Figure 1. Photographs remained consistent in size and location throughout the trial. At the beginning of each trial, the question ‘Who would you rather talk to about…’ with the selected topic for that trial (i.e. … ‘music?’) appeared on the screen for 3 s (‘choice phase’). Feedback was then provided about which person was chosen (the subject or the virtual peer) for 6 s (‘feedback phase’). The photograph of the person who was not chosen was superimposed with a gray ‘X’ and the photograph of the person who was chosen was highlighted around the border in gray. The participant was asked to press a button to indicate whether the person on the left or the right was chosen.
Stimuli were presented against a grey background, subtending 15.6° of visual angle. The photograph (1.7″ × 2.1″) of the agent was shown at the bottom left corner (9% from the left and 15% from the bottom) of the screen. The photographs (3.7″ × 6.2″ for each photograph) of the other two players were shown next to each other on the screen. The photographs of the two players were positioned at the slightly upper right-sided (37% and 77% from the left, respectively and 60% from the bottom of the screen) on the screen. The question sentence was presented at the bottom of the screen.
Debriefing questionnaire
Subjects were debriefed at the conclusion of the task and informed that in reality they had been playing with a preset computer program. They were also asked to rate how they had felt along six dimensions (happy, sad, angry, nervous, included and excluded) when they were chosen and when they were not chosen, as well as their level of interest in the task. Ratings were made on a 1–10 point Likert scale. Upon questioning, five participants reported that there was something suspicious about the task, but none reported guessing that the participants were not real.
Ecological momentary assessment
Participants completed an ecological momentary assessment (EMA) protocol designed to obtain information on children's social and emotional behavior in the naturalistic environment. Participants were given answer-only cellular phones on which they received calls from an interviewer 36 times between 4 p.m. Thursday and 10 p.m. Monday for three consecutive weekends preceding the laboratory visit. At each call, participants were asked to rate how connected they felt to any individuals with whom they were interacting, by answering the question, ‘How close or connected do you feel to [this person] right now?’ Ratings were made on a 1 to 5 Likert-type scale (1 = not at all, 5 = extremely). A score for peer connectedness was created by averaging ratings of closeness for all instances in which a participant was interacting with a peer. EMA analyses were limited to a subset of 42 participants who reported on at least three instances in which they were interacting with a peer (M = 8.27 ratings, SD = 4.97).
Plan of analyses
Pupil and eyetracking analyses were conducted in Matlab by comparing mean waveforms for reject and accept trials at each time-point along the waveform. For eye-tracking, the X-gaze coordinate was examined as an index of whether the participant was looking at their picture or the other person's; this was possible as the participant's picture was always on the left and the other person's picture was on the right. To control type I error, we used Guthrie and Buchwald's (1991) technique to identify regions of the waveform over which an entire series of contiguous point-by-point tests could be considered significant at P < 0.05, given the temporal autocorrelation of the waveform. This technique defines the size of a temporal window over which a series of contiguous point-by-point tests could be considered significant, controlling error across all tests at P < 0.05. Autocorrelation is accounted for via Monte Carlo simulations of the maximum length of adjacent significant tests present in <5% of simulated data with a similar autocorrelation structure to acquired empirical data. Correlation waveforms were computed to examine correlations among variables at each point along the waveform. Correlation waveforms were also subject to contiguity thresholds to control type I error rate. All analyses were replicated excluding the five participants who reported being suspicious about the task. Excluding these participants did not change the significance or pattern of findings for any of the hypotheses; therefore, results are presented below for the entire sample.
RESULTS
Demographics and subjective emotion
Participants showed high levels of interest in the task (M = 6.81, SD = 2.12). Paired t-tests comparing subjective ratings of emotions on acceptance versus rejection trials indicated significant differences for all dimensions with the exception of nervousness (t = 1.65, p = 0.11). Specifically, youth reported feeling angrier, sadder, more excluded, less happy and less included when the other subject was chosen (rejection) than when they were chosen (acceptance) (t's range from 4.48 to 9.17, all P's < 0.001).
Hypothesis 1: Youth will show increased pupil dilation to rejection vs acceptance.
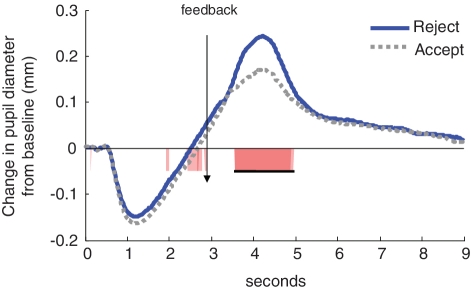
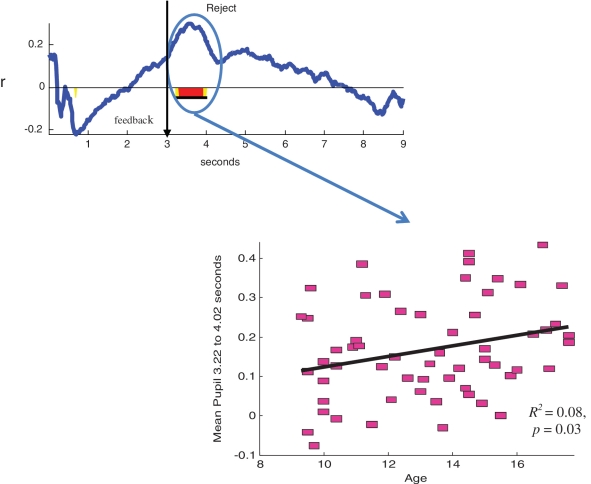
As shown in Figure 2, youth displayed increased pupil dilation to trials on which they were rejected compared to trials on which they were accepted immediately after receiving rejection or acceptance feedback [3.57–4.97 s (0.57–1.97 s following feedback); F(1,59) = 20.92, P < 0.001; d = 1.19; 1.3 contiguous seconds required to control type I error at P < 0.05]. To explore whether this differed by age, we computed correlation waveforms showing the correlations between participants’ age and pupil dilation to acceptance and rejection trials, focusing on the first 2 s following feedback (region containing the peak of the mean pupillary response waveform). As shown in Figure 3, age was positively correlated with pupil dilation from 3.2 to 4.02 s [0.2–1.02 s following rejection feedback] [F(1,59) = 4.92, R2 = 0.08, P = 0.03; 0.43 contiguous seconds required to control type I error at P < 0.05 for the examined 2-second interval]. This indicates that older adolescents had a stronger pupillary response to rejection than younger adolescents. Age was not related to pupillary response to acceptance feedback.
Fig. 2.
Pupillary response to rejection vs acceptance trials on the Chatroom Interact Task. Statistically significant t-tests are highlighted along the x-axis. Underlined area along the x-axis shows the length of statistically significant continguous t-tests exceeding the contiguity threshold.
Fig. 3.
Correlation waveform and scatterplot showing relation between pupillary response to rejection on the Chatroom Interact Task and youths’ age. Underlined area along the x-axis shows the length of statistically significant continguous t-tests exceeding the contiguity threshold.
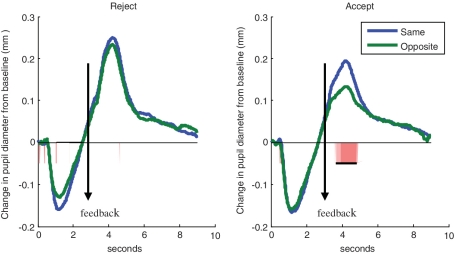
We next explored whether pupillary response to rejection or acceptance differed based on the gender of the rater. As shown in Figure 4, there were no significant differences in pupillary reactivity to rejection based on the gender of the rater. However, there was greater pupillary reactivity to acceptance from a same gender rater than to acceptance from an opposite gender rater [3.72–4.92 s (0.72 to 1.92 s following acceptance feedback): F(1,59) = 4.78, P = 0.03; d = 0.57; 1.3 contiguous seconds required to control type I error at P < 0.05]. Between subjects effects for participant's age and gender were added to repeated measures ANOVA comparing pupillary reactivity to same versus opposite gender raters to examine whether this effect differed based on participant characteristics. The interaction between gender of rater and gender of participant was nonsignificant [F(1,58) = 0.04, P = 0.85; d = 0.14] as was the interaction between gender of rater and participant age [F(1,58) = 1.82, P = 0.18; d = 0.35].
Hypothesis 2. Pupillary reactivity on the Chatroom Interact task will be related to real-world social interaction.
Fig. 4.
Differences in pupillary response to rejection vs acceptance trials based on rater gender. Regions of statistically significant differences are highlighted along the x-axis. Underlined area along the x-axis shows the length of statistically significant continguous t-tests exceeding the contiguity threshold.
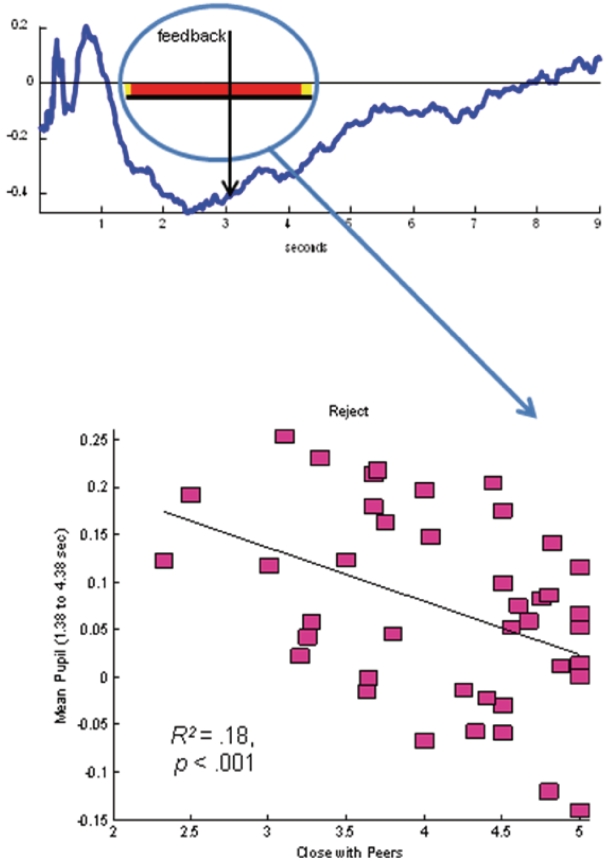
We examined relations between pupillary reactivity on the Chatroom Interact Task and feelings of closeness and connection during real-world social interactions with peers. We computed correlation waveforms showing the correlations between reports of closeness with peers and pupil dilation to acceptance and rejection trials. As shown in Figure 5, youth who reported lower levels of closeness/connection to their peers in everyday life also had greater pupillary response leading up to and following rejection [1.38–4.38 s (1.62 s prior to feedback to 1.38 s following feedback): (F(1,59) = 8.72, R2 = 0.18, P < 0.001; 1.3 contiguous seconds required to control type I error at P < 0.05]. Pupillary reactivity to acceptance trials was not significantly associated with levels of closeness/connection to peers in everyday life.
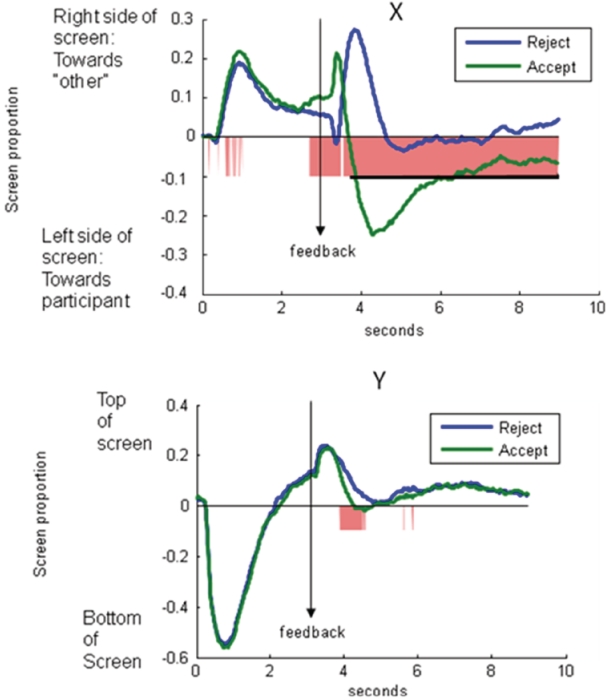
Hypothesis 3. Eyetracking data will reveal attentional shifts related to feedback. Figure 6 shows the X- and Y-coordinates of eye-position following accept and reject decisions.
Fig. 5.
Correlation waveform and scatterplot showing relation of pupillary response to rejection on the Chatroom Interact Task to youths’ feelings of social connectedness with peers in cell-phone EMA protocol conducted over a 5-day period outside the lab. Underlined area along the x-axis shows the length of statistically significant continguous t-tests exceeding the contiguity threshold.
Fig. 6.
Horizontal and vertical gaze patterns in response to rejection and acceptance on the Chatroom Interact Task. Underlined area along the x-axis shows the length of statistically significant continguous t-tests exceeding the contiguity threshold.
Eyetracking analyses indicated large differences in horizontal gaze patterns (i.e. looking at the picture of self vs other) to rejection vs acceptance for the majority of the trial [from 3.58 to 8.98 s (0.58 to 5.98 s following feedback); F(1,59) = 55.57, P < 0.001, d = 1.94; 1.2 contiguous seconds required to control type I error at P < 0.05]. As shown in Figure 6, when accepted, youth tended to glance very quickly at the rejected youth, then focus on themselves for the duration of the trial. When rejected, youth tended to avoid looking at themselves, looking first at the chosen youth and then toward the middle of the screen. Vertical gaze analyses indicated that gaze at the beginning of the trial (0-1) in both conditions went to the bottom right part of the screen containing the sentence describing the decision (e.g. ‘who would you rather talk to about parties?’). Minor differences in the Y coordinate did not exceed the contiguity threshold. Gaze position following feedback did not differ based on rater gender.
Figure 7 shows a ‘heat map’ of gaze position following accept and reject feedback to provide more fine-grained descriptive information about gaze position. For each trial, each pixel was colored by the number of samples in which gaze position fell within 10 pixels of that pixel. Trial-related gaze-maps were averaged within-subjects for each condition. These maps were then averaged across condition. As shown quantitatively in Figure 7, gaze centered around the self in the accept condition and the other youth in the reject condition.
Fig. 7.
Heat map showing eye position for 1.5 s following feedback.
We also computed correlation waveforms showing the correlations between participants’ age and gaze position to acceptance and rejection trials, focusing again on the first 2 s following feedback. Age was correlated with horizontal gaze position from 3.4 to 3.88 s (0.4–0.88 s following rejection feedback] [F(1,59) = 6.18, R2 = 0.10, P = 0.02; .47 contiguous seconds required to control type I error at P < 0.05 for the peak (3–5 s) region]. This correlation indicated that older youth were more likely to gaze at themselves when accepted than younger youth. Age was not related to pupillary response to rejection feedback.
Hypothesis 4. Pupillary reactivity and eye position will be correlated, such that greater pupil dilation to rejection will be associated with greater shift in attentional focus.
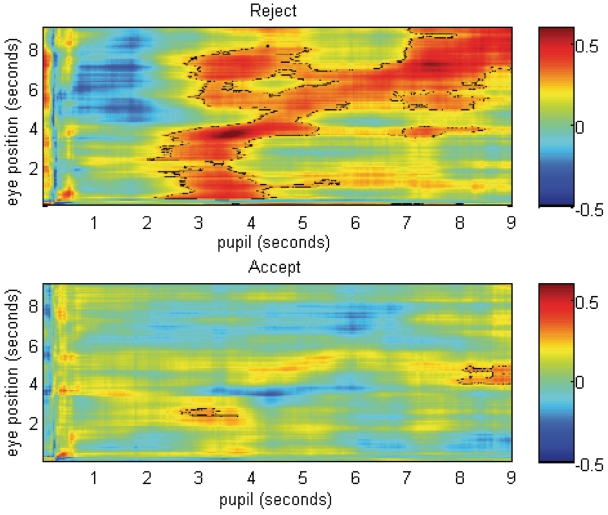
Finally, we examined whether gaze position was related to pupillary responses by computing a correlogram showing the correlations between pupil dilation and eyetracking at the same point in time as well as the correlation of earlier and later pupil dilation to eye position (Figure 8). This allowed us to examine whether gaze aversion following feedback was associated with earlier or later pupillary response. Type I error was controlled by identifying clusters of the matrix in which a correlation was significant (P < 0.05) and the correlated sample was surrounded by a large enough cluster of other correlated samples to infer that the correlation would not have occurred by chance. This was accomplished using Monte Carlo simulations to determine the number of contiguous correlated samples that would not have occurred by chance given the two-dimensional autocorrelation of the matrix, calculated using the 3dFWHMx and 3dAlphaSim routines from Cox (1996). Samples meeting these criteria are outlined in black in Figure 8.
Fig. 8.
Average correlation between pupillary response and horizontal gaze position across a trial. Strength of correlation at each point depicted by color. Clusters of correlated samples meeting criteria for Type 1 error control are outlined in black. The diagonal line represents correlations among pupillary response and eye gaze at the same point in time. Off diagonal samples reveal correlations between pupil dilation and eyetracking leading up to and following feedback.
Pupil dilation and gaze position were strongly related for rejection trials. The diagonal line on Figure 8 represents correlations among pupillary response and eye gaze at the same point in time. This analysis revealed a particularly strong correlation between pupillary responses and eye position within the first second following rejection feedback [3.5–3.75 s (0.5–0.75 s following rejection feedback); r = 0.62, P < 0.001]. As hypothesized, youth who had greater pupil dilation to rejection feedback also looked away from themselves more. As shown on Figure 8 (diagonal), this relation between greater gaze aversion from self and greater pupil dilation remained significant throughout the remainder of the trial.
Off diagonal samples reveal correlations between pupil dilation and eyetracking leading up to and following feedback. Figure 8 further reveals that youth whose pupils dilated immediately following rejection (i.e. 3.5–3.75 s on the x-axis) also had looked away prior to the rejection (i.e. 1–3 s on the y-axis) and following the rejection (i.e. 5–8 s on the y-axis). This suggests that these youth may have avoided beforehand, possibly associated with anticipation of rejection, and also remained avoidant following the rejection. The correlogram for acceptance trials revealed small correlations between pupil dilation and eye position, indicating that youth who looked toward the other participant immediately following acceptance feedback and at the end of the trial also had greater pupil dilation to the feedback.
DISCUSSION
Findings from this study suggest that the Chatroom Interact task provides useful information on how adolescents process social acceptance and rejection, and how the salience of peer rejection and acceptance increases across adolescence. The task elicited pupillary responses that differed for rejection and acceptance trials. These responses were related to a real-world index of social connectedness with peers in daily life. Eyetracking revealed marked differences in gaze patterns to acceptance and rejection feedback which were related to pupillary responses, suggesting that feedback on the task had a significant impact on adolescents’ attentional focus. Subjective ratings indicated that youth found the task interesting and responded in expected ways. They reported feeling angrier, sadder, more excluded, less happy and less included when they were rejected than when they were accepted.
As hypothesized, youth showed heightened peak pupillary reactivity in response to peer rejection compared to acceptance. The fine-grained temporal analysis afforded by the use of pupillometry indicates that this response occurs quickly, within 1 to 2 s following feedback, and is relatively brief. Following a growing literature linking pupillary reactivity to activity in prefrontal regions associated with executive control and emotion regulation, our findings likely suggest increased initial prefrontal activity in response to peer rejection. This is consistent with Masten et al.'s (2009) findings of greater activity in ventral prefrontal regions in response to social exclusion compared to inclusion among adolescents. Both studies suggest that youth may engage prefrontal areas of the brain in regulatory activity in response to social exclusion and/or rejection. We hypothesize that both ventral and dorsal prefrontal areas are activated in response to social rejection and negative social feedback during adolescence, and serve a regulatory function in the face of social distress. Future research using different types of social rejection stimuli is needed to test this hypothesis, and concurrent neuroimaging and pupillary studies are needed to rule out strong contributions of limbic reactivity to the observed pupillary responses.
We also found that older youth had a stronger pupillary response to rejection than younger youth. This suggests that peer rejection was more salient or resulted in the recruitment of greater prefrontal regulatory resources as children progressed through adolescence. This converges with evidence of the importance of the social sphere in the transition through adolescence (Steinberg and Morris, 2000). Adolescence is associated with enhanced sensitivity to social evaluation, complex social hierarchies, frequent social comparisons and increased desire for social affiliation (Brown and Lohr, 1987; O'Brien and Bierman, 1988; Steinberg and Morris, 2000; Brown, 2004). Because of the increased salience of peer rejection in adolescence, older youth likely needed to recruit more regulatory resources to cope with rejection than younger youth. It should be noted, however, that age effects observed in the present study were relatively modest (accounting for 8–10% of the variance in pupillary or eyetracking responses), and thus need to be replicated in future research to infer robust relationships.
We also found that greater pupillary reactivity to rejection was associated with lower feelings of connectedness in real-world peer interactions. This indicates that youth who responded more to rejection in the lab did not feel as close and connected to their peers in everyday life. This finding supports the ecological validity of the Chatroom Interact Task and is consistent with Eisenberger et al.'s (2007) finding that adults with greater ACC activity on the Cyberball task also felt less connected to others in their everyday lives. Taken together these results suggest that individuals differences in sensitivity to rejection may underlie feelings of social connectedness. Interestingly, we found that the correlation between social connectedness and pupil dilation was significant even during the period preceding delivery of rejection feedback for rejection trials. This can be explained by the blocked nature of the trials. Trials were arranged in blocks so that participants experienced two reject blocks in which they were rejected two-third of the time. For this reason, anticipation of rejection would be expected to be heightened during the rejection block. Individuals lower on social connectedness appear to particularly likely to show anticipatory pupillary reactivity during social rejection trials, suggesting that they were more sensitive to the priming effect of repeated rejection.
We found that, regardless of their own age and gender, youth displayed greater pupillary reactivity to acceptance from a same gender rater than to acceptance from an opposite gender rater. This findings is consistent with research on homophily which indicates that individuals tend to form stronger relationships with those most like themselves (McPherson, et al., 2001). Gender, in fact, is one of the most prevalent characteristic upon which individuals base social bonding preferences (Shrum et al., 1988; McPherson, et al., 2001). Given the links between pupil dilation and prefrontal activity, this finding may be driven by greater activation in prefrontal regions involved in self processing and social comparison when youth are accepted by a same gender compared to an opposite gender peer. Nevertheless, it was surprising that older adolescents were not more reactive to acceptance and rejection from an opposite gender peer given budding romantic interests during this period. Future research with larger samples of early, mid and late adolescents may be helpful in further clarifying this issue.
Youths’ gaze patterns during the task were consistent with attentional biases toward acceptance and away from rejection. During acceptance trials, youths’ visual attention was focused primarily on the photograph of themselves. We found that older youth were even more likely to gaze at their own pictures when accepted than younger youth. Self attributions become increasingly important during adolescence as self reflective processes such as identity, self esteem and self-worth crystallize (Damon and Hart, 1988; Harter, 1990). Furthermore, there is evidence that social comparative processes are integral to self worth in adolescence (Pfeifer et al., 2007, 2009). Thus, older youth in the midst of adolescence may be more likely to fixate on their own pictures following acceptance because of the increasing importance of positive social feedback for their own self-evaluations. This may also be a reflection of the continued maturation of structures such as the medial prefrontal cortex which support social cognitive processes like mentalizing and self concept (Sebastian et al., 2008; Burnett et al., 2011).
In contrast, youth tended to spend very little time looking at themselves during the reject trials. Looking at the self when rejected may be associated with negative emotions such as shame, embarrassment, or anger. Therefore, looking away may serve as an active self-regulatory mechanism to manage associated negative emotions (Posner and Rothbart, 1992). Relatedly, youth who had greater pupil dilation to rejection feedback were also more likely to look away from themselves when rejected. Furthermore, youth who looked away from themselves following rejection feedback also showed greater pupil dilation in the few seconds prior to receiving feedback. Since rejection trials occurred within a block in which two-third of trials ended in rejection, these youth were likely anticipating rejection prior to this negative feedback. These same youth also displayed greater pupil dilation following rejection feedback. Thus, rejection sensitive youth may anticipate rejection, reflected in a strong pupillary response prior to the rejection cue, and subsequently shift attention away from themselves to regulate strong self-directed negative emotion. Nevertheless, they continue to devote more resources to processing the rejection than youth who do not look away. These patterns may differ in important ways for youth with affective disorders such as anxiety and depression, and this task may be useful in revealing such emotional information processing patterns in clinical populations.
In designing tasks with strong ecological validity there is a trade-off in which the increased validity comes with some loss of experimental control. We opted in this experiment to use stimuli (peer profiles and topics) that are socially-relevant and familiar to subjects because we believe they are more likely to elicit meaningful and potent emotional responses. However, this approach does introduce within and between subject variability in responses to particular stimuli, and some youth may have had stronger reactions to aspects of a particular virtual peers’ profile or to a particular discussion topic. Using the present approach it would be impossible to generate contexts which are truly equivalent within and across all subjects, but we did attempt to minimize this impact by working with focus groups to create topics and youth profiles with broad general appeal and by allowing participants to choose their own virtual peers. By design, we expected that participants would choose virtual peers they viewed as most like them, and this was why it was important to have virtual peers with a variety of common interests available to choose from. It is also possible that the profiles could have been perceived as differing in social status, however, because the participants chose the virtual peers with whom they wished to interact, we assume that they chose participants whose acceptance would be valuable to them regardless of societal norms.
Several other limitations of the present study should be noted. First, as described above, pupillometry does not provide direct information about the brain regions involved in the observed response. Future neuroimaging research is needed to delineate the neural substrates underlying increased pupillary reactivity to peer rejection. Despite these limitations, pupillometry can be useful in generating hypotheses for future neurobiological investigations. Furthermore, pupillometry is cost-efficient, painless and noninvasive, making it a highly feasible approach to probing brain systems in adolescents. Combined with eyetracking, pupillometry provides information not only about where participants are looking but their level of engagement with those aspects of the scene they are looking at.
Second, because features of the faces were not in exactly the same position from trial to trial (i.e. the head/eyes/mouth were not in the exact same position in each photograph), we were not able to compare specific regions of the face. For this reason, we cannot rule out the possibility that differential pupil dilation to fixations on different regions of the face (i.e. whites of the eyes) could have influenced the present results. However, we were able to construct a heatmap showing average fixations superimposed on two typical photographs, which suggests that participants gazed at similar broad regions of the photographs in accept and reject conditions.
Since the task does not include measurement of pupillary and eyetracking responses to photographs of the self and others during a passive viewing or neutral condition (independent of feedback and evaluation), we were not able to measure the extent to which responses are associated with face viewing in general, or to rule out the possibility that differences in pupil dilation to acceptance and rejection could be related to possible differences in pupil dilation simply to viewing one's own versus another individual's face. Participants focused on the other person's face during different windows of time in the accept (2.72–3.52 s) and reject (3.58–8.98 s) conditions. Yet, peak pupil dilation in both conditions occurred during the same window of time ∼1 s following feedback. This gives us some confidence that pupillary response is driven largely by social feedback, rather than simply looking at one's own face versus another's face. Nevertheless, this possibility might warrant future consideration and study. Additionally, in order to maintain engagement in the task, participants were asked to press a button indicating on which side of the screen the accepted person appeared. This constraint could help to explain the appearance of gaze direction towards the accepted face. However, individuals tended to look first at the rejected person during accept trials, suggesting other influences on gaze direction in addition to the button press demand.
Despite these limitations, a major strength of the study is the development of a novel virtual peer interaction paradigm with applications for understanding socioemotional development in normative and clinical populations of adolescents. Validation of the task's utility for tapping into relevant real-world processes using EMA is also an important strength. We believe these findings extend previous research with youth by establishing relations of virtual laboratory measures of social rejection to real-world social interaction, reporting developmental differences in response to social rejection, showing differential effects based on the gender of the rater, and demonstrating attentional biases related to social acceptance and rejection. Our multi-method approach reveals that social rejection from peers, even virtual online peers that our participants have never met, has a strong effect on both the attentional focus and physiological response of typically developing youth. This effect is particularly marked for older adolescents.
Acknowledgments
The authors are grateful to Daniel Pine, M.D. for his input and assistance on this project, Marcie McCullough and Katie Burkhouse for their assistance in data acquisition, Wessyl Kelly for her assistance with data analysis, Harvey Iwamoto for task-related computer programming, and Ruth Stroud and Jennifer Sears for assistance with photography. The authors also thank the participants and their families. This research was supported by a National Institute of Drug Abuse grant R21DA024144 (J.S.S./R.E.D., PI's), the Staunton Farm Foundation, the Clinical and Translational Science Institute at the University of Pittsburgh (NIH/NCRR/CTSA Grant UL1 RR024153) and the NIMH intramural research program
REFERENCES
- American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Washington, DC: Author; 1994. [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117:497–529. [PubMed] [Google Scholar]
- Beatty J. Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychological Bulletin. 1982;91:276–92. [PubMed] [Google Scholar]
- Beatty J. The pupil system. In: Coles MGH, editor. Psychophysiology Systems Processes and Applications. New York: Guilford; 1986. pp. 44–50. [Google Scholar]
- Beatty J, Lucero Wagoner B. The pupillary system. In: Cacioppo JT, editor. Handbook of Psychophysiology. 2nd edn. New York, NY: Cambridge University Press; 2000. pp. 142–62. [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9:267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment and loss, Vol. 1: Attachment. New York: Basic Books; 1969. [Google Scholar]
- Brown BB. Adolescents’ relationships with peers. In: Lerner RM, Steinberg LD, editors. Handbook of Adolescent Psychology. 2nd edn. Hoboken, NJ: Wiley; 2004. pp. 363–394. [Google Scholar]
- Brown BB, Lohr MJ. Peer-group affiliation and adolescent self-esteem: An integration of ego-identity and symbolic-interaction theories. Journal of Personality and Social Psychology. 1987;52:47–55. doi: 10.1037//0022-3514.52.1.47. [DOI] [PubMed] [Google Scholar]
- Burnett S, Sebastian C, Cohen Kadosh K, Blakemore SJ. The social brain in adolescence: Evidence from functional magnetic resonance imaging and behavioural studies. Neuroscience and Biobehavioral Reviews. 2011;35:1654–64. doi: 10.1016/j.neubiorev.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly J, Furman W, Konarski R. The role of peers in the emergence of heterosexual romantic relationships in adolescence. Child Development. 2000;71:1395–408. doi: 10.1111/1467-8624.00235. [DOI] [PubMed] [Google Scholar]
- Cox R. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers in Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. Neuroimage. 2005;27:885–95. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Damon W, Hart D. Self-understanding in Childhood and Adolescence. New York: Cambridge University Press; 1988. [Google Scholar]
- Davey CG, Allen NB, Harrison BJ, Dwyer DB, Yucel M. Being liked activates primary reward and midline self-related brain regions. Human Brain Mapping. 2010;31:660–8. doi: 10.1002/hbm.20895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge KA, Lansford JE, Burks VS, et al. Peer rejection and social information-processing factors in the development of aggressive behavior problems in children. Child Development. 2003;74:374–93. doi: 10.1111/1467-8624.7402004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Gable SL, Lieberman MD. Functional magnetic resonance imaging responses relate to differences in real-world social experience. Emotion. 2007;7:745–54. doi: 10.1037/1528-3542.7.4.745. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Furman W. The emerging field of adolescent romantic relationships. Current Directions in Psychological Science. 2002;11:177–80. [Google Scholar]
- Gadow KD, Devincent CJ, Pomeroy J, Azizian A. Comparison of DSM-IV symptoms in elementary school-age children with PDD versus clinic and community samples. Autism. 2005;9:392–415. doi: 10.1177/1362361305056079. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J. Adolescent Symptom Inventory - 4: Norms Manual. Stony Brook, NY: Checkmate Plus; 1998a. [Google Scholar]
- Gadow KD, Sprafkin J. Child Symptom Inventory - 4: Screening Manual. Stony Brook, NY: Checkmate Plus; 1998b. [Google Scholar]
- Gross Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Social and Personality Psychology. 1998;74:224–37. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Guthrie D, Buchwald JS. Significance testing of difference potentials. Psychophysiology. 1991;28:240–4. doi: 10.1111/j.1469-8986.1991.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of General Psychiatry. 2008b;65:1303–12. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development. 2009;80:1000–15. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, et al. A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience. 2008a;20:1565–82. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter S. Developmental differences in the nature of self-representations: implications for the understanding, assessment, and treatment of maladaptive behavior. Cognitive Therapy and Research. 1990;14:113–42. [Google Scholar]
- Isaacowitz DM, Toner K, Neupert SD. Use of gaze for real-time mood regulation: effects of age and attentional functioning. Psychology and Aging. 2009;24:989–94. doi: 10.1037/a0017706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Unconscious processing of facial affect in children and adolescents. Social Neuroscience. 2007;2:28–47. doi: 10.1080/17470910701214186. [DOI] [PubMed] [Google Scholar]
- Kistner J, Balthazor M, Risi S, Burton C. Predicting dysphoria in adolescence from actual and perceived peer acceptance in childhood. Journal of Clinical Child Psychology. 1999;28:94–104. doi: 10.1207/s15374424jccp2801_8. [DOI] [PubMed] [Google Scholar]
- Koikegami H, Yoshida K. Pupillary dilation induced by stimulation of amygdaloid nuclei. Folia Pychiatrica Neurologica Japonica. 1953;7:109–25. doi: 10.1111/j.1440-1819.1953.tb00600.x. [DOI] [PubMed] [Google Scholar]
- Lopez C, DuBois DL. Peer victimization and rejection: investigation of an integrative model of effects on emotional, behavioral, and academic adjustment in early adolescence. Journal of Clinical Child and Adolescent Psychology. 2005;34:25–36. doi: 10.1207/s15374424jccp3401_3. [DOI] [PubMed] [Google Scholar]
- Maslow, A. H. Motivation and Personality. 3rd edn. New York: Addison-Wesley Educational Publishers, Inc; 1987. [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, et al. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc Cogn Affect Neurosci. 2009;4:143–57. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson M, Smith-Lovin L, Cook JM. Birds of a feather: homophily in social networks. Annual Review of Sociology. 2001;27:415–44. [Google Scholar]
- Monk CS, McClure EB, Nelson EE, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–8. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Moor BG, Crone EA, van der Molen MW. The Heartbrake of social rejection: heart rate deceleration in response to unexpected peer rejection. Psychological Science. 2010;29:1326–33. doi: 10.1177/0956797610379236. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure E, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–74. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- O'Brien SF, Bierman KL. Conceptions and perceived influence of peer groups: interviews with preadolescents and adolescents. Child Development. 1988;59:1360–5. doi: 10.1111/j.1467-8624.1988.tb01504.x. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman MD, Dapretto M. ‘I know you are but what am I?!’: Neutral bases of self- and social knowledge retrieval in children and adults. Journal of Cognitive Neuroscience. 2007;19:1323–37. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Borofsky LA, Dapretto M, Fuligni AJ, Lieberman MD. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Development. 2009;80:1016–38. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attentional mechanisms and conscious experience. In: Milner D, Rugg M, editors. The Neuropsychology of Consciousness. San Diego, CA: Academic Press; 1992. pp. 91–111. [Google Scholar]
- Rothbart MK, Ziaie H, O'Boyle CG. Self-regulation and emotion in infancy. New Directory for Child Development. 1992;55:7–23. doi: 10.1002/cd.23219925503. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C, Burge D. A Cognitive-interpersonal approach to depressive symptoms in preadolescent children. Journal of Abnormal Child Psychology. 1997;25:33–45. doi: 10.1023/a:1025755307508. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Burnett S, Blakemore S-J. Development of the self-concept during adolescence. Trends in Cognitive Sciences. 2008;12:441–6. doi: 10.1016/j.tics.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Shrum W, Cheek NH, Hunter SM. Friendship in school: gender and racial homophily. Sociology of Education. 1988;61:227–39. [Google Scholar]
- Siegle GJ, Steinhauer SR, Carter CS, Ramel W, Thase ME. Do the seconds turn into hours? Relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cognitive Therapy and Research. 2003a;27:365–82. [Google Scholar]
- Siegle GJ, Steinhauer SR, Friedman ES, Thompson WS, Thase ME. Remission prognosis for cognitive therapy for recurrent depression using the pupil: utility and neural correlates. Biological Psychiatry. 2011;69:726–33. doi: 10.1016/j.biopsych.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Stenger VA, Konecky R, Carter CS. Use of concurrent pupil dilation assessment to inform interpretation and analysis of fMRI data. Neuroimage. 2003b;20:114–124. doi: 10.1016/s1053-8119(03)00298-2. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME. Pupillary assessment and computational modeling of the Stroop task in depression. International Journal Psychophysiology. 2004;52:63–76. doi: 10.1016/j.ijpsycho.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Silk JS, Dahl RE, Ryan ND, et al. Pupillary reactivity to emotional information in child and adolescent depression: links to clinical and ecological measures. American Journal of Psychiatry. 2007;164:1873–80. doi: 10.1176/appi.ajp.2007.06111816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Whalen DJ, Ostapenko L, Ladouceur CD, Dahl RE. Pubertal changes in emotional information processing: pupillary, behavioral, and subjective evidence during emotional word identification. Development and Psychopathology. 2009;21:7–16. doi: 10.1017/S0954579409000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9:1007–8. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Morris A. Adolescent development. Annual Review of Psychology. 2000;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Silk JS. Parenting adolescents. In: Bornstein MH, editor. Handbook of Parenting: Vol. 1: Children and Parenting. 2nd edn. Mahwah, NJ: Lawrence Erlbaum Associates; 2002. pp. 103–33. [Google Scholar]
- Szabadi E, Bradshaw CM. Autonomic pharmacology of 2-adrenoceptors. Journal of Psychopharmacology. 1996;10(Suppl. 3):6–18. [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Davidson RJ. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. Neuroimage. 2009;47:852–63. doi: 10.1016/j.neuroimage.2009.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]