Abstract
The status of glycoconjugates (protein bound hexose, hexosamine, sialic acid and fucose) in plasma or serum serve as potential biomarkers for assessing tumor progression and therapeutic interventions. Aim of the present study was to investigate the protective effect of two major soy isoflavones, genistein and daidzein, in combination on the status of glycoconjugates in plasma, erythrocyte membrane and mammary tissues during 7,12-dimethylbenz[a]anthracene (DMBA) induced mammary carcinogenesis in female Sprague-Dawley rats. A single subcutaneous injection of DMBA (25 mg rat−1) in the mammary gland developed mammary carcinoma in female Sprague-Dawley rats. Elevated levels of plasma and mammary tissue glycoconjugates accompanied by reduction in erythrocyte membrane glycoconjugates were observed in rats bearing mammary tumors. Oral administration of genistein + daidzein (20 mg + 20 mg kg−1 bw/day) to DMBA treated rats significantly (p< 0.05) brought back the status of glycoconjugates to near normal range. The present study thus demonstrated that genistein and daidzein in combination protected the structural integrity of the cell surface and membranes during DMBA-induced mammary carcinogenesis.
Keywords: Mammary carcinoma, Glycoconjugates, Membrane integrity, Genistein, Daidzein, DMBA
Introduction
Glycosylation of proteins has been shown to have significant impact on protein function and confirmation (Yang and Hancock, 2004). Hexoses, fucose and sialic acid form the monosaccharide units of the oligosaccharides that are attached to proteins. More than half of all proteins in human serum are glycosylated (Apweiler et al., 1999). Measurement of serum glycoconjugates serve as a potential source of disease biomarkers and provides insights into disease pathogenesis (Dube and Bertozzi, 2005). Altered glycoconjugates levels were reported in many pathological conditions including cancer (Narayana, 1994).
The sialo-glycoconjugates that are expressed on the plasma membrane reflects the surface properties of either normal or tumor cells (McDonagh and Nathan, 1990). Sialic acid-rich glycoconjugates are over expressed in tumor cells and was correlated with stages and metastatic potential of tumor cells (Manoharan et al., 2004). Fucose, one of the eight essential sugars in the body, is essential for optimum cell-to-cell communication (Listinsky et al., 2001). Fucose and mannose have prominent role in slowing the growth of cancer cells (Rao et al., 1998).
Breast cancer is the most common neoplasm amongst women worldwide (Jemal et al., 2009). Around 1.5 million new cases and 400, 000 deaths due to breast cancer are reported every year (Hortobagyi et al., 2005). 7,12-dimethylbenz[a]anthracene (DMBA), a potent organ-specific carcinogen, is commonly used for the induction of mammary cancer in female Sprague-Dawley rats. Altered glycosylation pattern of proteins has been reported in DMBA- induced mammary carcinogenesis (Ramprasath et al., 2007).
Genistein and daidzein, the two major isoflavones of soybeans, have been the focus of recent research due to their promising role in cancer prevention. Diverse pharmacological and biochemical effects of genistein and daidzein including anticancer potential have been well documented (Sarkar and Li, 2002: Mishra et al., 2009). Genistein and daidzein act as anticancer agents in part by their ability to scavenge reactive oxygen species generated during carcinogenesis (Ruiz-Larrea et al., 1997: Ruffer and Kulling, 2006). Previous study from our laboratory has demonstrated the chemopreventive potential of genistein and daidzein, in combination, during DMBA-induced mammary carcinogenesis. Genistein and daidzein, in combination, significantly (80%) prevented tumor formation in rats treated with DMBA (Pugalendhi and Manoharan, 2010). The present study demonstrates the protective effect of genistein and daidzein, in combination, on cellular integrity during DMBA-induced mammary carcinogenesis in Sprague-Dawley rats
Materials and Methods
Forty female Sprague-Dawley rats six weeks old were obtained from National Institute of Nutrition, Hyderabad and maintained in the Central Animal House, Rajah Muthiah Medical College and Hospital, Annamalai University. The experimental design (Proposal No. 578 dated. 25.07.2008) was approved by the Annamalai University animal ethical committee (Register number 160/1999/CPCSEA), Annamalainagar. The rats were housed in polypropylene cages at room temperature (27 ± 2°C) with relative humidity 55 ± 5%, in an experimental room. In Annamalainagar, the LD (light: dark) cycle is almost 12:12h. The rats were maintained as per the principles and guidelines of the ethical committee for animal care of Annamalai University in accordance with the Indian National Law on animal care and use. The rats were provided with standard pellet diet (Amrut Laboratory Animal Feed, Mysore Feeds Limited, Bangalore, India) and water ad libitum.
Chemicals
Genistein and daidzein were purchased from Shaanxi Sciphar Biotechnology Co. Ltd, China. DMBA was obtained from Sigma-Aldrich Chemicals Pvt. Ltd., Bangalore, India. Other chemicals and solvents used were of analar grade.
Induction of mammary carcinogenesis
Mammary carcinogenesis was induced in Sprague-Dawley rats using a single subcutaneous injection of 25 mg of DMBA in 1 ml emulsion of sunflower oil (0.75 ml) and physiological saline (0.25 ml) to each rat (Kolanjiappan and Manoharan, 2005).
Experimental Design
Forty rats were divided into four groups of ten rats per group. Group 1 rats received the excipient (single dose of 1 ml of emulsion of sunflower oil and physiological saline, s.c) and 1 ml of 2% DMSO (p.o) throughout the experimental period and served as vehicle treated control. Rats in groups 2 and 3 were induced mammary carcinogenesis by providing single subcutaneous injection of 25 mg of DMBA in 1 ml of sunflower oil and physiological saline. Group 2 rats received no other treatment. Group 3 rats were orally administered with genistein + daidzein (20 mg + 20 mg kg−1 bw/day, dissolved in 2% DMSO) starting one week before the exposure of the carcinogen and continued till the experimental period. Group 4 rats were orally administered with genistein + daidzein (20 mg + 20 mg kg−1 bw/day, dissolved in 2% DMSO) alone throughout the experimental study. The experiment was terminated at 16th week and all rats were sacrificed by cervical dislocation. Plasma and processed tissues samples were used for various biochemical estimations.
Biochemical estimations
Blood samples were collected into heparinized tubes. Plasma was separated by centrifugation at 1000 × g for 15 min. Tissue samples from rats were washed with ice cold saline and dried between folds of filter paper, weighed and homogenized using Tris-HCl buffer (0.1 M, pH 7.4) and used for biochemical estimations. The precipitate obtained after treating the plasma with 95% ethanol was used for the estimation of protein bound hexose and hexosamine. After plasma separation, the erythrocyte membrane was isolated by the method of Dodge et al., (1968) modified by Quist (1980). Similarly, the precipitate obtained after treating the mammary tissues and erythrocyte membranes with 1% phosphotungstic acid followed by 5% TCA was used for the estimation of protein found hexose and hexosamine. The protein bound hexose, hexosamine, total sialic acid and fucose were estimated by the methods of Niebes (1972), Wagner (1979), Warren (1959) and Dische and Shettles (1948) respectively. Plasma lipid bound sialic acid level was determined by the method of Katopodis and Stock (1980).
Periodic acid-Schiff (PAS) staining
For histopathological studies, tumor and normal mammary tissues were fixed in 10% formalin and were routinely processed and paraffin embedded 2–3 µm sections were cut in a rotary microtome. The slides containing tissue sections were immersed in a solution of 0.5% periodic acid for 10 min at 56°C. The slides were washed in running tap water and immersed in Schiff's reagent for 40 min. Subsequently, the sections were washed in running tap water for 10 min, counterstained with hematoxylin, dehydrated in graded ethanol, cleared in xylene and mounted in resinous medium.
Results
The levels of protein bound hexose, hexosamine, total sialic acid, lipid bound sialic acid and fucose in plasma and mammary tissues of control and experimental rats in each group is shown in table 1 and 2 respectively. The levels of glycoconjugates were significantly increased in tumor bearing rats as compared to control rats. Oral administration of genistein + daidzein to DMBA-induced mammary cancer rat significantly reduced the levels of glycoconjugates. Rats treated with genistein + daidzein alone showed no significant difference as compared to control rats.
Table 1.
Protein bound hexose, hexosamine, total sialic acid, lipid bound sialic acid and fucose in plasma of control and experimental rats.
| Groups | Protein bound hexose (mg/dl) |
Protein bound hexosamine (mg/dl) |
Total sialic acid (mg/dl) |
Lipid bound sialic acid (mg/dl) |
Fucose (mg/dl) |
| Control (Vehicle) |
104.8±10.2a | 65.40±6.0a | 41.80±4.0a | 12.2±1.3a | 12.64±0.98a |
| DMBA | 135.5±12.62b | 94.61±8.8b | 67.40±7.1b | 23.4±2.4b | 21.50±1.9b |
| DMBA + Genistein + Daidzein | 116.4±10.1c | 73.2±7.9c | 47.1±4.8c | 13.9±1.5c | 14.10±1.6c |
| Genistein+ Daidzein alone | 103.5±10.4a | 65.10±6.5a | 41.3±4.1a | 12.2±1.2a | 12.55±1.0a |
Values are expressed as mean ± SD for 10 rats in each group. Values that are not sharing a common superscript letter in the same column differ significantly differ at p < 0.05 (DMRT).
Table 2.
Protein bound hexose, hexosamine, total sialic acid levels in mammary tissues of control and experimental rats.
| Groups | Protein bound hexose (mg/ g protein) |
Protein bound hexosamine (mg/ g protein) |
Total sialic acid (mg/ g protein) |
||
| Control (Vehicle) |
3.08±0.25a | 2.10±0.21a | 1.55±0.12a | ||
| DMBA | 5.24±0.51b | 4.14±0.34b | 3.10±0.29b | ||
| DMBA+ Genistein+ Daidzein |
3.40±0.35c | 2.36±0.24c | 1.74±0.18c | ||
| Genistein+ Daidzein alone |
3.08±0.28a | 2.09±0.20a | 1.54±0.14a | ||
Values are expressed as mean ± SD for 10 rats in each group. Values that are not sharing a common superscript letter in the same column differ significantly differ at p < 0.05 (DMRT).
The levels of protein bound hexose, hexosamine, and total sialic acid, in erythrocyte membranes of control and experimental rats in each group is shown in table 3. The levels of protein bound hexose, hexosamine, and total sialic acid were significantly decreased in erythrocyte membranes of tumor bearing rats as compared to control rats. Oral administration of genistein + daidzein to DMBA-induced mammary cancer rats significantly increased the status of glycoconjugates in erythrocyte membranes. Rats treated with genistein + daidzein alone showed no significant difference as compared to control rats.
Table 3.
Protein bound hexose, hexosamine, total sialic acid levels in erythrocyte membranes of control and experimental rats.
| Groups | Protein bound hexose (µg/mg protein) |
Protein bound hexosamine (µg/mg protein) |
Total sialic acid (µg/mg protein) |
||
| Control (Vehicle) |
106.8±10.24a | 70.14±6.2a | 24.04±2.1a | ||
| DMBA | 82.5±9.10b | 53.42±2.7b | 16.18±1.7b | ||
| DMBA+ Genistein+ Daidzein |
96.2±9.26c | 63.20±5.9c | 21.65±2.0c | ||
| Genistein+ Daidzein alone |
107.1±10.50a | 71.06±6.1a | 24.10±2.2a | ||
Values are expressed as mean ± SD for 10 rats in each group. Values that are not sharing a common superscript letter in the same column differ significantly differ at p < 0.05 (DMRT).
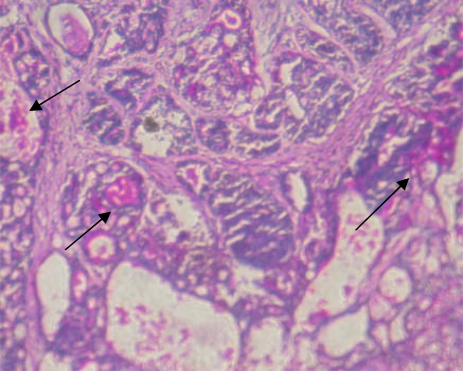
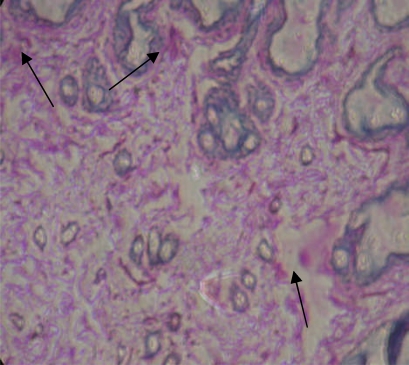
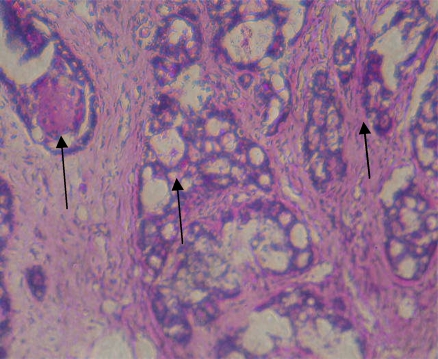
The glycoconjugates expression pattern using Periodic acid-Schiff (PAS) staining is given in fig 1 (a–c). Over expression of glycoconjugates was observed in the mammary tissues of tumor bearing rats (fig 1b) as compared to control rats (fig 1a). Oral administration of genistein + daidzein to DMBA-induced mammary cancer rats decreased the expression of glycoconjugates (fig 1c). Rats treated with genistein + daidzein alone showed similar pattern of expression as compared to control rats.
Figure 1b).
Over expression of glycoconjugates in the mammary tissues of tumor bearing rats
Figure 1a).
Glycoconjugates expression pattern in mammary tissues of the control rats
Figure 1c).
Decreased expression of glycoconjugates in DMBA-induced mammary cancer rats treated with genistein + daidzein.
Statistical analysis
The values are expressed as mean ± SD. The statistical comparisons were performed by one way analysis of variance (ANOVA) followed by Duncan's multiple range test (DMRT), using SPSS version 12.0 for windows. The values are considered statistically significant if the p value was less than 0.05.
Discussion
Breast cancer incidence is low in Asian populations than their American counterparts; this has been linked with high soy consumption. Soy isoflavones, genistein and daidzein have strong antioxidant activity (Guo et al., 2002). Combination of isoflavones including genistein and daidzein has more pronounced inhibitory effects on breast cancer cell proliferation (Verma and Goldin, 1998). Flavonoids and isoflavonoids have great potential to modulate the levels of glycoproteins (Elangovan et al., 1994).
Altered cell surface glycosylation plays crucial role in malignant transformation. Aberrant glycosylation is also involved in the key steps of neoplastic progression including tumor invasion and evasion of host immunosurveillance (Hakomori, 2002). Cell surface glycosylation could be used as a source of biomarkers as well as targets for immunotherapy of tumors (Kobata and Amano, 2005). Ramprasath et al (2007) demonstrated that the levels of hexose and hexosamine were elevated in DMBA-induced mammary carcinogenesis. Increase in plasma protein-bound hexose, hexosamine and total sialic acid were reported in cancer patients as well as DMBA-induced experimental carcinogenesis (Patel et al., 1989: Manoharan et al., 2009).
It has been suggested that alterations may occur in cell surface and cell membrane glycoproteins, glycolipids, and enzymic components that synthesize or degrade complex membrane carbohydrate molecules during neoplastic transformation (Nachbar et al., 1974). In the malignant tumors of the breast, the activities of glycosidases were increased 2–3 times as compared to normal tissue (Bossmann and Hall, 1974). Increased levels of glycoproteins in tumor cells are probably due to increased synthesis during carcinogenesis. Previous studies from our laboratory have reported decreased levels of erythrocyte membrane glycoconjugates in experimental and human cancers (Manoharan et al., 2004 and 2009). The depletion of erythrocyte membrane glycoconjugates could be due to increased degradation of membranes or decreased synthesis or as a result of increased shedding into circulation. Increased levels of plasma glycoprotein in tumor bearing rats could therefore be due to increased shedding from tumor tissues or erythrocytes membrane occurring during carcinogenesis.
Profound studies have demonstrated that sialic acid content was doubled in malignant tumors. Sialic acid (total, lipid bound or protein bound) levels were higher in patients with cancer as compared to normal subjects. Increased concentrations of serum and tumor tissues sialic acid were also reported in experimental carcinogenesis (Celil et al., 2003; Senthil et al, 2007; Suresh et al., 2007). Increased turnover of membrane sialoglycoproteins of tumor cell surface increase their concentration in blood. Fucose plays a significant role in cancer and its spread. Fucose status has potential applications as diagnostic and prognostic markers (Patel et al., 1994). Fucose helps to inhibit the growth of tumors. Elevated fucose content in serum and tumor tissues were reported in cancer patients and experimental cancer (Rao et al., 1998: Manoharan et al., 2009). Increased turnover of sialic acid and fucose could account for increased levels of plasma sialic acid and fucose.
Oral administration of genistein + daidzein to DMBA treated rats significantly brought back the status of glycoconjugates in plasma, erythrocyte membrane and mammary tissues. Our results thus suggest that genistein and daidzein, in combination, significantly protected the structural integrity of cell surface and membranes during DMBA-induced carcinogenesis.
References
- 1.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochem Biophys Acta. 1999;1473:4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 2.Bossmann HB, Hall TC. Enzyme activity in invasive tumours of human breast and colon. Proc Natl Acad Sci, USA. 1974;71:1833–1837. doi: 10.1073/pnas.71.5.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Celil U, Seyithan T, Fatih A, Yavuz S, Nuri B. Serum free and bound sialic acid and Alpha-1-acid glycoprotein in patients with laryngeal cancer. Annals of Clinical & Laboratory Science. 2003;33:156–159. [PubMed] [Google Scholar]
- 4.Dische L, Shettles LB. Specific color reactions of methyl pentoses and spectrophotometric micromethod for their determination. J Biol Chem. 1948;175:595–604. [PubMed] [Google Scholar]
- 5.Dodge JF, Michell G, Hanahan DJ. The preparation of hemoglobin free ghosts of human red blood cells. Arch Biochem Biophys. 1968;110:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- 6.Dube DH, Bertozzi CR. Glycans in cancer and inflammation—Potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 7.Elangovan V, Nalini R, Balasubramanian S, Sekar N, Govindasamy S. Studies on the antiproliferative effective of some naturally occurring bioflavonoidal compounds against human carcinoma of larynx and sarcoma- 180 cell lines. Indian J Pharmacol. 1994;26:266–269. [Google Scholar]
- 8.Guo Q, Rimbach G, Moini H, Weber S, Packer L. ESR and cell line culture studies on free radicalscavenging and antioxidant activities of isoflavonoids. Toxicology. 2002;179:171–180. doi: 10.1016/s0300-483x(02)00241-x. [DOI] [PubMed] [Google Scholar]
- 9.Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci U S A. 2002;99:10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hortobagyi GN, Jde SL, Pritchard K. The global breast cancer burden: variations in epidemiology and survival. Clin Breast Cancer. 2005;6:391–401. doi: 10.3816/cbc.2005.n.043. [DOI] [PubMed] [Google Scholar]
- 11.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 12.Katopodis NN, Stock CC. Improved method to determine lipid bound sialic acid in plasma. Res Commun Chem Pathol Pharmacol. 1980;30:171–180. [PubMed] [Google Scholar]
- 13.Kobata A, Amano J. Altered glycosylation of proteins produced by malignant cells, and application for the diagnosis and immunotherapy of tumours. Immunol Cell Biol. 2005;83:429–439. doi: 10.1111/j.1440-1711.2005.01351.x. [DOI] [PubMed] [Google Scholar]
- 14.Kolanjiappan K, Manoharan S. Chemopreventive efficacy and anti-lipid peroxidative potential of Jasminum grandiflorum Linn. on 7,12-dimethylbenz(a)anthracene-induced rat mammary carcinogenesis. Fund Clin Pharm. 2005;19:687–693. doi: 10.1111/j.1472-8206.2005.00376.x. [DOI] [PubMed] [Google Scholar]
- 15.Listinsky JJ, Listinsky CM, Alapati V. Cell surface fucose ablation as a therapeutic strategy for malignant neoplasms. Adv Anat Pathol. 2001;8:330–337. doi: 10.1097/00125480-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Manoharan S, Padmanabhan M, Kolanjiappan K, Ramachandran CR, Suresh K. Analysis of glycoconjugates in patients with oral squamous cell carcinoma. Clin Chim Acta. 2004;339:91–96. doi: 10.1016/j.cccn.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Manoharan S, Panjamurthy K, Pugalendhi P, Balakrishnan S, Rajalingam K, Vellaichamy L, Mary alias L. Protective role of withaferin-A on red blood cell integrity during 7,12- dimethylbenz(a)anthracene induced oral carcinogenesis. Afr J Trad CAM. 2009;6:94–102. doi: 10.4314/ajtcam.v6i1.57079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonagh JC, Nathan RD. Sialic acid and the surface charge of delayed rectifier potassium channels. J Mol Cell Cardiol. 1990;22:1305–1316. doi: 10.1016/0022-2828(90)90066-b. [DOI] [PubMed] [Google Scholar]
- 19.Mishra P, Kar A, Kale RK. Prevention of chemically induced mammary tumorigenesis by daidzein in pre-pubertal rats: the role of peroxidative damage and antioxidative enzymes. Mol Cell Biochem. 2009;325:149–157. doi: 10.1007/s11010-009-0029-1. [DOI] [PubMed] [Google Scholar]
- 20.Nachbar MS, Oppenheim JD, Aull F. Cell surface contributions to the malignant process. Am J Med Sci. 1974;268:122–138. doi: 10.1097/00000441-197409000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Narayana S. Sialic acid as a tumor marker. Ann Clin Lab Sci. 1994;24:376–384. [PubMed] [Google Scholar]
- 22.Niebes P. Determination of enzymes and degradation product of glycosaminoglycan metabolism in the serum of health and various subjects. Clin Chim Acta. 1972;42:399–408. [Google Scholar]
- 23.Patel PS, Adhvaryu SG, Balar DB, Parikh BJ, Shah PM. Clinical application of serum levels of sialic acid, fucose and seromucoid fraction as tumour markers in human leukemias. Anticancer Res. 1994;14:747–752. [PubMed] [Google Scholar]
- 24.Patel PS, Baxi BR, Balar PB. Significance of serum sialoglycoproteins in patients with lung cancer. Neoplasma. 1989;36:53–59. [PubMed] [Google Scholar]
- 25.Pugalendhi P, Manoharan S. Chemopreventive potential of genistein and daidzein in combination during 7,12-dimethylbenz(a)anthracene (DMBA) induced mammary carcinogenesis in Sprague-Dawley rats. Pak J Biol Sci. 2010;13:279–286. doi: 10.3923/pjbs.2010.279.286. [DOI] [PubMed] [Google Scholar]
- 26.Quist EE. Regulation of erythrocyte membrane shape by Ca2+ Biochem Biophys Res Commun. 1980;92:631–637. doi: 10.1016/0006-291x(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 27.Ramprasath VR, Akila G, Shanthi P, Sachdanandam P. Biochemical evaluation of glycoprotein components, Lysosomal enzymes and marker enzymes upon kalpaamruthaa administration in experimental mammary carcinoma rats. Journal of Health Sciences. 2007;53:644–654. [Google Scholar]
- 28.Rao VR, Krishnamoorthy L, Kumaraswamy SV, Ramaswamy G. Circulating levels in serum of total sialic acid, lipid-associated sialic acid and fucose in precancerous lesions and cancer of the oral cavity. Cancer Detect Prev. 1998;22:237–240. doi: 10.1046/j.1525-1500.1998.0oa04.x. [DOI] [PubMed] [Google Scholar]
- 29.Ruffer CE, Kulling SE. Antioxidant activity of isoflavones and their major metabolites using different in vitro assays. J Agric Food Chem. 2006;54:2926–2931. doi: 10.1021/jf053112o. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Larrea MB, Mohan AR, Paganga G, Miller NJ, Bolwell GP, Rice-Evans CA. Antioxidant activity of phytoestrogenic isoflavones. Free Radic Res. 1997;26:63–70. doi: 10.3109/10715769709097785. [DOI] [PubMed] [Google Scholar]
- 31.Sarkar FH, Li Y. Mechanisms of cancer chemoprevention by soy isoflavone genistein. Cancer and Metastasis Reviews. 2002;21:265–280. doi: 10.1023/a:1021210910821. [DOI] [PubMed] [Google Scholar]
- 32.Senthil N, Manoharan S, Balakrishnan S, Ramachandran CR, Muralinaidu R, Rajalingam K. Modifying effects of Piper longum on cell surface abnormalities in 7,12-dimethylbenz[a]anthracene induced hamster buccal pouch carcinogenesis. Int J Pharmacol. 2007;3:290–294. [Google Scholar]
- 33.Suresh K, Manoharan S, Panjamurthy K, Senthil N. Modifying effects of Annona squamosa on glycoconjugates levels in 7,12-dimethylbenz(a)anthracene induced hamster buccal pouch carcinogenesis. J Med Sci. 2007;7:100–105. [Google Scholar]
- 34.Verma SP, Goldin BR. Effect of soy-derived isoflavonoids on the induced growth of MCF-7cells by estrogenic environmental chemicals. Ntr Cancer. 1998;30:232–239. doi: 10.1080/01635589809514669. [DOI] [PubMed] [Google Scholar]
- 35.Wagner WD. A more sensitive assay discriminating galactosamine and glucosamine in mixture. Anal Biochem. 1979;94:369–394. doi: 10.1016/0003-2697(79)90379-8. [DOI] [PubMed] [Google Scholar]
- 36.Warren L. Thiobarbituric acid and assay of sialic acid. J Biol Chem. 1959;30:171–180. [PubMed] [Google Scholar]
- 37.Yang Z, Hancock WS. Approach to the comprehensive analysis of glycoproteins isolated from human serum using a multi-lectin affinity column. J Chromatogr A. 2004;1053:79–88. [PubMed] [Google Scholar]