Abstract
BACKGROUND:
Eicosapentaenoic acid (EPA) is the principal ω-3 fatty acids in marine oils. Fasting blood sugar (FBS), HbA1c and some of the plasma lipids and lipoproteins has been negatively related to the intake of ω-3 fatty acids and ascorbic acid, in some studies. Therefore, the purpose of this study was to assess the effects of EPA and/or vitamin C on glycemic indices, blood pressure, and plasma lipids in type 2 diabetic Iranian males.
METHODS:
Sixty five men with type 2 diabetes were enrolled into the study between April 2 and June 27, 2008. Venous blood samples were obtained from all participants after 10 hours of fasting, at the baseline and after the intervention. Subjects received 500 mg EPA and/or 200 mg vitamin C and/or placebo depending on their groups. For eight weeks, 15 participants received EPA supplements with vitamin C (group 1), 16 took EPA supplements and vitamin C placebo (group 2), 17 took EPA placebo and vitamin C (group 3), and 17 received EPA placebo and vitamin C placebo (group 4), daily.
RESULTS:
There were significant decreases in FBS, HbA1C, LDL-C and TG in groups 1, 2, 3 and 4 (p < 0.01), but significant decreases in TC were shown only in groups 1, 2 and 3 (p < 0.01). There was a significant increase in HDL-C in all groups (p < 0.01).
CONCLUSIONS:
In summary, it is concluded that, eight weeks of taking EPA + vitamin C supplementation improved the plasma levels of cardiovascular markers but didn’t reduce BP.
KEYWORDS: Eicosapentaenoic Acid, Ascorbic Acid, Fasting, Blood Glucose, HbA1C, Glycosylated, Lipids, Blood Pressure, Diabetes Mellitus
Diabetes is a common chronic disease and a serious medical and social problem. Diabetic men and women have over a 1.5 and 2.5 times risk of cardiovascular disease (CVD) in Iran, respectively.1
Eicosapentaenoic acid (EPA; 22:5n-3) is the principal ω-3 fatty acids in fish oils. Recent evidence demonstrated that this fatty acid have different effects on plasma glucose,2 serum lipids and lipoproteins,2,3 and blood pressure.4
EPA supplementation increased insulin sensitivity in animal models and in some human studies,5 therefore fasting blood sugar and HbA1c has been negatively correlated with intake of ω-3 fatty acids, in some studies.6 Results of some prospective studies, however, did not show a relation.7
On the other hand, some reports from few studies, however, raised the possibility of an adverse effect of high doses of fish oils on glycemic control in type 2 diabetes.8
However, there are significant evidences for a beneficial effect of dietary ω-3 fatty acids in the prevention of heart disease,9 in high-risk populations, especially.10
Insulin resistance, hyperlipidemia and hypertension are major metabolic disorders in patients with type 2 diabetes and therefore these patients have an increased risk of cardiovascular diseases.11
Consequently, some experts discourage the use of ω-3 fatty acids in type 2 diabetic patients, whereas others encourage such use, although at low doses.
The different studies in the past decade indicated that diabetes is associated with oxidative stress. It is therefore possible that dietary supplementation with antioxidants would be useful.12
The diabetes may increase free radicals generation. However, incorporation of the highly unsaturated fatty acids in membranes may increase the membranes’ susceptibility to lipid peroxidation.13 Vitamin C is reported to decrease diabetes induced lipid peroxidation.14 To this time, no study has been done to assess the effects of supplementation with EPA and/or vitamin C on cardiovascular risk factors in type 2 diabetic patients. The purpose of this study was to evaluate the effects of EPA and/or vitamin C on glycemic indices, blood pressure, and serum lipids in type 2 diabetic Iranian males.
Methods
In this randomized double blind placebo controlled clinical trial, 65 men with type 2 diabetes, aged 33-63 years, were enrolled into the study between April 2 and June 27, 2008. Ethical approval was obtained from the Medical Ethics Committee of Tehran University of Medical Sciences and informed consent was obtained from all individuals. The participants were instructed not to take any ω-3 fatty acids and antioxidant supplements during, and 2 weeks preceding the study. Exclusion criteria included onset of nephropathy, myocardial infarction, coronary bypass grafting and inflammatory diseases.
Venous blood samples were obtained from all participants at 8:00-9:00 A.M., after 10 hours of fasting, at the baseline and after intervention. Subjects received 500 mg EPA and/or 200 mg vitamin C or placebo depending on their groups. For a period of 8 weeks, 15 participants received daily EPA supplements with vitamin C (group 1), 16 took EPA supplements and vitamin C placebo (group 2), 17 took EPA supplement placebo and vitamin C (group 3), and 17 received EPA supplement placebo and vitamin C placebo (group 4). EPA and EPA placebo soft gels were supplied from Minami Nutrition (Belgium). The vitamin C and their placebo soft gels were obtained from Darou Pakhsh Pharma Chem. Co. (Iran).
Plasma glucose was determined by a glucose analyzer (YSI, Yellow Springs, OH). HbA1c levels were measured by immuneturbidimetric immunoassay (Unimate; Roche Diagnostics, Indianapolis, IN) with a normal range of 4.5-6.1%. Serum TG and TC levels were determined using the enzymatic method. HDL-C levels were measured by the turbidimetric method.
LDL-cholesterol (LDL-c) was calculated using Friedwald's formula. Anthropometric indices included height and weight measured with a Seca standard tools. BMI was calculated from this formula: [weight (kg)/hight2 (m)]. Blood pressure (BP) was measured twice in a seated position after 15 minutes of rest using a standard mercury sphygmomanometer.
Microsoft Excel was used for data handling and SPSS (version 10; SPSS Inc., Chicago, IL, USA) was used for statistical analysis . Values are expressed as means and standard errors for each group. The significant level was set at p < 0.05.
Results
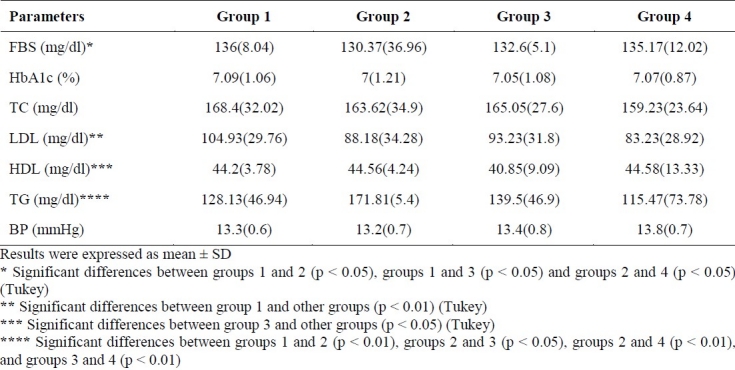
Sixty-nine diabetic men with a mean age of 48 years (range: 33-63 years) were recruited. Sixty-five participants completed the 8-week intervention. Some of the participants’ characteristics are shown in table 1. Mean ± SD plasma levels of FBS, HbA1C, TC, LDL-C, HDL-C, TG and BP are presented in table 2. There were significant decreases in FBS, HbA1C, LDL-C and TG in groups 1 (p < 0.01), 2 (p < 0.01), 3 (p < 0.01) and 4 (p < 0.01), but significant decreases in TC was shown in groups 1 (p < 0.01), 2 (p < 0.01) and 3 (p < 0.01), not in group 4. There was a significant increase in HDL-C in all groups (p < 0.01). There were significant differences in FBS between groups 1 and 2 (p < 0.05), groups 1 and 3 (p < 0.05) and groups 2 and 4 (p < 0.05). LDL-C was significantly different between group 1 and other groups (p < 0.01). There were significant differences in HDL-C level between group 3 and other groups (p < 0.05), and TG level was significantly different between groups 1 and 2 (p < 0.01), groups 2 and 3 (p < 0.05), groups 2 and 4 (p < 0.01), and groups 3 and 4 (p < 0.01), but no difference in HbA1C, TC and BP was seen between the groups after 8 weeks of intervention (Table 3).
Table 1.
Characteristics of subjects [mean (SD)]
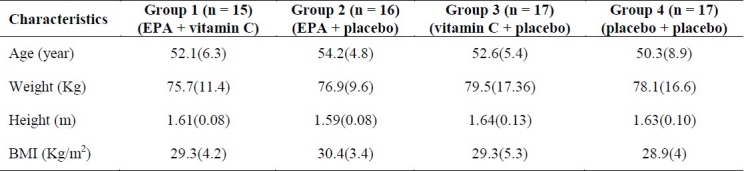
Table 2.
Comparisons of parameters in the groups before and after 8 weeks of supplementation [mean (SD)]
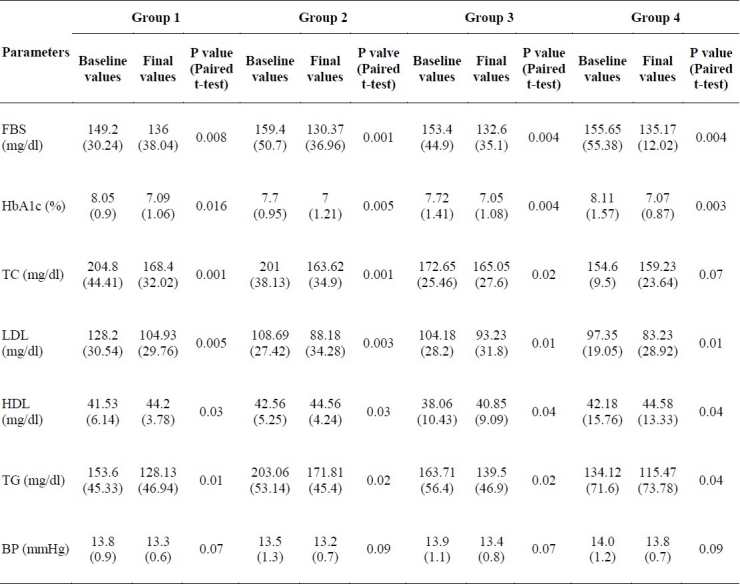
Table 3.
Multiple comparisons of parameters among groups after 8 weeks of supplementation [mean (SD)]
Discussion
This randomized double-blind placebo controlled clinical trial in diabetic patients demonstrated a decrease in all variables in four groups except for TC in group 4 and an increase in HDL-C in all groups, although BP did not change in any group, following 8 weeks of supplementation. These results suggest that EPA with or without vitamin C can improve the cardiovascular markers in plasma, but alone or along with vitamin C cannot reduce the blood pressure. These findings are consistent with the results of some previous studies.15,16 Others however, reported no effect or a harmful effect17 for increased ω-3 fatty acid intake.
Potential mechanisms for the cardio-protective effects of ω-3 fatty acids include anti-thrombotic, anti-inflammatory and anti-arrhythmic effects, as well as its effects on lowering blood pressure (BP), and reducing plasma triglycerides (TG) levels.18
In addition, higher EPA quantities in the phospholipid cell membranes could increase insulin sensitivity.19
Some studies showed that fish oil rich in EPA and DHA lowers blood pressure. Metaanalyses of controlled trials on fish oil supplementation suggested that the BP-lowering effect in hypertension is both dose- (> 3 g/d) and time-dependent.20,21
Many epidemiologic and interventional studies have assessed the relations and effects of the intake of ω-3 fatty acids22,23 on CVD biomarkers in a variety of populations. Healthy subjects and those at high risk for CHD, including diabetic patients and patients who had suffered a myocardial infarction (MI) have been evaluated. Many different study designs for both studies have been used. Reductions in CVD have been observed with two servings of fish per week, 1.8 g/day of EPA as an ethyl ester and 1 g/day of an 85% EPA + DHA concentrate in the form of an ethyl ester.24,25
The results of the present study also showed significant differences in FBS between groups 1 and 2 (p < 0.05), groups 1 and 3 (p < 0.05) and groups 2 and 4 (p < 0.05). Furthermore, there were significant differences in LDL-C between group 1 and other groups (p < 0.01). A significant difference in HDL-C was seen between group 3 and other groups (p < 0.05) and in TG between groups 1 and 2 (p < 0.01), groups 2 and 3 (p < 0.05), groups 2 and 4 (p < 0.01), and groups 3 and 4 (p < 0.01), too.
Individual fatty acids have large effects on physiologic functions that are determined by a collection of chain length, number and position of double bonds, and isomerism around these bonds. One of the possible mechanisms is that ω-3 fatty acids vary from ω-6 fatty acids in their effects on plasma lipids, BP, and other biomarkers of CVD risk such as lipoprotein (a), inflammatory markers, fasting plasma glucose and insulin, and clotting factors.26
In many studies, mechanisms potentially responsible for these effects have been examined. EPA constitutes part of the cell membrane, replacing other mostly unsaturated fatty acids there, and so modifying cellular function. Hence, a number of variations of cell function can be observed upon inclusion of EPA into the cell membrane. Among them are the shift of the eicosanoid system towards vasodilatation and less inflammation, a lowering of blood TG and anti-arrhythmic effects.27
Clinical trial data provide evidence on the favorable effects of ω-3 fatty acids in reducing the risk of CHD. The GISSI-Prevention Trial is one of the largest clinical trials to test the efficacy of ω-3 fatty acid supplementation for secondary prevention of CHD.28 The trial included 11,324 subjects with a recent MI who were assigned to either ω-3 fatty acid supplements (850 mg/d), tocopherol (300 mg/d), both or none for 3.5 years along with a Mediterranean diet. The Mediterranean diet emphasizes less meat consumption with more bread, vegetables, fruit, and fish intake. ω-3 fatty acid supplementation decreased all-cause death by 20% and stroke by 15%. On the other hand, evidence from three epidemiologic studies, the NHANES I Follow-up Study29 and the JPHC Study Cohort I30 did not show any significant decrease in CHD incidence with increasing intakes of fish.
Conclusion
In conclusion, this study showed that 8 weeks of taking EPA + vitamin C supplementation improved the plasma levels of cardiovascular markers but didn’t reduce BP. Further studies are needed to confirm these results.
Authors’ Contributions
MMSM did the laboratory procedures and statistical analysis and prepared the first version of manuscript. MD has planned the study and finalized the manuscript. SAD, SAK, MRE, AASY, GA, and RG supervised the project with association of MD. MZ provided the help in the laboratory analysis.
Acknowledgments
This project was supported by the Vice-Chancellery for Research of Tehran University of Medical Sciences; Grant No. 7330.
Footnotes
Conflict of Interests Authors has no conflict of interests.
References
- 1.Harati H, Hadaegh F, Saadat N, Azizi F. Population-based incidence of Type 2 diabetes and its associated risk factors: results from a six-year cohort study in Iran. BMC Public Health. 2009;9:186. doi: 10.1186/1471-2458-9-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wanjihia VW, Kiplamai FK, Waudo JN, Boit MK. Post-prandial glucose levels and consumption of omega 3 fatty acids and saturated fats among two rural populations in Kenya. East Afr Med J. 2009;86(6):259–66. doi: 10.4314/eamj.v86i6.54135. [DOI] [PubMed] [Google Scholar]
- 3.Zuliani G, Galvani M, Leitersdorf E, Volpato S, Cavalieri M, Fellin R. The role of polyunsaturated fatty acids (PUFA) in the treatment of dyslipidemias. Curr Pharm Des. 2009;15(36):4087–93. doi: 10.2174/138161209789909773. [DOI] [PubMed] [Google Scholar]
- 4.Geleijnse JM, Giltay EJ, Schouten EG, de Goede J, Oude Griep LM, Teitsma-Jansen AM, et al. Effect of low doses of n-3 fatty acids on cardiovascular diseases in 4,837 post-myocardial infarction patients: design and baseline characteristics of the Alpha Omega Trial. Am Heart J. 2010;159(4):539–546. doi: 10.1016/j.ahj.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 5.Thorseng T, Witte DR, Vistisen D, Borch-Johnsen K, Bjerregaard P, Jørgensen ME. The association between n-3 fatty acids in erythrocyte membranes and insulin resistance: the Inuit Health in Transition Study. Int J Circumpolar Health. 2009;68(4):327–36. doi: 10.3402/ijch.v68i4.17373. [DOI] [PubMed] [Google Scholar]
- 6.Muramatsu T, Yatsuya H, Toyoshima H, Sasaki S, Li Y, Otsuka R, et al. Higher dietary intake of alpha-linolenic acid is associated with lower insulin resistance in middle-aged Japanese. Prev Med. 2010;50(5-6):272–6. doi: 10.1016/j.ypmed.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Gerstein H, Yusuf S, Riddle MC, Ryden L, Bosch J ORIGIN Trial Investigators. Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: the ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention) (32.e1-6).Am Heart J. 2008;155(1):26–32. doi: 10.1016/j.ahj.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Olza J, Mesa MD, Aguilera CM, Moreno-Torres R, Jiménez A, Pérez de la Cruz A, et al. Influence of an eicosapentaenoic and docosahexaenoic acid-enriched enteral nutrition formula on plasma fatty acid composition and biomarkers of insulin resistance in the elderly. Clin Nutr. 2010;29(1):31–7. doi: 10.1016/j.clnu.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Manerba A, Vizzardi E, Metra M, Dei Cas L. n-3 PUFAs and cardiovascular disease prevention. Future Cardiol. 2010;6(3):343–50. doi: 10.2217/fca.10.19. [DOI] [PubMed] [Google Scholar]
- 10.Origasa H, Yokoyama M, Matsuzaki M, Saito Y, Matsuzawa Y. JELIS Investigators. Clinical importance of adherence to treatment with eicosapentaenoic acid by patients with hypercholesterolemia. Circ J. 2010;74(3):510–7. doi: 10.1253/circj.cj-09-0746. [DOI] [PubMed] [Google Scholar]
- 11.Bianchi C, Penno G, Miccoli R, Del Prato S. Blood glucose control and coronary heart disease. Herz. 2010;35(3):148–59. doi: 10.1007/s00059-010-3340-4. [DOI] [PubMed] [Google Scholar]
- 12.Vassort G, Turan B. Protective role of antioxidants in diabetes-induced cardiac dysfunction. Cardiovasc Toxicol. 2010;10(2):73–86. doi: 10.1007/s12012-010-9064-0. [DOI] [PubMed] [Google Scholar]
- 13.Rathod NR, Raghuveer I, Chitme HR, Chandra R. Free radical scavenging activity of calotropis gigantea on strepto-zotocin-induced diabetic rats. Indian J Pharm Sci. 2009;71(6):615–21. doi: 10.4103/0250-474X.59542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lautt WW, Ming Z, Legare DJ. Attenuation of age- and sucrose-induced insulin resistance and syndrome X by a synergistic antioxidant cocktail: the AMIS syndrome and HISS hypothesis. Can J Physiol Pharmacol. 2010;88(3):313–23. doi: 10.1139/Y09-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Degirolamo C, Kelley KL, Wilson MD, Rudel LL. Dietary n-3 LCPUFA from fish oil but not alpha-linolenic acid-derived LCPUFA confers atheroprotection in mice. J Lipid Res. 2010;51(7):1897–905. doi: 10.1194/jlr.M005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cayli S, Sati L, Seval-Celik Y, Tuncer MA, Yaymaci B, Berkman Z, et al. The effects of eicosapentaenoic acid on the endothelium of the carotid artery of rabbits on a high-cholesterol diet. Histol Histopathol. 2010;25(2):141–51. doi: 10.14670/HH-25.141. [DOI] [PubMed] [Google Scholar]
- 17.Matsuzaki M, Yokoyama M, Saito Y, Origasa H, Ishikawa Y, Oikawa S, et al. Incremental effects of eicosapentaenoic acid on cardiovascular events in statin-treated patients with coronary artery disease. Circ J. 2009;73(7):1283–90. doi: 10.1253/circj.cj-08-1197. [DOI] [PubMed] [Google Scholar]
- 18.Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Omega-3 fatty acids: how can they be used in secondary prevention? Curr Atheroscler Rep. 2008;10(6):510–7. doi: 10.1007/s11883-008-0079-y. [DOI] [PubMed] [Google Scholar]
- 19.Kuda O, Jelenik T, Jilkova Z, Flachs P, Rossmeisl M, Hensler M, et al. n-3 fatty acids and rosiglitazone improve insulin sensitivity through additive stimulatory effects on muscle glycogen synthesis in mice fed a high-fat diet. Diabetologia. 2009;52(5):941–51. doi: 10.1007/s00125-009-1305-z. [DOI] [PubMed] [Google Scholar]
- 20.Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 1993;88(2):523–33. doi: 10.1161/01.cir.88.2.523. [DOI] [PubMed] [Google Scholar]
- 21.Cicero AF, Ertek S, Borghi C. Omega-3 polyunsaturated fatty acids: their potential role in blood pressure prevention and management. Curr Vasc Pharmacol. 2009;7(3):330–7. doi: 10.2174/157016109788340659. [DOI] [PubMed] [Google Scholar]
- 22.Roth EM, Harris WS. Fish oil for primary and secondary prevention of coronary heart disease. Curr Atheroscler Rep. 2010;12(1):66–72. doi: 10.1007/s11883-009-0079-6. [DOI] [PubMed] [Google Scholar]
- 23.Shukla SK, Gupta S, Ojha SK, Sharma SB. Cardiovascular friendly natural products: a promising approach in the management of CVD. Nat Prod Res. 2010;24(9):873–98. doi: 10.1080/14786410903417378. [DOI] [PubMed] [Google Scholar]
- 24.Levitan EB, Wolk A, Mittleman MA. Fatty fish, marine omega-3 fatty acids and incidence of heart failure. Eur J Clin Nutr. 2010;64(6):587–94. doi: 10.1038/ejcn.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Sullivan TA, Ambrosini G, Beilin LJ, Mori TA, Oddy WH. Dietary intake and food sources of fatty acids in Australian adolescents. Nutrition. 2010 Mar 23; doi: 10.1016/j.nut.2009.11.019. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Massaro M, Scoditti E, Carluccio MA, Campana MC, De Caterina R. Omega-3 fatty acids, inflammation and angiogenesis: basic mechanisms behind the cardioprotective effects of fish and fish oils. Cell Mol Biol (Noisy-le-grand) 2010;56(1):59–82. [PubMed] [Google Scholar]
- 27.Allayee H, Roth N, Hodis HN. Polyunsaturated fatty acids and cardiovascular disease: implications for nutrigenetics. J Nutrigenet Nutrigenomics. 2009;2(3):140–8. doi: 10.1159/000235562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchioli R, Di Pasquale A. The biochemical, pharmacological and epidemiological reference picture of the GISSI-Prevention.The Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico. G Ital Cardiol. 1993;23(9):933–64. (Italian) [PubMed] [Google Scholar]
- 29.Osler M, Andreasen AH, Hoidrup S. No inverse association between fish consumption and risk of death from all causes, and incidence of coronary heart disease in middle-aged, Danish adults. J Clin Epidemiol. 2003;56(3):274–9. doi: 10.1016/s0895-4356(02)00600-5. [DOI] [PubMed] [Google Scholar]
- 30.Iso H, Kobayashi M, Ishihara J, Sasaki S, Okada K, Kita Y, et al. Intake of fish and n-3 fatty acids and risk of coronary heart disease among Japanese: the Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation. 2006;113(2):195–202. doi: 10.1161/CIRCULATIONAHA.105.581355. [DOI] [PubMed] [Google Scholar]


