Abstract
Pistacia lentiscus fatty oil (PLFO) is a well known natural remedy in eastern Algeria folk medicine. It is widely used in the treatment of respiratory disorders and dermal burns. The present study has been carried out to investigate effects of this oil on fasting glucose and some functional parameters of the liver and kidney in white male New Zealand rabbits (Initial mean weight 1.95 Kg). PLFO was applied to tested rabbits (PLFO group) via rectal route, once daily 5-day per week, for six consecutive weeks at the dose of 1ml/Kg body weight. The same number of animals (n=6) was not treated and served as control (CRL group). The results showed that PLFO was tolerated by rectal route. No significant differences were observed in body weights of the two groups. Biochemical analysis showed that aspartate transaminase (AST) and alanine transaminase (ALT) were significantly decreased in blood plasma at (P< 0.05) and (P< 0.01) respectively in PLFO group (Mann-Whitney test). On the other hand, the fasting glucose level (GLU) was significantly increased (Mann-Whitney test, P< 0.05), while the rest of the tested parameters (Albumin, total proteins, creatinine, urea) was not significantly affected. However, these variations have not biologic signification toxicity. The study concludes that PLFO is tolerable via rectal route; it is safe with no adverse effect on liver functions and renal functions with possible anti-glycogenesis activity.
Keywords: Pistacia lentiscus, fatty oil, glycemic index, LFT, RFT, rabbits
Introduction
Pistacia lentiscus L. is an evergreen shrub or small tree growing to 1–8 m tall belonging to the Anacardiaceous family (Lauk et al., 1996). It appears in dry open woods and scrublands in the Mediterranean region, and in guarrigues, maquis on sandy soils and dry rocky slopes (Dogan et al., 2003). It is a traditional natural remedy that has been used by very ancient Mediterranean civilizations like Greeks and Egyptians (Pellecuer et al., 1980). All parts of these plant posses medicinal uses. The leaves are extensively used in folk medicine for the treatment of eczema, diarrhea, and throat infections, and as a potent antiulcer agent (Ali-Shtayeh et al., 1998). The aerial parts have been used in the treatment of hypertension and possess stimulant and diuretic properties (Bently and Trimen, 1980). The mastic gum has been used for the relief of upper abdominal discomfort, stomachaches, dyspepsia and peptic ulcer (Al-Habbal et al., 1984). The essential oil extracted from the aerial parts has been proven to exhibit antioxidant, anti-inflammatory, antimicrobial (Benhammou et al., 2008), antifungal (Duru et al., 2003; Kordali et al., 2003) and antiatherogenic activities (Dedoussis et al. 2004). However, medicinal virtues of the fatty fruit's oil are particularly known in North Africa, in the eastern region of Algeria to Tunisia. The people of these regions have used this fruit's oil externally to treat sore throats, locally to remedy burns and wounds and internally for respiratory allergies (Boukef and Souissi, 1982). Benhammou et al. (2008) reported that this fatty oil has good nutritive quality because of its content in unsaturated fatty acids (Oleic + linoleic = 73%) and saturated fatty acids (Palmitic + stearic = 25.8%). We have demonstrated in a previous study the beneficial effect of this oil in healing process (Djerrou et al., 2010). In eastern Algeria, some families have inherited, from their ancestors, medication by this oil especially in respiratory disorders. These people use this oil at the dose of two teaspoons to two tablespoons per day according to age. Noting that the production of this vegetable oil and its use for therapeutic purposes are increasing in recent years. Despite this wide spread use, no scientific studies are available to ascertain the safety of this traditional remedy. Hence, the present study has been undertaken to investigate PLFO effects on fasting glucose and some functional parameters of the liver and kidney in rabbit model.
Materials and Methods
The present study was carried out at Department of Biology, University of Mentouri, Constantine, Algeria.
Extraction of Pistacia lentiscus fatty oil
Thirty kg of ripe fruits (black berries) of Pistacia lentiscus L. were collected in December 2009 in Skikda region, located in eastern Algeria. The oil was extracted from fruits in a traditional press and was stored in vials worm away from light until use.
Animals and husbandry
Healthy rabbits (New-Zealand, white, male, 4 months old, initial mean weight 1.95 Kg) were purchased from a local supplier (Hama Bouziane, Constantine, Algeria) and used for this study. Animals were kept in individual standard cages. A temperature of 22 ± 2° C, 50 – 75% relative humidity and a 12 h light-dark cycle were maintained in all times of experiment. Food and water were provided ad libitum. Animals were acclimated for laboratory conditions for a period of 7 days prior to initiation of the experiment.
Experimental protocol and drug administration
The study was carried out on 12 New Zealand (male, white, initial mean weight 1.95 Kg, age 16 wk) rabbits. After an acclimatization of 7 days in laboratory conditions, the rabbits were divided randomly into two groups of six each. Animals of first group were not treated and served as control (CRL group), the others served as tested group (PLFO group). In all animals for this group, PLFO was applied, once daily, 5-day per week, at the dose of 1 ml/Kg body weight, via rectal route for 6 consecutive weeks. The most important advantage suggested for the rectal administration is the possibility of avoiding the hepatic first-pass metabolism and the gastrointestinal tract (Choi et al., 1998; Khafagy, 2007). The choice of the dose of 1ml/kg body weight per day was based on doses used in folk medicine, as described above, in humans by oral consumption. This dose is usually used in repeat dose oral toxicity study (Limit-test) adopted by OECD (2006). The experimental protocol was approved by the Ethical Committee of the Faculty of Sciences of Constantine University.
Clinical alterations and determination of body weights
Rabbits were clinically evaluated daily; the observation was carried on anal region and anal sphincter, clinical signs
(e.g. sliding on the back-side, signs of pain) and feces (e.g. blood and mucus). Sacrifice of animals after administration period, necropsy and macroscopic examination of rectal mucosa (CPMP, 2001). The animals were weighted at the beginning of the experiment and repeated weekly until their sacrifice.
Biochemical analysis
At the end of experimentation, animals were fasted overnight, and blood samples were performed on heparinized tubes from marginal ear vein of rabbits. Plasma was obtained by blood centrifugation at 3000 rpm for 5 min and then stored at −20° C until used for analysis. The selected blood parameters were performed, in the Hormonal Chemistry Laboratory of Constantine, by a biochemical analyzer (Architect CI 8200) and included aspartate amino transferase (AST), alanine amino transferase (ALT), blood creatinine (CREA), fasting glucose (GLU), total proteins (TP), albumin (ALB) and urea.
Statistical analysis
Data obtained from body weight measurements were expressed as mean ± SD, the biochemical analyses were presented as median ± IQR (Inter quartile range). Parametric variables were analyzed by one-way ANOVA. Non parametric variables were analyzed with Mann-Whitney U test. All statistical tests were performed with SPSS 10.0 program. Differences between treated group and control were considered significant at P<0.05.
Results
During the experimentation period, no mortality was seen in the animals. All rabbits remained healthy and they were available for assessment.
Clinical alterations and body weights
All the animals (CRL group and PLFO group) were clinically normal throughout the experimental period. Only one rabbit from PLFO group, in the second week of experiment, had diarrhea for 3 days. Clinical observations of rectal mucosa showed a slight erythema from the second week until end of experiment. Administration of PLFO to rabbits daily for 6 weeks had no significantly effect on body weight (Fig. 1). The weight gains obtained after 52 days from the beginning of experiment were 990±72.5 g/rabbit in PLFO group and 923±141.5 g/rabbit for the untreated rabbits.
Figure 1.
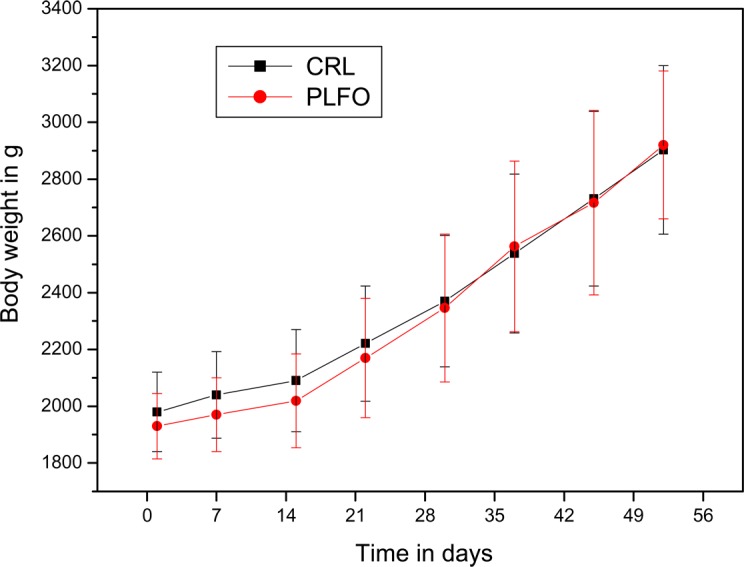
Trends in mean body weights of male New Zealand rabbits treated in PLFO and control group (CRL).
Clinical chemistry
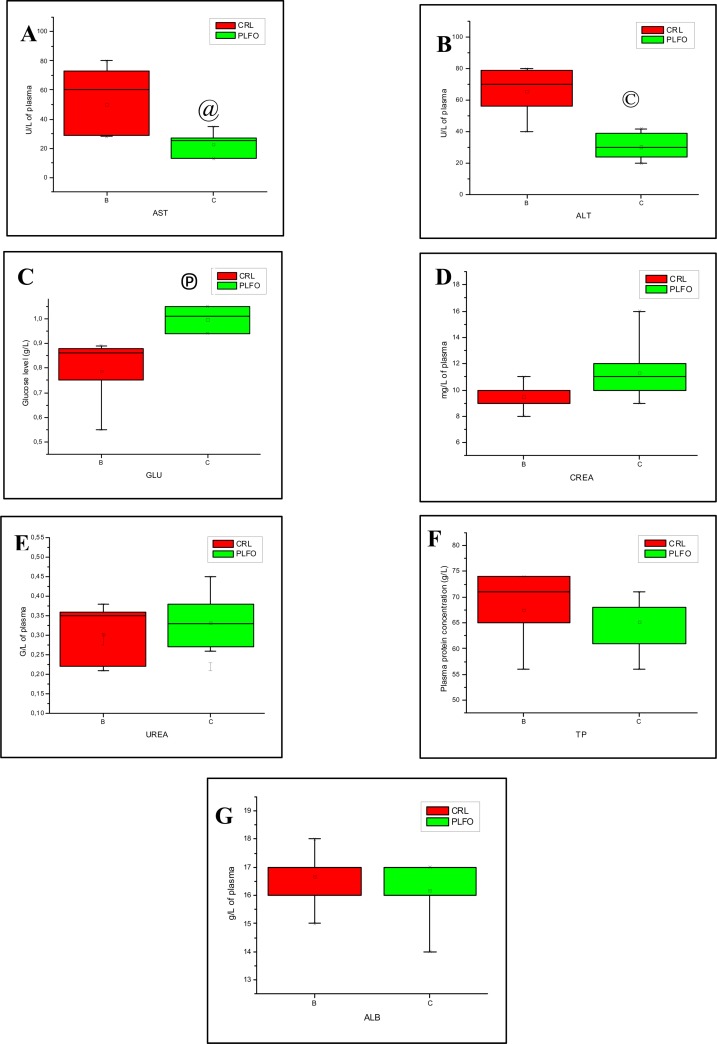
Statistical analysis of clinical chemistry data was registered in table 1. Liver enzymes levels (median ± IQR, n = 6) were significantly reduced in rabbits treated by PLFO. Aspartate aminotransferase (AST) showed a significant decrease (Mann-Whitney test, P< 0.05) in PLFO group compared to control group. Similarly, alanine aminetransferase (ALT) was reduced significantly (Mann-Whitney test, P< 0.01) in tested rabbits compared to control animals (Fig. 2A). Plasmatic fasting glucose level (median ± IQR, n = 6) was significantly higher (Mann-Whitney test, P< 0.01) in PLFO group compared to control group (Fig. 2B). In the other hand, creatinine was found to be slightly higher, but none significantly in PLFO group, than those detected in control. Urea was found to be slightly lower but none significantly in PLFO group than those detected in control (Fig. 2C and D). The results showed also that total proteins and albumin concentrations in PLFO group were very slightly lower but none significantly compared to control (Fig. 2E and F).
Table 1.
Plasma chemistry values in New Zealand male rabbits treated for 6-weeks by PLFO and control group.
| Blood parameters | Control (n=6) (median ± IQR) |
PLFO (n=6) (median ± IQR) |
U | Z | P |
| AST (U/L) | 45 (28 – 80) | 24 (13 – 35) | 33 | 2.40192 | 0.01515152@ |
| ALT (U/L) | 69.5 (40 – 80) | 28,5 (20 –42) | 35 | 2.72218 | 0.004329© |
| CREA (mg/L) | 9.7 (8 – 11) | 10.1 (9 – 16) | 27.5 | 1.52122 | 0.1320346 |
| GLU (g/L) | 0.82 (0.55 – 0.89) | 0.995 (0.94 – 1.05) | 36 | 2.88231 | 0.0021645© |
| TP (g/L) | 68 (56 – 74) | 67.5 (56 – 71) | 22 | 0.640513 | 0.588744 |
| ALB (g/L) | 17 (15 – 18) | 16.5 (14 – 17) | 22.5 | 0.720577 | 0.484848 |
| UREA (g/L) | 0.325 (0.21 – 0.38) | 0.315 (0.26 – 0.45) | 21.5 | 0.560449 | 0.588744 |
Significant at P<0.05(two-tailed test).
Significant at P<0.01 (two-tailed test)
Figure 2.
Comparison of some biochemical parameters in the plasma of rabbits from control group (CRL) and rabbits treated in Pistacia lentiscus fatty oil (PLFO) for 6 consecutive weeks. (A) Enzymatic activity of AST. (B) Enzymatic activity of ALT. (C) Glucose level. (D) Creatinine concentration. (E) Urea concentration. (F) Total protein concentration. (G) Albumin concentration. Values are expressed as median ± IQR (n = 6). @ Significant at P < 0.05 (Mann-Whitney, Z=2.40192, P= 0.01515152), © Significant at P<0.01(Mann-Whitney, Z=2.72218, p=0.004329), Ⓟ Significant at P<0.01 (Mann-Whitney, Z=2.88231, p=0.0021645).
Discussion
In the present study, rabbits have not presented signs of toxicity in all the experimental period. The animals of PLFO group have exhibited a normal behavior in comparison to CRL group. Any macroscopically lesions were observed in rectal mucosa. No significant differences were observed in body weight gain of the two groups. In view of these partial results, we can assert that PLFO did not cause clinical alterations for rabbits during its application for 6 consecutive weeks.
In several organs, cell damage is followed by released of a number of cytoplasmic enzymes to the blood, phenomena that provides the basis for clinical diagnosis (Sundberg et al., 1994). In our study, hepatic function was evaluated by measuring plasma ALT and AST activities, albumin and total proteins concentrations. Renal function was evaluated by measuring plasma creatinine and urea concentrations (Davis and Berdt, 1994; Finco, 1997; Correges et al., 1998). Regarding biochemical data, all biochemical blood parameters investigated in this study were within the physiological range as reported by Archetti et al. (2008). Additionally, our results showed a significant decrease of transaminases; AST and ALT concentrations were 1.8 and 2.4 times respectively lower than those observed in the control group. It is known that damage to the structural intergrity of liver is reflected by increase in the liver hepato-specific enzymes (ALP, ALT and AST) in the serum, because they are cytoplasmic in location and are released into circulation after cellular damage (Janbaz and Gilani, 1995; Venkateswaran et al., 1995). These transaminases play important roles in amino acids metabolism and providing necessary intermediates in gluconeogenesis (Hanley et al., 1986). According to this, the significant decrease of plasma transaminases (ALT and AST) activities of treated rabbits could indicate an improvement in liver function due to a possible hepatoprotective activity of PLFO. This pharmacological property has been investigated in Pistacia lentiscus plant in earlier study. It has been reported by Janakat and Al-Merie (2002) that aqueous extract of P. lentiscus (both boiled and non-boiled) showed marked antihepatotoxic activity against CCL4 (Carbon tetrachloride) by reducing the activity of the three enzymes (ALP, ALT and AST) and the level of bilirubin. They concluded that P. lentiscus is effective in the treatment of hepatic jaundice in the rat.
According to literature, the hepatoprotective medicinal preparations must: possess antioxidant properties; stabilize cell membranes; inhibit microsomal cytochrome P-450-dependent system activity, as the primary source of oxygen free radicals, and have anti-inflammatory properties (Lukivskaya et al. 2006). Its worth asking whether compounds of PLFO were implicated in the amelioration of liver functions. Preliminary studies indicated that this fatty oil contains two fractions: the unsaponifiable fraction presented mainly by tocopherols and phytosterols; the saponifiable fraction which is rich in unsaturated fatty acids (UFA) and saturated fatty acids (SFA). Phytosterols have been found to exhibit anti-inflammatory and anti-oxidant activities (Moreno, 2003). Tayal et al. (2007) reported that alpha-tocopherol (Vitamin E) is a potent antioxidant that provides hepatoprotection by scavenging free radicals. Hernández et al. (2005) reported that treatment with a balanced diet rich in olive oil contributed to the recovery of the liver from hepatic steatosis, by decreasing activation of hepatic stellate cells by MUFAs, which are less susceptible to lipid peroxidation compared to PUFAs. Moreover, previous studies carried out in fibrotic rats showed that olive oil, in contrast to polyunsaturated oils, could protect against the development of fibrosis (Szende et al., 1994). In view of these cited studies, the variations of transaminases in PLFO group may be essentially due to tocopherols, phytosterols and unsaturated fatty acids compounds.
Fasting glucose level was found to be increased (21.3%) in PLFO group compared to value of untreated rabbits. Values in the two groups were correspondent to standards. It is known that liver is the main organ of glycogen metabolism. The decrease of glycogen in liver is a common result of hepatic insufficiency after liver lesions caused by toxic compounds. This hepatic insufficiency can cause an increase of insulin in blood, and a consecutive reduction of the blood sugar content, which is consistent with glycogen/glucose level in liver (Wu et al., 2005). In our study, elevation of glucose level may be explained by an effect of PLFO on liver cell metabolism. This later may affect directly the synthesis, the storage and the decomposition of glycogen in liver. In addition, this fatty oil was known for its antioxidant properties due to tocopherols and phytosterols. The inhibition of fatty acids oxidation can lead to a decrease in glycogenesis due to the inhibition of acetyl CoA (Golden and Kean, 1984).
Regarding renal function, variations of creatinine and urea were found to be none significant between the two groups; consecutively renal function was not alliterated by PLFO treatments. Decreases in concentration of whole plasma proteins and albumin have been proposed as indicators of the alteration of protein synthesis (Kubena et al., 1993). A drop in serum albumin level is usually the result of decreased protein synthesis by the liver or increased protein loss trough the gut of the kidney, other possible cause of decrease in albumin may include mal absorption (Orhue et al., 2005). In the present study, total proteins and albumin were reduced very slightly and none significantly in tested group. This is why we report that PLFO treatment had no significant impact on proteins and albumin concentrations.
Conclusion
Results from this study demonstrated that PLFO is well tolerated by rectal route. It is safe and no adverse effect on LFT and RFT was seen with possible anti-glycogenesis activity.
Acknowledgements
The authors gratefully acknowledge the C.H.U. hospital staff of Constantine, for their collaboration and their assistance in the biochemical analysis. We thank also Zaïer A.H. (Fundamental Department, Faculty of Sciences, Skikda University) for his skilful help.
References
- 1.Al-Habbal MJ, Al-Habbal Z, Huwez FU. A double-blind controlled clinical trial of mastic and placebo in the treatment of duodenal ulcer. J Clin Exp Pharmacol Physiol. 1984;11:19–23. doi: 10.1111/j.1440-1681.1984.tb00864.x. [DOI] [PubMed] [Google Scholar]
- 2.Ali-Shtayeh MS, Yaghmour RM, Faidi YR, Salem K, Al-Nuri M. Antimicrobial activity of 20 plants used in folkloric medicine in the Palestinian area. Journal of Ethnopharmacology. 1998;60:265–271. doi: 10.1016/s0378-8741(97)00153-0. [DOI] [PubMed] [Google Scholar]
- 3.Archetti I, Tittarelli C, Cerioli M, Brivio R, Grilli G, Lavazza A. Serum chemistry and hematology values in commercial rabbits: Preliminary data from industrial farms in northern Italy; 9th World Rabbit Congress; June 1013, 2008; Verrona-Italy. 2008. pp. 1147–1151. [Google Scholar]
- 4.Benhammou N, Atik Bekkara F, Panovska TK. Antioxidant and antimicrobial activities of the Pistacia lentiscus and atlantica extracts. African Journal of Pharmacy and Pharmacology. 2008;2(2):022–028. [Google Scholar]
- 5.Bentley RY, Trimen H. Medicinal plants. London: J. and A Churchill; 1980. p. 68. [Google Scholar]
- 6.Boukef K, Souissi HR. Contribution à l'étude des plantes médicinales en médecine populaire en Tunisie. Rev Soc Pham Tunisie. 1982;2(3):34–35. (Fre). [Google Scholar]
- 7.Choi H G, Oh Y K, Kim C K. In-situ gelling and mucoadhesive liquid suppository containing acetaminophen: enhanced bioavailability. Int J Pharm. 1998;165:23–32. [Google Scholar]
- 8.Correges J-P, Becha J, Abood E, André L, Lamarka R. Renal artery stenosis and chronic renal failure in NIDDM. Arch Mal Coeur Vaiss. 1998;91:1077–1088. [PubMed] [Google Scholar]
- 9.CPMP/SWP/2145/00 (Committee for proprietary medicinal products), author Note for guidance on non-clinical local tolerance testing of medicinal products. EMEA; 2001. pp. 1–6. [Google Scholar]
- 10.Davis ME, Berdt WD. Renal methods for toxicology. In: Hayes AW, editor. Principles and methods of toxicology. 3th Ed. New York: Raven; 1994. pp. 871–894. [Google Scholar]
- 11.Dedoussis GVZ, Kaliora AC, Psarras S, Chiou A, Mylona A, Papadopoulos NG, Andrikopoulos NK. Antiatherogenic effect of Pistacia lentiscus via GSH restoration and down regulation of CD36 mRNA expression. Atherosclerosis. 2004;174:293–303. doi: 10.1016/j.atherosclerosis.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Djerrou Z, Maamari Z, Hamdi-Pacha Y, Serakta M, Riachi F, Djaalab H, Boukeloua A. Effect of virgin fatty oil of Pistacia lentiscus on experimantal burn wound's healing in rabbits. Afr J Trad CAM. 2010;7(3):258–263. doi: 10.4314/ajtcam.v7i3.54788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dogan Yunus, Baslar Suleyman, Aydin halil, Mert Hasan Huseyin. A study of the soil-plant interactions of Pistacia lentiscus L. distributed in the western Anatolian part of Turkey. Acta Bot Croat. 2003;62(2):73–88. [Google Scholar]
- 14.Duru ME, Cakir A, Kordali S, Zengin H, Harmandar M, Izumi S, Hirata T. Chemical composition and antifungal properties of essential oils of three Pistacia species. Fitoterapia. 2003;74:170–176. doi: 10.1016/s0367-326x(02)00318-0. [DOI] [PubMed] [Google Scholar]
- 15.Finco DR. Kidney function. In: Kanetto JJ, Harvey JW, Bruce. ML, editors. Clinical Biochemistry of domestic animal. 5th ed. San Diego, CA: Academic Press; 1997. pp. 462–478. [Google Scholar]
- 16.Golden KD, Kean EA. The biogenesis of dicarboxylic acids in rats given hypoglycerin. Biochem Biophys Acta. 1984;794:83–88. doi: 10.1016/0005-2760(84)90300-x. [DOI] [PubMed] [Google Scholar]
- 17.Hernández R, Martínez-Lara E, Cañuelo A, del Moral ML, Blanco S, Siles E, Jiménez A, Pedrosa JA, Peinado MA. Steatosis recovery after treatment with a balanced sunflower or olive oil-based diet: involvement of perisinusoidal stellate cells. World J Gastroenterol. 2005;11:7480–7485. doi: 10.3748/wjg.v11.i47.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sana Janakat, Hela Al-Merie. Evaluation of hepatoprotective effect of Pistacia lentiscus, Phillyrea latifolia and Nicotiana glauca. Journal of Ethnopharmacology. 2002;83:135–138. doi: 10.1016/s0378-8741(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 19.Janbaz KH, Gilani AH. Evaluation of the protective potential of Artemisia maritima extraction acetaminophen- and CCL4-induced liver damage. Journal of Ethnopharmacology. 1995;47:43–47. doi: 10.1016/0378-8741(95)01252-9. [DOI] [PubMed] [Google Scholar]
- 20.Hanley KS, Schmidt E, Schmidt FM. Enzymes in serum, their volume in diagnosis. Springfield Illinois: Charles Thomas; 1986. pp. 70–81. [Google Scholar]
- 21.Khafagy E, Morishita M, Onuki Y, Takayama K. Current challenges in noninvasive insulin delivery systems: A comparative review. Adv Drug Deliv Rev. 2007;59(15):1521–1546. doi: 10.1016/j.addr.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Kordali S, Cakir A, Zengin H, Duru ME. Antifungal activities of the leaves of three Pistacia species growth in Turkey. Fitoterapia. 2003;74:164–167. doi: 10.1016/s0367-326x(02)00320-9. [DOI] [PubMed] [Google Scholar]
- 23.Kubena LF, Harvey RB, Huff WE, Elissalde MH, Yersin AG, Phillips TD, Rottinghaus GE. Efficacy of hydrated sodium calcium aluminosilicate to reduce the toxicity of aflatoxin and diacetoxyscirpenol. J Poult Sci. 1993;72:51–59. doi: 10.3382/ps.0720051. [DOI] [PubMed] [Google Scholar]
- 24.Lauk L, Ragusa S, Rapisarda A, Franco S, Nicolosi VM. In vitro antimicrobial activity of Pistacia lentiscus L. extracts: preliminary report. J Chemother. 1996;8(3):207–209. doi: 10.1179/joc.1996.8.3.207. [DOI] [PubMed] [Google Scholar]
- 25.Lukivskaya O, Zavodnik L, Knas M, Buko V. Antioxidant mechanism of hepatoprotection by ursodeoxycholic acid in experimental alcoholic steatohepatitis. Advances in Medical Sciences. 2006;51:54–59. [PubMed] [Google Scholar]
- 26.Moreno J. Effects of olive oil minor compounds on oxidative stress and arachidonic acid mobilization and metabolism by macrophages RAW 267.4. Free Radical Biology and Medicine. 2003;35:1073–1081. doi: 10.1016/s0891-5849(03)00465-9. [DOI] [PubMed] [Google Scholar]
- 27.OECD, author. Report of the Validation of the Updated Test Guideline 407: Repeat Dose 28-day Oral Toxicity Study in Laboratory Rats. 2006. Series on Testing and Assessment No 59, ENV/JM/MONO (2006)26. [Google Scholar]
- 28.Orhue NEJ, Nwanze EAC, Okafor A. Serum total protein, albumin and globulin levels in Trypanosoma brucei-infected rabbits: Effect of orally administered Scoparia dulcis. African Journal of Biotechnology. 2005;4(10):1152–1155. [Google Scholar]
- 29.Pellecuer J, Jacob M, Simeon DM, Dusart G, Attisto M, Barthez M, Gourgas L, Pascal B, Tomei R. Essais d'utilisations d'huiles essentielles de plantes aromatiques Méditerranéennes en odontologie conservatrice. Plant Medicin Phytother. 1980;14:83–98. (Fre). [Google Scholar]
- 30.Sundberg A, Appelkwist EL, Dallner G, Nilsson R. Glutathione transferase in the urine: sensitive methods for detection of kidney damage induced by nephrotoxic agents in humans. Environ Health Perspect. 1994;102:293–296. doi: 10.1289/ehp.94102s3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szende B, Timár F, Hargitai B. Olive oil decreases liver damage in rats caused by carbon tetrachloride (CCl4) Exp Toxicol Pathol. 1994;46:355–359. doi: 10.1016/S0940-2993(11)80116-8. [DOI] [PubMed] [Google Scholar]
- 32.Vandana Tayal, Kalra Bhupinder Singh, Sarita Agarwal, Nita Khuranna, Usha Gupta. Hepatoprotective effect of tocopherol against ioniazid and rifampicin induced hepatotoxicity in albino rabbits. Indian Journal of Experimental Biology. 2007 Dec;45:1031–1036. [PubMed] [Google Scholar]
- 33.Venkateswaran S, Pari L, Viswanathan P, Menon V. Protective effect of livex, an herbal formulation against erythromycin estolate induced hepatotoxicity in rats. Journal of Ethnopharmacology. 1995;57:161–167. doi: 10.1016/s0378-8741(97)00062-7. [DOI] [PubMed] [Google Scholar]
- 34.Wu Huifeng, Zhang Xiaoyu, Li Xiaojing, Wu Yijie, Pei Fengkui. Acute biochemical effects of La (NO3)3 on liver and kidney tissues by magic-angle spinning H nuclear magnetic resonance spectroscopy and pattern recognition. Analytical Biochemistry. 2005;399:242–248. doi: 10.1016/j.ab.2005.01.021. [DOI] [PubMed] [Google Scholar]