Abstract
The high potential variability of chemical composition of the plant material involved in the manufacture of homoeopathic mother tinctures (a common source of homoeopathic medicines), renders both their quality control and assurance a significant challenge (Pande and Pathak, 2006). The absence of significant regulations regarding the quality of Complementary and Alternative Medicines (CAM) in South Africa contributes to this challenge (Gqaleni et al, 2007). In order to assess any quality differences between local and international manufacturers, the following homoeopathic mother tinctures, Artemisia absinthium, Rosmarinus officinalis e foliis recentibus, Salvia officinalis and Sambucus nigra, were chosen on the basis that they can be grown both locally in South Africa and internationally and are prepared according to the German Homoeopathic Pharmacopoeia (GHP), method 3a. Colour analysis was followed by thin layer chromatographic (TLC) analysis on each selected sample and relevant reference sample using both aluminum-backed TLC plates and glass-backed HPTLC plates. Photographs were taken of the resultant chromatograms, active components were identified, comparisons to the reference chromatograms were made and the overall quality of each homoeopathic mother tincture deduced. The quality of all nine of the selected samples manufactured internationally complied with the minimum quality standards set by the GHP. Five out of the six local samples complied with the minimum standards of the GHP._Notwithstanding the minimum GHP standards, the superior number of high quality international samples implies that their quality exceeded that of the locally manufactured tinctures. Greater regulation regarding the quality of these types of products has therefore been identified.
Keywords: Artemisia absinthium, Rosmarinus officinalis e foliis recentibus, Sambucus nigra, Salvia officinalis, thin layer chromatography, quality, homoeopathic, mother tincture, South Africa
Introduction
During the past few decades, the use of homoeopathic preparations and herbal medicines in the developed world has become a popular and highly demanded form of medicinal treatment, which has been facilitated by less stringent regulations than other medications (WHO, 2009). As the international costs of mother tinctures have begun to escalate, due to various factors, the use of local manufacturers has become an imperative source option. A need therefore has arisen to regularly assess whether there is a difference in quality between locally and internationally manufactured mother tinctures and whether the quality of the local and international manufactured homoeopathic mother tinctures comply with the standard stipulated by the Homoeopathic Pharmacopoeia (Lee et al., 2007).
A mother tincture is a liquid preparation resulting from the extraction of a suitable source, namely plant or animal substance material with alcohol or water mixture within a specific ratio (Banerjee, 2002).
Many techniques have been used to determine the quality of mother tinctures, including most commonly thin layer chromatography (TLC) and mass spectroscopy, while high performance liquid chromatography allows for both the quantitative and qualitative analysis of substances (Waksmundzka-Hajnos et al., 2008). TLC allows for an affordable yet reliable and effective technique in the determination of the active components within a herbal product. Due to lack of quality regulation in South Africa, it is uncertain how many products comply with the intended quality levels stipulated in the various pharmacopoeiae.
Through TLC analysis of selected homoeopathic mother tinctures, one is able to determine whether the active components of the samples are present and furthermore deduce whether the sample complies with the standard quality stipulated in the German Homoeopathic Pharmacopoeia and by implication, Good Manufacturing Practice (GMP).
Material and Methods
Research Samples
Samples of the various homoeopathic mother tinctures were sourced from both local and international manufacturers and were all prepared according to the GHP, method 3a. In order to avoid bias no samples were procured from Natura Laboratories, Pretoria and all samples were repackaged and re-labeled by way of random allocation to a coding system by a dispensary assistant at the University of Johannesburg. All samples were decanted into identical unlabeled 50ml clear amber glass bottles and randomised by a research assistant according to a precise labelling coding system to ensure correct tracking of samples and to avoid bias.
Colour Analysis
5ml of each sample of each mother tincture was measured and placed into a clear polytop and labeled. The samples were then visualized with the naked eye and the colour recorded. Pictures of each sample were taken with a Sony Cyber-shot DSC W300, set at 13 megapixels on manual exposure shooting, with no flash.
Thin Layer Chromatography
Reference chromatograms were developed using the active components stipulated for each mother tincture according to the German Homoeopathic Pharmacopoeia (GHP), or utilising a standardised reference sample verified to contain the active components (as for Artemisia absinthium). These reference chromatograms were then compared to the respective sample chromatograms, which were produced according to the methodology stipulated in the GHP. Other than the reference chromatogram of Artemisia absinthium (a standardised sample), all chromatograms were produced on both aluminum-backed TLC plates and glass-backed HPTLC plates. All chromatograms were verified and interpreted in conjunction with two experts in the field of chromatography and phytochemical analysis.
Artemisia absinthium (Samples D and E)
10ml of mother tincture was concentrated to 7ml under reduced pressure at 35°C. The solution was shaken with three 10ml portions of a mixture of 7ml ethyl acetate and 93ml of toluene and the combined organic phases were evaporated to dryness under reduced pressure. The residual was dissolved in 1ml of the mixture of 7ml ethyl acetate and 93ml of toluene (GHP, 2003).
Reference solution: A reference standard sample which contains the active components of Artemisia absinthium is not stipulated in the German Homoeopathic Pharmacopoeia but there is rather stipulation of expected colours of the active components. In order to mitigate this potential subjective influence a reference sample was developed and photographed by Dr E Prozesky, of Natura Laboratories, Pretoria, utilizing the same procedure, mentioned above and used for comparison against the sample plate produced. 30 µl of the test solution was applied to the silica plate. The sample was developed to a distance of 100mm (reduced from 150mm in order to standardise all sample development distances) with a mixture of 10ml of anhydrous acetic acid, 10ml of acetone and 80ml of toluene. The mobile phase was allowed to evaporate, then the chromatograms were sprayed with acetic anhydride-sulphuric acid, heated to 105°C for 5–10 minutes and examined in daylight within 10 minutes.
Rosmarinus officinalis e foliis recentibus (Samples B, C, D and F)
10ml of mother tincture was shaken with three 10ml portions of hexane. The combined organic phases were filtered, evaporated to dryness under reduced pressure at 30°C and the residue was dissolved in 1ml of methanol (GHP, 2003).
Reference solution: 10mg of borneol, 20mg of bornyl acetate and 20ul of cineole was dissolved in 10ml of methanol. 30µl of the test solution and 10 µl of the reference solution was applied separately to the silica plates. All samples were developed to a distance of 100mm with a mixture of 20ml of diisopropyl ether and 80ml of toluene. The mobile phase was allowed to evaporate, the chromatograms were then sprayed with anisaldehyde solution, heated at 105°C for 5–10 minutes and examined in daylight within 10 minutes.
Sambucus nigra (Samples A, B, D, E and F)
10ml of mother tincture was shaken with two 10ml portions of ethyl acetate. The combined organic phases were dried over anhydrous sodium sulphate and evaporated to dryness. The residue was dissolved in 0.5ml of methanol (GHP, 2003).
Reference solution: 10mg of each chlorogenic acid, caffeic acid and rutoside was dissolved in 10ml of methanol.
30 µl of test solution and 20 µl of the reference solution were applied separately to the silica plates. Each sample was developed to a distance of 100mm with a mixture of 7ml anhydrous formica acid, 7ml of glacial acetic acid, 18ml of water and 68ml of ethyl acetate. The mobile phase was allowed to evaporate in a current of warm air. The chromatogram was sprayed with a 10ml/l solution of diphenylboric acid aminoethyl ester in methanol and then with a 50ml/l solution of macrogol 400 in methanol. The chromatogram was left for 30 minutes and examined in ultraviolet light at 365nm.
Salvia officinalis (Samples A, B, C, D and E)
10ml of mother tincture were shaken with three 5ml portions of pentane. The organic phases were dried over anhydrous sodium sulphate and filtered. The filtrate was evaporated to dryness under reduced pressure and the residue was dissolved in 1ml of methanol (GHP, 2003).
Reference solution: 10mg of borneol, 20mg of bornyl acetate and 30ul of cineole was dissolved in 10ml of methanol.
10µl of the test solution and 10 µl of the reference solution were applied separately to the silica plates. Each sample was developed to the distance of 100mm with a mixture of 20ml of diisopropyl ether and 80ml of toluene. The mobile phase were allowed to evaporate, then the chromatograms were sprayed with anisaldehyde solution, heated at 105°C for 5–10 minutes and examined in daylight within 10 minutes.
Results and Discussion
Colour Analysis
Colour variations of homoeopathic mother tinctures are dependent on various factors including the time of harvest, the amount of rainfall and even small differences in the manufacturing process can cause a change in the colour of the mother tincture. A high chlorophyll concentration in the plant at the time of harvest may render a tincture showing a more green colouring whereas a high flavonoid glycoside concentration will show a more yellowish colouring of the mother tincture (WHO, 2003).
Thin Layer Chromatography
Artemisia absinthium
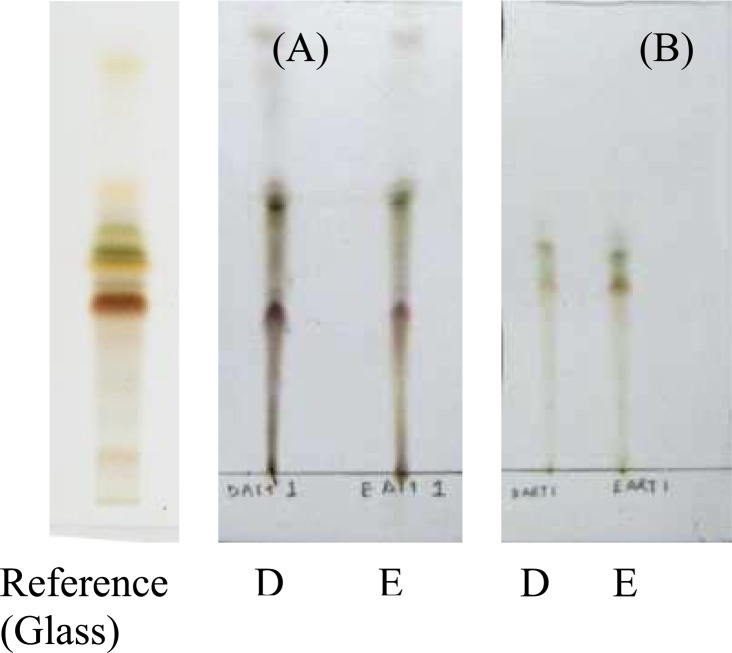
Artemisia absinthium contains three significant active constituents, which play a role in the quality of the mother tincture: artabsin, thujone and absinthin. When analyzing the standardized reference sample, Figure 3.1 (Reference), artabsin can be identified at the Rf 0.53 with a yellow orange colour, thujone at the Rf 0.44 with a dark brown colour and absinthin at the Rf 0.39 with a light brown colour.
Figure 3.1.
Thin layer chromatograms of Artemisia absinthium
Eluent: toluene: acetone: acetic acid (80:10:10, v/v/v). Sprayed with acetic anhydride sulphuric acid solution. (a) Aluminum-backed TLC plate. (b) Glass-backed HPTLC plate
After derivatization of the aluminum-backed TLC plate (Plate A) Sample E showed three less spots than Sample D, however all three active components were present, indicating no significant effect on the quality of the sample. On the glass-backed HPTLC plate (Plate B) after derivatization, Samples D and E both showed the same number of spots with the same colouring throughout as well as the presence of all three actives.
The concentration of the active components, assessed through the darker colouring of the respective spots as well as the size of the spots, was greater in Sample E than that of Sample D. Sample E was therefore selected as the sample with the greater quality when compared to Sample D.
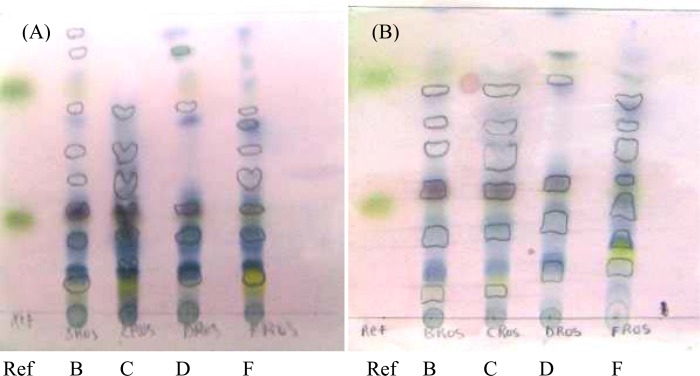
Rosmarinus officinalis e foliis recentibus
Borneol, rosmarinic acid, 1,8-cineole and bornyl acetate are the four active components essential for the medicinal properties of Rosmarinus officinalis e foliis recentibus.
On Plate A (Figure 3.2), the reference sample showed borneol at the Rf 0.31, 1,8-cineole at the Rf 0.34 and bornyl acetate at the Rf 0.75. All the samples, except Sample C, showed the presence of all three active components at similar Rf values. Sample C does not contain bornyl acetate at the mean Rf 0.75, therefore indicating a poorer quality of this sample when compared to the other samples.
Figure 3.2.
Thin layer chromatograms of Rosmarinus officinalis e foliis recentibus
Eluent: diisopropyl ether: toluene (20:80, v/v). Sprayed with anisaldehyde solution. (a) Aluminum-backed TLC plate. (b) Glass-backed HPTLC plate
On Plate B, Sample B, D and F contain all three active components; however Sample C did not show the presence of the bornyl acetate at the Rf 0.76, again indicating a poorer quality.
Figure 3.2 clearly shows the inadequate quality of Sample C, when compared to the other samples. The separation of Sample C is poor with a lack of components above the first active component.
Both Samples B and F have a superior quality when compared to Samples C and D. When assessing the size of the spots as well as the intensity of their colour to determine the concentration of the active components, Sample F had a greater concentration of borneol and rosmarinic acid whereas Sample B contained a higher concentration of 1,8-cineole and bornyl acetate. Sample F was declared as having the greatest quality.
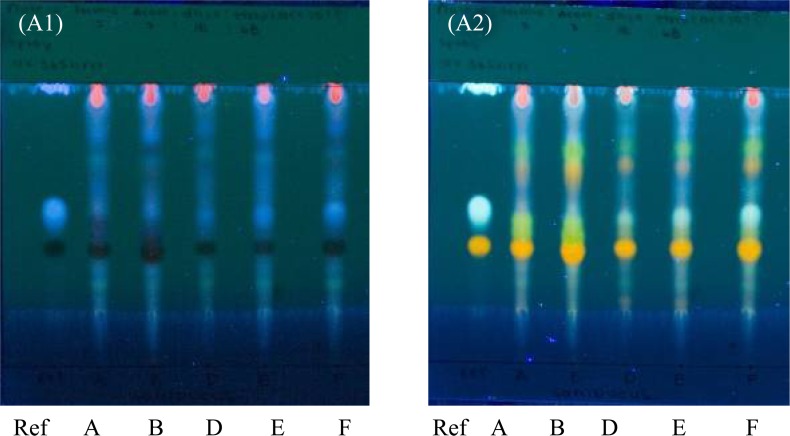
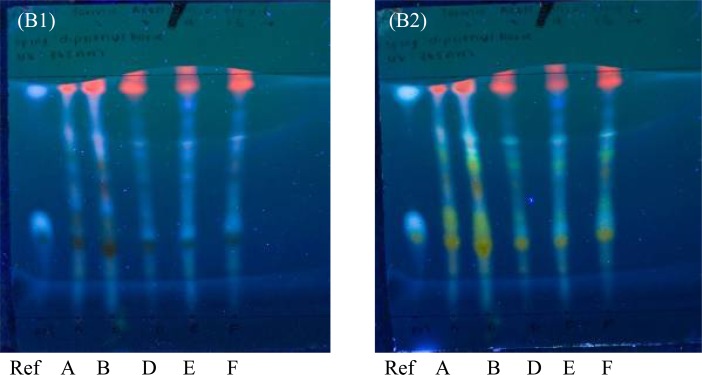
Sambucus nigra
The three spots present in the reference sample, are indicative of the active components and can be identified as rutin, chlorogenic acid and caffeic acid. On Plate A2, the aluminum-backed TLC plate; the reference sample showed the Rf 0.42 for rutin, the Rf 0.56 for chlorogenic acid and the Rf 0.99 for caffeic acid. All the samples (A, B, D, E and F) showed a presence of all three actives with similar Rf values.
On plate B2, the glass-backed HPTLC plate, the reference sample showed the Rf 0.34 for rutin, the Rf 0.43 for chlorogenic acid and the Rf 0.93 for caffeic acid. All Samples A, B, D, E and F showed the presence of rutin, however Samples A and B did not show a spot at the Rf 0.43, indicating that these samples did not show the presence of chlorogenic acid. For caffeic acid, Samples D, E and F did not show any spots.
UV detection for Sambucus nigra is important as the majority of components in the samples are fluorescent compounds. When assessing Figure 3.3 (Plate A1 and B1), rutin shows a dark black spot, while chlorogenic acid and caffeic acid show a white colour. Samples A and B however do show less fluorescent spots than the other samples. On comparison of Plate A1 and Plate B1, there is little difference in the separation quality of the fluorescent components between the two different types of plates.
Figure 3.3.
Thin layer chromatograms of Sambucus nigra on aluminum-backed TLC plate.
Eluent: formic acid: acetic acid: distilled water: ethyl acetate (7:7:18:68, v/v/v/v).
(a) UV 365nm. (b) Sprayed with diphenylboric acid, UV 365nm
All the samples show the presence of all three actives on the aluminum-backed TLC plate (Plate A2), yet not all active components appear on the glass-backed HPTLC plate (Plate B2). This is not indicative of a quality deficiency, but the effect of the use of a different type of plate.
Samples D, E and F appear to have a superior quality when compared to Samples A and B. When assessing the size of the spots as well as the intensity of their colour to determine the concentration of the active components, Sample F had a greater concentration of rutin and chlorogenic acid whereas Samples D and E contained a higher concentration of caffeic acid. Sample F was selected as the best sample based on its clear separation throughout both plates and the presence of all three actives.
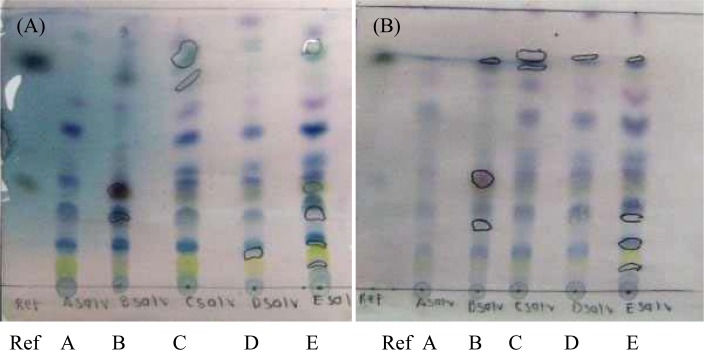
Salvia officinalis
Borneol, 1,8 cineole and bornyl acetate are the three active components essential for the medicinal properties of Salvia officinalis. On Plate A (Figure 3.5), the reference sample showed borneol at the Rf 0.36, 1,8-cineole at the Rf 0.39 and bornyl acetate at the Rf 0.79. Only Samples B and C showed the presence of all three active components, with Samples A, D and E lacking the presence of bornyl acetate at the Rf 0.79. On Plate B, Samples C, D and E contain all three active components, with Samples A and B not showing a spot at the Rf 0.43 for 1,8-cineole.
Figure 3.5.
Thin layer chromatograms of Salvia officinalis
Eluent: diisopropyl ether: toluene (20:80, v/v). Spayed with anisaldehyde solution.
(a) Aluminum-backed TLC plate. (b) Glass-backed HPTLC plat
Figure 3.5 clearly shows the variation between the samples, with the presence or lack thereof of the active component, the changes of colour between the plates and the varying spot sizes. As no one sample lacks an active component in both the aluminum-backed TLC plate (Plate A) and the glass-backed HPTLC plate (Plate B), one cannot state that there is a sample with an inadequate quality. Sample C was, however, consistent throughout both plates, showing the presence of all three actives with a clear separation of the other components.
Both Samples C and E have a superior quality when compared to Samples A, B and D. When assessing the size of the spots as well as the intensity of their colour to determine the concentration of the active components, Sample E had a greater concentration of borneol and rosmarinic acid whereas Sample C contained a higher concentration of 1,8-cineole and bornyl acetate.
Conclusion
As suggested by the experimental findings, while bearing in mind the limitations of the study with respect to numbers of homoeopathic tinctures tested, the selected internationally manufactured homoeopathic mother tinctures demonstrated a superior quality when compared to the selected locally manufactured homoeopathic mother tinctures. This indicates the need for increased regulation and quality assurance of all complementary and alternative medicines manufactured in South Africa.
Figure 3.4.
Thin layer chromatogram of Sambucus nigra on glass-backed HPTLC plate.
Eluent: formic acid: acetic acid: distilled water: ethyl acetate (7:7:18:68, v/v/v/v).
(a) UV 365nm. (b) Sprayed with diphenylboric acid, UV 365nm
Table 3.1.
Colour compliance of all samples of all homoeopathic mother tinctures when compared to the standard colour stipulated in the GHP (Poor, Acceptable, Good)*
| Standard GHP Colour |
Samples | ||||||
| A | B | C | D | E | F | ||
|
Artemisia absinthium |
Yellowish to green brown |
- | - | - | Good | Good | - |
|
Rosmarinus officinalis e foliis recentibus |
Yellowish to red brown |
- | Good | Good | Good | - | Good |
|
Sambucus nigra |
Brownish green |
Acceptable | Acceptable | - | Good | Acceptable | Good |
|
Salvia officinalis |
Greenish brown |
Good | Poor | Good | Good | Good | |
Poor = colour was distinctly different to GHP standard, Acceptable = colour was different from GHP standard but close enough to be considered acceptable, Good = colour matched GHP standard
Table 3.2.
Summary of quality assessment of selected homoeopathic mother tinctures
| Manufacturer | Sample | Homoeopathic Mother Tincture Quality | |||
|
Artemisia absinthium |
Rosmarinus officinalis e foliis recentibus |
Sambucus nigra | Salvia officinalis | ||
| Local | A | N/A | N/A | Acceptable | Good |
| B | N/A | Good | Acceptable | Good | |
| C | N/A | Poor | N/A | Good (Best) |
|
| International | D | Good | Good | Good | Good |
| E | Good (Best) |
N/A | Good | Good | |
| F | N/A | Good (Best) |
Good (Best) |
N/A | |
* Poor = active components not present thus not meeting minimum GHP standards, Acceptable = all active components were present and met minimum GHP standards, Good = all active components present and exceeded minimum GHP standards in terms of active component spot size and colour, Best = the best quality sample
Table 3.3.
Summary of overall quality performance of the local and international manufacturers
| Manufacturer | Quality* | ||||
| Poor | Acceptable | Good | Best | ||
| Local | 1 | ✓✓ | |||
| 2 | ✓ | ✓ | ✓ | ||
| 3 | ✓ | ✓ | |||
| International | 1 | ✓✓✓ | ✓ | ||
| 2 | ✓✓ | ✓ | |||
| 3 | ✓ | ✓ | |||
Poor = active components not present thus not meeting minimum GHP standards, Acceptable = all active components were present and met minimum GHP standards, Good = all active components present and exceeded minimum GHP standards in terms of active component spot size and colour, Best = the best quality sample
Acknowledgements
Dr. Erwin Prozesky, Natura Laboratories, Pretoria, South Africa for co-supervising the research. Prof. Bradley Williams, Department of Chemistry, University of Johannesburg, South Africa for the use of his laboratory and his assistance throughout the experimental procedure.
References
- 1.Banerjee DD. A text book of homoeopathic pharmacy. India: B Jain Publishers; 2002. p. 10. [Google Scholar]
- 2.German Homoeopathic Pharmacopoeia. Germany: Medpharm Scientific Publishers; 2003. Translated from German by Stephen Benyunes. [Google Scholar]
- 3.Gqaleni N, Moodley I, Kruger H, Ntuli A, McLeod H. Traditional and complementary medicine. South African Health Review. 2007:175–188. Chapter 12. [Google Scholar]
- 4.Lee G, Vallender M, Young S. Setting the standards for medicine - the British pharmacopoeia. The Pharmaceutical Journal. 2007;279:566–567. [Google Scholar]
- 5.Pande M, Pathak A. Physico-chemical evaluations of homeopathic mother tincture of Mimosa pudica Linn (lajvanti) 2006. [11 November 2009]. Available from: http://www.similima.com/thesis90.html.
- 6.Waksmundzka-Hajnos M, Sherma J, Kowalska T. Thin layer chromatography in phytochemistry. Vol. 99. USA: CRC Press; 2008. pp. 406–410. 595. [Google Scholar]
- 7.World Health Organization, author. Safety issues in the preparation of homoeopathic medicines. Spain: World Health Organization; 2009. pp. vii–x. 1–45. [Google Scholar]
- 8.World Health Organization, author. WHO guidelines on good agricultural and collection (GACP) for medicinal plants. Geneva: World Health Organization; 2003. pp. 9–25. [Google Scholar]