Abstract
Validation of new therapeutic targets calls for the advance in innovative assays that probe both spatial and temporal relationships in signaling networks. Cell death assays have already found a widespread use in pharmacological profiling of anticancer drugs. Such assays are, however, predominantly restricted to end point DEAD/LIVE parameter that provides only a snapshot of inherently stochastic process such as tumor cell death. Development of new methods that can offer kinetic real-time analysis would be highly advantageous for the pharmacological screening and predictive toxicology.
In the present work we outline innovative protocols for the real-time analysis of tumor cell death, based on propidium iodide (PI) and SYTOX Green probes. These can be readily adapted to both flow cytometry and time-lapse fluorescence imaging. Considering vast time savings and kinetic data acquisition such assays have the potential to be applied in a number of areas including accelerated anticancer drug discovery and high-throughput screening routines.
Keywords: Cytotoxicity, Real-time assays, Antitumor drugs, Flow cytometry, Time-lapse microscopy
1. Introduction
Tumor cell death serves as a useful end point in pharmacological profiling of anticancer drugs (1). Despite the large variety of techniques that have been developed so far to detect cell death, technological innovations can make the deployment of these assays more effective (2). The permeability of plasma membrane to charged fluorescent probes is an accepted marker that distinguishes LIVE from DEAD. Since it is generally assumed that such probes are inherently cytotoxic their use is mostly restricted to end point assays (3). The major drawback of such analysis is, however, capturing only a snapshot of the incidence of cell death which is inherently a stochastic process. Therefore, development of new methods that can provide kinetic quantification of drug induced cytotoxicity would be highly advantageous for the pharmacological screening and predictive toxicology (3). In such assays, markers applied supravitally, should have minimal effects on the structure, function, and survival of cells (4).
Recently, we have provided new evidence that many plasma membrane integrity markers such as propidium iodide (PI), SYTOX Green, SYTOX Red, and YO-PRO 1 can be used to dynamically probe and quantify cytotoxicity in real time (2, 3). Such assays meet the following criteria of dynamic and high-throughput analysis: (1) the straightforward staining and adaptability for automated dispensing; (2) the lack of side-effects on cellular viability, proliferation or cell migration; and (3) the lack of interference with the assay readout (2, 3).
Reduction of sample processing achieved with these protocols is important for the preservation of fragile apoptotic cells. Our data indicate that such simple bioassays can be readily adapted for novel microfluidic chip-based (Lab-on-a-Chip) platforms with minimal protocol modifications (5, 6).
2. Materials
2.1. Dynamic Detection of Cell Death Using Flow Cytometry
Cell suspension (1–5 × 105 cells/ml).
1 × PBS.
1 mg/ml PI stock solution in PBS. Store protected from light at +4°C. Stable for over 12 months. Caution: PI is a DNA binding molecule and thus can be considered as a potential carcinogen. Always handle with care and use protective gloves.
Optional: 1 mM SYTOX Green stock solution in DMSO. Store protected from light at −20°C. Stable for over 12 months. Caution: although there are no reports on SYTOX Green toxicity, appropriate precautions should always be applied when handling SYTOX Green solutions.
Optional: 10 μM SYTOX Green working solution in PBS (prepare fresh as required).
1.5-ml Eppendorf tubes.
12 × 75 mm polystyrene FACS tubes.
2.2. Real-Time Detection of Cell Death Using Time-Lapse Imaging
Cell suspension (1–5 × 105) or cell monolayer.
1 × PBS.
1 mg/ml PI stock solution in PBS. Store protected from light at +4°C. Stable for over 12 months. Caution: PI is a DNA binding molecule and thus can be considered as a potential carcinogen. Always handle with care and use protective gloves.
Optional: 1 mM SYTOX Green stock solution in DMSO. Store protected from light at −20°C. Stable for over 12 months. Caution: although there are no reports on SYTOX Green toxicity, appropriate precautions should always be applied when handling SYTOX Green solutions.
Optional: 100 μM SYTOX Green working solution in PBS (prepare fresh as required).
1.5-ml Eppendorf tubes.
Optical grade cell culture plates or cell culture chambers.
3. Methods
3.1. Dynamic Detection of Cell Death Using Flow Cytometry
The near real-time detection of cell death is based on a continuous presence of the fluorescent probe in the culture medium and performing sequential specimen sampling by flow cytometry (2, 3). As presence of the fluorescent dye has no impact on cellular viability, proliferation or cell migration, method presented here is a single-step and time saving assay (2, 3). Elimination of washing steps enhances preservation of fragile apoptotic cells in an intact state without compromising assay sensitivity (1–3).
Seed cells at a desired concentration in 24-well culture plates (see Note 1).
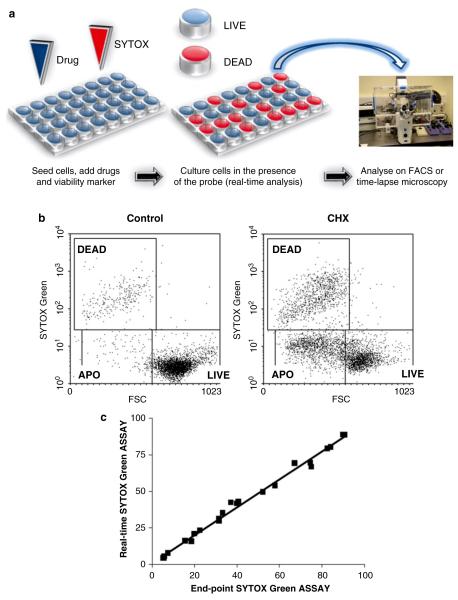
Add drug into the culture as appropriate (Fig. 1a).
- Add 1 μL of 1 mg/ml PI stock solution (final concentration 1 μg/ml; see Note 2).
- – Optional: instead of PI use 1.5 μL of 100 μM SYTOX Green working solution (final concentration 150 nM, see Notes 3 and 4).
Culture cells in the presence of PI or SYTOX Green and collect sample aliquots into 12 × 75 mm Falcon FACS at desired time-points (see Note 5).
Analyze on a flow cytometer with 488 nm excitation line (Argon-ion laser or blue solid-state laser) with emissions collected at 530 nm (SYTOX Green) or 575–610 nm (PI). Adjust the logarithmic amplification scale to distinguish between viable cells (bright PI− and SYTOX−) from late apoptotic and/or necrotic cells with compromised plasma membranes (PI+/SYTOX+) (Fig. 1b; see Notes 5–7).
Fig. 1.
Dynamic analysis of cytotoxicity using simplified real-time protocols: (a) Workflow of a modified real-time (no-wash) protocol. Note that viability marker SYTOX Green is continuously present in the culture medium as opposed to a standard, end-point staining procedure. (b) Viability marker SYTOX Green was applied to dynamically track drug-induced cytotoxicity using flow cytometry. Human promyelocytic leukemia HL60 cells were exposed to a pro-apoptotic drug cycloheximide (CHX; 50 μg/ml) for 24 h in the continuous presence of SYTOX Green (100 nM). Fluorescent probe was excited using 488 nm Argon-ion laser. SYTOX Green fluorescence signal was logarithmically amplified using 530 nm band-pass filter. Debris was excluded electronically. Analysis based on bivariate dot plots FSC vs. SYTOX Green is shown. LIVE – viable cells, APO – apoptotic cells, DEAD – late apoptotic/necrotic cells. (c) Comparison between percentages of cell death estimated using standard SYTOX Green end-point vs. new kinetic protocol. Human promyelocytic leukemia HL60 cells were exposed to a range of pro-apoptotic drugs cycloheximide (CHX), campthothecin (CAM), and staurosporine (STS) for 24 h. Data were acquired using BD FACS Calibur flow cytometer equipped with 488 nm excitation line and 530 nm band-pass filter. Note excellent agreement between results obtained with both assays (R2 ≥ 0.98 for p < 0.05 in Pearson and Lee linear correlation test).
3.2. Real-Time Detection of Cell Death Using Time-Lapse Imaging
The principle of this assay is similar to the previously described protocol for flow cytometry. The main advantage of this protocol is a true real-time detection of cell death based on a time-lapse fluorescent microscopy (3, 5, 6).
Seed cells at a desired concentration in optical grade culture plates.
Add drug into the culture as appropriate.
- Add 1 μL of 1 mg/ml PI stock solution (final concentration 1 μg/ml; see Note 2).
- – Optional: instead of PI use 1.5 μL of 100 μM SYTOX Green working solution (final concentration 150 nM, see Notes 3 and 4).
Position cell carrier on a time-lapse microscope stage.
Culture cells in the presence of PI or SYTOX Green and collect time-lapse images at desired time-points (see Note 8).
4. Notes
Cell seeding densities should be empirically adjusted to a particular type of cell line and/or primary cell culture.
Continuous presence of PI in the culture medium does not affect cellular viability, proliferation or cell migration. Our results indicate that human promyelocytic HL60 cells remain viable and reproductively competent even when challenged with PI concentrations up to 5 μg/ml for up to 72 h (3). Similar results were obtained on a panel of diverse tumor cell lines (suspension: U937, HL60, K562, MOLT-4, and Jurkat; adherent: U2OS, Saos2, MDA-MB-231, and 3T3). Importantly, presence of the probe does not appear to affect cell cycle and long-term cell proliferation as estimated using (methyl-3H)-thymidine incorporation and Trypan Blue assays (3). We recommend, however, initial titration of PI to find an optimal concentration for a particular cell line.
A green fluorescent SYTOX Green (Ex max: 504/Em max: 523 nm) probe can be continently substituted for PI. Remaining fluorescent channels can be utilized e.g. for multiparameter analysis of apoptotic markers such as calcium flux, caspase activation, or externalization of phosphatidyl serine residues (5, 6).
Similar to PI, SYTOX Green does not display any side effects on cellular viability, proliferation, or cell migration when used in concentrations up to 1 mM (3).
Cell suspension can be collected and analyzed without any centrifugation and washing steps (3). The continuous labeling procedure not only provided similar results to a standard end point staining protocol, but also allows for a straightforward adaptation for high-throughput screening (HTS) (3).
Cells cultured in the presence of PI or SYTOX Green exhibit an overall increase of background florescence as compared to end point protocols. Adjust the logarithmic amplification scale to distinguish between viable cells (bright PI− and SYTOX−) from late apoptotic and/or necrotic cells with compromised plasma membranes (PI+ /SYTOX+) as depicted in Fig. 1b (3).
Flow cytometry allows quantitative measurements of laser light scatter characteristics that reflect morphological features of cells (1). Cell shrinkage due to the dehydration can be detected at early stages of apoptosis as a decrease in intensity of forward light scatter (FSC) signal (1). Depending on a cell line model and stimuli being used, analysis based on FSC and SYTOX bivariate dotplots can provide additional information about apoptotic cells (APO – FSClow/SYTOX−) as depicted in Fig. 1b. It should be noted, however, that observable changes in light scattering are not a reliable marker of apoptosis and should be always confirmed by other dedicated assays (1).
A wide variety of optical grade cell carries can be exploited that include optical culture plates, cell culture chambers, and microfluidic chip-based devices (3, 5, 6). Program time-lapse protocol to collect images at desired time intervals. PI and SYTOX Green display substantial resistance to photobleaching. Therefore specimens can be repeatedly imaged for extended periods of time (3, 5, 6). No phototoxic reactions have been observed so far, but we recommend a careful assessment of selected fluorescent probes for a particular experimental protocol and biological specimen being used (3).
Acknowledgments
Supported by BBSRC, EPSRC and Scottish Funding Council, funded under RASOR (DW, SF, JMC) and NCI CA RO1 28 704 (ZD). Views and opinions described in this chapter were not influenced by any conflicting commercial interests.
References
- 1.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology: analysis of apoptosis and accidental cell death (necrosis) Cytometry. 1997;27:1–20. [PubMed] [Google Scholar]
- 2.Wlodkowic D, Skommer J, Faley S, Darzynkiewicz Z, Cooper JM. Dynamic analysis of apoptosis using cyanine SYTO probes: from classical to microfluidic cytometry. Exp Cell Res. 2009;315:1706–14. doi: 10.1016/j.yexcr.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wlodkowic D, Skommer J, McGuinness D, Faley S, Kolch W, Darzynkiewicz Z, Cooper JM. Chip-based dynamic real-time quantification of drug-induced cytotoxicity in human tumor cells. Anal Chem. 2009 Jul 2; doi: 10.1021/ac9010217. (Epub ahead of print), DOI: 10.1021/ac9010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wlodkowic D, Darzynkiewicz Z. Please do not disturb: Destruction of chromatin structure by supravital nucleic acid probes revealed by a novel assay of DNA-histone interaction. Cytometry A. 2008;10:877–879. doi: 10.1002/cyto.a.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wlodkowic D, Faley S, Zagnoni M, Wikswo JP, Cooper JM. Microfluidic single-cell array cytometry for the analysis of tumor apoptosis. Anal Chem. 2009;81:5517–23. doi: 10.1021/ac9008463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faley S, Copland M, Wlodkowic D, Kolch W, Seale KT, Wikswo JP, Cooper JM. Microfluidic single-cell arrays to interrogate signalling dynamics of individual, patient-derived hematopoietic stem cells. Lab Chip. 2009 doi: 10.1039/b902083g. (in press), DOI: 10.1039/b902083g. [DOI] [PubMed] [Google Scholar]