Abstract
BACKGROUND:
The aim of this study was to investigate the risk factors of low vision in type 2 diabetic patients and the prevalence of ischemic heart diseases and nephropathy for different visual acuities.
METHODS:
In this cross-sectional study, data from 738 type 2 diabetic patients including evidences for nephropathy and ischemic heart disease, demographic characteristics, blood pressure and body mass index were collected, and then patients were divided into 3 groups based on their best corrected visual acuity in the better-seeing eye. Analysis of variance was used to compare basic characteristics according to different levels of visual acuity.
RESULTS:
The prevalence of blindness and low vision was 5.5% and 13.3% respectively, and as age, duration of diabetes, systolic blood pressure and body mass index increased, the visual acuity decreased. The prevalence of hypertension and obesity in patients with visual disabilities was significantly higher than in patients with not impaired visions (p = 0.008 and p = 0.02, respectively). We also found that with greater decline in visual acuity, the prevalence of neph-ropathy and ischemic heart diseases increased.
CONCLUSIONS:
The factors related to retinopathy play a role in affecting the degree of visual impairment in diabetic patients. Therefore, controlling risk factors can be useful in decreasing impairment of vision and blindness.
KEYWORDS: Diabetes Mellitus, Type 2, Visual Acuity, Diabetic Retinopathy, Blindness, Diabetic Nephropathies, Cardiovascular Diseases
Diabetes mellitus is one of the most common non-communicable diseases in the world. The WHO has predicted that, “the number of diabetic patients in the world will reach 300 millions by 2025, and more than 75% of these patients will be living in developing countries.1”
Diabetic retinopathy is one of the most common complications of diabetes,2 and one of the leading causes of blindness and visual impairment.3–6
According to statistics from a decade ago, 40 million people were blind and 110 million had low vision.7 The American Diabetes Association claims that diabetes is responsible for 8% of legal blindness.8
Diabetic retinopathy and resulting blindness are responsible for a major disability in patients, and they lead to a high financial and social burden on the community indicating the importance of increased efforts in this field.
Diabetic retinopathy is the principal cause of one-third of all cases of blindness,9 and also, according to some trials, a factor which is asso ciated to increased prevalence of cardiovascular diseases.10–12 These trials supposed that the pathogenesis of diabetic retinopathy and cardiovascular diseases are both microvascular.
On the other hand, microalbuminuria which is another microvascular complication of diabetes has a connection with both, the development and progression of retinopathy.13
Taking into account the possibility that retinopathy, nephropathy and cardiovascular diseases may have similar pathogenesis in diabetic patients, and considering the role of retinopathy on vision acuity,14–16 it can be assumed that the prevalence of chronic complications of diabetes is different for different visual acuities, and risk factors related to diabetic retinopathy are possibly connected to visual impairment in diabetic patients. Since there are limited data on this field in Iran, and considering the fact that these data are essential for setting up of Iranian blindness prevention program, and also the clear effect of early screening and suitable intervention on reducing incidence of blindness and low vision related to diabetic retinopathy,17,18 this study was performed in an Iranian population of type 2 diabetic patients at the Isfahan Endocrine and Metabolism Research Center with the aim of investigating our assumptions, and collecting as much information as possible concerning the prevalence of blindness and low vision and their related risk factors in our diabetic patients.
Methods
This cross-sectional study was conducted in April 2009, at the Endocrine and Metabolism Research Center of Isfahan University of Medical Sciences which covers 40% of diabetic patients of Isfahan. Study protocol was approved by the ethics committee of the center (Project No. 87015). The study complied with the current version of the Declaration of Helsinki.
For this study we used routinely collected and registered data from patients registered at this center. These data were initially collected by an endocrinologist and subsequently via next follow-up visits to physicians at the center, and then the standard check lists that were filled out by these persons entered into the central computer system of the center.
This information included: demographic characteristics, past history, family history, blood pressure, lipid profile, blood urea nitrogen (BUN), creatinin, HbA1c (glycosylated hemoglobin), urine albumin, the results of eye exams and of cardiovascular follow-ups.
Lipid profile, BUN, creatinin, BP and body weight were usually monitored monthly and the ophthalmic, cardiovascular and renal condition of patients at least yearly.
All lab tests were performed by the laboratory at the Endocrine and Metabolism Research Center.
HbA1c was measured by spectrophotometer (DS5) and urine albumin by the sopt collection method. Creatinin was measured by the photometric method and albumin by the imunoturbidimetric method on a DB 3000 appliance.
Eye Examination
Eye examination was performed by an experienced ophthalmologist and included: testing of visual acuity (VA), corrected and uncorrected visual acuities were determined using a Snellen chart and a Canon autorefractometer (R-50m, Japan), best corrected visual acuity (BCVA) was defined as the visual acuity after subjective refraction in the better eye, and also intraocular pressure was measured using Goldman applanation tonometry (Haag-streit AG1A 900, Switzerland).
The pupil of each eye was dilated using tropicamide 1% followed by a dilated fundus examination using a 78-dioptre non-contact fundus viewing lens (Volk). If needed, indirect ophthalmoscopy was performed. All findings were registered in special checklists.
Cardiac Condition Follow-Up
Ischemic heart disease was diagnosed by taking history and an ECG test. If there were electrocardiographic abnormalities as diagnosed by the Minesota-code or symptoms of ischemic heart disease,19 the patient was referred to a cardiologist for the exercise tolerance test and other diagnostic and therapeutic procedures.
Patients
Our target population was type 2 diabetic patients of Isfahan. As it has been mentioned before, the center covers 40% of diabetic patients of Isfahan. Between 2008 and 2009, 1300 diabetic patients came to the center for their follow-up visits. Of these patients, 1000 persons had type 2 diabetes and 300 persons had type 1.
For this study we used data collected from 900 conveniently-selected patients with type 2 diabetes who had a complete eye exam during that period. Patients with incomplete records on their ophthalmologic exam or on evidences of nephropathy and ischemic heart disease were excluded from the study
Definitions
The patients were divided into 3 groups based on their best corrected visual acuity (BCVA) in the better seeing eye, using the North American definition.20
Best Corrected Visual Acuity: Not impaired was defined as best corrected visual acuity better than or equal to 20/40 (5/10), low vision as worse than 20/40 but better than 20/200 (2/10-4/10) and blind was defined as 2/200 or worse (1/10 or worse).
Ischemic Heart Disease: The patients who have been put on treatment or undergone surgery like coronary artery bypass graft (CABG) surgery or percutaneous transluminal coronary angioplasty (PTCA) after diagnostic tests
Nephropathy: Urine albumin/creatinin more than 30mg/g21
Hypertension: (in diabetic patients) Systolic/diastolic blood pressure ≥ 130/80 mmHg22
Obesity: Body mass index (weight in kg/squared of height in m) ≥ 30 kg/m23
Statistical Analysis
We used t-test to compare continuous variables, chi-square test to compare categorical variables, and analysis of variance (ANOVA) test to compare basic characteristics according to different levels of visual acuity. In all instances, p values < 0.05 were considered significant. Data analyses were done using the SPSS version 13 (Chicago, USA).
Results
Of the 900 investigated files, 162 were excluded because of incomplete information. The study included data from 738 patients. Of these patients with the mean age of 60.29 ± 10.3 years, 41 patients (5.5%) were blind (95% Confidence Interval (CI): 2.5-13.2) and 98 patients (13.3%) had low vision, (95% CI: 4.3-32.3).
Tables 1 and 2 show the demographic characteristics for patients divided into 3 groups and results of post hoc tests for continuous variables among 3 groups of patients with different visual acuities, respectively. According to these data, there was a significant difference among 3 groups in terms of mean age, body mass index (BMI), duration of diabetes and systolic blood pressure. Presence of any of the following conditions was associated with corresponding decrease in visual acuity: increasing age, longer duration of diabetes, higher systolic blood pressure, and increased body mass index.
Table 1.
Basic clinical and demographic characteristics and mean of HbA1c of patients with different visual acuities
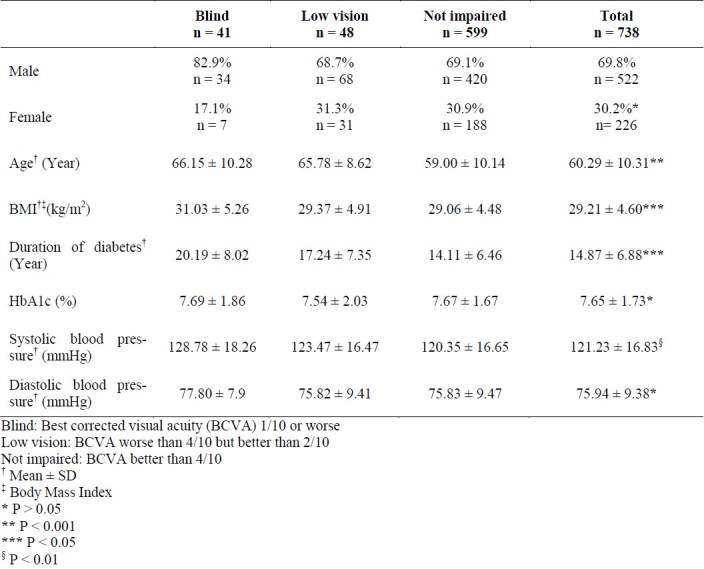
Table 2.
Results of post hoc tests for basic clinical and demographic characteristics and mean of HbA1c among 3 groups of patients with different visual acuities
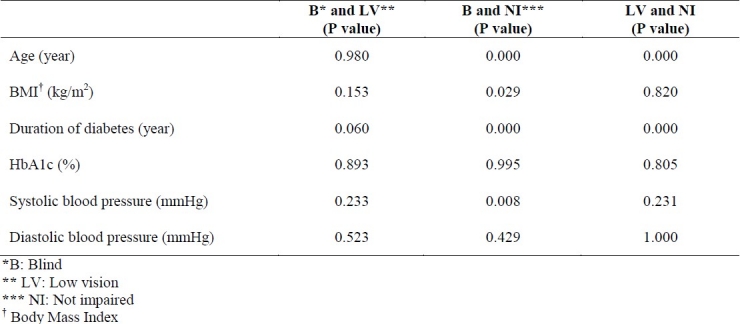
Table 3 shows the prevalence of different levels of visual acuity by the presence of hypertension or obesity. According to this table, when patients in blind and low vision groups are considered together, it is evident that 20.3% of hypertensive patients and 5.5% of non-hypertensive ones have visual disability (p = 0.008), and 22.9% of obese patients and 16.2% of non-obese ones have visual impairment (p = 0.02).
Table 3.
Prevalence of different levels of visual acuity in type 2 diabetic patients with and without hypertension or obesity
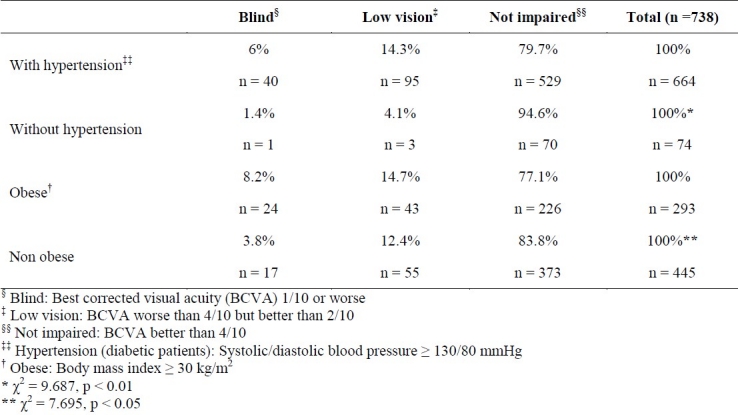
Table 4 shows the prevalence of ischemic heart diseases (IHD) and nephropathy in 3 groups.
Table 4.
Prevalence of ischemic heart disease and nephropathy in 3 groups of type 2 diabetic patients with different levels of visual acuity
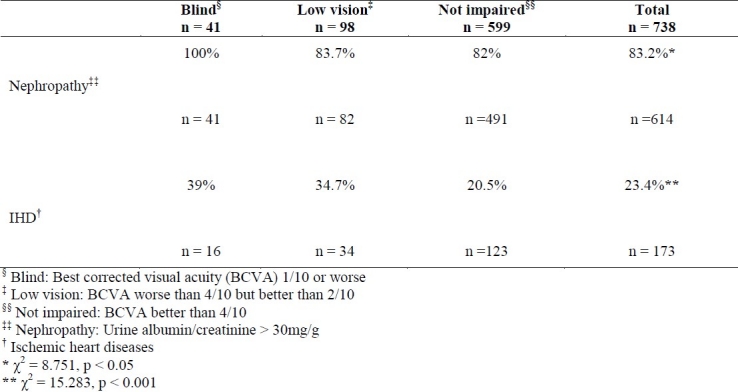
Considering these data, there is a significant difference between the 3 groups in terms of the prevalence of IHD and nephropathy.
The prevalence of nephropathy is significantly higher in the group with blindness than in both groups with not impaired vision (p = 0.003) and those with low vision (p = 0.01). Also the prevalence of IHD was significantly lower in patients with not impaired vision than those with visual disabilities.
Discussion
In this cross-sectional study carried out on 738 registered type 2 diabetic patients, the prevalence of blindness and low vision was 5.5% and 13.3%, respectively. It was observed that the visual acuity decreased with increasing age, duration of diabetes, systolic blood pressure, and body mass index. It was also showed that with greater decline in visual acuity, the prevalence of nephropathy and ischemic heart diseases increased.
Reported prevalence of blindness and low vision is very different in various societies. The prevalence of blindness in type 2 diabetic patients in Yemen was 16%,24 in Taiwan was 1.6%,25 in Denmark was 1.5%26 and in England was 1.16%.27 It has been claimed by the Center for Disease Control that among adults with diabetes, 2.9% suffered from mild vision impairment and 1% had severe visual impairment after correction.28
One reason for the higher prevalence of visual impairment and blindness in our study in comparison with other studies was the nature and the type of groups studied. The Taiwan study was a population-based diabetic retinopathy screening program. The study in England was a population-based study of diabetic patients in a district in the northwest of England. The Denmark study included blind cases registered in a database of diabetic patients, but our study has been carried out at a referral center for diabetic patients, which is affiliated to Isfahan University of Medical Sciences, and the prevalence of each chronic complication may be affected by this referral pattern, because the number of patients with more severe complications is greater, they also make more frequent visits and are more vigilant.
Other possible reasons for high prevalence of low vision and blindness in our study was primarily the long duration of diabetes (more than 15 years in 30.5%) and secondly, high prevalence of hypertension and nephropathy (89.8% and 83% respectively) in our studied population.
Different studies claimed that, the duration of diabetes,29–31 age,29,32 high blood pressure,33–35 and albuminuria,14,27 are directly related to the onset and progression of diabetic retinopathy, and diabetic retinopathy is one of the major causes of visual impairment in diabetic patients,13,36 so it is reasonable to suppose that risk factors related to it are also related to the status of visual acuity.
The results of our research revealed that our supposition was well-founded, and age, duration of diabetes, body mass index and systolic blood pressure were also related to the severity of visual impairment. In addition, in our study, hypertension and obesity were significantly more prevalent in patients with visual disabilities than in patients with unimpaired visions. Some other trials have also shown that there is a relationship between systolic blood pressure and diabetic retinopathy,32 and have claimed that the severity of retinopathy in type 2 diabetic patients is positively associated with systolic blood pressure28 and body mass index.34
In our study there was no relationship between different levels of visual acuity and sex. In an independent study in Isfahan, Janghorbani et al also reached the conclusion that there was no association between retinopathy and sex,32 which was in agreement with the results of other studies.24,33
We also found a relationship between severity of visual impairment and prevalence of ischemic heart disease and nephropathy.
In the Ossama et al study, there was also a relationship between ischemic heart disease and retinopathy, but after adjustment for risk factors, the relationship was no longer significant.33 A limited number of studied ischemic heart disease cases (44/500) was mentioned as the possible reason for this finding, but in our study, the number of ischemic heart disease cases was not limited (Table 4); so, it seems that the prevalence of ischemic heart disease does not have an independent relationship with visual acuity. The reason is possibly the difference in pathogenesis of ischemic heart disease and retinopathy; the former is macro-vascular in nature and the latter has a micro-vascular pattern. Nephropathy, which was in direct relationship with visual acuity has also a microvascular basis.
We are fortunate at our center to have access to some of the most comprehensive data of its kind in the developing world. Based upon information from earlier studies, it is clear that our patients are a representative sample of known diabetics in Isfahan.32,37 In addition, a positive aspect of our diagnosis of ischemic heart disease, nephropathy, hypertension and obesity was that it has not been based on a single examination but on continued examinations during follow-ups. However, this study has also some limitations. The study was of course clinic-based rather than population-based. Clinic-based estimates of the prevalence of complications are most likely to be affected by referral patterns, since patients at our clinic are more likely to have complications, and it has been showed by experience that once a complication occurs, patients are more likely to come regularly for treatment, and this factor affects the estimated prevalence of complications. Considering the above-mentioned reasons, it seems that a population-based study will be needed regarding the matter in the future.
Conclusion
The factors related to retinopathy play a role in affecting the degree of visual impairment in diabetic patients. Considering the fact that the high prevalence of low vision is a serious threat to the health of diabetic patients in Isfahan, control of risk factors can be useful in decreasing impairment of vision and blindness.
Authors’ Contributions
NH was the principal investigator of the research project, carried out the design, coordinated the study, participated in all of the research stages, and also in manuscript preparation. MF assisted in designing the study and coordinated and assisted in carrying out all the research stages. MG assisted in designing the study and assisted in carrying out the research. SH assisted in designing the study, coordinated in carrying out the research, and participated in manuscript preparation and revision. MA assisted principally in designing the study and participated in manuscript preparation and revision.
Acknowledgments
The authors would like to thank Ms. Zahra Khani, Mrs. Mehri Foroughifar and Mr. Abyar for their technical assistance in computer affairs.
Footnotes
Conflict of Interests Authors has no conflict of interests.
References
- 1.Khatib Oussama MW. Cairo: WHO Regional Office for the Eastern Mediterranean; 2006. Guidelines for the Prevention, Management and Care of Diabetes Mellitus; p. 81. [Google Scholar]
- 2.Davidson MB. The continually changing natural history of diabetes mellitus. J Chronic Dis. 1981;34(1):5–10. doi: 10.1016/0021-9681(81)90076-x. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE, Moss SE. The Wisconsin epidemiological study of diabetic retinopathy: a review. Diabetes Me-tab Rev. 1989;5(7):559–70. doi: 10.1002/dmr.5610050703. [DOI] [PubMed] [Google Scholar]
- 4.Kahn HA, Hiller R. Blindness caused by diabetic retinopathy. Am J Ophthalmol. 1974;78(1):58–67. doi: 10.1016/0002-9394(74)90010-5. [DOI] [PubMed] [Google Scholar]
- 5.Ghafour IM, Allan D, Foulds WS. Common causes of blindness and visual handicap in the west of Scotland. Br J Ophthalmol. 1983;67(4):209–13. doi: 10.1136/bjo.67.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munier A, Gunning T, Kenny D, O’Keefe M. Causes of blindness in the adult population of the Republic of Ireland. Br J Ophthalmol. 1998;82(6):630–3. doi: 10.1136/bjo.82.6.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thylefors B, Negrel AD, Parajasegaram R, Dadzie KY. Global data on blindness. Bull World Health Organ. 1995;73(1):115–21. [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes Association: facts and figures: Diabetes and Eye Disease [article online] 2003. [Accessed June 20, 2009]. Available from http.://WWW.Diabetes. Org/main/info/facts/eye/default. JSP .
- 9.American Diabetes Association. Diabetic retinopathy. Diabetes Care. 1998;21:157–9. [PubMed] [Google Scholar]
- 10.Targher G, Bertolini L, Zenari L, Lippi G, Pichirit I, Zoppinit G, et al. Diabetic retinopathy is associated with an.increased incidence of cardiovascular events among type 2 diabetic patients. Diabet Med. 2008;25(1):45–50. doi: 10.1111/j.1464-5491.2007.02327.x. [DOI] [PubMed] [Google Scholar]
- 11.Targher G, Bertolini L, Tessari R, Zenari L, Arcaro G. Retinopathy predicts future cardiovascular events among type 2 diabetic patients. Diabetes Care. 2006;29(5):1178. doi: 10.2337/diacare.2951178. [DOI] [PubMed] [Google Scholar]
- 12.Cheung N, Wang JJ, Klein R, Coper DJ, Sharrett AR, Wong TY. Diabetic retinopathy and the risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Diabetes Care. 2007;30(7):1742–6. doi: 10.2337/dc07-0264. [DOI] [PubMed] [Google Scholar]
- 13.Van Leiden HA, Dekker JM, Moll AC, Nijpels G, Heine RJ, Bouter LM, et al. Risk factors for incident retinopathy in a diabetic and non diabetic population: The Hoorn Study. Arch ophthalmol. 2003;121(2):245–51. doi: 10.1001/archopht.121.2.245. [DOI] [PubMed] [Google Scholar]
- 14.Moss SE, Klein R, Klein BE. The incidence of vision loss in a diabetic population. Ophthalmology. 1988;95(10):1875–95. doi: 10.1016/s0161-6420(88)32991-x. [DOI] [PubMed] [Google Scholar]
- 15.Keen H. Prevalence of blindness in diabetics. J Roy Coll Physicans London. 1972;7(1):53–60. [PMC free article] [PubMed] [Google Scholar]
- 16.Dwyer MS, Melton LJ, III, Ballard DJ, Palumbo PY, Trautmann JC, Chu CP. Incidece of diabetic retinopathy and blindness : A population - based study in Rochester, Minnesota. Diabetes Care. 1985;8(4):316–22. doi: 10.2337/diacare.8.4.316. [DOI] [PubMed] [Google Scholar]
- 17.Fairchild JM, Hing SJ, Donaghue KC, Bonney MA, Fung AT, Stephens MM, et al. Prevalence and risk factor for diabetic retinopathy in adolescents with type 1 diabetes. Med J Aust. 1994;160(12):757–61. doi: 10.5694/j.1326-5377.1994.tb125943.x. [DOI] [PubMed] [Google Scholar]
- 18.Sundling V, Gulbrandsen P, jervell J, Straand J. Care of vision and ocular health in diabetic members of a national diabetes organization: a cross sectional study. BMC Health Serv Res. 2008;8:159. doi: 10.1186/1472-6963-8-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diabetes Drafting Group. Prevalence of small vessel and large vessel disease in diabetic patients from 14 centers. The World Health Organization Multinational Study of Vascular Disease in Diabetics. Diabetologia. 1985;28(8):615–40. doi: 10.1007/BF00290267. [DOI] [PubMed] [Google Scholar]
- 20.Maberley DAL, Hollands H, Chang A, Adilman S, Chakraborti B, Kliever G. The prevalence of low vision and blindness in a Canadian inner city. Eye (Lond) 2007;21(4):528–33. doi: 10.1038/sj.eye.6702257. [DOI] [PubMed] [Google Scholar]
- 21.Mogensen CE, Keane WF, Bennett PH, Jerums G, Parving HH, Passa P, et al. Prevention of diabetic renal disease with special reference to microalbuminuria. Lancet. 1995;346(8982):1080–4. doi: 10.1016/s0140-6736(95)91747-0. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association. Standards of medical care in diabetes-2009. Diabetes Care. 2009;32(Suppl. 1):S6–S12. doi: 10.2337/dc09-S006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filer JS, Filer EM. Obesity. In: Kasper DL, editor. Harrison's Principles of Internal Medicine. New York: McGraw-Hill; 2005. pp. 422–9. [Google Scholar]
- 24.Bamashmus MA, Gunaid AA, khandekar RB. Diabetes retinopathy, visual impairment and ocular status among patients with diabetes mellitus in Yemen: A hospital – based study. Indian J Ophthalmol. 2009;57(4):293–8. doi: 10.4103/0301-4738.53055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tung TH, Liu JH, Lee FL, Chen SJ, Li AF, Chou P. Population – Based study of nonproliferative diabetic retinopathy among type 2 diabetic patients in Kinmen, Taiwan. Jpn J ophthalmaol. 2006;50(1):44–52. doi: 10.1007/s10384-005-0269-x. [DOI] [PubMed] [Google Scholar]
- 26.Jeppesen P, Bek T. The occurrence and causes of registered blindness in diabetes patients in Arhus County, Denmark. Acta ophthalmol Scand. 2004;82(5):526–30. doi: 10.1111/j.1600-0420.2004.00313.x. [DOI] [PubMed] [Google Scholar]
- 27.Prasad S, Kamath GG, Jones K, Clearkin LG, Phillips RP. Prevalence of blindness and visual impairment in a population of people with diabetes. Eye (Lond) 2001;15(Pt 5):640–3. doi: 10.1038/eye.2001.200. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Gregg EW, Cheng YJ, Thompson T, Geiss LS, Quenas MR. Correctable visual impairment among persons with diabetes – United States, 1999 – 2004. JAMA. 2007;297(1):34–6. [Google Scholar]
- 29.el Haddad OAW, Saad MK. Prevalence and risk factors for diabetic retinopathy among Omani diabetics. Br J Ophthalmol. 1998;82(8):901–6. doi: 10.1136/bjo.82.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Javitt JC, Aiello LP, Chiang Y, Ferris FL, Canner jk, Greenfield S. Preventive eye care in people with diabetes is cost-saving to the federal government: implications for health care reform. Diabetes care. 1994;17(8):909–17. doi: 10.2337/diacare.17.8.909. [DOI] [PubMed] [Google Scholar]
- 31.Backlund LB, Algvere PV, Rosenqvist U. New blindness in diabetes reduced by more than one – third in Stockholm County. Diabet Med. 1997;14(9):732–40. doi: 10.1002/(SICI)1096-9136(199709)14:9<732::AID-DIA474>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 32.Janghorbani M, Amini M, Ghanbari H, Safaiee H. Incidence of and risk factors for diabetic retinopathy in Isfahan, Iran. Ophthalmic Epidemiol. 2003;10(2):81–95. doi: 10.1076/opep.10.2.81.13893. [DOI] [PubMed] [Google Scholar]
- 33.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. Arch ophthalmol. 1984;102(4):527–32. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 34.Van leiden H A, Dekker JM, Moll AC, Nijpels G, Heine RJ, Bouter LM, et al. Blood pressure, lipid and obesity are associated with retinopathy, the Hoorn study. Diabetes Care. 2002;25(8):1320–5. doi: 10.2337/diacare.25.8.1320. [DOI] [PubMed] [Google Scholar]
- 35.Kohner EM, Aldington SJ, Stratton IM, Manley SE, Holman RR, Matthews DR, et al. United Kingdom Prospective Diabetes Study, 30: diabetic retinopathy at diagnosis of non-insulin-dependent diabetes mellitus and associated risk factors. Arch Ophthalmol. 1998;116(3):297–303. doi: 10.1001/archopht.116.3.297. [DOI] [PubMed] [Google Scholar]
- 36.Stefansson E, Bek T, Porta M, Larsen N, Kristinsson JK, Agardh E. Screening and prevention of diabetic blindness. Acta ophthalmol Scand. 2000;78(4):374–85. doi: 10.1034/j.1600-0420.2000.078004374.x. [DOI] [PubMed] [Google Scholar]
- 37.Amini M, Afshin-Nia F, Bashardoost N, Aminorroaya A, Shahparian M, Kazemi M. Prevalence and risk factors of diabetes mellitus in the Isfahan city population (aged 40 or over) in 1993. Diabetes Res Clin Pract. 1997;38(3):185–90. doi: 10.1016/s0168-8227(97)00099-5. [DOI] [PubMed] [Google Scholar]


