ABSTRACT
Gastrointestinal disturbances are commonly reported in children with autism and may be associated with compositional changes in intestinal bacteria. In a previous report, we surveyed intestinal microbiota in ileal and cecal biopsy samples from children with autism and gastrointestinal dysfunction (AUT-GI) and children with only gastrointestinal dysfunction (Control-GI). Our results demonstrated the presence of members of the family Alcaligenaceae in some AUT-GI children, while no Control-GI children had Alcaligenaceae sequences. Here we demonstrate that increased levels of Alcaligenaceae in intestinal biopsy samples from AUT-GI children result from the presence of high levels of members of the genus Sutterella. We also report the first Sutterella-specific PCR assays for detecting, quantitating, and genotyping Sutterella species in biological and environmental samples. Sutterella 16S rRNA gene sequences were found in 12 of 23 AUT-GI children but in none of 9 Control-GI children. Phylogenetic analysis revealed a predominance of either Sutterella wadsworthensis or Sutterella stercoricanis in 11 of the individual Sutterella-positive AUT-GI patients; in one AUT-GI patient, Sutterella sequences were obtained that could not be given a species-level classification based on the 16S rRNA gene sequences of known Sutterella isolates. Western immunoblots revealed plasma IgG or IgM antibody reactivity to Sutterella wadsworthensis antigens in 11 AUT-GI patients, 8 of whom were also PCR positive, indicating the presence of an immune response to Sutterella in some children.
IMPORTANCE
Autism spectrum disorders affect ~1% of the population. Many children with autism have gastrointestinal (GI) disturbances that can complicate clinical management and contribute to behavioral problems. Understanding the molecular and microbial underpinnings of these GI issues is of paramount importance for elucidating pathogenesis, rendering diagnosis, and administering informed treatment. Here we describe an association between high levels of intestinal, mucoepithelial-associated Sutterella species and GI disturbances in children with autism. These findings elevate this little-recognized bacterium to the forefront by demonstrating that Sutterella is a major component of the microbiota in over half of children with autism and gastrointestinal dysfunction (AUT-GI) and is absent in children with only gastrointestinal dysfunction (Control-GI) evaluated in this study. Furthermore, these findings bring into question the role Sutterella plays in the human microbiota in health and disease. With the Sutterella-specific molecular assays described here, some of these questions can begin to be addressed.
Introduction
Autism spectrum disorders (ASD) are pervasive developmental disorders that depend on triadic presentation of social abnormalities, communication impairments, and stereotyped and repetitive behaviors for diagnosis (DSM-IV-TR criteria, American Psychiatric Association, 2000). Gastrointestinal (GI) symptoms are commonly reported in children with autism and may correlate with autism severity (1, 2). Intestinal disturbances in autism have been associated with macroscopic and histological abnormalities, altered inflammatory parameters, and various functional disturbances (3–9).
In a previous study, we showed that a complex interplay exists between human intestinal gene expression for disaccharidases and hexose transporters and compositional differences in the mucoepithelial microbiota of children with autism and gastrointestinal disease (AUT-GI children) compared to children with GI disease but typical neurological status (Control-GI children). Significant compositional changes in Bacteroidetes, Firmicutes/Bacteroidetes ratios, and Betaproteobacteria in AUT-GI intestinal biopsy samples have been reported (10). Although others have demonstrated changes in fecal bacteria of children with autism (2, 11–15), our study differed from these by investigating mucoepithelial microbiota (10). The GI microbiota plays an essential role in physiological homeostasis in the intestine and periphery, including maintaining resistance to infection, stimulating immunological development, and perhaps even influencing brain development and behavior (16–19). Thus, disruption of the balanced communication between the microbiota and the human host could have profound effects on human health.
In our previous metagenomic study, we found sequences corresponding to members of the family Alcaligenaceae in the class Betaproteobacteria that were present in ileal and cecal biopsy samples from 46.7% (7/15) of AUT-GI children. Alcaligenaceae sequences were completely absent from biopsy samples from Control-GI children (10). Members of the family Alcaligenaceae inhabit diverse habitats, ranging from humans and animals to soil (20). Several members of Alcaligenaceae cause clinically relevant infections or are suspected opportunistic pathogens in humans and animals, including members of the genus Bordetella (including the human respiratory pathogens B. pertussis and B. parapertussis, the mammalian respiratory pathogen B. bronchiseptica, and the poultry respiratory pathogen B. avium), a member of the genus Alcaligenes (the human opportunistic pathogen A. faecalis), members of the genus Achromobacter (the human opportunistic pathogens A. xylosoxidans and A. piechaudii), members of the genus Oligella (the potential opportunistic genitourinary species O. urethralis and O. ureolytica), a member of the genus Taylorella (the equine urogenital pathogen, T. equigenitalis), and a member of the genus Pelistega (the pigeon respiratory pathogen P. europaea) (20).
In some cases, the pathogenic potential of Alcaligenaceae members is unclear. The genus Sutterella represents one such member. Members of the genus Sutterella are anaerobic or microaerophilic, bile-resistant, asaccharolytic, Gram-negative, short rods (21). Members of the genus Sutterella have been isolated from human infections below the diaphragm (22, 23). Sutterella 16S rRNA gene sequences have also been identified in intestinal biopsy and fecal samples from individuals with Crohn’s disease and ulcerative colitis (24, 25). Whether the presence of Sutterella species at sites of human infection and inflammation represents cause or consequence or whether Sutterella is a normal part of the microbiota in some individuals remains unclear. The dearth of knowledge concerning the epidemiology and pathogenic potential of Sutterella derives in part from the lack of specific, culture-independent assays to detect and characterize members of this genus.
Here we further characterize Alcaligenaceae sequences identified in AUT-GI children and describe PCR assays for detection, quantitation, and genotyping of Sutterella as well as serological assays for detection of immunological responses to Sutterella.
RESULTS
High levels of Sutterella in a subset of AUT-GI patients identified by pyrosequencing.
Our previous pyrosequencing results (10) demonstrated a high abundance of sequences from the family Alcaligenaceae in nearly half of AUT-GI children (patients 1 to 15) and the absence of corresponding sequences in Control-GI children (patients 16 to 22) and prompted a more detailed investigation of these taxa of bacteria. Genus-level analysis of pyrosequencing reads revealed that all sequences of Alcaligenaceae found in AUT-GI patients’ biopsy samples were classified as members of the genus Sutterella. The average confidence estimate of all genus-level Ribosomal Database Project (RDP)-classified Sutterella sequences was high (99.1%), with the majority of sequences classified at 100% confidence.
Comparison of Sutterella abundance from pyrosequencing reads revealed significant increases in Sutterella in the ilea (Fig. 1A) (Mann-Whitney, tied P value = 0.022) and ceca (Fig. 1B) (Mann-Whitney, tied P value = 0.037) of AUT-GI children compared to Control-GI children. Individual analysis of AUT-GI patients revealed that 46.7% (7/15) of AUT-GI patients (patients 1, 3, 5, 7, 10, 11, and 12) had high levels of Sutterella 16S rRNA gene sequences in both the ileum (Fig. 1C; see Table S1 in the supplemental material) and cecum (Fig. 1D; see Table S1). Sutterella sequences were absent from all Control-GI samples (patients 16 to 22). In those seven AUT-GI patients with Sutterella sequences, ileal Sutterella sequence abundance ranged from 1.7 to 6.7% of total bacterial reads (Fig. 1C; see Table S1). For the same patients, cecal Sutterella sequence abundance ranged from 2.0 to 7.0% of total bacterial reads (Fig. 1D; see Table S1).
FIG 1 .
Presence of Sutterella sequences in a subset of AUT-GI patients. Shown are the results from detection by pyrosequencing of the V2 region of the 16S rRNA gene. (A and B) Distribution of Sutterella sequences as a percentage of total bacterial 16S rRNA gene reads from ileal (A; Mann-Whitney, tied P = 0.022) and cecal (B; Mann-Whitney, tied P = 0.037) biopsy samples from AUT-GI and Control-GI patients. (C to F) Distribution of Sutterella sequences by individual patient as a percentage of total bacteria (C and D) or total Betaproteobacteria (E and F) 16S rRNA reads from ileal (C and E) and cecal (D and F) biopsy samples from AUT-GI (patients 1 to 15) and Control-GI (patients 16 to 22) patients. *, P < 0.05.
To put the levels of Sutterella in these patients into perspective, we ranked the abundance of all ileal and cecal genus-level classifications from our pyrosequencing results. In the ileum, Sutterella sequences represented the fourth most abundant genus for patient 1, the sixth most abundant genus for patient 3, the fifth most abundant genus for patient 5, the fifth most abundant genus for patient 7, the third most abundant genus for patient 10, the eighth most abundant genus for patient 11, and the fifth most abundant genus for patient 12 (see Fig. S1 and S2 in the supplemental material). Similar rankings were obtained in the cecum of these patients (data not shown).
Sutterella sequences represented the majority of sequences present in the class Betaproteobacteria in these seven AUT-GI patients. In ileal biopsy samples from the seven AUT-GI patients with Sutterella sequences, Sutterella sequences accounted for 75.6% to 97.8% of all Betaproteobacteria sequences (Fig. 1E; see Table S1 in the supplemental material). In cecal biopsy samples, Sutterella sequences accounted for 92.1% to 98.2% of all Betaproteobacteria sequences (Fig. 1F; see Table S1).
OTU and sequence analysis of Sutterella sequences in AUT-GI children.
Operational taxonomic unit (OTU) analysis of V2 pyrosequencing reads in ileum (see Fig. S3A in the supplemental material) and cecum (see Fig. S3B) revealed that sequences from patients 1, 3, 10, 11, and 12 clustered together with OTU 2 containing the majority of Sutterella sequences, and sequences from patients 5 and 7 clustered together with OTU 1 containing the majority of Sutterella sequences. OTU 2 accounted for 87% and 84% for patient 1, 85% and 87% for patient 3, 66% and 66% for patient 10, 87% and 85% for patient 11, and 81% and 81% for patient 12 of all Sutterella sequences obtained by pyrosequencing of the 16S rRNA gene in ileum and cecum, respectively (Fig. 2). OTU 1 accounted for 88% and 86% for patient 5 and 88% and 83% for patient 7 of all Sutterella sequences obtained by pyrosequencing of the V2 region of the 16S rRNA gene in ileum and cecum, respectively (Fig. 2). Subdominant OTUs may represent true phylotypes, but they could also arise from PCR or sequencing artifacts. We focused our analysis on those OTUs containing the majority of Sutterella sequences, namely, OTU 1 and OTU 2.
FIG 2 .
Pie chart indicating the percentage of Sutterella sequences in the dominant OTU (either OTU 1 or OTU 2) relative to sequences from subdominant Sutterella OTUs in ileum and cecum of the seven Sutterella-positive patients. The percentage of the dominant OTU is shown per patient.
The representative sequences from OTU 1 and OTU 2 were aligned and used for phylogenetic analysis (see Fig. S4 in the supplemental material). The representative sequence from OTU 1 was phylogenetically most closely related to the species S. wadsworthensis; the representative sequence from OTU 2 was most closely related to S. stercoricanis. Although some branches in the tree are clearly differentiated by high bootstrap values, others are differentiated poorly by low bootstrap values. Furthermore, members of the genera Comamonas and Burkholderia were grouped with members of the genus Sutterella. This suggests that sequences from the V2 region alone may be insufficient for accurate species-level phylogenetic analysis of Sutterella sequences.
Confirmation and quantitation of Sutterella sequences using novel PCR assays.
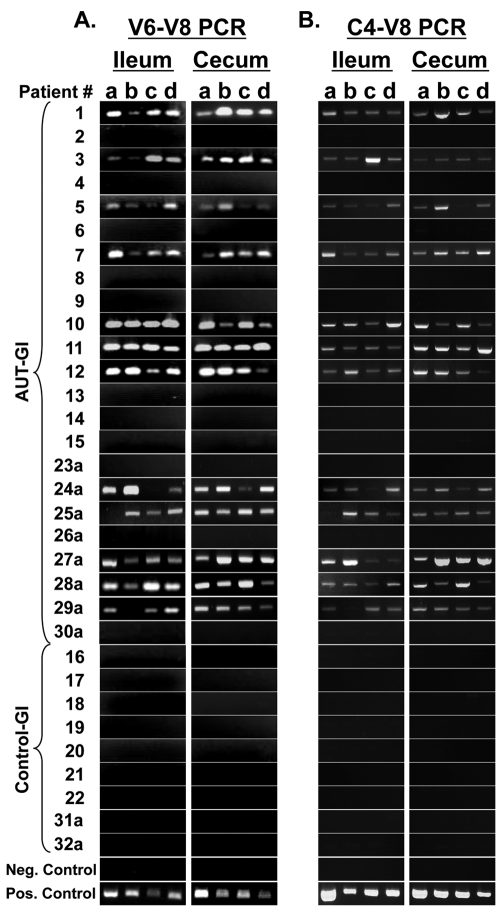
To independently verify V2 pyrosequencing results for Sutterella, we designed Sutterella-specific PCR assays that could be used in both conventional and real-time PCR, using primers that amplify a 260-bp region spanning the regions from V6 to V8 (V6–V8 PCR) of the 16S rRNA gene (SuttFor and SuttRev primers) (Fig. 3A to 3D). Conventional PCR analysis using DNA from each of 4 ileal and 4 cecal biopsy samples per patient showed that the same individuals identified as having high levels of Sutterella by V2 pyrosequencing (patients 1, 3, 5, 7, 10, 11, and 12) were also positive by our novel V6–V8 Sutterella-specific PCR (Fig. 4A). All four biopsy samples from ileum and cecum, in all seven Sutterella-positive patients, showed Sutterella products. A single 260-bp product was observed in positive amplifications, and nonspecific products were never observed. No products were observed in any Control-GI patients that were evaluated by pyrosequencing (patients 16 to 22), the AUT-GI patients that were negative for Sutterella sequences by V2 pyrosequencing (patients 2, 4, 6, 8, 9, 13, 14, and 15), or water/reagent controls (Fig. 4A). Furthermore, our positive control (DNA from a cultured S. wadsworthensis isolate) was positive by PCR. In addition to those patients evaluated by pyrosequencing, we have assessed ileal and cecal biopsy samples from eight male AUT-GI children (patients 23a to 30a) and two male Control-GI children (patients 31a and 32a) using our V6–V8 Sutterella PCR. Of these additional samples, 5 of the 8 AUT-GI patients were positive for Sutterella in ileal and cecal biopsy samples (patients 24a, 25a, 27a, 28a, and 29a). All biopsy samples from the two additional Control-GI patients were PCR negative (patients 31a and 32a). In summary, whereas 12 of 23 (52%) AUT-GI children were PCR positive for Sutterella, 0 of the 9 Control-GI children were PCR positive for Sutterella.
FIG 3 .
Sutterella-specific PCR assays. (A) Schematic representation showing the location of PCR primers and products evaluated in this study. (B) Sutterella-specific 16S rRNA gene (V6–V8) PCR amplification of 10-fold dilutions of Sutterella plasmid DNA standards spiked into ileal DNA from a Sutterella-negative Control-GI patient. Note the linear amplification down to 5 × 102 copies and the endpoint detection limit of 5 × 101 copies. (C) Real-time PCR amplification plot of 10-fold serial dilutions of Sutterella plasmid DNA standards. ΔRn, magnitude of the signal generated by the PCR conditions. (D) Standard curve generated from our Sutterella-specific quantitative real-time PCR assay.
FIG 4 .
PCR-based detection of Sutterella 16S rRNA gene sequences (V6–V8 region and C4–V8 region) in biopsies from AUT-GI and Control-GI patients. (A) Agarose gel detection of 260-bp Sutterella products in ileal (4 biopsy samples/patient) and cecal (4 biopsy samples/patient) biopsy DNA using SuttFor and SuttRev primers (V6–V8 region) in conventional PCR assays. (B) Agarose gel detection of 715-bp Sutterella products in ileal and cecal biopsy DNA using pan-bacterial primer 515For and SuttRev primer (C4–V8) in conventional PCR assays. The negative control is PCR reagents with water substituted for DNA. The positive control is DNA isolated from cultured S. wadsworthensis (ATCC 51579).
In addition, we used the broadly conserved, pan-bacterial primer 515For in combination with the SuttRev primer in conventional PCR assays (Fig. 4B). These primers amplify a 715-bp region of the 16S rRNA gene from conserved region 4 to variable region 8 (C4–V8 assay) (Fig. 3A). Results of the C4–V8 amplification were identical to those of the V6–V8 assay. All products were confirmed to represent Sutterella by sequencing of V6–V8 and C4–V8 products. These results suggest that the SuttRev primer is sufficient to confer specificity for Sutterella amplification.
In addition, we quantified Sutterella 16S rRNA gene sequences in biopsy samples from AUT-GI and Control-GI patients using real-time PCR (Fig. 3C and D). Real-time PCR analysis using the SuttFor and SuttRev (V6–V8) primers and a high-coverage TaqMan probe revealed similar results to conventional PCR assays. By real-time PCR, Sutterella was detected in patients 1, 3, 5, 7, 10, 11, 12, 24a, 25a, 27a, 28a, and 29a (Fig. 5), consistent with both pyrosequencing and conventional PCR results. Sutterella was undetectable in all Control-GI and Sutterella-negative AUT-GI patients’ samples. Mean Sutterella copy numbers were high in both the ileum and cecum (in the range of 103 to 105 copies) of Sutterella-positive patients.
FIG 5 .
Quantitation of Sutterella sequences in ileal and cecal biopsy samples from AUT-GI and Control-GI patients using a novel Sutterella-specific real-time PCR assay. Bars in the graph show mean copy number in 4 biopsy samples from ileum (blue) and 4 biopsy samples from cecum (red) + the standard error of the mean (SEM) for each patient.
Phylogenetic analysis of Sutterella sequences obtained by novel PCR assays.
Phylogenetic analysis of V6–V8 sequences obtained by library cloning of PCR products revealed similar results to those obtained by V2 pyrosequencing. While most V6–V8 sequences matched most closely with either S. wadsworthensis or S. stercoricanis, bootstrap values were low at many branches in phylogenetic trees (data not shown). Thus, neither the V2 nor V6–V8 regions appear to provide sufficient information for accurate species-level differentiation.
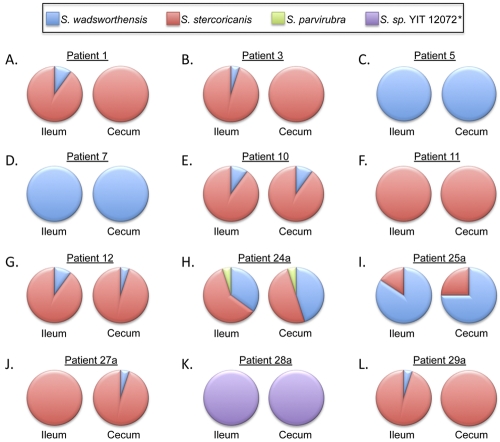
As C4–V8 PCR assays provide the longest 16S products containing the most variable regions, we also analyzed 480 sequences (40 sequences per patient, consisting of 20 ileal sequences and 20 cecal sequences) obtained from clone libraries of C4–V8 products from the 12 Sutterella-positive patients (Fig. 6). No sequences were obtained from any genus other than Sutterella from any cloned PCR products. Although one species predominated in each patient, mixed populations of S. wadsworthensis and S. stercoricanis were detected in several patients. The majority or all of the C4–V8 sequences from patients 1, 3, 10, 11, 12, 24a, 27a, and 29a matched most closely with S. stercoricanis, the majority or all of the C4–V8 sequences obtained from patients 5, 7, and 25a matched most closely with S. wadsworthensis, and all sequences obtained from patient 28a matched most closely with Sutterella sp. strain YIT 12072. The predominant Sutterella 16S rRNA gene sequences identified in ileal biopsy samples were identical to the predominant Sutterella sequences in cecal biopsy samples for each of the patients. Thus, a single predominant sequence was further assessed for each patient.
FIG 6 .
Distribution of Sutterella species in ileal and cecal biopsy samples from AUT-GI patients based on C4–V8 products. The closest sequence match to known Sutterella isolates was determined using the RDP seqmatch tool. The frequency of Sutterella species matches in ileal and cecal clone libraries are shown as pie charts for patients 1 (A), 3 (B), 5 (C), 7 (D), 10 (E), 11 (F), 12 (G), 24a (H), 25a (I), 27a (J), 28a (K), and 29a (L). *, Sutterella 16S sequences obtained from patient 28a were less than 97% similar to the 16S sequence of all known isolates of Sutterella species.
Alignment of the predominant C4–V8 sequence from each patient revealed that patients 1 and 24a had identical predominant sequences, but these were distinct from all other patients, patients 3, 10, 11, 12, 27a, and 29a had identical sequences, distinct from all other patients, patients 5, 7, and 25a had identical sequences that were distinct from all other patients, and patient 28a had a unique sequence (see Fig. S5 in the supplemental material).
Comparison of percentages of sequence similarity between these groups (Table 1) revealed 99.9% similarity between sequences of patients 1 and 24a and those of patients 3, 10, 11, 12, 27a, and 29a. This value is above the cutoff value of 97% similarity, commonly applied for bacterial species definition (26), suggesting that the predominant sequences from these two groups are likely the same species.
TABLE 1 .
Sequence similarity between 16S rRNA genes (C4–V8 region) of Sutterella from AUT-GI children and other Sutterella isolatesa
| Sutterella species | % similarity to rRNA gene from: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Sutterella from patients |
S. stercoricanis
(AJ566849) |
S. wadsworthensis
(GU585669) |
S. parvirubra
(AB300989) |
S. sanguinus
(AJ748647) |
Sutterella sp.
strain YIT 12072 (AB491210) |
||||
| 1 and 24a |
3, 10, 11, 12, 27a, and 29a |
5, 7, and 25a |
28a | ||||||
| Sutterella from patient(s): | |||||||||
| 1 and 24a | 99.9 | 94.8 | 93.8 | 98.5 | 94.8 | 95.4 | 96.3 | 93.2 | |
| 3, 10, 11, 12, 27a, and 29a | 94.7 | 93.6 | 98.4 | 94.7 | 95.4 | 96.4 | 93.3 | ||
| 5, 7, and 25a | 92.8 | 94.7 | 100 | 96.6 | 93.6 | 92.9 | |||
| 28a | 93.0 | 92.8 | 93.6 | 92.0 | 95.3 | ||||
| S. stercoricanis (AJ566849) | 94.7 | 94.7 | 96.6 | 93.2 | |||||
| S. wadsworthensis (GU585669) | 96.6 | 93.6 | 92.9 | ||||||
| S. parvirubra (AB300989) | 95.0 | 92.6 | |||||||
| S. sanguinis (AJ748647) | 92.2 | ||||||||
| Sutterella sp. strain YIT 12072 (AB491210) | |||||||||
The highest sequence similarities are shown in boldface. Accession numbers are given in parentheses.
The predominant sequences from patients 1 and 24a and patients 3, 10, 11, 12, 27a, and 29a had the highest percent similarity to the S. stercoricanis isolate (98.5% and 98.4% similarity, respectively) and below 97% similarity compared to other Sutterella isolates (Table 1). In addition, the 16S rRNA gene sequence from patients 1 and 24a shared 100% similarity with 16S rRNA gene sequences from uncultured bacteria in GenBank, such as those derived from intestinal biopsy samples from an ulcerative colitis patient (i.e., accession no. FJ512128) (27) and mucosal biopsy samples from the intestinal pouch of a familial adenomatous polyposis patient (i.e., accession no. GQ159316). Similarly, the sequences from patients 3, 10, 11, 12, 27a, and 29a shared 100% similarity with 16S rRNA gene sequences from uncultured bacteria in GenBank, including sequences derived from intestinal biopsy samples from a patient with ulcerative colitis (i.e., accession no. 512152) (27) and fecal samples from bovines (i.e., accession no. FJ682648) (28).
Sequences from patients 5, 7, and 25a had 100% sequence similarity to S. wadsworthensis and below 97% sequence similarity to all other Sutterella isolates (Table 1). The sequences from patients 5, 7, and 25a also shared 100% sequence similarity to 16S rRNA sequences in GenBank, such as those derived from intestinal biopsy samples from an ulcerative colitis patient (i.e., accession no. FJ509042) (27).
The unique sequence found in patient 28a matched most closely with the isolate Sutterella sp. strain YIT 12072; however, the similarity was only 95.3% (Table 1). Thus, based on sequence analysis alone, Sutterella sequences from patient 28a cannot be classified as Sutterella sp. strain YIT 12072 or any of the other known isolates. The 16S rRNA gene sequence from patient 28a shared 100% similarity with 16S rRNA gene sequences from uncultured bacteria in GenBank that were derived from intestinal biopsy samples from a Crohn’s disease patient (i.e., accession no. FJ503635) (27), human skin popliteal fossa swab (i.e., accession no. HM305996), and feces from a 95-year-old woman (i.e., accession no. EF401376) (29). Thus, the 16S rRNA gene sequences from patient 28a and identical GenBank sequences likely represent an uncharacterized species of Sutterella.
Phylogenetic analysis of the predominant sequences obtained from patient biopsy samples using the C4–V8 PCR assay revealed high bootstrap values at most branches and good grouping of members of the genus Sutterella from other Alcaligenaceae family members and other Burkholderiales order members (Fig. 7). Thus, sequences obtained by C4–V8 PCR can be used for accurate species-level classification of Sutterella sequences. This tree demonstrates that sequences from patients 1, 24a, 3, 10, 11, 12, 27a, and 29a grouped most closely with S. stercoricanis (supported by a bootstrap resampling value of 92%), sequences from patients 5, 7, and 25a grouped most closely with S. wadsworthensis (supported by a bootstrap resampling value of 99%), and sequences from patient 28a grouped most closely with the isolate Sutterella sp. strain YIT 12072 (supported by a bootstrap resampling value of 97%) but formed a distinct phylogenetic lineage.
FIG 7 .
Phylogenetic tree based on predominant 16S rRNA gene sequences obtained by C4–V8 Sutterella PCR from AUT-GI patients, Sutterella species isolates, and related species. The tree was constructed by the neighbor-joining method. Bootstrap values (>60%) based on 1,000 replicates are shown next to the branches. There were a total of 653 positions in the final data set. The evolutionary distances were computed using the Jukes-Cantor method and are in units representing the number of base substitutions per site. The optimal tree with the sum of branch length of 0.66371685 is shown. The tree is rooted to the outgroup Escherichia coli. Accession numbers are shown in parentheses. AUT-GI patient sequences are boxed in red.
AUT-GI plasma antibodies bind to S. wadsworthensis proteins.
We also sought to determine whether systemic antibody responses to Sutterella were present in this cohort. The antigens used for our Western blot analysis were whole-protein lysates from cultured S. wadsworthensis containing a wide range of proteins, as observed on Coomassie-stained SDS-polyacrylamide gels (data not shown). Individual patient’s plasma was assessed for IgG (Fig. 8A) and IgM (Fig. 8B) antibody immunoreactivity against the bacterial antigens. Immunoreactive bands were visible for 11 out of 23 (48%) AUT-GI patients. In 10 AUT-GI children, the immunoreactive antibodies were IgG (Fig. 8A); one child (patient 26a) had IgM antibodies (Fig. 8B). In contrast, only 1 of the 9 (11%) Control-GI patients (patient 21) had weak immunoreactivity to 84-kDa and 41-kDa Sutterella proteins. A total of 11 distinct immunoreactive protein bands were identified, based on size (104, 89, 84, 62, 56, 50, 48, 44, 41, 30, and 27 kDa). AUT-GI patients 1 and 5 (both positive by PCR) had the most immunoreactive protein bands, with four protein bands in common (89, 62, 56, and 41 kDa). The 89-kDa band was detected by IgG or IgM antibodies in seven AUT-GI patients. The 56-, 41-, and 30-kDa bands were detected by IgG antibodies in each of three patients. The other bands (104, 84, 62, 50, 48, and 44 kDa) were less frequent.
FIG 8 .
Western immunoblot analysis of AUT-GI and Control-GI patients’ plasma antibody immunoreactivity against S. wadsworthensis antigens. (A) Patients’ plasma IgG antibody immunoreactivity against S. wadsworthensis antigens. (B) Patients’ IgM antibody immunoreactivity against S. wadsworthensis antigens. 2°, secondary antibody control.
Of the 12 AUT-GI patients that were PCR positive for Sutterella, 8 (66.7%) had plasma IgG antibodies against S. wadsworthensis proteins (patients 1, 3, 5, 7, 10, 11, 24a, and 25a). Three AUT-GI patients (patients 4, 23a, and 26a) had IgG or IgM antibodies against S. wadsworthensis proteins, but were PCR negative. In total, 15 out of 23 (65.2%) AUT-GI children had evidence of Sutterella by either PCR or serology (Table 2).
TABLE 2 .
Summary of results from PCR assays and Western immunoblot analysis
| Patient | PCR | IgG | Molecular mass(es) (kDa) of bands with IgG | IgM | Molecular mass (kDa) of bands with IgM | Any Ig positive | Any PCR or lg positive |
|---|---|---|---|---|---|---|---|
| AUT-GI | |||||||
| 1 | + | ++ | 89, 62, 56, 41 | − | Yes | Yes | |
| 2 | − | − | − | No | No | ||
| 3 | + | + | 30 | − | Yes | Yes | |
| 4 | − | ++ | 89 | − | Yes | Yes | |
| 5 | + | ++ | 89, 62, 56, 48, 44, 41 | − | Yes | Yes | |
| 6 | − | − | − | No | No | ||
| 7 | + | ++ | 50, 44 | − | Yes | Yes | |
| 8 | − | − | − | No | No | ||
| 9 | − | − | − | No | No | ||
| 10 | + | ++ | 30 | − | Yes | Yes | |
| 11 | + | + | 89, 48 | − | Yes | Yes | |
| 12 | + | − | − | No | Yes | ||
| 13 | − | − | − | No | No | ||
| 14 | − | − | − | No | No | ||
| 15 | − | − | − | No | No | ||
| 23a | − | ++ | 104, 30, 27 | − | Yes | Yes | |
| 24a | + | + | 89 | − | Yes | Yes | |
| 25a | + | ++ | 89, 56 | − | Yes | Yes | |
| 26a | − | − | ++ | 89 | Yes | Yes | |
| 27a | + | − | − | No | Yes | ||
| 28a | + | − | − | No | Yes | ||
| 29a | + | − | − | No | Yes | ||
| 30a | − | − | − | No | No | ||
| Control-GI | |||||||
| 16 | − | − | − | No | No | ||
| 17 | − | − | − | No | No | ||
| 18 | − | − | − | No | No | ||
| 19 | − | − | − | No | No | ||
| 20 | − | − | − | No | No | ||
| 21 | − | + | 84, 41 | − | Yes | Yes | |
| 22 | − | − | − | No | No | ||
| 31a | − | − | − | No | No | ||
| 32a | − | − | − | No | No | ||
| % positive | |||||||
| AUT-GI | 52 | 43 | 4 | 48 | 65 | ||
| Control-GI | 0 | 11 | 0 | 11 | 11 |
DISCUSSION
We previously reported detection by pyrosequencing of Alcaligenaceae sequences in AUT-GI children (10). More focused analysis revealed that this finding reflects the presence of Sutterella species. Whereas 12 of 23 AUT-GI patients (52%) were PCR positive in both ileum and cecum, 0 of 9 Control-GI children were PCR positive for Sutterella. Sutterella abundance in the seven Sutterella-positive AUT-GI patients, assessed by pyrosequencing, ranged from 1 to 7% of total bacterial sequences. Novel real-time PCR assays confirmed high copy numbers of Sutterella species in DNA from ileal and cecal biopsy samples from Sutterella-positive patients, with averages ranging from 103 to 105 Sutterella 16S rRNA gene copies amplified from only 25 ng of total genomic biopsy DNA.
OTU analysis of V2 region pyrosequencing reads indicated that only two OTUs accounted for the majority of Sutterella sequences in the seven AUT-GI patients that were Sutterella positive by pyrosequencing. Sequencing of PCR products from V6–V8 and C4–V8 Sutterella-specific PCR assays corroborated this finding. Our analysis also suggests that C4–V8 Sutterella products can be accurately classified at the species level. Classification with RDP and phylogenetic analysis of Sutterella sequences obtained from C4–V8 Sutterella-specific PCR indicated that the predominant sequences obtained from patients 1, 3, 10, 11, 12, 24a, 27a, and 29a were most closely related to the isolate S. stercoricanis, supported by a sequence similarity of over 98%. The predominant C4–V8 sequences obtained from patients 5, 7, and 25a were most closely related to the isolate S. wadsworthensis, supported by a sequence similarity of 100%. Our results suggest that these two species of Sutterella are the dominant phylotypes present at high levels in the intestines of AUT-GI children in this cohort. Of the known isolates, the predominant C4–V8 sequence obtained from patient 28a was most closely related to Sutterella sp. strain YIT 12072. However, the low sequence similarity (95.3%) between sequences from patient 28a and Sutterella sp. strain YIT 12072 suggests that these are not likely to be the same species. Sequences from patient 28a did have 100% sequence similarity to uncultured Sutterella sequences in GenBank, suggesting that this undefined species has been detected previously in human samples by nonspecific techniques.
Sutterella species have been isolated from human and animal feces (30–32) and have also been isolated from human infections below the diaphragm; most often from patients with appendicitis, peritonitis, or rectal or perirectal abscesses (22, 23). Sutterella sequences have been identified in fecal samples and intestinal biopsy samples from individuals with Crohn’s disease and ulcerative colitis but also from apparently healthy adults (24, 25, 27, 33). Thus, based on these previous findings, it remains unclear whether Sutterella species contribute to inflammation and infection or are simply normal inhabitants of the human microbiota in some individuals. Even if the latter is the case, our results demonstrate that Sutterella is a major component of the mucoepithelial microbiota in some children, accounting for up to 7% of all bacteria. Relative to all other bacterial genera identified in biopsy samples, Sutterella ranged from the third to eighth most abundant genus in the patients assessed by pyrosequencing. Only the most abundant Bacteroidetes and Firmicutes genera outnumbered Sutterella sequences. This result is remarkable given that Sutterella is not reported as a major component of the microbiota (34).
Loss of commensals in the intestine can affect immune responses and disrupt colonization resistance to potentially pathogenic bacteria (17, 19). In our previous study, we found a significant loss of commensals, namely, members of the phylum Bacteroidetes, in AUT-GI biopsy samples (10). Thus, the loss of Bacteroidetes in AUT-GI children could facilitate the growth of opportunistic pathogens. Whether Sutterella is pathogenic in AUT-GI children cannot be determined from current data. However, the observation that some AUT-GI children have antibodies that react with S. wadsworthensis proteins is generally consistent with infection. We detected either IgG or IgM antibodies against S. wadsworthensis proteins in ~48% (11/23) of AUT-GI children. Only one Control-GI child had very weak IgG immunoreactivity against S. wadsworthensis proteins. Of the 12 patients that were positive for Sutterella by PCR, 8 (66.7%) demonstrated plasma IgG antibodies against S. wadsworthensis proteins. In total, 65.2% (15 out of 23) of AUT-GI children were either positive by PCR assays or had immunoglobulin reactivity to S. wadsworthensis proteins. Three AUT-GI patients were negative by PCR but had IgG or IgM antibodies against S. wadsworthensis proteins. As we only examined ileal and cecal biopsy samples in this cohort, we cannot exclude the possibility that Sutterella species were present in other regions of the small or large intestine or elsewhere in the body of these three patients. This could explain the presence of Sutterella-specific antibodies without detection of the agent by PCR. Alternatively, IgG antibodies may persist long after antigenic exposure; thus, the presence of IgG antibodies may indicate past exposure in some children. The IgM immunoreactivity of patient 26a suggests recent or current exposure to Sutterella antigen in this patient. It is perhaps not surprising that proteins recognized by different patients’ plasma were variable. It is well recognized that the use of different strains and species as antigen leads to variations in the immunoreactive profile of immunogenic proteins (35). Several Sutterella-positive patients in this study had S. stercoricanis as the dominant Sutterella species. We recognize this limitation of our Western blot analysis, as the only antigen available to us was lysate from an S. wadsworthensis isolate.
The nature of intestinal damage in autism has not been fully defined. Abnormalities in intestinal permeability in children with autism have been reported in two studies (8, 9). In Crohn’s disease, a condition associated with increased intestinal permeability, a generalized enhancement of antimicrobial IgG to many members of the intestinal microbiota is reported (36). A defective epithelial barrier could lead to enhanced contact between many members of the microbiota and antigen-presenting cells in the lamina propria. If this turns out to be the case in autism, then antibodies against Sutterella proteins may reflect interindividual, compositional variation in the microbiota, rather than being an indication of Sutterella infection. Additional studies are warranted in order to draw definitive conclusions from this immunological analysis.
In conclusion, we have identified Sutterella 16S rRNA gene sequences in mucoepithelial biopsy samples from AUT-GI children using nonspecific, pan-microbial pyrosequencing. We have further designed and applied novel Sutterella-specific PCR assays that confirmed high levels of Sutterella species in over half of AUT-GI children and the complete absence of Sutterella in Control-GI children tested in this study. The Sutterella-specific molecular assays reported in this study will enable more directed studies to detect, quantify, and classify this poorly understood bacterium in biological and environmental samples. With such specific techniques, we can begin to understand the epidemiology of this bacterium and its associations with human infections and inflammatory diseases, the role Sutterella plays in the microbiota, and the extent to which Sutterella may contribute to the pathogenesis of GI disturbances in children with autism.
MATERIALS AND METHODS
Clinical samples.
The clinical procedures used for this study population were previously described (10, 37). For more details, see Text S1 in the supplemental material. The Institutional Review Board (IRB) at Columbia University Medical Center reviewed and approved the use of deidentified residual ileal and cecal samples, obtained as described in an earlier publication (37), and waived the need for patient consent for these analyses, as all samples were analyzed anonymously. All patients underwent upper and/or lower endoscopic procedures based on clinical imperative. Pinch biopsy samples were obtained, by an experienced gastroenterologist, from the terminal ileum (4 per patient) and cecum (4 per patient) during endoscopy. Endoscopic biopsy samples were immediately placed in coded tubes, snap-frozen in liquid nitrogen, and stored at −70°C until processed. All AUT-GI and Control-GI patients and their biopsy samples evaluated in this study were derived from the initial cohort (37). Patients assessed by pyrosequencing were restricted to male children between 3 and 5 years of age to control for confounding effects of gender and age on the microbiota (10, 37). This subset comprised 15 AUT-GI (patients 1 to 15) and 7 Control-GI (patients 16 to 22) children. For assessment of Sutterella sequences in ileal and cecal biopsy samples, we also included 8 additional male AUT-GI children (patients 23a to 30a: 6 children between 6 and 7 years of age and 2 children between 8 and 10 years of age) and 2 additional male Control-GI children (patients 31a and 32a: 1 child between 6 and 7 years of age and 1 child between 8 and 10 years of age) from the initial cohort (37).
Bacterial culture.
S. wadsworthensis was obtained from American Type Culture Collection (ATCC 51579). The isolate was grown in chopped meat broth in Hungate capped tubes (Anaerobe Systems, Morgan Hill, CA), supplemented with sodium formate and fumaric acid at a final concentration of 0.3% each. Inoculated cultures were incubated at 37°C, and growth was monitored at 0, 6, 12, 24, and 48 h using a Sutterella-specific real-time PCR assay (see below).
DNA extraction.
DNA was extracted from individual ileal and cecal biopsy samples (total of 256 biopsy samples: 128 ileal and 128 cecal, 8 biopsy samples per patient [4 from ileum and 4 from cecum], 23 AUT-GI patients and 9 Control-GI patients) and bacterial cultures of S. wadsworthensis in TRIzol (Invitrogen, Carlsbad, CA) using standard protocols. DNA concentrations and integrity were determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE) and Bioanalyzer (Agilent Technologies, Foster City, CA) and stored at –80°C.
Bar-coded pyrosequencing of the bacterial V2 region of the 16S rRNA gene and analyses are previously described for ileal and cecal biopsy samples from AUT-GI patients 1 to 15 and Control-GI patients 16 to 22 (10). The pan-bacterial bar-coded V2 primers, designated V2For and V2Rev, amplify a region of the 16S rRNA gene from nucleotide positions 27 to 338 (38) (Fig. 3A). For more details, see Text S1 in the supplemental material.
Sutterella-specific PCR assay design.
Sutterella-specific 16S rRNA PCR primers were designed against the 16S rRNA gene sequence for S. wadsworthensis (accession no. L37785) using Primer Express 1.0 software (Applied Biosystems, Foster City, CA). Genus specificity of candidate primers was evaluated using the RDP (Ribosomal Database Project) probe match tool. Several potential primer pairs were identified, but only one pair showed high specificity for Sutterella. These primers are designated here as SuttFor (nucleotide positions 936 to 956 of S. wadsworthensis; accession no. L37785) and SuttRev (nucleotide positions 1177 to 1195 of S. wadsworthensis; accession no. L37785) (see Table S2 in the supplemental material). SuttFor and SuttRev primers amplify a 260-bp region between variable regions 6, 7, and 8 (V6–V8) of the 16S rRNA gene of Sutterella (Fig. 3A). For more details on primer and probe specificity see Text S1 in the supplemental material.
Conventional PCR assays.
Conventional PCR for detection of Sutterella was carried out in 25-µl reaction mixtures consisting of 25 ng of biopsy DNA or 25 pg of genomic DNA from cultured S. wadsworthensis (ATCC 51579; positive control), 300 nM each of the SuttFor and SuttRev primers (for V6–V8 amplification) or 515For and SuttRev (for C4–V8 amplification), 2 µl deoxynucleoside triphosphate (dNTP) mix (10 mM; Applied Biosystems, Foster City, CA), 2.5 µl of 10× PCR buffer (Qiagen, Valencia, CA), 5 U of HotStarTaq DNA polymerase (Qiagen), and 5 µl Q-solution (Qiagen). Cycling parameters consisted of an initial denaturation step at 95°C for 15 min, followed by 30 cycles of 94°C for 1 min, 60°C for 1 min, 72°C for 1 min, and a final extension at 72°C for 5 min. The amplified product was detected by electrophoresis on a 1.5% agarose gel stained with ethidium bromide. To confirm specificity of PCR amplification, V6–V8 products were gel extracted and sent for direct sequencing with SuttFor and SuttRev primers. Additionally, V6–V8 and C4–V8 products were subcloned into the vector pGEM-T Easy (Promega, Madison, WI), and bacterial libraries were created. One hundred twenty V6–V8 plasmid clones were sequenced. A total of 480 C4–V8 colonies were sequenced and analyzed (40 sequences from each of the 12 PCR-positive patients, 20 sequences each from ileal and cecal biopsy samples). All V6–V8 and C4–V8 plasmid clones were found to contain Sutterella sequences, using the RDP classifier tool with a minimum 80% bootstrap confidence estimate. The closest sequence match to Sutterella isolates was determined using the RDP seqmatch tool. Sequences from each patient were aligned using MacVector, and a consensus sequence was determined from the predominant Sutterella species in each patient. For details on Sutterella V6–V8 conventional PCR sensitivity, linearity, and endpoint detection limit, see Text S1 in the supplemental material.
Quantitative real-time PCR assay.
PCR standards for determining copy numbers of bacterial 16S rRNA genes were prepared from products of the partial 16S rRNA gene (V6–V8 region) of S. wadsworthensis (accession no. GU585669). A representative product with high sequence similarity to the Bacteroides intestinalis (accession no. NZ[lowem]ABJL02000007) 16S rRNA gene was used with broadly conserved total bacterial primers (10, 39). Products were cloned into the vector pGEM-T Easy (Promega), and 10-fold serial dilutions of linearized plasmid standards were created ranging from 5 × 105 to 5 × 10° copies. Amplification and detection of DNA by real-time PCR were performed with the ABI StepOne Plus real-time PCR system (Applied Biosystems). Linearity and sensitivity of plasmid standards were tested with the SuttFor and SuttRev primers and the SuttProbe. Amplification plots of plasmid standards indicated sensitivity of detection down to 5 copies of plasmid (Fig. 3C), and standard curves generated from plasmid dilutions had correlation coefficients of 0.996 (Fig. 3D). For detailed protocols for real-time PCR set up, analysis, and primers and probe used, see Text S1 in the supplemental material.
Bioinformatics analysis.
Operational taxonomic unit (OTU)-based analysis of pyrosequencing data was carried out in MOTHUR (version 1.8.0) and as previously described (10, 40). For a detailed description of OTU analysis, see Text S1 in the supplemental material.
Phylogenetic analysis of Sutterella sequences.
Phylogenetic analyses were conducted in MEGA4 (41). Sequence alignments were based on representative sequences from OTU 1 and OTU 2, obtained from pyrosequencing analysis of the V2 region of the 16S rRNA gene, as well as sequences of Sutterella from our V6–V8 (SuttFor and SuttRev amplifications) conventional PCR assay, and the predominant sequences obtained from clone libraries of the C4–V8 (515For and SuttRev amplifications) conventional PCR assay. Primer sequences were trimmed from the sequences. Classification was confirmed using the RDP classifier and seqmatch tools. Sutterella sequences obtained from ileal and cecal biopsy samples were aligned with sequences from 8 isolates of Sutterella found in the RDP database and sequences from 14 additional related species (members of the family Alcaligenaceae and order Burkholderiales). Sequences from Sutterella isolates and related species were trimmed to the length of the sequences obtained from ileal and cecal biopsy samples from AUT-GI patients. Phylogenetic trees were constructed according to the neighbor-joining method with evolutionary distances determined using the Jukes-Cantor method (42, 43). Trees were rooted to the outgroup Escherichia coli (accession no. X80725). The stability of the groupings was estimated by bootstrap analysis (1,000 replications) using MEGA4. The percentages of 16S rRNA gene sequence similarity were determined for Sutterella C4–V8 products and Sutterella isolates using the EzTaxon server 2.1 (http://www.eztaxon.org/) (44).
Western immunoblots.
Soluble proteins of cultured S. wadsworthensis (ATCC 51579) were extracted and used as the antigen in immunoblot assays. S. wadsworthensis antigens were separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked, incubated with each patient’s plasma (diluted 1:100 in blocking solution), probed with secondary antibodies (either peroxidase-conjugated goat anti-human IgG [Fcγ fragment-specific; Jackson ImmunoResearch, West Grove, PA] or peroxidase-conjugated goat anti-human IgM [Fc5μ fragment-specific; Jackson ImmunoResearch]), and developed with the ECL Plus Western blot detection system (Amersham Biosciences, Arlington Heights, IL). For detailed protocols for antigen preparation and immunoblotting see Text S1 in the supplemental material.
SUPPLEMENTAL MATERIAL
Supporting materials and methods. Download Text S1, DOC file, 0.1 MB.
Summary of total bacterial, Betaproteobacteria, and Sutterella sequences obtained by 16S rRNA gene (V2 region) pyrosequencing from ileal and cecal biopsy samples from AUT-GI and Control-GI children.
Primers and probes used for conventional PCR or real-time PCR amplification and quantitation of Sutterella species.
Abundance distribution of all genus-level classifications of sequences from pyrosequencing for patients 1, 3, 5, and 7. A bar graph shows all ileal genera, in order of highest abundance (top) to lowest abundance (bottom), from patient 1 (32 total genera) (A), patient 3 (35 total genera) (B), patient 5 (39 total genera) (C), and patient 7 (39 total genera) (D). The abundances of Sutterella sequences are indicated in red. Note that unclassified family members may represent more than one genus (i.e., “Unclassified Lachnospiraceae”). Download Figure S1, TIF file, 1 MB.
Abundance distribution of all genus-level classifications of sequences from pyrosequencing for patients 10, 11, and 12. The bar graph shows all ileal genera, in order of highest abundance (top) to lowest abundance (bottom) from patient 10 (32 total genera) (A), patient 11 (39 total genera) (B), and patient 12 (44 total genera) (C). The abundances of Sutterella sequences are indicated in red. Note that unclassified family members may represent more than one genus (i.e., “Unclassified Lachnospiraceae”). Download Figure S2, TIF file, 0.8 MB.
Sutterella OTU analysis. Shown is a heat map generated from OTU analysis of all Sutterella sequences by patient. Note patients 1, 3, 10, 11, and 12 cluster together, and the majority of Sutterella sequences are present in OTU 2. Patients 5 and 7 cluster together, and the majority of Sutterella sequences are present in OTU 1. The heat map scale represents OTU abundance (expressed as the percentage of total bacterial pyrosequencing reads per patient). Download Figure S3, TIF file, 0.7 MB.
Phylogenetic tree based on the representative 16S rRNA gene sequences obtained by V2 region pyrosequencing (OTU 1 and OTU 2) from AUT-GI patients, Sutterella species isolates, and related species. The tree was constructed by the neighbor-joining method. Bootstrap values based on 1,000 replicates are shown next to the branches (percentage of bootstrap support). There were a total of 218 positions in the final data set. The evolutionary distances were computed using the Jukes-Cantor method and are in units representing the number of base substitutions per site. The optimal tree with a sum of branch length of 1.01142743 is shown. The tree is rooted to the outgroup Escherichia coli. Accession numbers are shown in parentheses. The locations of AUT-GI patients’ representative OTU 1 and OTU 2 sequences are boxed in red. Download Figure S4, TIF file, 0.5 MB.
Sutterella sequence alignment. Shown is ClustalW alignment of the most abundant Sutterella 16S rRNA gene (C4-to-V8 region) sequences in the 12 Sutterella-positive patients. Sequences have had the 515For and SuttRev primer sequences removed. The positions of the beginning (nucleotide position 501) and end (nucleotide position 1176) of the sequences are relative to the 16S rRNA gene of S. wadsworthensis (accession no. L37785). Download Figure S5, TIF file, 1.4 MB.
ACKNOWLEDGMENTS
This work is supported by National Institutes of Health awards AI57158 and NS047537 and the Defense Threat Reduction Agency, Department of Defense.
We thank Timothy Buie and Margaret Bauman for their role in sample collection and characterization. We also thank Ivan Wick, Omar Jabado, Craig Street, and Komal Jain for data management and Ashlee Bennett for technical support.
Footnotes
Citation Williams BL, Hornig M, Parekh T, Lipkin WI. 2012. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. mBio 3(1):e00261-11. doi:10.1128/mBio.00261-11.
REFERENCES
- 1. Buie T, et al. 2010. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics 125(Suppl. 1):S1–S18 [DOI] [PubMed] [Google Scholar]
- 2. Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. 2011. Gastrointestinal flora and gastrointestinal status in children with autism—comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 11:22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. White JF. 2003. Intestinal pathophysiology in autism. Exp. Biol. Med. (Maywood) 228:639–649 [DOI] [PubMed] [Google Scholar]
- 4. Horvath K, Papadimitriou JC, Rabsztyn A, Drachenberg C, Tildon JT. 1999. Gastrointestinal abnormalities in children with autistic disorder. J. Pediatr. 135:559–563 [DOI] [PubMed] [Google Scholar]
- 5. Ashwood P, Wills S, Van de Water J. 2006. The immune response in autism: a new frontier for autism research. J. Leukoc. Biol. 80:1–15 [DOI] [PubMed] [Google Scholar]
- 6. Enstrom AM, Onore CE, Van de Water JA, Ashwood P. 2010. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav. Immun. 24:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jyonouchi H, Geng L, Ruby A, Zimmerman-Bier B. 2005. Dysregulated innate immune responses in young children with autism spectrum disorders: their relationship to gastrointestinal symptoms and dietary intervention. Neuropsychobiology 51:77–85 [DOI] [PubMed] [Google Scholar]
- 8. D’Eufemia P, et al. 1996. Abnormal intestinal permeability in children with autism. Acta Paediatr. 85:1076–1079 [DOI] [PubMed] [Google Scholar]
- 9. de Magistris L, et al. 2010. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J. Pediatr. Gastroenterol. Nutr. 51:418–424 [DOI] [PubMed] [Google Scholar]
- 10. Williams BL, et al. 2011. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One 6:e24585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Finegold SM, et al. 2002. Gastrointestinal microflora studies in late-onset autism. Clin. Infect. Dis. 35:S6–S16 [DOI] [PubMed] [Google Scholar]
- 12. Song Y, Liu C, Finegold SM. 2004. Real-time PCR quantitation of clostridia in feces of autistic children. Appl. Environ. Microbiol. 70:6459–6465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parracho HM, Bingham MO, Gibson GR, McCartney AL. 2005. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 54:987–991 [DOI] [PubMed] [Google Scholar]
- 14. Wang L, et al. 2011. Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl. Environ. Microbiol. 77:6718–6721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finegold SM, et al. 2010. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 16:444–453 [DOI] [PubMed] [Google Scholar]
- 16. O’Hara AM, Shanahan F. 2006. The gut flora as a forgotten organ. EMBO Rep. 7:688–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Macpherson AJ, Harris NL. 2004. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 4:478–485 [DOI] [PubMed] [Google Scholar]
- 18. Heijtz RD, et al. 2011. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. U. S. A. 108:3047–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vollaard EJ, Clasener HA. 1994. Colonization resistance. Antimicrob. Agents Chemother. 38:409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Busse HJ, Auling G. 2005. Family III. Alcaligenaceae, p 647–653. In Brenner DJ, Krieg NR, Staley JT, Bergey’s manual of systematic bacteriology, vol 2, part C. The Proteobacteria. Springer-Verlag, New York, NY. [Google Scholar]
- 21. Wexler HM. 2005. Genus VIII. Sutterella, p 682–683. Brenner DJ, Krieg NR, Staley JT, Bergey’s manual of systematic bacteriology, vol 2, part C. The Proteobacteria. Springer-Verlag, New York, NY. [Google Scholar]
- 22. Wexler HM, et al. 1996. Sutterella wadsworthensis gen. nov., sp. nov., bile-resistant microaerophilic Campylobacter gracilis-like clinical isolates. Int. J. Syst. Bacteriol. 46:252–258 [DOI] [PubMed] [Google Scholar]
- 23. Molitoris E, Wexler HM, Finegold SM. 1997. Sources and antimicrobial susceptibilities of Campylobacter gracilis and Sutterella wadsworthensis. Clin. Infect. Dis. 25(Suppl. 2):S264–S265 [DOI] [PubMed] [Google Scholar]
- 24. Mangin I, et al. 2004. Molecular inventory of faecal microflora in patients with Crohn’s disease. FEMS Microbiol. Ecol. 50:25–36 [DOI] [PubMed] [Google Scholar]
- 25. Gophna U, Sommerfeld K, Gophna S, Doolittle WF, Veldhuyzen van Zanten SJ. 2006. Differences between tissue-associated intestinal microfloras of patients with Crohn’s disease and ulcerative colitis. J. Clin. Microbiol. 44:4136–4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stackebrandt E, Goebel BM. 1994. A place for DNA-DNA reassociation and 16S ribosomal-RNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44:846–849 [Google Scholar]
- 27. Walker AW, et al. 2011. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 11:7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Durso LM, et al. 2010. Animal-to-animal variation in fecal microbial diversity among beef cattle. Appl. Environ. Microbiol. 76:4858–4862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li M, et al. 2008. Symbiotic gut microbes modulate human metabolic phenotypes. Proc. Natl. Acad. Sci. U. S. A. 105:2117–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Engberg J, On SL, Harrington CS, Gerner-Smidt P. 2000. Prevalence of Campylobacter, Arcobacter, Helicobacter, and Sutterella spp. in human fecal samples as estimated by a reevaluation of isolation methods for campylobacters. J. Clin. Microbiol. 38:286–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sakon H, Nagai F, Morotomi M, Tanaka R. 2008. Sutterella parvirubra sp. nov. and Megamonas funiformis sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 58:970–975 [DOI] [PubMed] [Google Scholar]
- 32. Greetham HL, et al. 2004. Sutterella stercoricanis sp. nov., isolated from canine faeces. Int. J. Syst. Evol. Microbiol. 54:1581–1584 [DOI] [PubMed] [Google Scholar]
- 33. Hong PY, Croix JA, Greenberg E, Gaskins HR, Mackie RI. 2011. Pyrosequencing-based analysis of the mucosal microbiota in healthy individuals reveals ubiquitous bacterial groups and micro-heterogeneity. PLoS One 6:e25042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arumugam M, et al. 2011. Enterotypes of the human gut microbiome. Nature 473:174–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mavin S, et al. 2011. Interpretation criteria in western blot diagnosis of Lyme borreliosis. Br. J. Biomed. Sci. 68:5–10 [DOI] [PubMed] [Google Scholar]
- 36. Adams RJ, et al. 2008. IgG antibodies against common gut bacteria are more diagnostic for Crohn’s disease than IgG against mannan or flagellin. Am. J. Gastroenterol. 103:386–396 [DOI] [PubMed] [Google Scholar]
- 37. Hornig M, et al. 2008. Lack of association between measles virus vaccine and autism with enteropathy: a case-control study. PLoS One 3:e3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R. 2008. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat. Methods 5:235–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Frank DN, et al. 2007. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. U. S. A. 104:13780–13785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schloss PD, et al. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75:7537–7541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 42. Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 43. Jukes T, Cantor C. 1969. Evolution of protein molecules. Academic Press, New York, NY. [Google Scholar]
- 44. Chun J, et al. 2007. EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int. J. Syst. Evol. Microbiol. 57:2259–2261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting materials and methods. Download Text S1, DOC file, 0.1 MB.
Summary of total bacterial, Betaproteobacteria, and Sutterella sequences obtained by 16S rRNA gene (V2 region) pyrosequencing from ileal and cecal biopsy samples from AUT-GI and Control-GI children.
Primers and probes used for conventional PCR or real-time PCR amplification and quantitation of Sutterella species.
Abundance distribution of all genus-level classifications of sequences from pyrosequencing for patients 1, 3, 5, and 7. A bar graph shows all ileal genera, in order of highest abundance (top) to lowest abundance (bottom), from patient 1 (32 total genera) (A), patient 3 (35 total genera) (B), patient 5 (39 total genera) (C), and patient 7 (39 total genera) (D). The abundances of Sutterella sequences are indicated in red. Note that unclassified family members may represent more than one genus (i.e., “Unclassified Lachnospiraceae”). Download Figure S1, TIF file, 1 MB.
Abundance distribution of all genus-level classifications of sequences from pyrosequencing for patients 10, 11, and 12. The bar graph shows all ileal genera, in order of highest abundance (top) to lowest abundance (bottom) from patient 10 (32 total genera) (A), patient 11 (39 total genera) (B), and patient 12 (44 total genera) (C). The abundances of Sutterella sequences are indicated in red. Note that unclassified family members may represent more than one genus (i.e., “Unclassified Lachnospiraceae”). Download Figure S2, TIF file, 0.8 MB.
Sutterella OTU analysis. Shown is a heat map generated from OTU analysis of all Sutterella sequences by patient. Note patients 1, 3, 10, 11, and 12 cluster together, and the majority of Sutterella sequences are present in OTU 2. Patients 5 and 7 cluster together, and the majority of Sutterella sequences are present in OTU 1. The heat map scale represents OTU abundance (expressed as the percentage of total bacterial pyrosequencing reads per patient). Download Figure S3, TIF file, 0.7 MB.
Phylogenetic tree based on the representative 16S rRNA gene sequences obtained by V2 region pyrosequencing (OTU 1 and OTU 2) from AUT-GI patients, Sutterella species isolates, and related species. The tree was constructed by the neighbor-joining method. Bootstrap values based on 1,000 replicates are shown next to the branches (percentage of bootstrap support). There were a total of 218 positions in the final data set. The evolutionary distances were computed using the Jukes-Cantor method and are in units representing the number of base substitutions per site. The optimal tree with a sum of branch length of 1.01142743 is shown. The tree is rooted to the outgroup Escherichia coli. Accession numbers are shown in parentheses. The locations of AUT-GI patients’ representative OTU 1 and OTU 2 sequences are boxed in red. Download Figure S4, TIF file, 0.5 MB.
Sutterella sequence alignment. Shown is ClustalW alignment of the most abundant Sutterella 16S rRNA gene (C4-to-V8 region) sequences in the 12 Sutterella-positive patients. Sequences have had the 515For and SuttRev primer sequences removed. The positions of the beginning (nucleotide position 501) and end (nucleotide position 1176) of the sequences are relative to the 16S rRNA gene of S. wadsworthensis (accession no. L37785). Download Figure S5, TIF file, 1.4 MB.