Abstract
Pharmacological manipulation of the type 5 metabotropic glutamate (mGlu5) receptor alters various addiction related behaviors such as drug self-administration and the extinction and reinstatement of drug-seeking behavior. However, the effects of pharmacological modulation of mGlu5 receptors on brain reward function have not been widely investigated. We examined the effects of acute administration of positive and negative allosteric modulators (PAMs and NAMs, respectively) on brain reward function by assessing thresholds for intracranial self-stimulation (ICSS). In addition, when acute effects were observed, we examined changes in ICSS thresholds following repeated administration. Male Sprague-Dawley rats were implanted with bipolar electrodes into the medial forebrain bundle and trained to respond for ICSS, followed by assessment of effects of mGlu5 ligands on ICSS thresholds using a discrete trials current–intensity threshold determination procedure. Acute administration of the selective mGlu5 NAMs MTEP (0, 0.3, 1, or 3 mg/kg) and fenobam (0, 3, 10, or 30 mg/kg) dose-dependently increased ICSS thresholds (∼70% at the highest dose tested), suggesting a deficit in brain reward function. Acute administration of the mGlu5 PAMs CDPPB (0, 10, 30, and 60 mg/kg) or ADX47273 (0, 10, 30, and 60 mg/kg) was without effect at any dose tested. When administered once daily for five consecutive days, the development of tolerance to the ability of threshold-elevating doses of MTEP and fenobam to increase ICSS thresholds was observed. We conclude that mGlu5 PAMs and NAMs differentially affect brain reward function, and that tolerance to the ability of mGlu5 NAMs to reduce brain reward function develops with repeated administration. These brain reward deficits should be taken into consideration when interpreting acute effects of mGlu5 NAMs on drug self-administration, and repeated administration of these ligands may be an effective method to reduce these deficits.
Keywords: mGluR5, glutamate, allosteric modulator, intracranial self-stimulation, brain reward
Introduction
The type 5 metabotropic glutamate (mGlu5) receptor has been implicated in numerous CNS functions including synaptic plasticity, learning and memory, cognition, nociception, affect regulation, and motivated behaviors (Niswender and Conn, 2010). mGlu5 receptors are also involved in numerous CNS diseases such as depression, anxiety, schizophrenia, epilepsy, chronic pain, Fragile X syndrome, and drug addiction (Spooren et al., 2001; Bird and Lawrence, 2009a,b; Krystal et al., 2010; Niswender and Conn, 2010). With regards to drug addiction, genetic deletion of the mGlu5 receptor in mice results in indifference to the reinforcing and locomotor stimulant effects of cocaine (Chiamulera et al., 2001) and reduced ethanol consumption (Bird et al., 2008). In addition, a substantial literature exists with a general consensus that negative allosteric modulation of mGlu5 receptors reduces self-administration of most drugs of abuse including cocaine, heroin, methamphetamine, nicotine, and ethanol, as well as the reinstatement of drug-seeking behavior (reviewed in Kenny and Markou, 2004; Bird and Lawrence, 2009b; Olive, 2009; Cleva and Olive, in press).
The mechanism by which pharmacological antagonism of mGlu5 receptors reduces drug intake is not well understood. Several recent studies have demonstrated that a potential site of action of mGlu5 antagonists in reducing drug intake is the nucleus accumbens (Cozzoli et al., 2009; Gass and Olive, 2009b; Besheer et al., 2010), a primary component of the brain’s reward circuitry. A well-established method for assessing brain reward circuitry function is the intracranial self-stimulation (ICSS) paradigm, where animals are trained to perform an operant response in order to obtain electrical stimulation of the medial forebrain bundle (Kornetsky and Esposito, 1979; Kornetsky and Bain, 1992; Markou and Koob, 1992). Antagonism of mGlu5 receptors with the negative allosteric modulator (NAM) 2-methyl-6-(phenylethynyl) pyridine (MPEP) was first demonstrated to reduce thresholds for ICSS by Harrison et al. (2002). These findings were subsequently replicated by Kenny et al. (2003, 2005) who advanced the hypothesis that inhibition of mGlu5 receptor function may reduce cocaine self-administration by reducing brain reward function. Although MPEP has typically been the prototypical ligand of choice in many studies for inhibiting mGlu5 receptor function, some studies have revealed that this ligand has off-target effects on NMDA receptors, monoamine oxidase, and the norepinephrine transporter (O’Leary et al., 2000; Heidbreder et al., 2003; Lea and Faden, 2006). More recently, mGlu5 receptor NAMs that exhibit increased selectivity for mGlu5 receptors with fewer off-target effects have been developed, including 3-((2-methyl-4-thiazolyl)ethynyl)pyridine (MTEP; Cosford et al., 2003) and 1-(3-chlorophenyl)-3-(3-methyl-5-oxo-4H-imidazol-2-yl)urea (fenobam; Porter et al., 2005; Montana et al., 2009).
Conversely, systemically active positive allosteric modulators (PAMs) of mGlu5 such as 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB; Lindsley et al., 2004; Kinney et al., 2005) and (S)-(4-fluoro-phenyl)-(3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]piperidin-1-yl)methanone (ADX47273; Liu et al., 2008) have been developed as novel cognition enhancing agents and potential novel treatments for schizophrenia (Niswender and Conn, 2010). Studies by our laboratory and others have shown that CDPPB facilitates the extinction of cocaine-seeking behavior following intravenous self-administration (Cleva et al., 2011; Nic Dhonnchadha and Kantak, 2011) as well as the extinction of a cocaine-induced conditioned place preference (Gass and Olive, 2009a). However, no studies to date have examined the effects of mGlu5 PAMs on brain reward function.
The goals of the present study were to (1) determine if the more recently developed mGlu5 NAMs MTEP and fenobam, at doses that have been shown to reduce self-administration of drugs of abuse, produce decrements in brain reward function as indicated by increases in ICSS thresholds, (2) determine if mGlu5 PAMs alter brain reward function, and (3) determine if any observed effects of mGlu5 PAMs or NAMs on brain reward function would change after repeated administration, which would be more relevant to clinical use of such ligands.
Materials and Methods
Animals
Male Sprague-Dawley rats (250–275 g upon arrival) that were obtained from Harlan Laboratories (Indianapolis, IN, USA) were used for this study. Food and water were freely available at all times except during behavioral testing. The animal housing room was maintained on a reversed 12 h light–dark cycle (lights off at 0800 h), with controlled temperature and humidity within NIH guidelines. All experimentation was conducted during the dark phase of the light–dark cycle. All experimental procedures conformed to the 2003 Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research, and were approved by an Institutional Animal Care and Use Committee.
Surgical procedures
Animals were anesthetized with isoflurane (2% v/v) vaporized in oxygen at a flow rate of 2 l/min and placed in a stereotaxic frame (Stoelting Co., Wood Dale, IL, USA). The skin overlying the skull was shaved and scrubbed with betadine and 0.1% v/v H2O2, and an incision was made to expose the skull surface. A bipolar electrode (#MS303/2, Plastics One, Roanoke, VA, USA) was then unilaterally implanted into the lateral hypothalamus (AP: −0.5, ML: ±1.7, DV: −8.3 mm from skull surface and bregma according to the atlas of Paxinos and Watson, 2007). The length of the electrode (10 mm) was insulated except at the ventral tip. Electrodes were secured to the skull with stainless steel screws and dental cement. The wound was then treated with 2% bacitracin/polymyxin B/neomycin and 5% xylocaine, and sutured closed with 3-0 Vicryl sutures. Animals received meloxicam (10 mg/kg s.c.) once daily for 5 days to minimize post-surgical pain and discomfort, and were allowed to recover for at least 5 days prior to ICSS training.
ICSS testing apparatus
Intracranial self-stimulation procedures were conducted in computer-interfaced behavioral testing chambers (ENV-007, 30.5 cm × 30 cm × 17 cm, Med Associates. St. Albans, VT, USA) housed in melamine sound-attenuating cubicles as described above. Each chamber contained a metal wheel manipulandum (5 cm wide), centered on one of the side walls, that required ∼0.2 N force to result in a quarter turn rotation. Electrical brain stimulation was delivered by constant current stimulators (Med Associates) controlled by MED-PC IV software. Animals were connected to the stimulators with bipolar leads (Plastics One) attached to gold-contact electrical commutators (model SL2C, Plastics One) mounted on counterbalanced lever arms located atop the chamber.
ICSS procedures
A discrete trials current–intensity threshold procedure was employed (Kornetsky and Esposito, 1979; Kornetsky and Bain, 1992; Markou and Koob, 1992) to determine ICSS thresholds. Animals were first trained to turn the wheel manipulandum one-quarter of a turn in order to receive a delivery of a 200-ms train of cathodal square-wave pulses (frequency 100 Hz, intensity 120 μA) on a fixed-ratio 1 (FR1) schedule of reinforcement. Training was conducted in 30 min daily sessions. Following successful acquisition of responding for stimulation (>100 reinforcements per 5 min), training on the discrete trials current–intensity threshold procedure commenced. Each trial began with a response-independent delivery of an electrical stimulus (see above for parameters), followed by a 7.5-s period during which the animal was given the opportunity to make a response to receive an identical stimulus. A response during this 7.5 s period was labeled as a positive response and was followed by a 2-s timeout period, whereas a lack of a response during this period was labeled as a negative response. Additional responses during the 2-s timeout period resulted in an additional 12.5 s delay of the onset of the next trial. The inter-trial interval (ITI) that followed either a positive response or the end of the 7.5-s period (in the case of a negative response) was 10 s in duration. Responses that occurred during the ITI had no consequences.
Animals were subsequently tested on the current–intensity threshold procedure in which stimulation intensities were varied according to the psychophysical method of limits. A test session consisted of five alternating series of descending and ascending current intensities, starting with a descending series. Blocks of five trials were conducted at a given stimulation intensity starting at 120 μA, and the current–intensity was changed by 5 μA steps between blocks of trials. Each test session typically lasted 30–40 min. To determine the current–intensity threshold for each animal, the stimulus intensity between the successful completion of a set of trials (positive responses during three or more of the five trials) and the stimulus intensity for which the animal failed to respond positively on two or more of the five trials were recorded. The mean of the thresholds for the five series was defined as the threshold for the session. The time between the beginning of the response-independent stimulation and a positive response was recorded as the response latency for each trial. No response latencies were determined from negative response trials. The response latency for each session was defined as the mean response latency for all trials with positive responses.
Drugs and treatment
MTEP hydrochloride was obtained from Ascent Scientific (Princeton, NJ, USA) and was dissolved in a vehicle consisting of distilled water. Fenobam, CDPPB, and ADX47273 were custom synthesized by Chemir Analytical Services (Maryland Heights, MO, USA) according to previously published methods (Lindsley et al., 2004; Kinney et al., 2005; Porter et al., 2005; Liu et al., 2008) and were suspended in a vehicle consisting of 0.3% v/v Tween 80 (Sigma-Aldrich, St. Louis, MO, USA). Drug treatment procedures commenced following stabilization of baseline current–intensity thresholds (approximately five to seven discrete trial sessions, <10% variability in absolute ICSS threshold values). mGlu5 ligands were administered via the s.c. route in a volume of 1 ml/kg 20 min prior to discrete trial current–intensity threshold determinations.
For acute dose response studies, a minimum of two drug-free days of regular ICSS threshold determination testing were conducted between dose, and each dose and vehicle were given in a randomized counterbalanced manner. Doses of each compound administered were as follows: MTEP (0.3, 1, or 3 mg/kg), fenobam (3, 10, or 30 mg/kg), CDPPB (10, 30, and 60 mg/kg), and ADX47273 (10, 30, and 60 mg/kg). Separate groups of animals were utilized for each compound administered.
For repeated administration studies, doses of MTEP and fenobam that were found to elevate ICSS thresholds (3 and 30 mg/kg, respectively) were administered once daily for five consecutive days, each given 20 min prior to threshold determination procedures. Separate groups of animals were utilized for each compound administered.
Histology
Animals were deeply anesthetized with sodium pentobarbital, 150 mg/kg i.p. and perfused transcardially with 100 ml of phosphate-buffered saline (PBS, pH = 7.4) followed by 200 ml 4% w/v paraformaldehyde in PBS (pH = 7.4). Brains were then removed, post-fixed at 4°C for 24 h, and placed in a 30% w/v sucrose in PBS cryoprotectant solution at 4°C for 48 h. Brains were then cut into 40 μm sections on a cryostat (Model CM1900, Leica Microsystems, Bannockburn, IL, USA), mounted onto gelatin-coated microscope slides, and stained using cresyl violet. The tip of the electrode was then verified to localized to the lateral hypothalamus under light microscopy. Data from animals with incorrect placement of the electrode were excluded from analysis.
Data analysis
Data were analyzed using SigmaPlot software (Version 12.0, Systat Software, San Jose, CA, USA). ICSS thresholds from individual animals were calculated from three consecutive pre-treatment sessions that showed <10% variability in current–intensity thresholds, and these values were averaged to obtain a baseline threshold value. ICSS thresholds obtained from the remaining sessions were transformed to a percentage of this baseline value for each individual animal. Effects of acute administration of vehicle or mGlu5 PAMs and NAMs and response latencies were analyzed by one-way between-subjects ANOVA, with drug dose as the main factor, followed by Holm–Sidak post hoc pairwise comparisons against values from vehicle treated animals. Effects of repeated administration of the 3-mg/kg dose of MTEP and the 30-mg/kg dose of fenobam on ICSS thresholds were analyzed by one-way repeated-measures ANOVA, with treatment day as the main factor, followed by Holm–Sidak post hoc pairwise comparisons against threshold values from the first day of treatment. P < 0.05 was considered statistically significant for all tests performed. All data are presented as mean ± SEM.
Results
Acute dose response for mGlu5 NAMs and PAMs
Prior to commencement of treatment, baseline ICSS thresholds were 90.11 + 6.90 μA (mean ± SEM). The effects of vehicle and MTEP (0.3, 1, or 3 mg/kg) and fenobam (3, 10, and 30 mg/kg) on ICSS thresholds are shown in Figures 1A,B. A significant effect of MTEP dose was observed [F(3,31) = 10.82, P < 0.001], and post hoc analyses revealed that the 3-mg/kg dose of MTEP produce a significant (∼70%) increase in ICSS thresholds as compared to those following vehicle treatment (P < 0.05). Similarly, a significant effect of fenobam dose was observed [F(3,28) = 3.18, P < 0.05], and post hoc analyses revealed that the 30-mg/kg dose of fenobam produced a significant (∼68%) increase in ICSS thresholds as compared to those following vehicle treatment (P < 0.05). MTEP and fenobam did not produce significant effects on response latencies during the discrete trials (P > 0.05 vs. vehicle).
Figure 1.
Dose-dependent increases in ICSS thresholds following acute administration of the mGlu5 NAMs MTEP [(A): n = 13] or fenobam [(B): n = 11]. *P < 0.05 vs. vehicle.
The effects of vehicle and the 10-, 30-, and 60-mg/kg doses of CDPPB and ADX47273 on ICSS thresholds are shown in Figures 2A,B. None of the doses tested for either mGlu5 PAM produced a significant effect on ICSS thresholds or response latencies (all Ps > 0.05 vs. vehicle). Due to the lack of effects observed with these compounds, further investigation with repeated administration was not conducted.
Figure 2.
Absence of effects of acute administration of various doses of the mGlu5 PAM CDPPB [(A): n = 15] or ADX47273 [(B): n = 15] on ICSS thresholds.
Effects of repeated administration of MTEP and fenobam
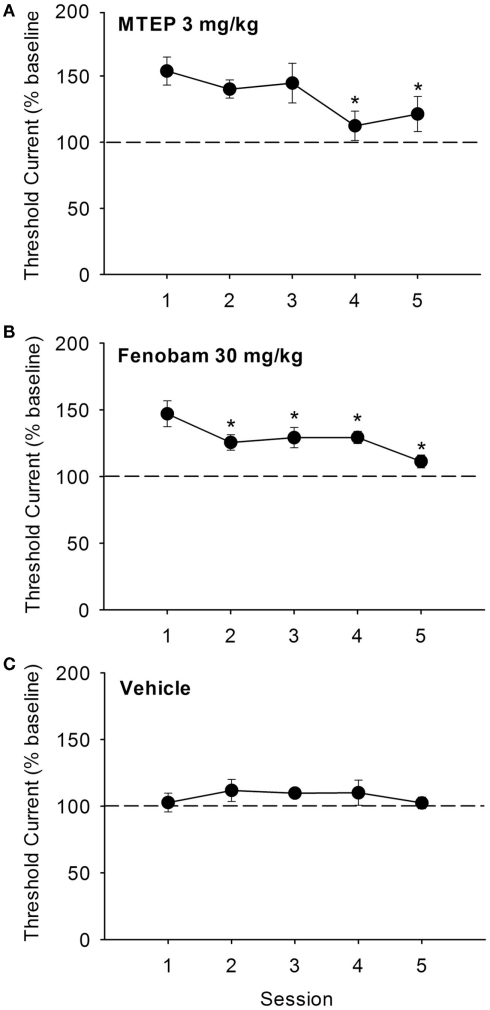
The effects of repeated (once daily for five consecutive days) administration of the threshold-elevating doses of MTEP (3 mg/kg) and fenobam (30 mg/kg) on ICSS thresholds are shown in Figures 3A,B. Effects of repeated administration of vehicle are shown in Figure 3C. In MTEP treated animals, a significant effect of session was observed [F(4,43) = 3.97, P < 0.01], and post hoc analyses revealed that ICSS thresholds during sessions 4 and 5 were significantly lower than those observed during session 1. Similarly, a significant effect of session dose was observed in fenobam treated rats [F(4,28) = 7.76, P < 0.001], and post hoc analyses revealed that ICSS thresholds during sessions 2, 3, 4, and 5 were significantly lower than those during session 1. Repeated administration of vehicle (10% v/v) produced no effects on ICSS thresholds across sessions, and response latencies for all three treatment groups were unaffected (all Ps > 0.05).
Figure 3.
Effects of repeated administration of MTEP [(A): 3 mg/kg, n = 12], fenobam [(B): 30 mg/kg, n = 9], or vehicle [(C): n = 4] for five consecutive threshold determination sessions conduced once daily. *P < 0.05 vs. Session 1.
Electrode placement
Examination of histological sections under bright microscopy demonstrated that three rats had incorrect placement of the ICSS electrode into the lateral hypothalamus. Data obtained from these animals were discarded. An additional seven rats were removed from the ICSS study due to loss of the cranial implant during the experiment. Tissue from all other animals demonstrated correct placement of the electrode.
Discussion
Our findings indicate that positive and negative allosteric modulation of mGlu5 receptors differentially modulates brain reward function as assessed by ICSS threshold determination procedures. Specifically, acute administration of the mGlu5 PAMs CDPPB and ADX47273 are without effect on ICSS thresholds, suggesting an absence of alteration in brain reward function. However, it should be noted that we tested doses up to 60 mg/kg of these mGlu5 PAMs, while other studies have shown that a median effective doses of 100 mg/kg ADX47273 was required to attenuate dopamine-mediated behaviors such as apomorphine-induced climbing and phencyclidine-, apomorphine-, and amphetamine-induced hyperlocomotion (Liu et al., 2008). Thus, the possibility exists that doses of ADX47273 and CDPPB higher than 60 mg/kg may alter brain reward function, and further studies are needed to confirm this possibility.
In contrast to the lack of observed effects of acute administration of mGlu5 PAMs, acute administration of the mGlu5 NAMs MTEP and fenobam dose-dependently increased ICSS thresholds, which is reflective of decreased brain reward function (Kornetsky and Esposito, 1979; Kornetsky and Bain, 1992; Markou and Koob, 1992). These latter observations are in agreement with previous studies showing that acute administration of the less selective mGlu5 NAM MPEP (3 and 9 mg/kg) also elevates ICSS thresholds (Harrison et al., 2002; Kenny et al., 2003, 2005). However, a more recent study found that these doses of MPEP did not alter brain stimulation reward (Gormley and Rompre, 2011). These discrepant results are likely due to differences in the ICSS procedures employed. In the present study and others (Harrison et al., 2002; Kenny et al., 2003, 2005), a current–intensity threshold determination procedure was used, which varies the intensity of the stimulation current delivered to the electrode while keeping the stimulation frequency constant. On the other hand, Gormley and Rompre (2011) utilized a rate–frequency analysis procedure which varies the frequency of the stimulation current delivered to the electrode while keeping the current–intensity constant. It is therefore of interest to conduct future studies to determine if MTEP or fenobam produce any effects on brain stimulation reward using this rate–frequency approach.
The fact that acute administration of MTEP and fenobam increased ICSS threshold suggests that the reported ability of these drugs to attenuate drug self-administration (Cowen et al., 2005, 2007; Adams et al., 2008; Osborne and Olive, 2008; Palmatier et al., 2008; Gass et al., 2009; Hao et al., 2010; Sidhpura et al., 2010) may result from decreases in baseline activity of the brain’s reward circuitry. In addition, elevations in ICSS threshold are generally associated with aversive or anhedonic states (Markou and Koob, 1992). Thus, mGlu5 NAM-induced suppression of drug intake may reflect a negative affective state of the animal as opposed to a reduction in the reinforcing and motivational effects of the self-administered drug. These factors need to be taken into consideration when interpreting the underlying mechanisms by which mGlu5 NAMs reduce drug intake.
We also found when the doses of MTEP and fenobam that elevated ICSS thresholds following acute administration (3 and 30 mg/kg, respectively) were administered repeatedly over the course of 5 days, a gradual attenuation of the ICSS threshold-elevating effects was observed. This development of tolerance may possibly be reflective of reduced expression of mGlu5 in forebrain regions that result from repeated mGlu5 NAM administration, as has previously been reported (Cowen et al., 2005). Regardless of the mechanism, since elevations in ICSS thresholds produced by MTEP and fenobam were significantly reduced after several days of treatment, it is of interest to discern whether repeated administration of either of these ligands results in tolerance to their ability to suppress drug intake. If tolerance to the potential therapeutic effects of MTEP or fenobam are absent, as has recently been reported with regards to the lack of ability of repeated fenobam administration to produce tolerance to its analgesic effects (Montana et al., 2011), it would therefore follow that repeated administration of mGlu5 NAMs may be a novel experimental approach to suppressing drug intake that circumvents the potential confounds of reduced brain reward function. Repeated drug administration also has greater face validity for pharmacotherapeutic approaches to reducing drug intake in human drug addicts than single dosing paradigms.
The ability of acute fenobam administration to increase ICSS thresholds, and the subsequent development of tolerance to these effects, has important clinical implications for medical conditions other than drug addiction. Fenobam was introduced more than 30 years ago as a potential novel non-benzodiazepine anxiolytic compound (Itil et al., 1978; Friedmann et al., 1980; Pecknold et al., 1980, 1982; Goldberg et al., 1983). However, these clinical trials were discontinued following reports of adverse dose-related side effects such as dizziness, paresthesias, sedation, and derealization. More recently, it has been demonstrated that lower doses of fenobam (50–150 mg/day) actually produces cognitive improvement in adult patients with Fragile X syndrome, with no CNS-related adverse side effects (Berry-Kravis et al., 2009). Additional reports of clinical efficacy and few side effects have been reported for other mGlu5 NAMs such as ADX10059 for the treatment of gastro-esophageal reflux disease (Keywood et al., 2009; Zerbib et al., 2011). With the possible exception of early studies with high doses of fenobam, clinical reports on fenobam administration to humans have not yet reported adverse side effects that would be consistent with decreased brain reward function, such as anhedonia, dysphoria, or other negative affective states. Nonetheless, based on our current findings, future clinical studies should monitor for possible occurrence of these effects, and if such effects resolve with repeated dosing.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by Public Health Services grants AA013852, DA024355, and DA025606 from the National Institutes of Health of the United States.
References
- Adams C. L., Cowen M. S., Short J. L., Lawrence A. J. (2008). Combined antagonism of glutamate mGlu5 and adenosine A2A receptors interact to regulate alcohol-seeking in rats. Int. J. Neuropsychopharmacol. 11, 229–241 10.1017/S1461145707007845 [DOI] [PubMed] [Google Scholar]
- Besheer J., Grondin J. J., Cannady R., Sharko A. C., Faccidomo S., Hodge C. W. (2010). Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biol. Psychiatry 67, 812–822 10.1016/j.biopsych.2009.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E. M., Hessl D., Coffey S., Hervey C., Schneider A., Yuhas J., Hutchison J., Snape M., Tranfaglia M., Nguyen D. V., Hagerman R. (2009). A pilot open-label single-dose trial of fenobam in adults with fragile X syndrome. J. Med. Genet. 46, 266–271 10.1136/jmg.2008.063701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird M. K., Kirchhoff J., Djouma E., Lawrence A. J. (2008). Metabotropic glutamate 5 receptors regulate sensitivity to ethanol in mice. Int. J. Neuropsychopharmacol. 11, 765–774 10.1017/S1461145708008572 [DOI] [PubMed] [Google Scholar]
- Bird M. K., Lawrence A. J. (2009a). Group I metabotropic glutamate receptors: involvement in drug-seeking and drug-induced plasticity. Curr. Mol. Pharmacol. 2, 83–94 10.2174/1874467210902010083 [DOI] [PubMed] [Google Scholar]
- Bird M. K., Lawrence A. J. (2009b). The promiscuous mGlu5 receptor – a range of partners for therapeutic possibilities? Trends Pharmacol. Sci. 30, 617–623 10.1016/j.tips.2009.09.008 [DOI] [PubMed] [Google Scholar]
- Chiamulera C., Epping-Jordan M. P., Zocchi A., Marcon C., Cottiny C., Tacconi S., Corsi M., Orzi F., Conquet F. (2001). Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat. Neurosci. 4, 873–874 10.1038/nn0901-873 [DOI] [PubMed] [Google Scholar]
- Cleva R. M., Hicks M. P., Gass J. T., Wischerath K. C., Plasters E. T., Widholm J. J., Olive M. F. (2011). mGluR5 positive allosteric modulation enhances extinction learning following cocaine self-administration. Behav. Neurosci. 125, 10–19 10.1037/a0022339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleva R. M., Olive M. F. (in press). Metabotropic glutamate receptors and drug addiction. Wiley Interdiscip. Rev. Membr. Transp. Signal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosford N. D., Tehrani L., Roppe J., Schwieger E., Smith N. D., Anderson J., Bristow L., Brodkin J., Jiang X., Mcdonald I., Rao S., Washburn M., Varney M. A. (2003). 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J. Med. Chem. 46, 204–206 10.1021/jm025570j [DOI] [PubMed] [Google Scholar]
- Cowen M. S., Djouma E., Lawrence A. J. (2005). The metabotropic glutamate 5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl)-pyridine reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems. J. Pharmacol. Exp. Ther. 315, 590–600 10.1124/jpet.105.090449 [DOI] [PubMed] [Google Scholar]
- Cowen M. S., Krstew E., Lawrence A. J. (2007). Assessing appetitive and consummatory phases of ethanol self-administration in C57BL/6J mice under operant conditions: regulation by mGlu5 receptor antagonism. Psychopharmacology (Berl.) 190, 21–29 10.1007/s00213-006-0583-0 [DOI] [PubMed] [Google Scholar]
- Cozzoli D. K., Goulding S. P., Zhang P. W., Xiao B., Hu J. H., Ary A. W., Obara I., Rahn A., Abou-Ziab H., Tyrrel B., Marini C., Yoneyama N., Metten P., Snelling C., Dehoff M. H., Crabbe J. C., Finn D. A., Klugmann M., Worley P. F., Szumlinski K. K. (2009). Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J. Neurosci. 29, 8655–8668 10.1523/JNEUROSCI.5900-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann C. T. H., Davis L. J., Ciccone P. E., Rubin R. T. (1980). Phase II double-blind controlled study of a new anxiolytic, fenobam (McN-3377) vs. placebo. Curr. Ther. Res. 27, 144–151 [Google Scholar]
- Gass J. T., Olive M. F. (2009a). Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol. Psychiatry 65, 717–720 10.1016/j.biopsych.2008.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass J. T., Olive M. F. (2009b). Role of protein kinase C epsilon (PKCε) in the reduction of ethanol reinforcement due to mGluR5 antagonism in the nucleus accumbens shell. Psychopharmacology (Berl.) 204, 587–597 10.1007/s00213-009-1490-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass J. T., Osborne M. P., Watson N. L., Brown J. L., Olive M. F. (2009). mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology 34, 820–833 10.1038/npp.2008.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. E., Salama A. I., Patel J. B., Malick J. B. (1983). Novel non-benzodiazepine anxiolytics. Neuropharmacology 22, 1499–1504 10.1016/0028-3908(83)90118-1 [DOI] [PubMed] [Google Scholar]
- Gormley S., Rompre P. P. (2011). Blockade of mGLUR5 receptors differentially alters amphetamine-induced enhancement of locomotor activity and of brain stimulation reward. J. Psychopharmacol. 25, 393–401 10.1177/0269881110367460 [DOI] [PubMed] [Google Scholar]
- Hao Y., Martin-Fardon R., Weiss F. (2010). Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol. Psychiatry 68, 240–248 10.1016/j.biopsych.2010.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A. A., Gasparini F., Markou A. (2002). Nicotine potentiation of brain stimulation reward reversed by DHβE and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl.) 160, 56–66 10.1007/s00213-001-0953-6 [DOI] [PubMed] [Google Scholar]
- Heidbreder C. A., Bianchi M., Lacroix L. P., Faedo S., Perdona E., Remelli R., Cavanni P., Crespi F. (2003). Evidence that the metabotropic glutamate receptor 5 antagonist MPEP may act as an inhibitor of the norepinephrine transporter in vitro and in vivo. Synapse 50, 269–276 10.1002/syn.10261 [DOI] [PubMed] [Google Scholar]
- Itil T. M., Seaman P. A., Huque M., Mukhopadhyay S., Blasucci D., Tat K., Ciccone P. E. (1978). The clinical and quantitative EEG effects and plasma levels of fenobam (McN-3377) in subjects with anxiety: an open rising dose tolerance and efficacy study. Curr. Ther. Res. 24, 708–724 [Google Scholar]
- Kenny P. J., Boutrel B., Gasparini F., Koob G. F., Markou A. (2005). Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology (Berl.) 179, 247–254 10.1007/s00213-004-2069-2 [DOI] [PubMed] [Google Scholar]
- Kenny P. J., Gasparini F., Markou A. (2003). Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J. Pharmacol. Exp. Ther. 306, 1068–1076 10.1124/jpet.103.052027 [DOI] [PubMed] [Google Scholar]
- Kenny P. J., Markou A. (2004). The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol. Sci. 25, 265–272 10.1016/j.tips.2004.03.009 [DOI] [PubMed] [Google Scholar]
- Keywood C., Wakefield M., Tack J. (2009). A proof of concept study evaluating the effect of ADX10059, a metabotropic glutamate receptor-5 negative allosteric modulator, on acid exposure and symptoms in gastro-esophageal reflux disease. Gut 58, 1192–1199 10.1136/gut.2008.162040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney G. G., O’Brien J. A., Lemaire W., Burno M., Bickel D. J., Clements M. K., Chen T. B., Wisnoski D. D., Lindsley C. W., Tiller P. R., Smith S., Jacobson M. A., Sur C., Duggan M. E., Pettibone D. J., Conn P. J., Williams D. L., Jr. (2005). A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. J. Pharmacol. Exp. Ther. 313, 199–206 10.1124/jpet.104.079244 [DOI] [PubMed] [Google Scholar]
- Kornetsky C., Bain G. (1992). Brain-stimulation reward: a model for the study of the rewarding effects of abused drugs. NIDA Res. Monogr. 124, 73–93 [PubMed] [Google Scholar]
- Kornetsky C., Esposito R. U. (1979). Euphorigenic drugs: effects on the reward pathways of the brain. Fed. Proc. 38, 2473–2476 [PubMed] [Google Scholar]
- Krystal J. H., Mathew S. J., D’Souza D. C., Garakani A., Gunduz-Bruce H., Charney D. S. (2010). Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs 24, 669–693 10.2165/11533230-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Lea P. M., Faden A. I. (2006). Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev. 12, 149–166 10.1111/j.1527-3458.2006.00149.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley C. W., Wisnoski D. D., Leister W. H., O’Brien J. A., Lemaire W., Williams D. L., Jr., Burno M., Sur C., Kinney G. G., Pettibone D. J., Tiller P. R., Smith S., Duggan M. E., Hartman G. D., Conn P. J., Huff J. R. (2004). Discovery of positive allosteric modulators for the metabotropic glutamate receptor subtype 5 from a series of N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamides that potentiate receptor function in vivo. J. Med. Chem. 47, 5825–5828 10.1021/jm049400d [DOI] [PubMed] [Google Scholar]
- Liu F., Grauer S., Kelley C., Navarra R., Graf R., Zhang G., Atkinson P. J., Wantuch C., Popiolek M., Day M., Khawaja X., Smith D., Olsen M., Kouranova E., Gilbert A., Lai M., Pausch M. H., Pruthi F., Pulicicchio C., Brandon N. J., Comery T. A., Beyer C. E., Logue S., Rosenzweig-Lipson S., Marquis K. L. (2008). ADX47273: a novel metabotropic glutamate receptor 5 selective positive allosteric modulator with preclinical antipsychotic-like and pro-cognitive activities. J. Pharmacol. Exp. Ther. 327, 827–839 10.1124/jpet.108.136580 [DOI] [PubMed] [Google Scholar]
- Markou A., Koob G. F. (1992). Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol. Behav. 51, 111–119 10.1016/0031-9384(92)90211-J [DOI] [PubMed] [Google Scholar]
- Montana M. C., Cavallone L. F., Stubbert K. K., Stefanescu A. D., Kharasch E. D., Gereau R. W. (2009). The metabotropic glutamate receptor subtype 5 antagonist fenobam is analgesic and has improved in vivo selectivity as compared to the prototypical antagonist 2-methyl-6-(phenylethynyl)-pyridine. J. Pharmacol. Exp. Ther. 330, 834–843 10.1124/jpet.109.154138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montana M. C., Conrardy B. A., Cavallone L. F., Kolber B. J., Rao L. K., Greco S. C., Gereau R. W. T. (2011). Metabotropic glutamate receptor 5 antagonism with fenobam: examination of analgesic tolerance and side effect profile in mice. Anesthesiology 115, 1239–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha B. A., Kantak K. M. (2011). Cognitive enhancers for facilitating drug cue extinction: insights from animal models. Pharmacol. Biochem. Behav. 99, 229–244 10.1016/j.pbb.2011.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender C. M., Conn P. J. (2010). Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 50, 295–322 10.1146/annurev.pharmtox.011008.145533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary D. M., Movsesyan V., Vicini S., Faden A. I. (2000). Selective mGluR5 antagonists MPEP and SIB-1893 decrease NMDA or glutamate-mediated neuronal toxicity through actions that reflect NMDA receptor antagonism. Br. J. Pharmacol. 131, 1429–1437 10.1038/sj.bjp.0703715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive M. F. (2009). Metabotropic glutamate receptor ligands as potential therapeutics for drug addiction. Curr. Drug Abuse Rev. 2, 83–98 10.2174/1874473710902010083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne M. P. H., Olive M. F. (2008). A role for mGluR5 receptors in intravenous methamphetamine self-administration. Ann. N. Y. Acad. Sci. 1139, 206–211 10.1196/annals.1432.034 [DOI] [PubMed] [Google Scholar]
- Palmatier M. I., Liu X., Donny E. C., Caggiula A. R., Sved A. F. (2008). Metabotropic glutamate 5 receptor (mGluR5) antagonists decrease nicotine seeking, but do not affect the reinforcement enhancing effects of nicotine. Neuropsychopharmacology 33, 2139–2147 10.1038/sj.npp.1301623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Watson C. (2007). The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press [Google Scholar]
- Pecknold J. C., Mcclure D. J., Appeltauer L. (1980). Fenobam in anxious outpatients. Curr. Ther. Res. 27, 119–123 [Google Scholar]
- Pecknold J. C., Mcclure D. J., Appeltauer L., Wrzesinski L., Allan T. (1982). Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double-blind standard (diazepam) placebo-controlled study. J. Clin. Psychopharmacol. 2, 129–133 10.1097/00004714-198204000-00010 [DOI] [PubMed] [Google Scholar]
- Porter R. H., Jaeschke G., Spooren W., Ballard T., Buettelmann B., Kolczewski S., Peters J. U., Prinssen E., Wichmann J., Vieira E., Muehlemann A., Gatti S., Mutel V., Malherbe P. (2005). Fenobam: a clinically validated non-benzodiazepine anxiolytic is a potent, selective and non-competitive mGlu5 receptor antagonist with inverse agonist activity. J. Pharmacol. Exp. Ther. 315, 711–721 10.1124/jpet.105.089839 [DOI] [PubMed] [Google Scholar]
- Sidhpura N., Weiss F., Martin-Fardon R. (2010). Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biol. Psychiatry 67, 804–811 10.1016/j.biopsych.2010.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooren W. P., Gasparini F., Salt T. E., Kuhn R. (2001). Novel allosteric antagonists shed light on mglu5 receptors and CNS disorders. Trends Pharmacol. Sci. 22, 331–337 10.1016/S0165-6147(00)01694-1 [DOI] [PubMed] [Google Scholar]
- Zerbib F., Bruley D. V. S., Roman S., Tutuian R., Galmiche J. P., Mion F., Tack J., Malfertheiner P., Keywood C. (2011). Randomised clinical trial: effects of monotherapy with ADX10059, a mGluR5 inhibitor, on symptoms and reflux events in patients with gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther. 33, 911–921 10.1111/j.1365-2036.2011.04646.x [DOI] [PubMed] [Google Scholar]