Abstract
The risks of unmasking and paradoxical forms of immune reconstitution disease in HIV-infected patients starting antiretroviral therapy (ART) are fuelled by a combination of the late presentation of patients with advanced immunodeficiency, the associated high rates of opportunistic infections (OIs) and the need for rapid initiation of ART to minimize overall mortality risk. We review the risk factors and our current knowledge of the immunopathogenesis of immune reconstitution disease, leading to a discussion of strategies for prevention. Initiation of ART at higher CD4 counts, use of OI-preventive therapies prior to ART eligibility, intensified screening for OIs prior to ART initiation and optimum therapy for OIs are all needed. In addition, use of a range of pharmacological agents with immunosuppressive and immunomodulatory activity is being explored.
Keywords: antiretroviral, HIV, immune reconstitution, immune reconstitution inflammatory syndrome, IRIS, prevention
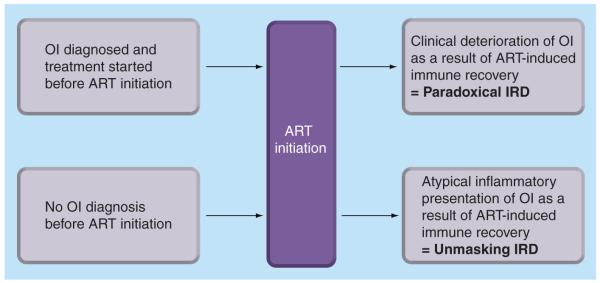
Immune reconstitution disease (IRD), also referred to as ‘immune reconstitution inflammatory syndrome’ or ‘immune restoration disease’, is a widely recognized phenomenon that may complicate antiretroviral therapy (ART) [1,2]. This occurs most commonly, although not exclusively, in those with opportunistic infections (OIs) and results from rapid restoration of pathogen-specific immune responses. IRD may occur in two different scenarios (Figure 1). The ‘paradoxical’ form of IRD occurs when an OI diagnosed pre-ART initially responds successfully to treatment but then deteriorates as a direct result of immune recovery following ART initiation. By contrast, the ‘unmasking’ form of IRD occurs when an OI present pre-ART remains undiagnosed and subsequent immune recovery following ART initiation triggers the presentation of OI with the development of unusual inflammatory features.
Figure 1. ‘Unmasking’ and ‘paradoxical’ forms of immune reconstitution disease associated with opportunistic infections during early antiretroviral therapy.
ART: Antiretroviral therapy; IRD: Immune reconstitution disease; OI: Opportunistic infection.
IRD is recognized to be associated with a wide spectrum of pathogens and typically occurs during the initial months of ART when the prevalence of OIs is highest and immune recovery is most rapid. The opportunistic diseases most commonly associated with IRD include mycobacterial diseases, including TB, Mycobacterium avium complex disease, leprosy and a wide range of other nontuberculous mycobacterial diseases; deep fungal infections, especially cryptococcal meningitis; herpes viruses, including cytomegalovirus (CMV) retinitis, herpes zoster and herpes simplex; Kaposi’s sarcoma (KS) and progressive multifocal leukoencephalopathy (PML). The diverse clinical manifestations and clinical sequelae of IRD have been well documented [1-10].
A systematic review and meta-analysis of data from 54 cohorts containing over 13,000 patients provided summary estimates of the frequency of some of the most common forms of paradoxical IRD and the associated mortality risk (Table 1). CMV retinitis was complicated by IRD in over one third of cases [11]. Paradoxical IRD occurred in one in five patients with cryptococcal meningitis and one in six patients with TB. Both cryptococcal IRD and TB-IRD were associated with mortality risk, although this was substantially greater for those with cryptococcal disease (20.8 vs 3.2%, respectively) (Table 1). Although IRD associated with M. avium complex was one of the most commonly recognized forms of IRD following the advent of ART in high-income countries [12], risk has subsequently diminished with initiation of ART at higher CD4+ cell counts. Moreover, with scale-up in resourcelimited settings, TB-IRD has become the predominant form of mycobacterial IRD [13].
Table 1. Results of a systematic review and meta-analysis of the incidence of immune reconstitution disease and resulting mortality associated with a range of opportunistic infections.
| Opportunistic infection | Pooled summary incidence, % (95% CI) |
Proportion dying, % (95% CI) |
|---|---|---|
| Unselected patients starting ART | ||
| Any type of IRD | 16.1 (11.1–22.9) | 4.5 (2.1–8.6) |
| Patients with previous AIDS-defning illness | ||
| TB | 15.7 (9.7–24.5) | 3.2 (0.7–9.2) |
| Cryptococcal meningitis | 19.5 (6.7–44.8) | 20.8 (5.0–52.7) |
| Cytomegalovirus retinitis | 37.7 (26.6–49.4) | |
| Herpes zoster | 12.2 (6.8–19.6) | |
| Kaposi’s sarcoma | 6.4 (1.2–24.7) | |
| Progressive multifocal leukoencephalopathy |
16.7 (2.3–50.7) | |
ART: Antiretroviral therapy; IRD: Immune reconstitution disease.
Data taken from [11].
In contrast to paradoxical forms of TB and cryptococcal IRD, the frequencies of unmasking forms of IRD and associated mortality remain poorly defined. This is largely because of the widely acknowledged limitations in case definitions for unmasking disease [4,6]. Cases of TB and cryptococcal meningitis presenting during the initial months of ART may arise either as a result of unmasking of subclinical prevalent disease or may be due to development of new incident disease in the context of persisting immunodeficiency. Clinical case definitions are largely unable to resolve these two etiologies in individual patients.
For many patients, IRD presents as a self-limiting complication with few consequences. For some, however, there may be considerable morbidity, requiring hospitalization, therapeutic procedures or immunosuppressive therapy. IRD can also lead to diagnostic uncertainty, considerably complicating clinical management. The potential complications of IRD are diverse, but include visual loss due to CMV-associated immune recovery uveitis; abscess formation within lymph nodes, soft tissues or solid organs due to TB-IRD; airway obstruction in those with nontuberculous mycobacteria, TB-IRD or KS-IRD; and respiratory failure and hepatic dysfunction in those with TB-IRD. In addition, manifestations may be life threatening, especially with IRD involving the CNS such as in those with tuberculous meningitis, cryptococcal meningitis or PML [9,10]. Overall, the two most important forms of IRD are paradoxical TB-IRD (owing to associated morbidity) and unmasking and paradoxical forms of cryptococcal IRD (owing to associated mortality).
Thus, IRD remains an important clinical problem and strategies for prevention are needed. In this article we first summarize what is known about the risk factors and immunopathogenesis of IRD. We then use these data to describe the rationale for known and potential interventions that might be implemented at programmatic and clinical levels for prevention.
Risk factors
Risk of unmasking IRD is directly related to the prevalence of undiagnosed OIs at baseline, which in turn will be related to the degree of immunodeficiency of patients, the methods used to screen for OIs and the prevailing frequency of specific OIs in the geographic location. Additional key risk factors associated with paradoxical IRD (some of which may also contribute to unmasking IRD risk) include the timing of ART during OI treatment, extent of the OI, baseline CD4 count and HIV viral load, and response of CD4 count and HIV viral load to ART. Each of these is considered in the following sections.
Timing of antiretroviral therapy
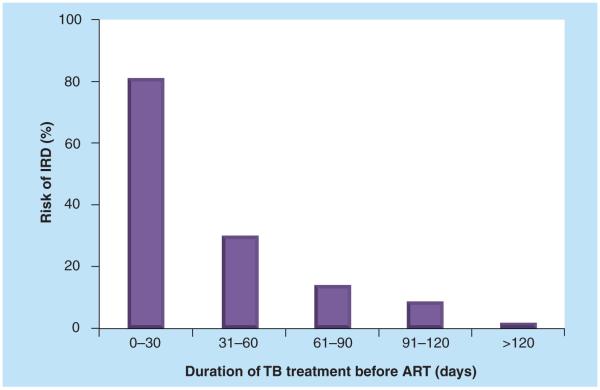
The residual amount of OI antigen present in the body tissues when ART is initiated is likely to be a key factor associated with risk of IRD. This, in turn, relates to the duration of effective treatment for the OI prior to ART. Many observational studies of TB [14-17] have found an association between earlier ART initiation during TB treatment and higher risk of paradoxical IRD. In a report from a South African cohort, the very strong relationship between duration of TB treatment prior to ART and risk of IRD (Figure 2) persisted in adjusted analyses [15]. Risk appears to be particularly heightened when starting ART during the first 2 months of TB treatment and this has recently been confirmed in randomized strategy trials of the optimum timing of ART during TB treatment [18,19].
Figure 2. Relationship between the proportion of patients with TB and CD4 cell counts <100 cells/μl who developed immune reconstitution disease and the timing of initiation of antiretroviral therapy during TB treatment.
For the periods 0–30, 31–60, 61–90, 91–120 and >120 days, the proportions of patients with IRD were 13/16, 9/30, 3/21 3/35 and 1/59, respectively.
ART: Antiretroviral therapy; IRD: Immune reconstitution disease.
Data taken from [4].
The impact of the timing of ART on risk of cryptococcal IRD is unclear and the literature is complicated in some retrospective studies by concurrent reporting of both unmasking and paradoxical forms of the disease [20,21]. Higher risk of cryptococcal IRD in patients receiving earlier ART observed in some retrospective studies [20,21] is not supported by the findings of other studies [22,23].
Extent of opportunistic infection
Patients with evidence of more extensive or disseminated OIs appear to be at increased risk of IRD, which is likely to be related to a greater antigen burden. Patients with advanced immunosuppression whose immune systems are poorly able to control microbial replication prior to initiating appropriate antimicrobial therapy are therefore at greater risk of IRD.
Risk of paradoxical TB-IRD is increased in those with extrapulmonary [16,24,25] or disseminated TB [26,27] and was positively correlated with mycobacterial antigen load in a rabbit model of TB-IRD [28]. Although the burden of Mycobacterium tuberculosis in humans cannot be directly measured, the major cell wall antigen lipoarabinomannan (LAM) is detectable in the urine of HIV-infected TB patients with advanced immunodeficiency and especially in those with disseminated TB [29,30]. LAM detection may therefore serve as a useful index of systemic antigen burden. Among 22 patients who were at risk for paradoxical TB-IRD when starting ART in a South African study, a positive urinary LAM assay at baseline was observed in all five patients who developed TB-IRD and in only one of 17 patients who did not [29]. Thus, urine LAM may be a useful predictive marker of those at highest risk of TB-IRD.
In studies of patients with cryptococcal meningitis, risk of IRD was associated with positive blood cultures [21] and higher cerebro spinal fluid (CSF) [20] and serum [23] cryptococcal antigen titer at diagnosis. Similarly, risk of IRD associated with KS was associated with detectable KS-associated herpesvirus [31]. IRD associated with CMV retinitis was positively associated with the clinical extent of disease [32].
Baseline CD4 count & HIV viral load & changes on ART
In a large meta-analysis, higher risk of paradoxical IRD was observed among those with lower baseline CD4 cell counts, especially those with counts <50 cells/μl [11]. This may potentially reflect an increased risk of high OI antigen burden, greater potential for rapid improvements in immune function or an increased risk of dysregulated immune recovery.
In studies of IRD associated with TB or cryptococcal meningitis, greater risk was not only observed in those with lower baseline CD4 cell counts [15,21,27] but also in those with the greatest increments during ART in CD4 cell count [22,27], CD4 cell count percentage and CD4/CD8 ratio [26]. Other studies of patients with a range of OIs have shown similar associations [33-36] although one study found that KS-associated IRD was associated with a higher CD4 cell count at KS diagnosis [8]. Higher baseline HIV-1 viral load and a more rapid fall in viral load during ART have also been identified as risk factors [17,20,33,34,37-39].
Other risk factors
Incidence rates of paradoxical TB-IRD vary widely according to setting from 0% in a Tanzania study [40] to 45% in a French cohort [41]. In addition to the variables discussed earlier, other possible explanations for heterogeneity in risk are differences in prevalent strains of M. tuberculosis, genetic factors or study design issues (especially case definitions). No association with TB strain type was demonstrated in two relatively small studies [42,43]. Genetic studies have found polymorphisms in the TNF-α and IL-6 genes to be associated with mycobacterial IRD [44] and certain HLA polymorphisms to be associated with CMV IRD [45]. One study in the USA also found TB-IRD to be associated with black race [16]. Others have hypothesized that vitamin D deficiency may play a role in the development of IRD since 1,25-dihydroxyvitamin D has a role in pathogen clearance, the inflammatory response and macrophage activation [46].
Most studies have shown no association between specific classes of antiretroviral drugs and risk of IRD. However, one study showed that patients receiving non-nucleoside reverse transcriptase inhibitors and protease inhibitors (PIs) together were more likely to develop KS-associated IRD [8]. One other study found ritonavir-boosted PIs to be associated with IRD [33].
Immunopathogenesis
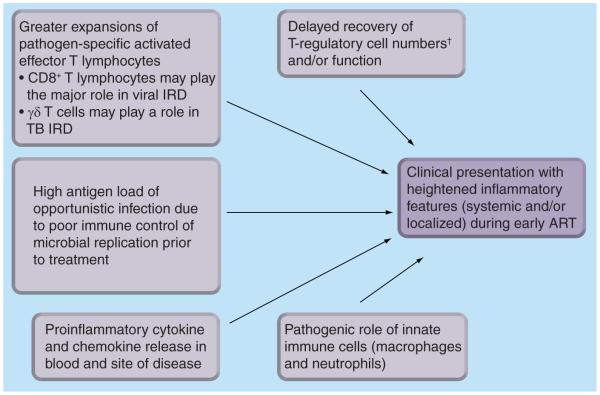
Immune reconstitution disease most commonly occurs during the early, most rapid phase of immune recovery and is characterized by exaggerated inflammatory responses. However, the precise mechanisms underlying the immunopathology of IRD are incompletely understood and may differ according to the OI involved. For example, mycobacterial and fungal IRD are characterized by granulomatous inflammation whereas CD8+ T-lymphocyte infiltrates predominate in IRD associated with viral infections [2,47]. Here, we review data concerning a range of different OIs. A summary of the key factors involved in pathogenesis is shown in Figure 3. Our understanding of these mechanisms is important for the development of immunomodulatory pharmacological interventions for treatment and prevention of IRD.
Figure 3. The immunopathogenesis of immune reconstitution disease: mechanisms that have been hypothesized and studied.
†No study has demonstrated deficient numbers of T-regulatory cells in IRD patients, although studies have suggested impaired function.
ART: Antiretroviral therapy; IRD: Immune reconstitution disease.
Mycobacterial immune reconstitution disease
The first reports of mycobacterial IRD in the early 1990s were in patients who developed localized forms of Mycobacterium avium complex disease, such as focal lymphadenitis, after starting zidovudine monotherapy [48]. These unusual presentations and subsequent reports of TB-IRD were associated with a switch in skin test responses to mycobacterial antigens from anergic to strongly positive, implicating recovery of delayed-type hypersensitivity responses [38,48]. This notion was supported by the observation that these clinical phenomena were typically, though not invariably, associated with increases in peripheral blood CD4 cell counts. In the minority of patients in whom no such increases are seen [12,38], early immune recovery could be associated with accumulation of pathogen-specific CD4 cells at the tissue site of disease without being reflected in peripheral blood.
Cytokine release assays have been used to explore the association between increases in mycobacterial-specific T cells in peripheral blood and paradoxical TB-IRD [41,49-53]. Bourgarit et al. reported that TB-IRD was associated with large expansions of purified protein derivative (PPD)-specific T cells producing IFN-γ [49] and similar findings have been reproduced in other studies [50,52]. Bourgarit et al. also recently reported that these PPD-specific T cells are highly activated, multifunctional (IFN-γ+ TNF-α+ IL2−) cells of the CD4+ effector memory phenotype [41]. TB-IRD patients were also found to have high levels of γδ T cells, which had reduced expression of killer Ig-related receptor at baseline and at the time of TB-IRD, suggesting a possible deficiency in immune regulation and a role in pathogenesis [41].
In contrast to the findings of Bourgarit et al., a cross-sectional study conducted in South Africa showed that while TB-IRD patients had greater T-cell expansions in response to a range of mycobacterial antigens compared with controls, responses in both cases and controls were heterogeneous [51]. Moreover, in longitudinal analyses, T-cell expansions and contractions were observed in both groups, raising the question as to whether cell expansions were the actual cause of TB-IRD or an epi-phenomenon [51].
It has also been suggested that paradoxical TB-IRD is precipitated by a ‘cytokine storm’ [54]. TB-IRD has been shown to be associated with elevated concentrations of a range of Th1 and inflammatory cytokines and chemokines, but not Th2 cytokines [49]. In a South African study, in vitro re-stimulation of peripheral blood mononuclear cells with mycobacterial antigens resulted in markedly increased production of a broad range of cytokines and chemokines in TB-IRD cases compared with controls [55]. However, TNF-α, IFN-γ and IL-6 were the most consistently elevated cytokines and these were also present in markedly increased concentrations in unstimulated serum samples [55].
It has been hypothesized that paradoxical TB-IRD may reflect a relative delay in recovery of regulatory T-cell numbers and function [54,56]. Two studies found no such deficiency [51,52], however, and Seddiki et al. demonstrated expansions of CD127lo Foxp3+ CD25+ T-regulatory cells and a higher ratio of T-regulatory: effector/memory subsets in TB-IRD patients [57]. However, in this study, in vitro suppression assays demonstrated reduced functional capacity and reduced IL-10 secretion from suppressor cells in TB-IRD patients. This suggested that although T-regulatory cell numbers were increased, their ability to downregulate aberrant immune responses was compromised. Another study of two patients with mycobacterial IRD demonstrated impaired IL-10 and increased IFN-γ production [56]. Thus, while no deficiency in T-regulatory cell numbers in mycobacterial IRD has been shown, data suggest there may be functional impairment that contributes to dysregulated inflammation.
The role of the innate immune system in IRD is unknown but it has been hypothesized that macrophages may be important [58]. A fatal case of unmasking pulmonary TB-IRD was found to have bronchiolitis obliterans organizing pneumonia at autopsy with an infiltrate consisting predominantly of macrophages [59]. Oliver et al. demonstrated that TB-IRD was associated with higher concentrations of CXCL10 and IL-18, also suggesting that innate immune responses contribute to pathogenesis [60]. Neutrophils are likely involved given that suppurative lymphadenitis and abscess formation are frequent features of TB-IRD.
Cryptococcal IRD
Higher cryptococcal antigen titer in CSF [20] and lower levels of inflammation at diagnosis [61] have been associated with development of IRD. This suggests that poorly controlled fungal replication prior to ART related to poor immune response may establish the conditions for subsequent IRD. Glucuronoxylomannan, a major constituent of the capsular wall of cryptococcus, has immunomodulatory effects and may contribute to establishing these conditions [62]. Cryptococcal antigen persists in the CNS for months despite antifungal treatment, providing a potent antigenic stimulus.
Cryptococcal IRD has been associated with an exacerbation of proinflammatory responses in CSF. In patients who developed cryptococcal IRD, CSF leukocyte counts and concentrations of IFN-γ, TNF-α, GCSF, VEGF and eotaxin have been reported to be higher at time of IRD compared with at the time of initial diagnosis of cryptococcosis [20,61]. However, this differs from the findings of a South African study [22].
Immune reconstitution disease associated with other opportunistic diseases
Kaposi’s sarcoma IRD is characterized by the enlargement of lesions, inflammation or edema, and emergence of new lesions during ART. Proinflammatory cytokine and chemokine production that increase during ART may upregulate expression of factors inducing angioproliferation and tumorigenesis in such patients [31,63,64]. However, KS IRD lesions may also spontaneously regress following development of IRD [65], suggesting that this may reflect the early stage of an ultimately beneficial immune response.
CMV immune recovery uveitis (IRU) is characterized by inflammatory cell infiltrates in the posterior chamber (vitritis), macular edema and epiretinal membrane formation [66]. Cells that may be involved include natural killer cells [62] and cytotoxic CD8+ T lymphocytes [67]. One study showed that CMV IRU could be distinguished from CMV retinitis that occurs in patients not on ART by presence of IL-12, lower levels of IL-6, and absence of detectable CMV replication in aqueous vitreal fluid [66]. CMV IRU is associated with an increase in plasma CMV-specific IgG [68].
Prevention: clinical interventions
In light of what is known of the risk factors and immunopathogenesis of IRD, it follows that a range of different interventions may reduce the risk and severity of unmasking and paradoxical forms of IRD. These include the initiation of ART much earlier in the course of HIV progression, preventive therapy for OIs prior to ART eligibility, pre-ART screening for OIs, use of optimized treatment for OIs, adjustment of the timing of starting ART during OI treatments, and immunosuppressive and immunomodulatory therapies.
Global interventions to prevent both unmasking & paradoxical IRD
Timing of ART initiation during HIV progression
Early ART is arguably the most important strategy to reduce the risk of both OIs and associated risks of unmasking and paradoxical IRD. There is substantial geographical variation in baseline CD4 cell counts. In patients (n = 24,444) starting ART in Europe between 1997 and 2006, the median CD4 cell count was 230 cells/μl [69]. By contrast, 18 cohorts containing 39,536 patients in sub-Saharan Africa had median CD4 cell counts typically in the range of 100–150 cells/μl [70], similar to cohorts in Latin America and the Caribbean [71]. However, the median CD4 cell count among 58,000 patients starting ART in Thailand between 2000 and 2005 was just 41 cells/μl [72].
ART guidelines now recommend earlier initiation of ART. WHO guidelines in 2002 initially only recommended ART for patients with WHO stage four disease (AIDS) and those with CD4 cell counts <200 cells/μl [201]. Current guidelines now recommend ART for all patients with CD4 cell counts <350 cells/μl and for all patients with WHO stage three or four disease (which includes all TB patients) regardless of CD4 cell counts [202]. In many high-income settings, it is recommended to start ART even earlier, including patients with CD4 cell counts between 350 and 500 cells/μl in the USA, for example [73].
Some evidence indicates that baseline CD4 counts in cohorts in sub-Saharan Africa and South America have increased over time during ART scale-up [74-76]. Despite such trends, late presentation is likely to remain an issue worldwide, especially in resourcelimited settings [77]. Here, a large majority of patients still have baseline counts <200 cells/μl and such patients are therefore at substantial risk of opportunistic disease and potential IRD.
Prevention of opportunistic infections pre-ART
Pre-ART care provides an opportunity to prevent OIs, including TB and cryptococcal disease, thereby reducing the risk of associated IRD.
Prevention of TB
Isoniazid-preventive therapy (IPT) reduces the risk of TB by 33% overall and by 64% (95% CI: 39–78%) among individuals who have a positive tuberculin skin test (TST); no benefit is derived by those with a negative TST [78]. Patients who sequentially receive IPT and then ART may derive an additive TB preventive effect as these two interventions have differing and complementary mechanisms of action [79-81].
Uptake of IPT among HIV-infected individuals worldwide has been extremely low, being provided for an estimated 0.2% of eligible individuals worldwide in 2008 [203]. One key hurdle to scaling up this intervention has been the difficulty in reliably excluding active TB disease prior to IPT. The WHO previously recommended a cough duration of 2–3 weeks as a symptom screen for TB [82,204]. This is now recognized as inadequate for HIV-associated TB, with the sensitivity frequently being found to be less than 50% in this patient group [83-85].
Screening tools that combine multiple symptoms have much higher sensitivity, albeit with low specificity. A meta-analysis of nearly 10,000 HIV-infected patients actively screened for TB has been conducted to derive a high-sensitivity symptom screening algorithm [86]. The optimum algorithm identifies patients who have one or more of four common symptoms (current cough, night sweats, weight loss or fever) with a sensitivity of 79% and a specificity of 50%. For individuals who are not yet eligible for ART and who have a TB prevalence less than 10%, the negative predictive value of this screening tool is very high and is regarded as sufficient to identify those with a very low probability of TB who can then start IPT [83]. Use of this algorithm may help increase uptake of IPT.
Prevention of cryptococcal disease
Routine primary fluconazole prophylaxis in both developed and developing world settings has been shown to reduce the number of cases of cryptococcal meningitis, yet it has not shown a consistent survival benefit [87]. Large numbers of patients require long-term medication, concerns exist around the development of drug resistance and cost–effectiveness analyses are unfavorable in many settings [88-90]. Thus, few national programs recommend this although the benefit may vary substantially between countries. A study from Cambodia reported that routine use of fluconazole prophylaxis during ART was associated with a 50% reduction in adjusted hazards of death among those with baseline CD4 cell counts of <100 cells/μl [91]. Routine fluconazole prophylaxis given in this way has not been shown to select for fluconazole resistance in cryptococcal isolates [92] although there is an association with development of fluconazole-resistant Candida [93].
In a large community-based placebo-controlled trial of fluconazole prophylaxis administered three-times weekly in Uganda, a significantly reduced risk of cryptococcal disease was observed. However, overall disease rates were very low because of the exclusion of patients found to have a positive serum cryptococcal antigen on screening at baseline [94]. Here, an approach of screening for cryptococcal antigenemia prior to starting ART and using targeted pre-emptive therapy may be a more cost-effective strategy, as discussed later [95,96].
In patients who have been treated for cryptococcal meningitis, secondary prophylaxis with fluconazole is very important. Lack of provision or poor patient adherence with such treatment results in high rates of secondary cases in South Africa [97] and high associated mortality risk during early ART [98,99].
Interventions to prevent unmasking IRD
Unmasking IRD arises in patients with undiagnosed OIs present at the time of ART initiation. Systematic screening for OIs represents the key means to prevent this.
Screening for TB pre-ART
There is a very strong rationale for screening and rapid TB diagnosis among patients accessing ART services to reduce morbidity, mortality and nosocomial transmission. Early diagnosis and rapid initiation of TB treatment are also likely to reduce risks of unmasking and paradoxical TB-IRD by limiting the extent of disease and mycobacterial antigen load. In contrast to unmasking cryptococcal IRD, the majority of unmasking TB disease has a clinically unremarkable presentation (therefore simply referred to as ‘unmasking TB’). However, a small subset of such cases present with overtly hyper-inflammatory features that might truly be considered true ‘unmasking TB-IRD’ [100] and such presentations are occasionally life-threatening and fatal [59,101]. In addition to patients referred to ART programs with known TB diagnoses, there is a large burden of undiagnosed TB among the remaining patients. The proportion detected is likely to vary greatly depending on the prevailing TB burden, the degree of immunodeficiency and the rigor with which patients are screened [102]. The prevalence of TB is established most accurately when all patients are screened for TB regardless of the presence or absence of symptoms and when high-sensitivity investigations, such as automated liquid culture, are used [85,102]. In two such studies of South African cohorts, patients had median CD4 cell counts of approximately 100 cells/μl and pulmonary TB was diagnosed in 19% of patients in Durban [103] and 25% of patients in Cape Town, South Africa [29] when sputum samples from all patients were examined using automated liquid culture. Prevalence was substantially higher among the subset of patients with CD4 cell counts <100 cells/μl [29].
In the Cape Town cohort, approximately 40% of cases of TB presenting during the first 4 months of ART were estimated to be due to unmasking TB [104]. Consistent with this, efficient baseline screening in the same cohort was associated with a substantial reduction in the incidence of TB (approximately halved) during the initial months of ART [105]. In effect, this high-sensitivity routine screening substantially reduced the risk of unmasking TB during early ART and this may well also minimize the risk of the more severe cases of unmasking IRD [59,101].
The great challenge of screening for TB in this patient population is that a large majority of the disease is sputum smear negative. Owing to the very low numbers of mycobacteria in sputum of many such patients, the time to positive culture is also prolonged, with a mean of over 3 weeks in one study in Cape Town [29]. Newer diagnostics are urgently required to provide simpler and more rapid diagnosis. A simple, commercially available ELISA is able to detect LAM excreted in the urine of patients with TB. Despite disappointing performance in non-HIV-infected patients [106], moderate sensitivity and high specificity has been observed in HIV-infected patients in South Africa [29,30,107].
In each of these studies of HIV-infected patients [29,30,107], the sensitivity of the LAM assay exceeded that of sputum smear microscopy and was highest in patients with the lowest CD4 cell counts. In ambulatory patients screened prior to ART, the sensitivity of the assay was 67% in those with CD4 cell counts <50 cells/μl and specificity was excellent [29]. LAM antigenuria at baseline was also predictive of the development of paradoxical TB-IRD [29] although larger studies are needed to evaluate this association further. A simplified lateral flow version (‘dip-stick’) of this urine LAM assay is currently being evaluated [106]. If the sensitivity and specificity of this assay is at least comparable to the ELISA, it could be used as a simple and cheap point-of-care test incorporated within a diagnostic screening algorithm for out-patients accessing ART or for HIV-infected in-patients.
Nucleic acid amplification tests (NAATs) represent the most promising development for rapid diagnosis of TB and rapid drug susceptibility testing [108]. Progress has been made in developing simplified versions of these tests with higher sensitivity for smear-negative disease. The most important of these to date is a sensitive and specific fully automated and commercially available NAAT assay, which has been developed for use outside reference laboratory centers [109,110]. The Xpert MTB/RIF Assay (Cepheid, Sunnyvale, CA, USA) uses real-time PCR technology to detect M. tuberculosis and the rpoB rifampicin resistance mutation. The cartridge-based system dispenses with the need for prior sputum processing, requires minimal laboratory expertise and results are available in less than 2 h, providing a specific TB diagnosis and a rapid assessment of rifampicin resistance. A large multicountry evaluation found excellent performance characteristics, including sensitivities of 72.5, 85.1 and 90.2% for sputum smear-negative disease when processing one, two or three sputum specimens, respectively [110]. This assay requires evaluation for TB screening in ART programs; in this setting it may be stretched to the limits of its diagnostic sensitivity [111].
In a study among patients initiating ART in Cambodia, positive IFN-γ-release assays (IGRAs) were found to be strongly predictive of unmasking TB [50], presumably due to association with un diagnosed TB at baseline. The study did not specify how patients were screened for TB pre-ART and what the incremental value of IGRAs was above other diagnostic tests. However, the inability of such assays to distinguish between active and latent disease limits their utility, especially in high TB burden settings.
Screening for cryptococcal disease pre-ART
The clinical course of patients with asymptomatic crypto coccal antigenemia remains poorly defined since all such patients who are identified typically receive treatment. However, if left untreated, clinical disease may develop as fungal burden increases in the context of persisting immunodeficiency [88]. Alternatively, if ART is commenced, rapid restoration of pathogen-specific immune responses may cause unmasking of subclinical disease. Such unmasking cryptococcal disease has a poor prognosis and contributes substantially to early mortality in ART programs in Africa [70,95,99]. Cryptococcal antigen screening pre-ART, however, offers a potential solution.
Two retrospective studies from Africa have found that cryptococcal antigen detected in stored serum samples obtained pre-ART was an independent predictor of mortality [95,112]. In the South African study, the prevalence of antigenemia was 13% among patients with CD4 cell counts <100 cells/μl and an antigen titer of ≥1:8 was 100% sensitive and 96% specific for predicting development of cryptococcal meningitis during the first year of ART [95]. Use of this as a screening test pre-ART might permit implementation of a targeted pre-emptive treatment strategy, preventing most, if not all, cases of unmasking cryptococcal disease during ART. Such a screening strategy was evaluated as being a highly cost-effective intervention in Uganda [96]. Simplified point-of-care methods for antigen testing are needed together with evaluation of the impact of preemptive therapy for those found to be antigen positive. The optimal management strategy for such patients remains to be defined.
Screening for CMV ophthalmic disease
Development of immune recovery uveitis during ART in patients with CMV retinitis at baseline may result in visual loss [7,113,114]. Screening for CMV retinitis is therefore of great importance, especially among treatment-naive patients with very low CD4 cell counts (<100 cells/μl). Ideally, retinal examination should form part of the normal standard of care of all HIV-infected individuals. The gold standard for detection of CMV retinitis is retinal examination through a dilated pupil by a skilled examiner (usually a trained ophthalmologist), using a binocular indirect ophthalmoscope and condensing lens [115]. However, this is impractical in many settings.
Approaches to simplify screening might include the initial use of symptom screening. However, the predictive value of symptom screening is highly variable with the proportion of patients with newly diagnosed CMV retinitis who have symptoms varying geographically, being higher in the USA and UK than in nonindustrialized countries. In India, for example, symptom screening had a sensitivity of just 7.7% and a positive predictive value of 18% [116]. Clearly, in many settings, ocular examination cannot be the sole domain of the ophthalmologist and non-ophthalmic personnel require training in ophthalmoscopy. Alternative approaches might be to use fundus cameras that could be systematically reviewed by trained personnel to select those cases requiring specialist review.
It is important to note that despite diagnosing and appropriately treating CMV retinitis prior to ART, a proportion of patients will still develop CMV immune recovery uveitis as a paradoxical IRD. However, limiting the extent of CMV retinitis by earlier treatment may reduce this risk.
Interventions to prevent paradoxical IRD
The risk and severity of paradoxical IRD are likely to be strongly associated with the residual antigen burden present at the time of rapid immune recovery following ART initiation. This might be reduced by effective OI treatment and by adjusting the timing of ART.
Effective treatment of OIs
The amount of residual OI antigen present at the time ART is started is a function not only of the preceding duration of OI treatment but also of the efficacy of that treatment. In a large case series of TB-IRD in Cape Town, undiagnosed rifampicinresistant TB (mostly multidrug resistant) in patients receiving first-line TB treatment was detected in 10.1% of cases and it is possible that reduced clearance of mycobacterial antigen in such patients predisposed to IRD [117]. Much more widespread availability of routine TB drug susceptibility testing is needed.
Optimal care for patients with cryptococcal meningitis includes the use of intravenous amphotericin B – a fungicidal drug that leads to rapid clearance of the organism from the CSF when measured by serial quantitative CSF cultures [118,119]. However, in many resource-limited settings, the standard of care for initial treatment of cryptococcal meningitis is oral fluconazole provided by the Pfizer free access program. Fluconazole, however, is essentially a fungistatic drug and high organism load persists in CSF during the first 2 weeks of fluconazole treatment [120]. This may in turn predispose to cryptoccal IRD, which may possibly be one among several factors contributing to the extremely high mortality observed among patients starting ART within 72 h of treatment for cryptococcal meningitis using oral fluconazole in a randomized controlled trial in Zimbabwe [121-123].
There is currently no other treatment available for PML other than ART, which triggers IRD in 16.7% of cases [11]. However, in vitro data and case reports suggest that the antimalarial drug mefloquine may have efficacy [124,125] and a Phase II multicenter clinical trial is underway [205].
Timing of ART during treatment of OIs
Risk of paradoxical IRD could theoretically be substantially reduced simply by deferral of ART initiation as suggested by Figure 2. However, observational studies have also shown that even short delays in the initiation of ART may be associated with substantial all-cause mortality risk, especially in resource-limited settings [98,126]. Risk of IRD and associated consequences is just one issue among a range of competing factors favoring either early or delayed initiation of ART during OI treatment [127]. Additional factors include the risks of drug co-toxicity, pharmacokinetic drug interactions, pill burden, treatment tolerability and overall risk of further morbidity and mortality.
Data on timing of ART in patients with OIs are now available from five randomized controlled trials in which mortality was included within the primary outcome (Table 2) [127]. The conclusions derived from these studies appear to differ according to whether or not the OI involves the CNS. A study of patients with acute OIs excluding TB (a multicenter trial that was largely based in the USA) [128] and two other studies of patients with TB in South Africa [19] and Cambodia [18] all showed an overall substantial mortality reduction among patients randomized to the earlier treatment arms (Figure 3). In the two studies of patients with TB, substantial mortality reduction was observed despite the approximately two- to threefold greater risk of IRD in the early treatment groups (Table 2) [18,19]. However, the study by Zolopa et al. of non-TB OIs did not find timing of ART to be associated with risk of IRD [37], but this may largely reflect the fact that most OIs represented were Pneumocystis jirovecii pneumonia and acute bacterial infections, neither of which is associated with substantial risk of IRD.
Table 2. Randomized clinical trials of early versus deferred initiation of antiretroviral therapy during treatment for opportunistic infections.
| Study (year), country |
Opportunistic infection | Details | Main results | IRIS events (%) | Ref. | |
|---|---|---|---|---|---|---|
|
Early
arm |
Deferred
arm |
|||||
| Zolopa (2009), Multi-country |
Pneumocystis jirovecii pneumonia (63%) Serious bacterial infections (12%) Cryptococcal meningitis (12%) Miscellaneous (13%) |
ART started within 14 days (median 12 days) vs ART after completing OI treatment (median 45 days) |
Early ART reduced risk of AIDS progression/death (OR:0.51; 95% CI: 0.27–0.94) |
5.7 | 8.5 | [128] |
| Abdool Karim et al. (2010), South Africa |
Smear-positive pulmonary TB | ART started during frst 3 months of TB treatment vs after completion of TB treatment CD4 count 0–500 cells/μl |
Mortality reduced in early arm (HR: 0.44) |
12.4 | 3.8 | [19] |
| Blanc et al. (2010), Cambodia |
Smear-positive pulmonary or extrapulmonary TB |
ART started 2 weeks after starting TB treatment vs 2 months CD4 counts <200 cells/μl |
Mortality higher in 2-month arm Adjusted HR: 1.52 |
33 | 15 | [18] |
| Torok et al. (2009), Vietnam |
Tuberculous meningitis | Immediate ART at start of TB treatment vs after 2 months |
No signifcant difference in mortality but more adverse events in immediate arm |
Not assessed | [129] | |
| Makadzange et al. (2010), Zimbabwe |
Cryptococcal meningitis | ART started within 72 h of diagnosis vs after 10 weeks Patients treated with fuconazole |
3-year cumulative mortality 88% in early arm vs 54% in delayed arm (p < 0.006) Adjusted HR: 2.85 |
Not assessed | [121] | |
ART: Antiretroviral therapy; HR: Hazard ratio; IRIS: Immune reconstitution infammatory syndrome; OI: Opportunistic infection; OR: Odds ratio.
In the study from Zimbabwe, patients with cryptococcal meningitis receiving initial fluconazole therapy and no per-protocol therapeutic interventions to control CSF pressure had much higher mortality when randomized to start ART within 72 h of antifungal treatment compared with starting after 10 weeks [121]. From clinical evaluation, the causes of death were ascribed largely to complications of cryptococcal meningitis, but clinical or postmortem data on IRD were lacking. In contrast to these data, the subset of patients with cryptococcal meningitis treated with amphotericin in the study by Zolopa et al., a strong trend towards lower risk of progression to AIDS and death among those receiving earlier ART was observed [128].
A study of patients with TB meningitis in Cambodia randomized to start ART immediately or after 2 months of TB treatment did not differ with regards to survival [129] but overall IRD events were not specifically reported (Table 2). In both studies of CNS OIs, it might be hypothesized that the consequences of IRD within the vital structures of the CNS confined within cranial cavity may be far worse than for IRD affecting structures outside the CNS [10]. In this case, risks and consequences of IRD may off-set or even outweigh the mortality benefit that is otherwise derived by HIV-infected patients receiving early ART [127].
In summary, risk of paradoxical IRD is just one factor in a complex clinical scenario that affects the optimal timing of ART during OIs. However, the overall mortality risk is the overriding determinant and this may differ for intra-cranial and extra-cranial OIs and according to the efficacy of the treatment received for the OI. The most recent revision of the WHO ART guidelines recommend that ART be started as soon as possible within 2–8 weeks for all patients with HIV-associated TB, but no specific recommendations were made with regard to TB meningitis or other OIs [202]. In light of these recommendations, it is clear that paradoxical TB-IRD will remain an important clinical issue and that strategies other than adjusting the timing of ART initiation will need to be sought in efforts to prevent this complication.
Prevention: pharmacological interventions
From the observations that the immunopathogenesis of IRD is associated with marked expansion of T lymphocytes, a marked proinflammatory cytokine drive and lack of regulatory immune function (Figure 3) , it is logical to conclude that immunosuppressive and immunomodulatory therapies may be beneficial with regards to the treatment and prevention of IRD.
Preventing complications of IRD
Case reports and case series describe a range of pharmacological interventions used in IRD case management, including oral corticosteroids, NSAIDs, thalidomide and leukotriene receptor antagonists [3,130,131]. Other immunomodulatory agents such as TNF-α inhibitors have also been suggested for use.
Only one study has formally assessed an intervention using a randomized controlled trial. In a study in Cape Town, South Africa, patients with moderately severe, non-life-threatening TB-IRD were randomized to receive either 4 weeks of oral corticosteroids or placebo [131]. Prednisone was given for 2 weeks at a dose of 1.5 mg/kg per day for 2 weeks followed by 0.75 mg/kg per day for 2 weeks. The composite primary endpoint was days of hospitalization and out-patient therapeutic procedures, the latter of which were counted as 1 day of hospitalization. Those receiving prednisone had a lower median number of days of hospitalization (0 vs 3 days; p = 0.04) and significantly greater improvements in symptoms, Karnofsky score and quality of life after 2 and 4 weeks despite a higher risk of nonsevere OI [131]. This study therefore provided important evidence of the benefit of oral corticosteroids for moderately severe IRD.
It would be unethical to perform such a randomized trial among those with life-threatening manifestations of TB-IRD, but the data from the trial by Meintjes et al. clearly suggest their likely important role in such patients [131]. This includes patients with TB involving the CNS in whom coadministration of corticosteroids is widely regarded as standard of care [132]. Optimum case management of other forms of IRD, especially that complicating cryptococcal disease, remains undefined. Specifically, the benefits of corticosteroids and repeated CSF lumbar draining to manage raised intracranial pressure are unknown. Use of other anti-inflammatory agents at present remains anecdotal.
Prevention of IRD
No clinical trials of pharmacological agents to prevent IRD have been completed. We are aware of just two clinical trials involving use of maraviroc and a NSAID. Statins, vitamin D and corticosteroids have also been suggested as other possible IRD prevention strategies.
Maraviroc is a CCR5 chemokine receptor antagonist that not only blocks the uptake of R5-tropic HIV into CD4 cells but also has immunomodulatory properties [133], possibly by interfering with leukocyte recruitment to sites of inflammation through CCR5 blockade [134]. Maraviroc was used in the treatment of one patient with PML IRD and a surprisingly rapid clinical response was reported [135]. In the CADIRIS trial [206], patients in South Africa and Mexico starting ART are being randomized to receive maraviroc or placebo in addition to standard triple-drug ART. The primary outcome is the incidence of all forms of IRD.
Another planned trial in South Africa plans to assess the use of a NSAID in the prevention of paradoxical TB-IRD. TB patients will be randomized to receive either meloxicam (a NSAID) or placebo in addition to standard TB treatment and ART [Nachega J, Pers. Comm.].
Vitamin D acts on diverse cell types, mediating pleiotropic effects on the immune system and modulating both the adaptive and innate immune responses [136]. Epidemiological studies have linked vitamin D deficiency to various immune-mediated disorders and a potential role in IRD has been proposed [46]. Similarly, in addition to their lipid-lowering properties, statins have anti-inflammatory properties and there is precedence for using these agents for autoimmune inflammatory disorders in an experimental model [137,138]. It has been proposed that statins and vitamin D may therefore also be of potential use in the treatment or prevention of IRD.
Corticosteroids have also been suggested for potential use for preventing paradoxical IRD. Although effective in case management [131], use in prevention does not appear well justified. Use of corticosteroids in HIV-infected people is associated with a range of adverse effects including reactivation of herpes virus infections, exacerbation of KS and precipitating other infections, including strongyloidiasis [131,139-141]. Given that paradoxical TB-IRD develops in around one in five patients with a TB diagnosis prior to ART, it would mean that four out of five patients would be unnecessarily exposed to the adverse effects of corticosteroids. Moreover, the ACTG5164 trial of the optimum timing of ART during treatment for OIs excluding TB found no association between use of corticosteroids and risk of IRD [37]. We think that corticosteroids are best reserved for treatment of certain forms of IRD, with the exception of patients with TB involving the CNS in whom routine use of corticosteroids is regarded as standard of care [132]. However, our experience is that cases of TB-IRD involving the CNS may nevertheless occur despite use of corticosteroids.
Expert commentary & five-year view
Despite guidelines recommending earlier initiation of ART, late presentation with advanced HIV is likely to persist as an issue in a substantial proportion of patients, especially in resource-limited settings. This, in turn, will continue to fuel high rates of OIs and associated risks of IRD. Furthermore, several recent randomized controlled trials have shown that survival is improved with earlier initiation of ART during treatment for OIs, despite the increased risk of IRD. Resulting shifts in recommendations for rapid initiation of ART during OIs will also continue to fuel rates of paradoxical IRD. Additional prevention strategies are needed.
Development of simple means to rapidly and reliably screen for key infections prior to ART such as TB and cryptococcosis is of paramount importance. While optimum screening for HIV-associated TB in this patient population is heavily reliant upon mycobacterial culture, which is expensive, slow and unavailable in many settings, real progress is now being made in the development of new near-patient technologies and point-of-care tests that will greatly simplify TB screening and early detection of drug-resistant disease. Similarly, subclinical cryptococcal disease can also be readily diagnosed by detection of cryptococcal antigen in serum and we anticipate that point-of-care tests to detect antigen in blood or urine will simplify this process further. Operational research will also develop simpler means to screen for CMV retinitis pre-ART.
Ongoing basic research will extend our understanding of the immunopathogenesis of IRD and may provide the rationale for new immunomodulatory interventions. Identification of transcriptional or proteomic signatures associated with IRD may facilitate diagnosis, overall simplifying clinical management. A single randomized clinical trial of corticosteroids has demonstrated reduced morbidity in patients with paradoxical TB-IRD, but additional data will arise from further clinical trials to prevent paradoxical IRD using a range of drugs with immunomodulatory properties but fewer adverse effects than corticosteroids. Currently, no evidence-based pharmacological strategy for the prevention of IRD can be recommended.
Key issues.
Unmasking and paradoxical forms of immune reconstitution disease (IRD) remain an important cause of morbidity, and patients with cryptococcal IRD in particular have substantial mortality risk.
Risk factors for paradoxical IRD include early initiation of antiretroviral therapy (ART) during opportunistic infection (OI) treatment, high antigen load in those with advanced OIs, low baseline CD4 cell counts, high baseline viral load and rapid immunological and virological responses to ART.
The immunopathogenesis of IRD remains incompletely defined but studies have shown associations with T-cell expansion, proinflammatory cytokine release and diminished regulatory T-cell activity.
Key to prevention of unmasking and paradoxical IRD is diminishing the risk of OIs through early HIV diagnosis and initiation of ART at CD4 cell counts greater than 200 cells/μl.
Unmasking TB may be prevented using isoniazid-preventive therapy and rigorous TB screening prior to ART using high-sensitivity TB diagnostics (such as liquid culture of sputum).
Unmasking cryptococcal IRD can be prevented by pre-ART screening for blood cryptococcal antigen and pre-emptive treatment.
Prevention of immune recovery uveitis associated with cytomegalovirus (CMV) requires careful screening for CMV retinitis among patients starting ART. Paradoxical CMV immune recovery uveitis may occur, however, despite treatment of CMV retinitis.
Risk of paradoxical IRD in patients with cryptococcal meningitis is likely to be reduced by using amphotericin B (a fungicidal drug that rapidly clears the organism from the cerebrospinal fluid) rather than fluconazole (a fungistatic drug), which is standard of care in many resource-limited settings.
Risk of paradoxical IRD could be reduced by delaying initiation of ART during OI treatment. However, several randomized controlled trials now show the overall survival benefit of early ART (with the possible exception of OIs involving the CNS). Mortality reduction by early initiation of ART is the over-riding priority.
Morbidity associated with TB IRD may be reduced using adjunctive corticosteroid therapy in patients with moderately severe disease. Optimum management of cryptococcal IRD, however, remains undefined.
Trials of the use of drugs with immunomodulatory properties are planned, including the use of maraviroc and nonsteroidal antiinflammatories. Others agents such as statins and vitamin D are also being considered.
Acknowledgments
Financial & competing interests disclosure
Stephen D Lawn and Graeme Meintjes are funded by the Wellcome Trust, London, UK. Graeme Meintjes has also received South African TB HIV Training (SATBAT) research training that was funded by the Fogarty International Center and the NIH (NIH/FIC U2RTW007373-01A1 and U2RTW007370-01A1). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject or materials discussed in this manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Shelburne SA, III, Hamill RJ. The immune reconstitution inflammatory syndrome. AIDS Rev. 2003;5:67–79. [PubMed] [Google Scholar]
- 2.French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. AIDS. 2004;18:1615–1627. doi: 10.1097/01.aids.0000131375.21070.06. [DOI] [PubMed] [Google Scholar]
- 3.Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect. Dis. 2005;5:361–373. doi: 10.1016/S1473-3099(05)70140-7. [DOI] [PubMed] [Google Scholar]
- 4.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect. Dis. 2008;8:516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ustianowski AP, Lawn SD, Lockwood DN. Interactions between HIV infection and leprosy: a paradox. Lancet Infect. Dis. 2006;6:350–360. doi: 10.1016/S1473-3099(06)70493-5. [DOI] [PubMed] [Google Scholar]
- 6.Haddow LJ, Colebunders R, Meintjes G, et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: literature review and proposed clinical case definitions. Lancet Infect. Dis. 2010;10:791–802. doi: 10.1016/S1473-3099(10)70170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otiti-Sengeri J, Meenken C, van den Horn GJ, Kempen JH. Ocular immune reconstitution inflammatory syndromes. Curr. Opin. HIV AIDS. 2008;3:432–437. doi: 10.1097/COH.0b013e328302cc3d. [DOI] [PubMed] [Google Scholar]
- 8.Bower M, Nelson M, Young AM, et al. Immune reconstitution inflammatory syndrome associated with Kaposi’s sarcoma. J. Clin. Oncol. 2005;23:5224–5228. doi: 10.1200/JCO.2005.14.597. [DOI] [PubMed] [Google Scholar]
- 9.Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9:425–437. doi: 10.1016/S1474-4422(10)70040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torok ME, Kambugu A, Wright E. Immune reconstitution disease of the central nervous system. Curr. Opin. HIV AIDS. 2008;3:438–445. doi: 10.1097/COH.0b013e328302ebd1. [DOI] [PubMed] [Google Scholar]
- 11.Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect. Dis. 2010;10:251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A meta-analysis of published data regarding the incidence, mortality risk and risk factors for immune reconstitution disease (IRD). Summary estimates show that in unselected cohorts of patients starting antiretroviral therapy (ART). IRD occurs in 16.1% of patients. Mortality is high among those with cryptococcal IRD (20.8%).
- 12.Phillips P, Bonner S, Gataric N, et al. Nontuberculous mycobacterial immune reconstitution syndrome in HIV-infected patients: spectrum of disease and long-term follow-up. Clin. Infect. Dis. 2005;41:1483–1497. doi: 10.1086/497269. [DOI] [PubMed] [Google Scholar]
- 13.Lawn SD, Lipman MC, Easterbrook PJ. Immune reconstitution disease associated with mycobacterial infections. Curr. Opin. HIV AIDS. 2008;3:425–431. doi: 10.1097/COH.0b013e3282fe99dc. [DOI] [PubMed] [Google Scholar]
- 14.Breen RA, Smith CJ, Bettinson H, et al. Paradoxical reactions during tuberculosis treatment in patients with and without HIV co-infection. Thorax. 2004;59:704–707. doi: 10.1136/thx.2003.019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335–341. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 16.Burman W, Weis S, Vernon A, et al. Frequency, severity and duration of immune reconstitution events in HIV-related tuberculosis. Int. J. Tuberc. Lung Dis. 2007;11:1282–1289. [PubMed] [Google Scholar]
- 17.Navas E, Martin-Davila P, Moreno L, et al. Paradoxical reactions of tuberculosis in patients with the acquired immunodeficiency syndrome who are treated with highly active antiretroviral therapy. Arch. Intern. Med. 2002;162:97–99. doi: 10.1001/archinte.162.1.97. [DOI] [PubMed] [Google Scholar]
- 18.Blanc F-X, Sok T, Laureillard D, et al. Significant enhancement in survival with early (2 weeks) vs late (8 weeks) initiation of highly active antiretroviral treatment (HAART) in severely immunosuppressed HIV-infected adults with newly diagnosed tuberculosis; Program and Abstracts of the XVIII International AIDS Conference; Vienna, Austria. 2010; Abstract THLBB1. [Google Scholar]; •• A randomized controlled trial of early (2 weeks) versus delayed (8 weeks) ART in HIV-infected TB patients in Cambodia. Early ART increased the incidence of TB-IRD, but more importantly reduced all-cause mortality by 35%.
- 19.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N. Engl. J. Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This randomized strategy trial demonstrated that HIV-infected patients with smear-positive TB and CD4 counts 0–500 cells/μl who start ART during TB treatment have a significantly lower mortality than patients who start ART after completion of TB treatment despite a threefold greater risk of IRD.
- 20.Shelburne SA, III, Darcourt J, White AC, Jr, et al. The role of immune reconstitution inflammatory syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin. Infect. Dis. 2005;40:1049–1052. doi: 10.1086/428618. [DOI] [PubMed] [Google Scholar]
- 21.Lortholary O, Fontanet A, Memain N, Martin A, Sitbon K, Dromer F. Incidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in France. AIDS. 2005;19:1043–1049. doi: 10.1097/01.aids.0000174450.70874.30. [DOI] [PubMed] [Google Scholar]
- 22.Bicanic T, Meintjes G, Rebe K, et al. Immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis: a prospective study. J. Acquir. Immune Defic. Syndr. 2009;51:130–134. doi: 10.1097/QAI.0b013e3181a56f2e. [DOI] [PubMed] [Google Scholar]
- 23.Sungkanuparph S, Filler SG, Chetchotisakd P, et al. Cryptococcal immune reconstitution inflammatory syndrome after antiretroviral therapy in AIDS patients with cryptococcal meningitis: a prospective multicenter study. Clin. Infect. Dis. 2009;49:931–934. doi: 10.1086/605497. [DOI] [PubMed] [Google Scholar]
- 24.Manosuthi W, Kiertiburanakul S, Phoorisri T, Sungkanuparph S. Immune reconstitution inflammatory syndrome of tuberculosis among HIV-infected patients receiving antituberculous and antiretroviral therapy. J. Infect. 2006;53:357–363. doi: 10.1016/j.jinf.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Serra FC, Hadad D, Orofino RL, et al. Immune reconstitution syndrome in patients treated for HIV and tuberculosis in Rio de Janeiro. Braz. J. Infect. Dis. 2007;11:462–465. doi: 10.1590/s1413-86702007000500004. [DOI] [PubMed] [Google Scholar]
- 26.Breton G, Duval X, Estellat C, et al. Determinants of immune reconstitution inflammatory syndrome in HIV type 1-infected patients with tuberculosis after initiation of antiretroviral therapy. Clin. Infect. Dis. 2004;39:1709–1712. doi: 10.1086/425742. [DOI] [PubMed] [Google Scholar]
- 27.Michailidis C, Pozniak AL, Mandalia S, Basnayake S, Nelson MR, Gazzard BG. Clinical characteristics of IRIS syndrome in patients with HIV and tuberculosis. Antivir. Ther. 2005;10:417–422. doi: 10.1177/135965350501000303. [DOI] [PubMed] [Google Scholar]
- 28.Manabe YC, Kesavan AK, Lopez-Molina J, et al. The aerosol rabbit model of TB latency, reactivation and immune reconstitution inflammatory syndrome. Tuberculosis (Edinb.) 2008;88:187–196. doi: 10.1016/j.tube.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawn SD, Edwards DJ, Kranzer K, Vogt M, Bekker LG, Wood R. Urine lipoarabinomannan assay for tuberculosis screening before antiretroviral therapy diagnostic yield and association with immune reconstitution disease. AIDS. 2009;23:1875–1880. doi: 10.1097/qad.0b013e32832e05c8. [DOI] [PubMed] [Google Scholar]; •• Detection of lipoarabinomannan (LAM) in urine was found to be a moderately sensitive diagnostic for TB in those patients with the most advanced immunodeficiency accessing an ART program in South Africa. LAM antigenuria was also associated with risk of subsequent paradoxical TB-IRD.
- 30.Shah M, Variava E, Holmes CB, et al. Diagnostic accuracy of a urine lipoarabinomannan test for tuberculosis in hospitalized patients in a high HIV prevalence setting. J. Acquir. Immune Defic. Syndr. 2009;52:145–151. doi: 10.1097/QAI.0b013e3181b98430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Letang E, Almeida JM, Miro JM, et al. Predictors of immune reconstitution inflammatory syndrome-associated with Kaposi sarcoma in mozambique: a prospective study. J. Acquir. Immune Defic. Syndr. 2010;53:589–597. doi: 10.1097/QAI.0b013e3181bc476f. [DOI] [PubMed] [Google Scholar]
- 32.Karavellas MP, Lowder CY, Macdonald C, Avila CP, Jr, Freeman WR. Immune recovery vitritis associated with inactive cytomegalovirus retinitis: a new syndrome. Arch. Ophthalmol. 1998;116:169–175. doi: 10.1001/archopht.116.2.169. [DOI] [PubMed] [Google Scholar]
- 33.Manabe YC, Campbell JD, Sydnor E, Moore RD. Immune reconstitution inflammatory syndrome: risk factors and treatment implications. J. Acquir. Immune Defic. Syndr. 2007;46:456–462. doi: 10.1097/qai.0b013e3181594c8c. [DOI] [PubMed] [Google Scholar]
- 34.French MA, Lenzo N, John M, et al. Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active antiretroviral therapy. HIV Med. 2000;1:107–115. doi: 10.1046/j.1468-1293.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 35.Ratnam I, Chiu C, Kandala NB, Easterbrook PJ. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin. Infect. Dis. 2006;42:418–427. doi: 10.1086/499356. [DOI] [PubMed] [Google Scholar]
- 36.Jevtovic DJ, Salemovic D, Ranin J, Pesic I, Zerjav S, Djurkovic-Djakovic O. The prevalence and risk of immune restoration disease in HIV-infected patients treated with highly active antiretroviral therapy. HIV Med. 2005;6:140–143. doi: 10.1111/j.1468-1293.2005.00277.x. [DOI] [PubMed] [Google Scholar]
- 37.Grant PM, Komarow L, Andersen J, et al. Risk factor analyses for immune reconstitution inflammatory syndrome in a randomized study of early vs. deferred ART during an opportunistic infection. PLoS ONE. 2010;5:e11416. doi: 10.1371/journal.pone.0011416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am. J. Respir. Crit. Care Med. 1998;158:157–161. doi: 10.1164/ajrccm.158.1.9712001. [DOI] [PubMed] [Google Scholar]
- 39.Cinque P, Pierotti C, Vigano MG, et al. The good and evil of HAART in HIV-related progressive multifocal leukoencephalopathy. J. Neurovirol. 2001;7(4):358–363. doi: 10.1080/13550280152537247. [DOI] [PubMed] [Google Scholar]
- 40.Shao HJ, Crump JA, Ramadhani HO, et al. Early versus delayed fixed dose combination abacavir/lamivudine/zidovudine in patients with HIV and tuberculosis in Tanzania. AIDS Res. Hum. Retroviruses. 2009;25:1277–1285. doi: 10.1089/aid.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bourgarit A, Carcelain G, Samri A, et al. Tuberculosis-associated immune restoration syndrome in HIV-1-infected patients involves tuberculin-specific CD4 Th1 cells and KIR-negative γδ T cells. J. Immunol. 2009;183:3915–3923. doi: 10.4049/jimmunol.0804020. [DOI] [PubMed] [Google Scholar]; • This study characterized the T-cell expansions that predominate in patients with TB-IRD as activated multifunctional effector memory CD4+ T lymphocytes. There was also evidence to suggest that γδ T cells have a role in TB-IRD pathogenesis.
- 42.Simonney N, Dewulf G, Herrmann JL, et al. Anti-PGL-Tb1 responses as an indicator of the immune restoration syndrome in HIV-TB patients. Tuberculosis (Edinb.) 2008;88:453–461. doi: 10.1016/j.tube.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Perrin FM, Breen RA, Lipman MC, Shorten RJ, Gillespie SH, McHugh TD. Is there a relationship between Mycobacterium tuberculosis strain type and TB paradoxical reaction? Thorax. 2005;60:706–707. doi: 10.1136/thx.2005.044321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price P, Morahan G, Huang D, et al. Polymorphisms in cytokine genes define subpopulations of HIV-1 patients who experienced immune restoration diseases. AIDS. 2002;16:2043–2047. doi: 10.1097/00002030-200210180-00009. [DOI] [PubMed] [Google Scholar]
- 45.Price P, Mathiot N, Krueger R, Stone S, Keane NM, French MA. Immune dysfunction and immune restoration disease in HIV patients given highly active antiretroviral therapy. J. Clin. Virol. 2001;22:279–287. doi: 10.1016/s1386-6532(01)00200-1. [DOI] [PubMed] [Google Scholar]
- 46.Conesa-Botella A, Mathieu C, Colebunders R, et al. Is vitamin D deficiency involved in the immune reconstitution inflammatory syndrome? AIDS Res. Ther. 2009;6:4. doi: 10.1186/1742-6405-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kestens L, Seddiki N, Bohjanen PR. Immunopathogenesis of immune reconstitution disease in HIV patients responding to antiretroviral therapy. Curr. Opin. HIV AIDS. 2008;3:419–424. doi: 10.1097/COH.0b013e328302ebbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.French MA, Mallal SA, Dawkins RL. Zidovudine-induced restoration of cell-mediated immunity to mycobacteria in immunodeficient HIV-infected patients. AIDS. 1992;6:1293–1297. doi: 10.1097/00002030-199211000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Bourgarit A, Carcelain G, Martinez V, et al. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20:F1–F7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]; • First major study to investigate the pathogenesis of TB-IRD and demonstrated that patients who developed TB-IRD had large expansions of purified protein derivative-specific T cells not seen in controls who did not develop TB-IRD.
- 50.Elliott JH, Vohith K, Saramony S, et al. Immunopathogenesis and diagnosis of tuberculosis and tuberculosis-associated immune reconstitution inflammatory syndrome during early antiretroviral therapy. J. Infect. Dis. 2009;200:1736–1745. doi: 10.1086/644784. [DOI] [PubMed] [Google Scholar]
- 51.Meintjes G, Wilkinson KA, Rangaka MX, et al. Type 1 helper T cells and FoxP3-positive T cells in HIV-tuberculosis-associated immune reconstitution inflammatory syndrome. Am. J. Respir. Crit. Care Med. 2008;178:1083–1089. doi: 10.1164/rccm.200806-858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Immunological study of patients with TB-IRD that demonstrated heterogeneous expansions and contractions of mycobacterial-specific T cells during the first 8 weeks of ART and thus called into question whether these cells are the cause of TB-IRD.
- 52.Tan DB, Yong YK, Tan HY, et al. Immunological profiles of immune restoration disease presenting as mycobacterial lymphadenitis and cryptococcal meningitis. HIV Med. 2008;9:307–316. doi: 10.1111/j.1468-1293.2008.00565.x. [DOI] [PubMed] [Google Scholar]
- 53.Tieu HV, Ananworanich J, Avihingsanon A, et al. Immunologic markers as predictors of tuberculosis-associated immune reconstitution inflammatory syndrome in HIV and tuberculosis coinfected persons in Thailand. AIDS Res. Hum. Retroviruses. 2009;25:1083–1089. doi: 10.1089/aid.2009.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruhwald M, Ravn P. Immune reconstitution syndrome in tuberculosis and HIV-co-infected patients: Th1 explosion or cytokine storm? AIDS. 2007;21:882–884. doi: 10.1097/QAD.0b013e3280b079c8. [DOI] [PubMed] [Google Scholar]
- 55.Tadokera R, Meintjes G, Skolimowska KH, et al. Hypercytokinaemia accompanies HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur. Respir. J. 2010 doi: 10.1183/09031936.00091010. DOI: 10.1183/09031936.00091010. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; • An immunological study that compared cytokine gene expression and concentrations in TB-IRD patients and controls. TNF-α, IFN-γ and IL-6 were the cytokines that were most consistently increased in TB-IRD patients.
- 56.Lim A, D’Orsogna L, Price P, French MA. Imbalanced effector and regulatory cytokine responses may underlie mycobacterial immune restoration disease. AIDS Res. Ther. 2008;5:9. doi: 10.1186/1742-6405-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seddiki N, Sasson SC, Santner-Nanan B, et al. Proliferation of weakly suppressive regulatory CD4+ T cells is associated with over-active CD4+ T-cell responses in HIV-positive patients with mycobacterial immune restoration disease. Eur. J. Immunol. 2009;39:391–403. doi: 10.1002/eji.200838630. [DOI] [PubMed] [Google Scholar]
- 58.Van den Bergh R, Vanham G, Raes G, De Baetselier P, Colebunders R. Mycobacterium-associated immune reconstitution disease: macrophages running wild? Lancet Infect. Dis. 2006;6:2–3. doi: 10.1016/S1473-3099(05)70302-9. [DOI] [PubMed] [Google Scholar]
- 59.Lawn SD, Wainwright H, Orrell C. Fatal unmasking tuberculosis immune reconstitution disease with bronchiolitis obliterans organizing pneumonia: the role of macrophages. AIDS. 2009;23:143–145. doi: 10.1097/QAD.0b013e32831d2a98. [DOI] [PubMed] [Google Scholar]
- 60.Oliver BG, Elliott JH, Saphonn V, Vun MC, French MA, Price P. Interferon-γ and IL-5 production correlate directly in HIV patients co-infected with Mycobacterium tuberculosis with or without immune restoration disease. AIDS Res. Hum. Retroviruses. 2010;26(12):1287–1289. doi: 10.1089/aid.2010.0004. [DOI] [PubMed] [Google Scholar]
- 61.Boulware DR, Bonham SC, Meya DB, et al. Paucity of initial cerebrospinal fluid inflammation in cryptococcal meningitis is associated with subsequent immune reconstitution inflammatory syndrome. J. Infect. Dis. 2010;202:962–970. doi: 10.1086/655785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Price P, Murdoch DM, Agarwal U, Lewin SR, Elliott JH, French MA. Immune restoration diseases reflect diverse immunopathological mechanisms. Clin. Microbiol. Rev. 2009;22:651–663. doi: 10.1128/CMR.00015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leidner RS, Aboulafia DM. Recrudescent Kaposi’s sarcoma after initiation of HAART: a manifestation of immune reconstitution syndrome. AIDS Patient Care STDs. 2005;19:635–644. doi: 10.1089/apc.2005.19.635. [DOI] [PubMed] [Google Scholar]
- 64.Mesri EA. Inflammatory reactivation and angiogenicity of Kaposi’s sarcoma-associated herpesvirus/HHV8: a missing link in the pathogenesis of acquired immunodeficiency syndrome-associated Kaposi’s sarcoma. Blood. 1999;93:4031–4033. [PubMed] [Google Scholar]
- 65.Martin J, Laker M, Clutter D, et al. Kaposi’s sarcoma-associated IRIS in Africa: initial findings from a prospective evaluation; Program and Abstracts of the 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009; Abstract 31. [Google Scholar]
- 66.Schrier RD, Song MK, Smith IL, et al. Intraocular viral and immune pathogenesis of immune recovery uveitis in patients with healed cytomegalovirus retinitis. Retina. 2006;26:165–169. doi: 10.1097/00006982-200602000-00007. [DOI] [PubMed] [Google Scholar]
- 67.Mutimer HP, Akatsuka Y, Manley T, et al. Association between immune recovery uveitis and a diverse intraocular cytomegalovirus-specific cytotoxic T cell response. J. Infect. Dis. 2002;186:701–705. doi: 10.1086/342044. [DOI] [PubMed] [Google Scholar]
- 68.Stone SF, Price P, Tay-Kearney ML, French MA. Cytomegalovirus (CMV) retinitis immune restoration disease occurs during highly active antiretroviral therapy-induced restoration of CMV-specific immune responses within a predominant Th2 cytokine environment. J. Infect. Dis. 2002;185:1813–1817. doi: 10.1086/340636. [DOI] [PubMed] [Google Scholar]
- 69.Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tuboi SH, Schechter M, McGowan CC, et al. Mortality during the first year of potent antiretroviral therapy in HIV-1-infected patients in 7 sites throughout Latin America and the Caribbean. J. Acquir. Immune Defic. Syndr. 2009;51:615–623. doi: 10.1097/QAI.0b013e3181a44f0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chasombat S, McConnell MS, Siangphoe U, et al. National expansion of antiretroviral treatment in Thailand, 2000–2007: program scale-up and patient outcomes. J. Acquir. Immune Defic. Syndr. 2009;50:506–512. doi: 10.1097/QAI.0b013e3181967602. [DOI] [PubMed] [Google Scholar]
- 73.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services; USA: 2011. pp. 1–166. [Google Scholar]
- 74.Keiser O, Anastos K, Schechter M, et al. Antiretroviral therapy in resource-limited settings 1996 to 2006: patient characteristics, treatment regimens and monitoring in sub-Saharan Africa, Asia and Latin America. Trop. Med. Int. Health. 2008;13:870–879. doi: 10.1111/j.1365-3156.2008.02078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boulle A, Van CG, Hilderbrand K, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24:563–572. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 76.Nglazi M, Lawn SD, Kaplan R, et al. Changes in programmatic outcomes during 7 years of scale-up at a community-based antiretroviral treatment service in South Africa. J. Acquir. Immune Defic. Syndr. 2011;56:e1–e8. doi: 10.1097/QAI.0b013e3181ff0bdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Battegay M, Fluckiger U, Hirschel B, Furrer H. Late presentation of HIV-infected individuals. Antivir. Ther. 2007;12:841–851. [PubMed] [Google Scholar]
- 78.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV-infected persons. Cochrane Database Syst. Rev. 2004 doi: 10.1002/14651858.CD000171.pub3. DOI: 10.1002/14651858.CD000171.pub2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–1448. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Golub JE, Pronyk P, Mohapi L, et al. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS. 2009;23:631–636. doi: 10.1097/QAD.0b013e328327964f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lawn SD, Wood R, De Cock KM, Kranzer K, Lewis JJ, Churchyard GJ. Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources. Lancet Infect. Dis. 2010;10:489–498. doi: 10.1016/S1473-3099(10)70078-5. [DOI] [PubMed] [Google Scholar]
- 82.World Health Organization . Treatment of Tuberculosis. Guidelines for National Programmes. 3rd Edition. WHO; Geneva, Switzerland: 2003. WHO/CDS/TB 2003.313. [Google Scholar]
- 83.Getahun H. Meta-analysis to inform the development of a standardised approach for TB screening in HIV-infected patients; Prsented at: 40th Union World Conference on Lung Health; Cancun, Mexico. 3-7 December 2009; Symposium 2. [Google Scholar]
- 84.Cain KP, McCarthy KD, Heilig CM, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. N. Engl. J. Med. 2010;362:707–716. doi: 10.1056/NEJMoa0907488. [DOI] [PubMed] [Google Scholar]
- 85.Reid MJ, Shah NS. Approaches to tuberculosis screening and diagnosis in people with HIV in resource-limited settings. Lancet Infect. Dis. 2009;9:173–184. doi: 10.1016/S1473-3099(09)70043-X. [DOI] [PubMed] [Google Scholar]
- 86.Getahun H, Kittikraisak W, Heilig CM, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8(1):e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang LW, Phipps WT, Kennedy GE, Rutherford GW. Antifungal interventions for the primary prevention of cryptococcal disease in adults with HIV. Cochrane Database Syst Rev. 2005;3:CD004773. doi: 10.1002/14651858.CD004773.pub2. [DOI] [PubMed] [Google Scholar]
- 88.Feldmesser M, Harris C, Reichberg S, Khan S, Casadevall A. Serum cryptococcal antigen in patients with AIDS. Clin. Infect. Dis. 1996;23:827–830. doi: 10.1093/clinids/23.4.827. [DOI] [PubMed] [Google Scholar]
- 89.Scharfstein JA, Paltiel AD, Freedberg KA. The cost–effectiveness of fluconazole prophylaxis against primary systemic fungal infections in AIDS patients. Med. Decis. Making. 1997;17:373–381. doi: 10.1177/0272989X9701700402. [DOI] [PubMed] [Google Scholar]
- 90.Yazdanpanah Y, Goldie SJ, Paltiel AD, et al. Prevention of human immunodeficiency virus-related opportunistic infections in France: a cost–effectiveness analysis. Clin. Infect. Dis. 2003;36:86–96. doi: 10.1086/344902. [DOI] [PubMed] [Google Scholar]
- 91.Madec Y, Laureillard D, Pinoges L, et al. Response to highly active antiretroviral therapy among severely immuno-compromised HIV-infected patients in Cambodia. AIDS. 2007;21:351–359. doi: 10.1097/QAD.0b013e328012c54f. [DOI] [PubMed] [Google Scholar]
- 92.Manosuthi W, Sungkanuparph S, Thongyen S, et al. Antifungal susceptibilities of Cryptococcus neoformans cerebrospinal fluid isolates and clinical outcomes of cryptococcal meningitis in HIV-infected patients with/without fluconazole prophylaxis. J. Med. Assoc. Thai. 2006;89:795–802. [PubMed] [Google Scholar]
- 93.Apisarnthanarak A, Mundy LM. The impact of primary prophylaxis for cryptococcosis on fluconazole resistance in Candida species. J. Acquir. Immune Defic. Syndr. 2008;47:644–645. doi: 10.1097/QAI.0b013e31815bad0c. [DOI] [PubMed] [Google Scholar]
- 94.Parkes-Ratanshi R, Kamali A, Wakeham K, et al. Successful primary prevention of cryptococcal disease using fluconazole prophylaxis in HIV-infected Ugandan adults; Program and Abstracts of the 16th Conference on Retroviruses and Opportunistic Infections (CROI); Montreal, Canada. 2009; Abstract 32. [Google Scholar]
- 95.Jarvis JN, Lawn SD, Vogt M, Bangani N, Wood R, Harrison TS. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin. Infect. Dis. 2009;48:856–862. doi: 10.1086/597262. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Investigators retrospectively analyzed stored plasma samples of patients starting on ART in South Africa for cryptococcal antigen: 7% were positive and antigenemia was 100% sensitive for predicting cryptococcal meningitis during ART. Routine antigen screening in patients with CD4 cell counts ≤100 cells/μl and pre-emptive therapy may be an effective way to prevent incident cryptococcal disease and associated IRD during ART.
- 96.Meya DB, Manabe YC, Castelnuovo B, et al. Cost–effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count 100 cells/μl who start HIV therapy in resource-limited settings. Clin. Infect. Dis. 2010;51:448–455. doi: 10.1086/655143. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Study conducted in Uganda; from study data and cost–effectiveness calculations serum cryptococcal antigen screening followed by pre-emptive therapy was found to be a cost-effective strategy for preventing cryptococcal disease in patients with advanced HIV.
- 97.Jarvis JN, Meintjes G, Williams Z, Rebe K, Harrison TS. Symptomatic relapse of HIV-associated cryptococcal meningitis in South Africa: the role of inadequate secondary prophylaxis. S. Afr. Med. J. 2010;100:378–382. doi: 10.7196/samj.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 99.Lawn SD, Bekker LG, Myer L, Orrell C, Wood R. Cryptococcocal immune reconstitution disease: a major cause of early mortality in a South African antiretroviral programme. AIDS. 2005;19:2050–2052. doi: 10.1097/01.aids.0000191232.16111.f9. [DOI] [PubMed] [Google Scholar]
- 100.Lawn SD, Wilkinson RJ, Lipman MC, Wood R. Immune reconstitution and “unmasking” of tuberculosis during antiretroviral therapy. Am. J. Respir. Crit. Care. Med. 2008;177:680–685. doi: 10.1164/rccm.200709-1311PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goldsack NR, Allen S, Lipman MC. Adult respiratory distress syndrome as a severe immune reconstitution disease following the commencement of highly active antiretroviral therapy. Sex. Transm. Infect. 2003;79:337–338. doi: 10.1136/sti.79.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kranzer K, Houben RM, Glynn JR, Bekker LG, Wood R, Lawn SD. Yield of HIV-associated tuberculosis during intensified case finding in resource-limited settings: a systematic review and meta-analysis. Lancet Infect. Dis. 2010;10:93–102. doi: 10.1016/S1473-3099(09)70326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bassett IV, Wang B, Chetty S, et al. Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clin. Infect. Dis. 2010;51:823–829. doi: 10.1086/656282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS. 2009;23:1717–1725. doi: 10.1097/QAD.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The proportion of TB cases presenting during the first 4 months of ART that is due to unmasking TB is unknown. Epidemiological analyses in this study suggest that under routine program conditions, this was approximately 40% in this cohort. These data were further substantiated by TB screening studies (see [109]).
- 105.Lawn SD, Kranzer K, Edwards DJ, McNally M, Bekker LG, Wood R. Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. AIDS. 2010;24:1323–1328. doi: 10.1097/QAD.0b013e3283390dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A study in an ART program in South Africa that demonstrated intensified screening with sputum TB culture in all patients was associated with an approximately twofold lower incidence of TB in the first 4 months of ART compared with historical data from the same cohort. This suggests that much unmasking TB can be prevented by effective baseline TB screening.
- 106.Peter J, Green C, Hoelscher M, Mwaba P, Zumla A, Dheda K. Urine for the diagnosis of tuberculosis: current approaches, clinical applicability, and new developments. Curr. Opin. Pulm. Med. 2010;16:262–270. doi: 10.1097/MCP.0b013e328337f23a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dheda K, Davids V, Lenders L, et al. Clinical utility of a commercial LAM-ELISA assay for TB diagnosis in HIV-infected patients using urine and sputum samples. PLoS ONE. 2010;5:e9848. doi: 10.1371/journal.pone.0009848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pai M, Minion J, Sohn H, Zwerling A, Perkins MD. Novel and improved technologies for tuberculosis diagnosis: progress and challenges. Clin. Chest Med. 2009;30:701–716. doi: 10.1016/j.ccm.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 109.Helb D, Jones M, Story E, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J. Clin. Microbiol. 2010;48:229–237. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N. Engl. J. Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lawn SD, Wood R. Tuberculosis screening in patients starting antiretroviral therapy: stretching diagnostics to the limits. Clin. Infect. Dis. 2011;52:276–277. doi: 10.1093/cid/ciq128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liechty CA, Solberg P, Were W, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop. Med. Int. Health. 2007;12:929–935. doi: 10.1111/j.1365-3156.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- 113.Karavellas MP, Plummer DJ, Macdonald JC, et al. Incidence of immune recovery vitritis in cytomegalovirus retinitis patients following institution of successful highly active antiretroviral therapy. J. Infect. Dis. 1999;179:697–700. doi: 10.1086/314639. [DOI] [PubMed] [Google Scholar]
- 114.Nguyen QD, Kempen JH, Bolton SG, Dunn JP, Jabs DA. Immune recovery uveitis in patients with AIDS and cytomegalovirus retinitis after highly active antiretroviral therapy. Am. J. Ophthalmol. 2000;129:634–639. doi: 10.1016/s0002-9394(00)00356-1. [DOI] [PubMed] [Google Scholar]
- 115.Heiden D, Ford N, Wilson D, et al. Cytomegalovirus retinitis: the neglected disease of the AIDS pandemic. PLoS Med. 2007;4:e334. doi: 10.1371/journal.pmed.0040334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pathai S, Deshpande A, Gilbert C, Lawn SD. Prevalence of HIV-associated ophthalmic disease among patients enrolling for antiretroviral treatment in India: a cross-sectional study. BMC Infect. Dis. 2009;9:158. doi: 10.1186/1471-2334-9-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Meintjes G, Rangaka MX, Maartens G, et al. Novel relationship between tuberculosis immune reconstitution inflammatory syndrome and antitubercular drug resistance. Clin. Infect. Dis. 2009;48:667–676. doi: 10.1086/596764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. AIDS. 2007;21:2119–2129. doi: 10.1097/QAD.0b013e3282a4a64d. [DOI] [PubMed] [Google Scholar]
- 119.Brouwer AE, Rajanuwong A, Chierakul W, et al. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet. 2004;363:1764–1767. doi: 10.1016/S0140-6736(04)16301-0. [DOI] [PubMed] [Google Scholar]
- 120.Bicanic T, Meintjes G, Wood R, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin. Infect. Dis. 2007;45:76–80. doi: 10.1086/518607. [DOI] [PubMed] [Google Scholar]
- 121.Makadzange AT, Ndhlovu CE, Takarinda K, et al. Early versus delayed initiation of antiretroviral therapy for concurrent HIV infection and cryptococcal meningitis in sub-saharan Africa. Clin. Infect. Dis. 2010;50:1532–1538. doi: 10.1086/652652. [DOI] [PubMed] [Google Scholar]
- 122.Grant PM, Aberg JA, Zolopa AR. Concerns regarding a randomized study of the timing of antiretroviral therapy in Zimbabweans with AIDS and acute cryptococcal meningitis. Clin. Infect. Dis. 2010;51:984–985. doi: 10.1086/656435. [DOI] [PubMed] [Google Scholar]
- 123.Boulware DR. Safety, censoring, and intent-to-treat analysis: dangers to generalizability. Clin. Infect. Dis. 2010;51:985–986. doi: 10.1086/656436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brickelmaier M, Lugovskoy A, Kartikeyan R, et al. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob. Agents Chemother. 2009;53:1840–1849. doi: 10.1128/AAC.01614-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gofton TE, Al-Khotani A, O’Farrell B, Ang LC, McLachlan RS. Mefloquine in the treatment of progressive multifocal leukoencephalopathy. J. Neurol. Neurosurg. Psychiatry. 2010 doi: 10.1136/jnnp.2009.190652. DOI:10.1136/jnnp.2009.190652. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 126.Bassett IV, Wang B, Chetty S, et al. Loss to care and death before antiretroviral therapy in Durban, South Africa. J. Acquir. Immune Defic. Syndr. 2009;51:135–139. doi: 10.1097/qai.0b013e3181a44ef2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lawn SD, Torok ME, Wood R. Optimum time to start antiretroviral therapy during HIV-associated opportunistic infections. Curr. Opin. Infect. Dis. 2010;24(1):34–42. doi: 10.1097/QCO.0b013e3283420f76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zolopa A, Andersen J, Powderly W, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS ONE. 2009;4:e5575. doi: 10.1371/journal.pone.0005575. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The first published strategy trial to assess the optimum time to start ART in patients diagnosed with opportunistic or bacterial infections. Mortality/AIDS progression was reduced in the early ART arm (within 2 weeks of diagnosing opportunistic or bacterial infection).
- 129.Torok ME, Yen NTB, Chau TTH, et al. Randomised controlled trial of immediate versus deferred antiretroviral therapy in HIV-associated tuberculous meningitis; Presented at: 49th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); San Francisco, CA, USA. 12-15 September (2009); Abstract H-1224. [Google Scholar]; • Randomized double-blind trial that demonstrated that in HIV-infected patients with TB meningitis starting ART, at the start of TB treatment or deferring 2 months did not result in differential mortality rates. IRD was not ascertained in this study.
- 130.Marais S, Wilkinson RJ, Pepper DJ, Meintjes G. Management of patients with the immune reconstitution inflammatory syndrome. Curr. HIV/AIDS Rep. 2009;6:162–171. doi: 10.1007/s11904-009-0022-z. [DOI] [PubMed] [Google Scholar]
- 131.Meintjes G, Wilkinson RJ, Morroni C, et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2010;24:2381–2390. doi: 10.1097/QAD.0b013e32833dfc68. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Randomized placebo-controlled trial of prednisone for the treatment of patients with paradoxical TB-IRD. The trial demonstrated that a 4-week course of prednisone reduced morbidity and led to more rapid improvement in symptoms. This is the first clinical trial to study treatment strategies for IRD.
- 132.Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N. Engl. J. Med. 2004;351:1741–1751. doi: 10.1056/NEJMoa040573. [DOI] [PubMed] [Google Scholar]
- 133.Funderburg N, Kalinowska M, Eason J, et al. Effects of maraviroc and efavirenz on markers of immune activation and inflammation and associations with CD4+ cell rises in HIV-infected patients. PLoS ONE. 2010;5:e13188. doi: 10.1371/journal.pone.0013188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Turner JE, Steinmetz OM, Stahl RA, Panzer U. Targeting of Th1-associated chemokine receptors CXCR3 and CCR5 as therapeutic strategy for inflammatory diseases. Mini Rev. Med. Chem. 2007;7:1089–1096. doi: 10.2174/138955707782331768. [DOI] [PubMed] [Google Scholar]
- 135.Martin-Blondel G, Cuzin L, Delobel P, et al. Is maraviroc beneficial in paradoxical progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome management? AIDS. 2009;23:2545–2546. doi: 10.1097/QAD.0b013e32833365f4. [DOI] [PubMed] [Google Scholar]
- 136.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr. Opin. Pharmacol. 2010;10:482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 137.Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat. Rev. Immunol. 2006;6:358–370. doi: 10.1038/nri1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sun HY, Singh N. Potential role of statins for the management of immune reconstitution syndrome. Med. Hypotheses. 2010;76(3):307–310. doi: 10.1016/j.mehy.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 139.Elliott AM, Halwiindi B, Bagshawe A, et al. Use of prednisolone in the treatment of HIV-positive tuberculosis patients. Q. J. Med. 1992;85:855–860. [PubMed] [Google Scholar]
- 140.Elliott AM, Luzze H, Quigley MA, et al. A randomized, double-blind, placebo-controlled trial of the use of prednisolone as an adjunct to treatment in HIV-1-associated pleural tuberculosis. J. Infect. Dis. 2004;190:869–878. doi: 10.1086/422257. [DOI] [PubMed] [Google Scholar]
- 141.Volkow PF, Cornejo P, Zinser JW, Ormsby CE, Reyes-Teran G. Life-threatening exacerbation of Kaposi’s sarcoma after prednisone treatment for immune reconstitution inflammatory syndrome. AIDS. 2008;22:663–665. doi: 10.1097/QAD.0b013e3282f4f223. [DOI] [PubMed] [Google Scholar]
Websites
- 201.WHO Scaling up antiretroviral therapy in resource-limited settings: guidelines for a public health approach. 2002 www.who.int/hiv/pub/prev_care/en/ScalingUp_E.pdf. [PubMed]
- 202.WHO Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a publc health approach. 2010 revision. www.who.int/hiv/pub/arv/adult/en/index.html. [PubMed]
- 203.WHO Global tuberculosis control. A short update to the 2009 report. www.who.int/tb/publications/global_report/2009/update/tbu_9.pdf.
- 204.WHO Improving the diagnosis and treatment of smear-negative pulmonary and extra-pulmonary tuberculosis among adults and adolescents. Recommendations for HIV-prevalent and resource-constrained settings. http://whqlibdoc.who.int/hq/2007/WHO_HTM_TB_2007.379_eng.pdf.
- 205.Study to explore the effect of mefloquine in subjects with progressive multifocal leukoencephalopathy (PML) http://clinicaltrials.gov/ct2/show/NCT00746941.
- 206.Maraviroc (CCR5) antagonism to decrease the incidence of the immune reconstitution inflammatory syndrome in HIV-infected patients (CADIRIS) http://clinicaltrials.gov/ct2/show/NCT00988780.