Abstract
The antiprotozoal activity in vivo against Trypanosoma cruzi of (8-hydroxymethylen)-trieicosanyl acetate was evaluated in BALB/c mice during the acute phase of Chagas' disease (15 days after infection). Animals were treated during 15 days at doses of 16.8 and 33.6 µg/g, reduced parasitemia of 77.6 and 64.1% was observed respectively, in comparison with positive control mice (allopurinol 8.5 µg/g) which reduced only 29.7%. Also, amastigote nests in cardiac tissue were significant reduced in treated mice groups. The regression of effect induced after the suppression of the treatment with the compound was evaluated; animals were infected and simultaneously began the treatment with the compound during 20 days (16.8 and 33.6 µg/g). Mice were monitored after the end of the treatment for one more week. A good antitrypanosomal response was observed (66.1 and 68.9% less than untreated mice) during treatment, but 8 days after suspension of treatment, parasitemia level increased, reducing only 58.6 and 56.29 % respectively in treated animals compared with no treated.
Keywords: Chagas' disease, antiprotozoal, in vivo, Senna villosa
Résumé
L'activité in vivo contre antiprotozoaires Trypanosoma cruzi de (8-hydroxymethylen)-acétate de trieicosanyl a été évaluée chez des souris BALB / c pendant la phase aiguë de la maladie de Chagas (15 jours après l'infection). Les animaux ont été traités pendant 15 jours à des doses de 16,8 et 33,6 mg / g, la parasitémie réduite de 77,6 et 64,1% a été observée, respectivement, en comparaison avec les souris de contrôle positif (allopurinol 8,5 mg / g), qui réduit que de 29,7%. En outre, les nids amastigote dans le tissu cardiaque ont été réduits dans les groupes importants chez les souris traitées. La régression de l'effet induit après la suppression du traitement avec le composé a été évalué, les animaux ont été infectés et, simultanément, a commencé le traitement avec le composé pendant 20 jours (16,8 et 33,6 mg / g). Les souris ont été suivis après la fin du traitement pour une semaine de plus. Une bonne réponse a été observée antitrypanosomal (66,1 et 68,9% de moins que les souris non traitées) en cours de traitement, mais 8 jours après la suspension du traitement, le niveau de la parasitémie a augmenté, réduisant seulement 58,6 et 56,29% respectivement chez les animaux traités par rapport à aucun traitement.
Introduction
American trypanosomiasis (Chagas' disease) is a zoonosis caused by the hemoflagelated protozoa Trypanosoma cruzi (T. cruzi). Specific treatment had been used since around three decades ago, based in the administration of nitrofurans, mainly benznidazole or Nifurtimox, but the results obtained had been very unsatisfactory (Guedes et al., 2006). For that reason, there are scientific interests in the searching of biomolecules with antiprotozoal activity besides botanical sources. Senna villosa (Mill.) (S. villosa) it is a Leguminoceae, widely distributed in the south of Mexico. It had been previously reported their in vitro antiprotozoal properties (Guzman et al., 2004), and from the leaves was isolated the compound (8hydroxymethylen)-trieicosanyl acetate, with antiprotozoal activity in vitro against T. cruzi (3.3 and 6.6 µg/mL) (Guzman et al., 2008) and in vivo, in infected mice (8.4 and 33.6 µg/g) beginning the treatment with the compound immediately after the inoculation (Jimenez-Coello et al., 2010), however, this condition was hypothetical, because in a real scenario, a patient would begin a treatment some days after of the infection. The aim of this study was to determine antiprotozoal activity in vivo against of T. cruzi (amastigotes and trypomastigotes) during the established acute phase of the Chagas' disease and the infection development after the suppression of treatment with the evaluated compound.
Materials and Methods
Parasites and Animals
Trypomastigotes of T. cruzi strain H4 were used. Eight weeks old BALB/c mice were maintained on a 12:12 h light-dark cycle and had access to food and water ad libitum. Each mouse was IP-inoculated with 5x104 trypomastigotes of T. cruzi.
Plant Material, Extraction and isolation of (8-hydroxymethylen)-trieicosanyl acetate.
Senna villosa (voucher number 10284 authenticated by Salvador Flores-Guido) was collected from Komchen Yucatan, Mexico. Extraction procedure and isolation of the evaluated compound were done as described by Guzman et al., 2008.
In vivo antiprotozoal activity against T. cruzi during acute phase of the disease.
Mice were infected and examined every 4 days for 28 days after infection to estimate the parasitemia. Fifteen days after infection, four groups of mice (n=10 mice each) were implemented: negative control, positive control and two different doses of (8-hydroxymethylen)-trieicosanyl acetate (16.8 and 33.6 µg/g). The compound was resuspended in 50 µL of phosphate buffered saline (PBS) per mouse, and administered orally every 24 h for 13 days. Positive control group was treated with allopurinol (8.5 µg/g) and negative control only with PBS. To determine the compound activity against amastigote form, cardiac tissue samples from all mice were collected, processed and evaluated at the end of the bioassay as previously described by Jimenez-Coello et al., (2010). In a second bioassay, the regression of the effect from the treatment with the compound was evaluated, in mice groups infected (day 1) and treated (16.8 and 33.6 µg/g) from day 1 to 20 post infection, with treatment suppression at day 20 and monitoring of parasitaemia curve until day 28 post infection. Parasitemia curve, amount of amastigote nests and mortality were also recorded. All experimental bioassays were performed in triplicate and the results were compared with those of controls infected without treatment and those treated with allopurinol. Statistical analysis. Data are expressed as means ± S.E.M. and statistical analyses were performed using the Student's t-test (p<0.05), and ANOVA followed by Tukey's multiple comparison test was used to compare more than two groups.
Results
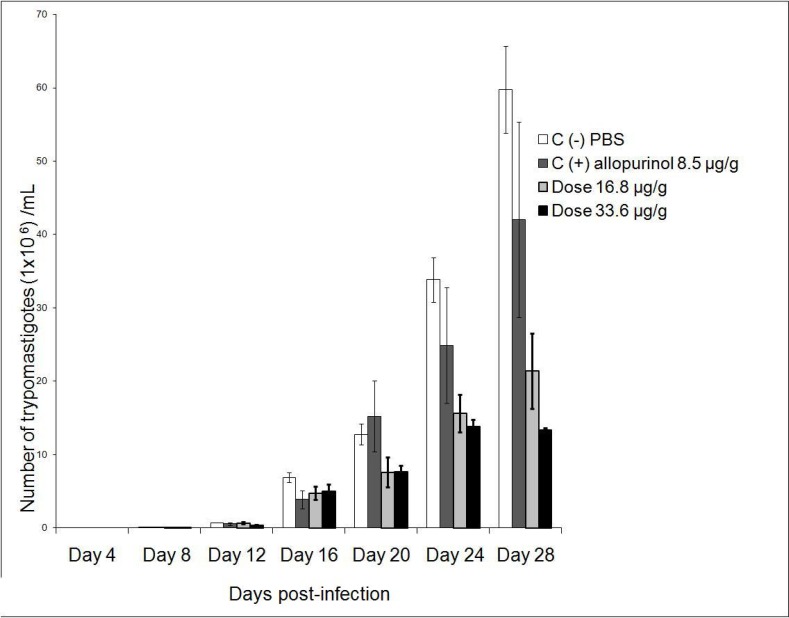
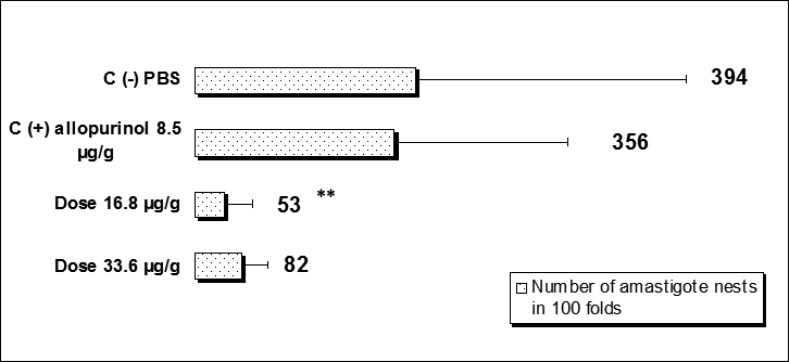
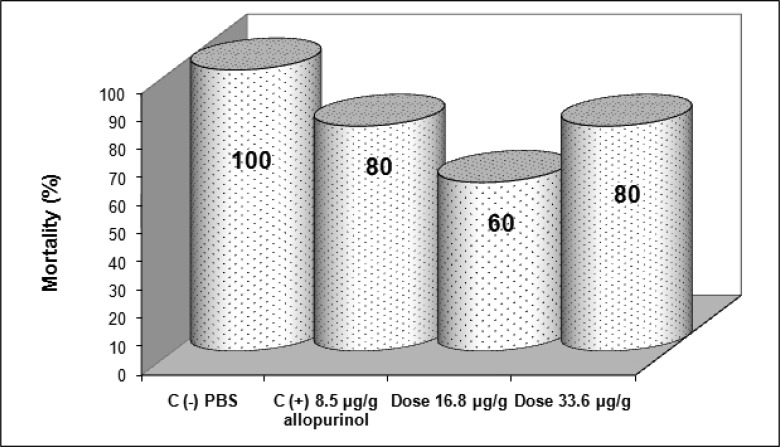
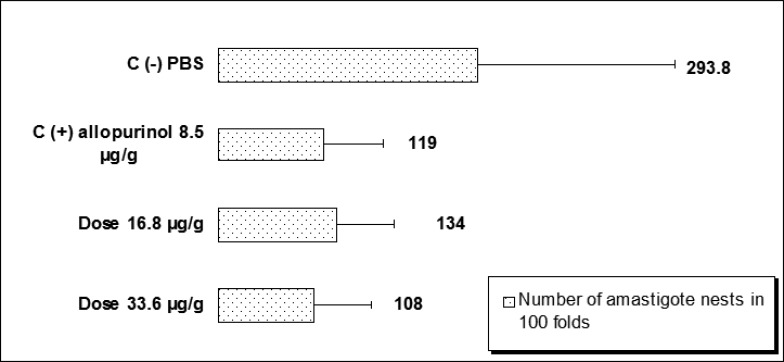
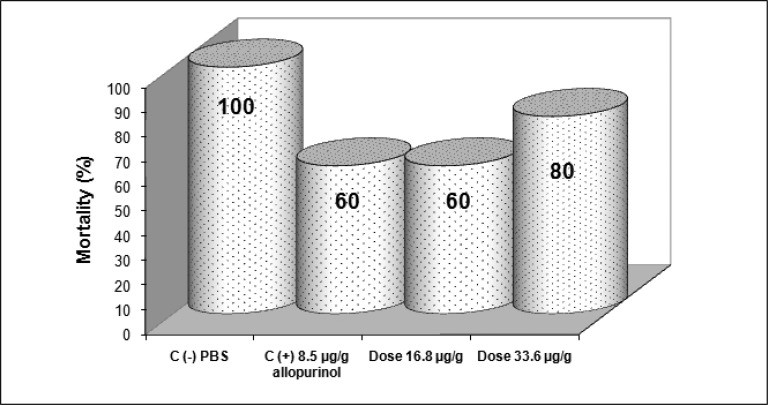
Mice treated with (8-hydroxymethylen)-trieicosanyl acetate at the concentration of 33.6 µg/g, 15 days after parasite inoculation showed the strongest antiprotozoal activity (p<0.05). However, both doses (16.8 and 33.6 µg/g) were able to diminish bloodstream parasites in 64.1 and 77.6 % respectively compared with no treated mice (negative control). On the other hand, in this same bioassay, mice treated with allopurinol at a concentration of 8.5 µg/g reduced only 29% the parasitemia (Fig 1). When evaluated the intracellular form of the parasite in heart tissue of all groups, the number of amastigote nests were significant reduced (p<0.05) in mice treated with the compound at dose 16.8 µg/g, reducing 6.6 times the amount of amastigote nests than untreated mice and 3.25 times when were compared with mice from positive control group. Also there were observed that the dose of 33.6 µg/g decreases the number of amastigote nests in comparison with the control groups but not statistically significant (Fig 2). The mortality recorded until the end of the bioassay in mice treated with the evaluated compound was minor than untreated mice and similar to showed by positive control group (Fig 3).
Figure 1.
Parasitaemia of mice BALB/c during the acute phase of the Chagasɴ disease and treated by oral route with (8-hydroxymethylen)-trieicosanyl acetate at doses 16.8 and 33.6 µg/g. and allopurinol (8.5 µg/g) during 28 days. Values are the mean + SD.
Figure 2.
Effect of (8-hydroxymethylen)-trieicosanyl acetate over the number amastigote nest observed in mice BALB/c during the acute phase of the Chagas' disease and treated at doses 16.8 and 33.6 µg/g (**p<0.05).
Figure 3.
Effect of (8-hydroxymethylen)-trieicosanyl acetate on the mortality rate of BALB/c mice after 28 days of infection with T. cruzi and treated at doses 16.8, and 33.6 µg/g.
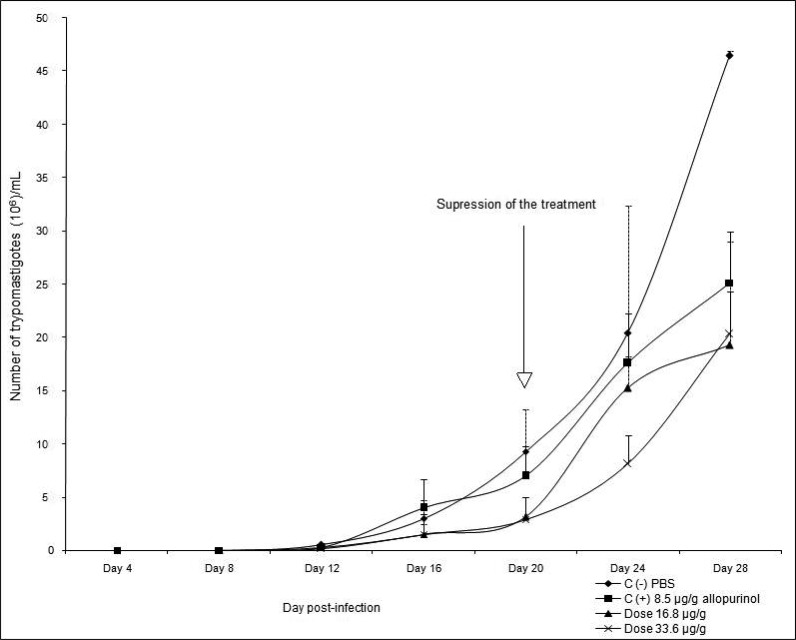
The aim of the second bioassay was to observe the parasitemia replication when the treatment was suppressed. Animals infected and treated with doses of 16.8 and 33.6 µg/g, showed a reduction in parasitemia 66.1 and 68.9% (respectively) less than untreated mice (Fig 4). When treatment was suspended, parasitemia increased in all groups. However the number of parasites observed in blood in both treated groups with the compound was 60% lower than in the untreated mice and lower than the parasitemia observed in mice treated with allopurinol. In contrast, the number of amastigote nests in mice when the treatment was suspended, in both groups treated with the evaluated compound, a 2.7 times reduction was observed when compared with untreated mice but similar to animals treated with allopurinol (Fig 5). Though mortality was lower in both groups treated with (8-hydroxymethylen)-trieicosanyl acetate than in the untreated mice, mortality was similar to the observed in the positive control group (Fig 6).
Figure 4.
Parasitaemic curve of T.cruzi BALB/c infected mice orally treated (since day 1) with (8-hydroxymethylen)-trieicosanyl acetate during 20 days from the beginning of the infection. Values are the mean + SD.
Figure 5.
Effect of (8-hydroxymethylen)-trieicosanyl acetate over the number amastigote nest observed in mice BALB/c infected with T. cruzi after only 20 days of treatment and sacrificed one week after treatment suppression.
Figure 6.
Effect of (8-hydroxymethylen)-trieicosanyl acetate on the mortality rate of BALB/c mice after 28 days of infection with T. cruzi and treated during 20 days at doses of 16.8, and 33.6 µg/g.
Discussion
The lack of alternatives for treatment of Chagas' disease is a major problem in Latin America where the disease is still present. Primarily diagnosed patients are generally treated with benznidazole and nifurtimox (now discontinued). However, in many countries like Mexico, both drugs are not available. Allopurinol has been reported as an agent with activity against blood trypomastigotes and amastigotes of Trypanosoma cruzi (Berens et al., 1982) but there are conflicting views about its effectiveness, as well as other pharmaceutical options used to treat the disease. Carignani et al., (2000) describes that under experimental conditions, allopurinol is not able to eliminate the presence of Trypanosoma cruzi in from infected triatomines. In contrast, Coura (2009) found that the use of allopurinol in infected patients may be an alternative choice (like the beznidazol) when is administered (8 mg/Kg/day) for at least for 60 days and preferably in combination with another drug with antiprotozoal activity towards Trypanosoma cruzi (as benznidazole and ketoconazole). Also Apt (1998) described that only 44% of patients treated with this same dose elimination of the parasite was observed. These results differ from the observed in this study because no complete elimination of parasitemia in infected animals treated with allopurinol was observed. In contrast, during the first 5 days after beginning of treatment (day 20 bioassay) the parasitemia was higher even than in the negative control animals, and antiprotozoal activity was observed until day 24 and 28 post infection. However, it should be noted that the duration of this trial was only 28 days and we can not compare the activity at 60 days as described by the authors mentioned. Similarly, Gobbi et al., (2007) mentioned that treatment with allopurinol 15 mg / kg in infected mice showed obvious reduction of parasitemia and absence of electrocardiographic changes. Probably because a lower dose of this drug was used in this study, there was not an entirely appropriate response towards it.
On the other hand, there are few in vivo studies evaluating the antiprotozoal activity of molecules obtained from natural products. The activity of (8-hydroxymethylen)-trieicosanyl acetate observed is similar to other studies where a reduction of the parasitemia has been observed when treatments with plant crude extracts were used, like Aderbauer et al., (2008) who studied the root extract of Securidaca longepedunculata and the leaf extract of Guiera senegalensis demonstrating than were able to reduce parasitaemia in mice, experimentally infected with Trypanosoma brucei brucei at the dose of 150 mg/kg b.w. intraperitoneally, two times daily for 3 days but only by 48 and 42% respectively for each extract. Also, Caceres et al., (1998) reported than crude extracts from Neurolaena lobata, Solanum americanum Acalypha guatemalensis, Petiveria alliacea and Tridax procumbens have an in vitro and in vivo activity against T. cruzi, but the administration to mice orally of S. americanum showed intraperitoneal subacute toxicity. In the present study the advantage of the evaluated compound is that no toxicity is observed for in vitro and in vivo studies (Guzman et al., 2004; Jimenez-Coello et al., 2010).
Results about antiprotozoal activity of (8-hydroxymethylen)-trieicosanyl acetate under in in vivo conditions is also similar with the reported by Sülsen et al., (2008); they identify and isolated a pair of compounds from the medicinal plant Ambrosia tenuifolia Sprengel (Asteraceae), but in these experiment infection in mice included 5 × 103 trypomastigotes, in contrast with this bioassay the inoculation to infect mice included 50 × 106 parasites with a highly pathogenic strain of Trypanosoma cruzi (H4), demonstring a clear antriprotozoal activity of the evaluated compound. Cunha et al., 2006, reported a triterpene isolated from Miconia species showing a good antiprotozoal activity in vivo against T. cruzi. However, the activity of those compound over the intracellurar stage of the parasite was not reported in contrast with the activity observed with (8-hydroxymethylen)-trieicosanyl acetate over the amastigote nests. Another in vivo study has been conducted to evaluate indirect derivates from plants as crude extract of brazilian green propolis, but there were used as immunostimulant, searching a better immunological response against the infection towards the parasite. However, as observed in many drugs including the (8-hydroxymethylen)-trieicosanyl acetate, the crude extract was not able to eliminate completely the parasites from bloodstream (Dantas et al., 2006).
For (8-hydroxymethylen)-trieicosanyl acetate, the best results were observed when treatment started simultaneously with the infection (Jimenez-Coello et al., 2010), and the evaluated compound showed a high antiprotozoal activity than other crude extract such as Zanthoxylum naranjillo (Bastos et al., 1999), even the amount of parasites for inoculation used in the present study was 10 times higher than in that study, with the mentioned virulent strain of T. cruzi (H4 strain). In the present study, when the compound was administrated 15 days after infection, an inhibition of the replication of the parasite was observed even when the infection was already well established. Results showed a reduction in parasitemia and a significant reduction in the number of amastigote nests in heart tissue (p<0.05), these results are difficult to compare with other studies because not many reports from crude extracts or medicinal isolated compounds includes the evaluation of developmental forms of the parasite. However, it is important to mention that the evaluated compound appears to have an effect over the replicative intracellular stage of T. cruzi.
On the other hand, results observed in mice infected and treated simultaneously (in the second bioassay), during the first 20 days of treatment, the parasitemia in treated mice with the compound was similar to those previously reported (Jimenez-Coello et al., 2010) however, it was observed that parasitemia level is increased when the administration of the compound was suppressed, demonstrating that in the treated animals with the compound a reduction in the number of amastigote nests in comparison with control groups may occurs.
It is necessary to evaluate the effectiveness of (8-hydroxymethylen)-trieicosanyl acetate during longer periods of time, during the chronic phase of the disease and maybe in combination with other antitrypanosomal drugs.
References
- 1.Aderbauer B, Clausen PH, Kershaw O, Melzig MF. In vitro and in vivo trypanocidal effect of lipophilic extracts of medicinal plants from Mali and Burkina Faso. J Ethnopharmacol. 2008;119:225–231. doi: 10.1016/j.jep.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Apt W, Aguilera X, Arribada A, Perez C, Miranda C, Sandez G, Zulantay I, Cortes P, Rodrigues J, Iuri D. Treatment of chronic Chagas disease with itraconazole and allopurinol. Am J Trop Med Hyg. 1998;59:133–138. doi: 10.4269/ajtmh.1998.59.133. [DOI] [PubMed] [Google Scholar]
- 3.Bastos JK, Albuquerque S, Silva ML. Evaluation of the trypanocidal activity of lignans isolated from the leaves of Zanthoxylum naranjillo. Planta Med. 1999;65:541–514. doi: 10.1055/s-1999-14012. [DOI] [PubMed] [Google Scholar]
- 4.Berens RL, Marr JJ, Steele da Cruz FS, Nelson DJ. Effect of allopurinol on Trypanosoma cruzi: metabolism and biological activity in intracellular and bloodstream forms. Antimicrob Agents Chemother. 1982;22:657–661. doi: 10.1128/aac.22.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cáceres A, López B, González S, Berger I, Tada I, Maki J. Plants used in Guatemala for the treatment of protozoal infections. I. Screening of activity to bacteria, fungi and American trypanosomes of 13 native plants. J Ethnopharmacol. 1998;62:195–202. doi: 10.1016/s0378-8741(98)00140-8. [DOI] [PubMed] [Google Scholar]
- 6.Carignani FL, Braz LM, Neto VA, de Souza ER. Evaluation of antiparasitic activity of allopurinol, against Trypanosoma cruzi, in experimental system using infected triatomines. Rev Soc Bras Med Trop. 2000;33:613–615. doi: 10.1590/s0037-86822000000600016. [DOI] [PubMed] [Google Scholar]
- 7.Coura JR. Present situation and new strategies for Chagas disease chemotherapy: a proposal. Mem Inst Oswaldo Cruz. 2009;104:549–554. doi: 10.1590/s0074-02762009000400002. [DOI] [PubMed] [Google Scholar]
- 8.Cunha W R, Crevelin E J, Arantes G M, Crotti A E, Andrade e Silva M L, Furtado N A, Albuquerque S, Ferreira Dda S. A study of the trypanocidal activity of triterpene acids isolated from Miconia species. Phytother Res. 2006;20:474–478. doi: 10.1002/ptr.1881. [DOI] [PubMed] [Google Scholar]
- 9.Dantas A P, Olivieri B P, Gomes F H, De .Castro S L. Treatment of Trypanosoma cruzi-infected mice with propolis promotes changes in the immune response. J Ethnopharmacol. 2006;103:187–193. doi: 10.1016/j.jep.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Gobbi P, Lo Presti M S, Fernández A R, Enders J E, Fretes R, Gea S, Paglini-Oliva P A, Rivarola H W. Allopurinol is effective to modify the evolution of Trypanosoma cruzi infection in mice. Parasitol Res. 2007;101:1459–1462. doi: 10.1007/s00436-007-0644-2. [DOI] [PubMed] [Google Scholar]
- 11.Guedes P M M, Fietto J L R, Lana M, Bahia M T. Advances in Chagas Disease Chemotherapy. Anti-Infective Agents in Medicinal Chemistry. 2006;5:175–186. [Google Scholar]
- 12.Guzman E, Perez C, Zavala M A, Acosta-Viana K Y, Perez S. Antiprotozoal activity of (8hydroxymethylen)-trieicosanyl acetate isolated from Senna villosa. Phytomedicine. 2008;15:892–895. doi: 10.1016/j.phymed.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Guzmán E S, González R, Flores S, Zavala J, Rosado M, Pérez S. Activity of Senna villosa against Trypanosoma cruzi. Pharm Biol. 2004;42:504–507. [Google Scholar]
- 14.Jimenez-Coello M, Acosta-Viana K Y, 1-Marin E S, Pérez G C, Pérez G M S. Antitrypanosomal activity of (8-hydroxymethylen)-trieicosanyl acetate against infective forms of Trypanosoma cruzi. Pharm Biol. 2010;48:666–671. doi: 10.3109/13880200903241853. [DOI] [PubMed] [Google Scholar]
- 15.Saraiva J,C, Vega M, Rolon R, da Silva M L, E Silva P M, Donate J K, Bastos, Gomez-Barrio A, de Albuquerque S. In vitro and in vivo activity of lignan lactones derivatives against Trypanosoma cruzi. Parasitol Res. 2007;100:791–795. doi: 10.1007/s00436-006-0327-4. [DOI] [PubMed] [Google Scholar]
- 16.Sülsen V P, Frank F M, Cazorla S I, Anesini C A, Malchiodi E L, Freixa B, Vila R, Muschietti L V, Martino V S. Trypanocidal and leishmanicidal activities of sesquiterpene lactones from Ambrosia tenuifolia Sprengel (Asteraceae) Antimicrob Agents Chemother. 2008;52:2415–2419. doi: 10.1128/AAC.01630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]