Abstract
c-Jun N-terminal kinase (JNK) is activated by dual phosphorylation of both threonine and tyrosine residues in the phosphorylation loop of the protein in response to several stress factors. However, the precise molecular mechanisms for activation after phosphorylation remain elusive. Here we show that Pin1, a peptidyl-prolyl isomerase, has a key role in the JNK1 activation process by modulating a phospho-Thr-Pro motif in the phosphorylation loop. Pin1 overexpression in human breast cancer cell lines correlates with increased JNK activity. In addition, small interfering RNA (siRNA) analyses showed that knockdown of Pin1 in a human breast cancer cell line decreased JNK1 activity. Pin1 associates with JNK1, and then catalyzes prolyl isomerization of the phospho-Thr-Pro motif in JNK1 from trans- to cis-conformation. Furthermore, Pin1 enhances the association of JNK1 with its substrates. As a result, Pin1−/− cells are defective in JNK activation and resistant to oxidative stress. These results provide novel insights that, following stress-induced phosphorylation of Thr in the Thr-Pro motif of JNK1, JNK1 associates with Pin1 and undergoes conformational changes to promote the binding of JNK1 to its substrates, resulting in cellular responses from extracellular signals.
Keywords: c-Jun N-terminal kinase, peptidyl-prolyl cis/trans-isomerase, apoptosis
A variety of extracellular stimuli induce cellular activities such as survival, proliferation, differentiation, and apoptosis through the activation of a family of mitogen-activated protein kinases (MAPKs). Extracellular signal-regulated kinase (ERK), p38 MAPK, and c-Jun N-terminal kinase (JNK) are the members of MAPKs.1, 2 MAPKs are activated by phosphorylation of Thr and Tyr residues with the motif Thr-X-Tyr (X is Glu in ERK; Gly in p38; Pro in JNK) within a conserved phosphorylation loop. JNK, also known as stress-activated protein kinase, serves as a phosphorylation substrate for MAP kinase kinases (MAP2Ks) such as MKK4/7, which are activated in turn by phosphorylation via MAP2K kinases.3, 4, 5 JNK is activated by phosphorylation of Thr and Tyr residues in the phosphorylation loop in response to several different stress factors including oxidative stress, inflammatory cytokines, protein synthesis inhibitors, growth factor withdrawal, chemotherapeutic drugs, and ultraviolet (UV) irradiation.6 JNK phosphorylates and thereby regulates a variety of transcription factors, including c-Jun, ATF-2, Elk-1, p53, T-cell factor β1 (TCFβ1), and c-Myc, as well as other factors such as BAD and Bcl-2.7 However, how the duration of JNK activation is regulated has not been fully elucidated.
The peptidyl-prolyl cis/trans-isomerase (PPIase) Pin1 is a small protein with an N-terminal WW domain and a C-terminal PPIase domain.8 The WW domain binds phosphorylated Ser/Thr-Pro (pSer/Thr-Pro) motifs8, 9, 10, 11 and the PPIase domain catalyzes cis/trans-isomerization of such proline-containing peptides.8, 12 Peptidyl-prolyl isomerization of phosphorylated moieties makes it possible to switch the conformation of a protein, thereby modulating protein activity, phosphorylation status, protein–protein interactions, subcellular localization, and stability. A recent study revealed that Pin1 is overexpressed in breast cancer;13 the authors of this study suggested that Pin1 overexpression promotes oncogenesis due to the interaction of Pin1 with c-Jun, which increases the transcriptional activity of c-Jun, resulting in increased cellular levels of cyclin D1. Conversely, Pin1 has also been shown to have a potential anti-cancer role by regulating the apoptotic response to genotoxic insults via its interaction with the tumor suppressor protein p53.14, 15, 16
Here we show that Pin1 interacts with and activates phosphorylated JNK1. The binding of Pin1 to JNK1 increases the binding affinity of JNK1 for its downstream substrates. Both JNK1 binding and isomerase activities of Pin1 are required for JNK1 stimulation. Moreover, JNK1 treated with Pin1 is resistant to proteolysis by subtilisin and to dephosphorylation by the trans-pSer/Thr-Pro isomer-specific phosphatase PP2A. Our findings elucidate a novel mechanism of control of JNK1 activity and thus of the biological roles of JNK1.
Results
Overexpression of Pin1 in human breast tumors correlates with activation of JNK
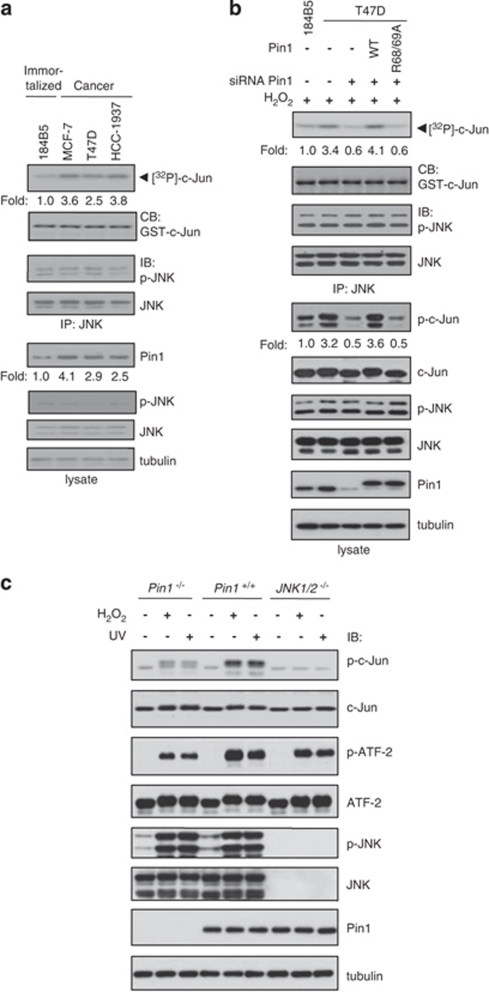
Pin1 is overexpressed in human breast tumors and its levels are correlated with the tumor grade.13 We examined whether Pin1 expression levels correlate with JNK activity in human breast cancer cell lines (Figure 1a). The levels of Pin1 in breast cancer cell lines are higher than that in mammary cells derived from normal mammary epithelial cells, whereas levels of phospho-JNK or JNK in those cells are similar. When in vitro kinase assays using c-Jun as a JNK substrate were performed with the immunoprecipitated endogenous JNK1, the kinase activities of JNK correlate with the levels of Pin1. When Pin1 was knocked down in T47D cells, both in vitro JNK activities and in vivo phospho-c-Jun levels were reduced without changing JNK expression level and phosphorylation status (Figure 1b). In addition, in vitro JNK activities and in vivo phospho-c-Jun levels were recovered when Pin1 was overexpressed by re-introducing small interfering RNA (siRNA)-resistant Pin1 wild-type (WT) expression plasmid. However, overexpression of Pin1 mutant (R68/69A) that has no isomerase activity resulted in no enhancement of JNK activities or in vivo phospho-c-Jun levels. Taken together, the results suggest that Pin1 has a role in enhancing JNK activity in breast cancer.
Figure 1.
Correlation between Pin1 level and JNK activity. (a) The same amounts of total lysates prepared from spontaneously immortalized normal human mammary epithelial cell lines (immortalized) and human breast carcinoma-derived cell lines (cancer) were subjected to immunoblot analysis with Pin1 or tubulin antibodies. For JNK kinase activity assays, cells were lysed and endogenous JNK1 was immunoprecipitated with an anti-JNK antibody. Immunoprecipitates were subjected to an in vitro kinase assay as described in Materials and Methods. Kinase activity was normalized to the expression level of JNK and presented as fold increase. CB, coomassie blue staining; IB, immunoblot; IP, immunoprecipitation. (b) 184B5 and T47D cells were transfected with Pin1 siRNA (targeting non-coding region) or FLAG-Pin1 (WT or R68/69A) plasmids and then exposed to 1 mM H2O2 for 1 h. After cell lysis, endogenous JNK1 was immunoprecipitated with an anti-JNK antibody. Immunoprecipitates were subjected to an in vitro kinase assay using 1 μg His-c-Jun as a substrate. Total amounts of nucleic acids for each transfection were equalized with the addition of scrambled siRNA or empty plasmid. (c) Lysates from Pin1+/+, Pin1−/− MEFs, and JNK1/2−/− cells treated with 1 mM H2O2 or irradiated with UV were analyzed by immunoblotting with antibodies as indicated. The experiments were repeated three times with similar results
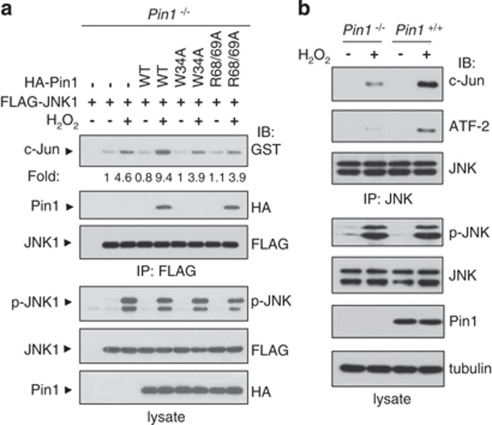
We then measured in vivo JNK activity in Pin1−/− mouse embryonic fibroblasts (MEFs) to examine the effect of Pin1 depletion on JNK activity. Given that activated JNK phosphorylates c-Jun and ATF-2, their in vivo phosphorylation levels were determined by immunoblot analysis. On exposure to H2O2 or UV to activate JNK1, the levels of phosphorylated c-Jun and ATF-2 were significantly reduced in Pin1−/− MEFs compared with Pin1+/+ cells (Figure 1c). To further examine whether the phosphorylation levels of c-Jun and ATF-2 are dependent on JNK, we measured the phosphorylation levels of these proteins in JNK1/2−/− cells. The phosphorylation of c-Jun was significantly decreased in JNK1/2−/− cells, whereas that of ATF-2 decreased moderately on H2O2 or UV treatment when compared with Pin1+/+ cells. The moderate decrease of ATF-2 phosphorylation might be due to the presence of p38, which also phosphorylates ATF-2. Taken together, our results indicate that Pin1 is critical for JNK1 activity in vivo.
Pin1 binds to phosphorylated Thr-183 of JNK1 in a JNK1 phosphorylation-dependent manner
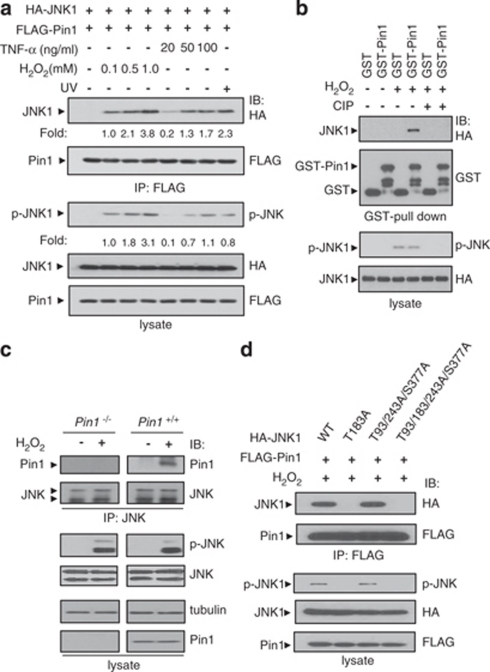
JNK not only preferentially phosphorylates Ser/Thr-Pro motifs but also contains a phosphorylatable Thr-Pro-Tyr motif in its phosphorylation loop that is critical for activation. We tested whether Pin1 could associate with JNK1 by using coimmunoprecipitation assays after cells were treated with H2O2, TNF-α, or UV. JNK1–Pin1 association was observed in H2O2, TNF-α, and UV-treated human embryonic kidney (HEK) 293 cell lysates and increased in a dose-dependent manner, concomitant with an increase in the amount of phospho-JNK1 (Figure 2a). Other MAPKs, ERK, and p38, which do not have Thr-Pro motifs in their phosphorylation loops, did not associate with Pin1. To investigate whether phosphorylation of JNK1 is responsible for this association, we performed an in vitro interaction analysis after phosphorylated JNK1 was dephosphorylated by calf intestine phosphatase (CIP). Dephosphorylation of JNK1 by CIP abolished the interaction between Pin1 and JNK1, indicating that this interaction is highly dependent on JNK1 phosphorylation (Figure 2b). Endogenous association between Pin1 and JNK1 was detected in H2O2-treated Pin1+/+ MEFs but not in Pin1−/− or untreated Pin1+/+ MEFs (Figure 2c). Taken together, these results indicate that Pin1 interacts specifically with JNK1 that is phosphorylated in response to several stress factors.
Figure 2.
Pin1 interacts specifically with activated JNK1 in a phosphorylation-dependent manner. (a) HEK 293 cells transfected with plasmids as indicated were treated for 1 h with H2O2, UV (25 J/m2), or TNF-α at the concentrations indicated. Immunoprecipitation and immunoblotting were performed as described in Materials and Methods. HA, hemagglutinin. (b) Lysates from HEK 293 cells transfected with the HA-JNK1 expression plasmid and treated with 1 mM H2O2 for 1 h or left untreated were incubated with CIP in the absence of phosphatase inhibitors before incubation with GST or GST-Pin1. GST pulled-down complexes were subjected to SDS-PAGE and then immunoblotted with an anti-HA antibody. (c) Lysates from H2O2-treated or untreated Pin1−/− and Pin1+/+ MEFs were analyzed by immunoprecipitation and then immunoblotting as described in Materials and Methods. (d) HEK 293 cells transfected with plasmids as indicated were treated with 1 mM H2O2 for 1 h. Immunoprecipitation and immunoblotting were performed as described in Materials and Methods
There are four Ser/Thr-Pro motifs in human JNK1; three Thr-Pro (Thr-93, Thr-183, and Thr-243) motifs and one Ser-Pro (Ser-377) motif. As phosphorylation of Thr-183 on JNK1 is critical for JNK1 activation and a candidate for the Pin1-binding sites, we tested whether Thr-183 is involved in the association. JNK1 WT was detected in the pulled-down Pin1 complexes, but not the mutant T183A (Figure 2d). The mutations at the other Ser/Thr sites had no effect on the association between the JNK1 and Pin1, suggesting that Thr-183 is the only Pin1-binding site in JNK1. In addition, pTyr-185 of JNK1 might be involved in the association between JNK1 and Pin1, because the JNK1 (Y185F) mutant had lower binding affinity for Pin1 than JNK1 WT (Supplementary Figure 1a).
The interaction required the WW domain, as demonstrated by the lack of binding when the WW domain was deleted (Supplementary Figure 1b). Pin1 mutant that is defective in substrate binding (W34A) lost most of its binding activity to JNK1, whereas a PPIase mutant (C113A) interacted with JNK1 (Supplementary Figure 1c).
Both the JNK1 binding and isomerase activities of Pin1 WT are required for the regulation of JNK1 activity
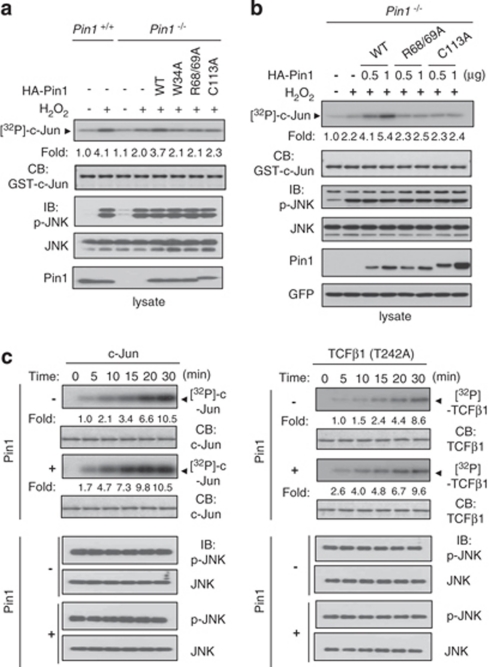
We next carried out in vitro kinase assays to investigate whether Pin1 regulates JNK1 kinase activity. JNK1 kinase activity was increased to a greater extent in Pin1+/+ than in Pin1−/− cells (Figure 3a). Transient expression of Pin1 WT in Pin1−/− cells resulted in enhanced c-Jun phosphorylation, whereas Pin1 (W34A) or Pin1 (C113A) showed no effect on JNK1 activity, implying that both the JNK1 binding and isomerase activities of Pin1 are required for regulating JNK1. Another Pin1 mutant (R68/69A) also did not have any effect on JNK1 activity. H2O2 did not interfere with Pin1 activity under our experimental conditions (Supplementary Figure 2). Furthermore, Pin1 WT increased JNK1 activity in a dose-dependent manner, but catalytically inactive Pin1 mutants (C113A and R68/69A) had no effect on JNK1 activity (Figure 3b).
Figure 3.
Pin1 enhances JNK1 kinase activity. (a and b) Pin1−/− and Pin1+/+ MEFs from passages from 15 to 20 were untransfected or transfected with empty or HA-Pin1 (WT, W34A, R68/69A, or C113A) plasmids. Cells were lysed and endogenous JNK1 was immunoprecipitated with an anti-JNK antibody. Immunoprecipitates were subjected to an in vitro kinase assay using 1 μg of His-c-Jun as a substrate as described in Materials and Methods. Kinase activity was normalized to the expression level of JNK1 and presented as the fold increase. (b) Green fluorescent protein (GFP) expression plasmid was co-transfected into cells to show similar transfection efficiency. (c) Active recombinant JNK1 was incubated in the absence or presence of 1 μg Pin1 for 1 h at room temperature. JNK1 in vitro kinase assays, using 1 μg GST-c-Jun (left) or 1 μg His-TCFβ1 (T242A; right) as a substrate, were allowed to proceed for the indicated times. The experiments were repeated at least three times with similar results
Because c-Jun is both a JNK1 substrate and a Pin1 substrate,13 it is possible that the Pin1-induced increase in JNK1 activity may have resulted from the action of Pin1 as an adapter. To exclude this possibility, we searched for a JNK1 substrate in which Ser/Thr motifs without Pro are phosphorylated by JNK1. Although most JNK targets are phosphorylated on Ser/Thr-Pro motifs by JNK,7 a few proteins, including TCFβ1, retinoic receptor RXRα, and IRS-1 have non-conserved sequences that are phosphorylated by JNK.7 We chose TCFβ1 for further investigation because TCFβ1 has only two sites (Ser-232 and Thr-242) that are phosphorylated by JNK,17 whereas the other proteins listed have more than three JNK target sites. Although Thr-242 of TCFβ1 is a candidate site for Pin1 binding, Ser-232 is not a Pin1 target site because of the absence of a proline next to Ser-232. We mutated Thr-242 to Ala (T242A) to eliminate any possibility of Pin1 binding to TCFβ1, even though Thr-242 is not involved in binding to Pin1 as shown by coimmunoprecipitation assays (Supplementary Figure 3a). Mutation of Thr-242 to Ala in TCFβ1 did not change the phosphorylation level of TCFβ1 (Supplementary Figure 3b) or TCFβ1 transactivation activity (Figure 6b and Supplementary Figure 6), suggesting that Thr-242 is not a major site for phosphorylation by JNK1 and does not have a major role in transactivating TCFβ1. However, mutation of Ser-232 of TCFβ1 to Ala (S232A) decreased both JNK1-mediated TCFβ1 phosphorylation and transactivation activity significantly, suggesting that Ser-232 is the major JNK1 target site and is involved in transactivation. We used recombinant c-Jun and TCFβ1 (T242A) proteins as substrates for in vitro JNK1 kinase assays (Figure 3c). Active recombinant JNK1 phosphorylated both c-Jun and TCFβ1 (T242A) more rapidly in the presence of Pin1 than in the absence of Pin1. The finding that phosphorylation of TCFβ1 (T242A) by JNK1 was enhanced in the presence of Pin1 suggests that Pin1-induced JNK1 activation is due to the action of Pin1 on JNK1 rather than on JNK1 substrates.
Pin1 induces conformational changes in JNK1, and Pin1-stimulated JNK1 is resistant to dephosphorylation by the trans-pSer/Thr-Pro isomer-specific phosphatase PP2A
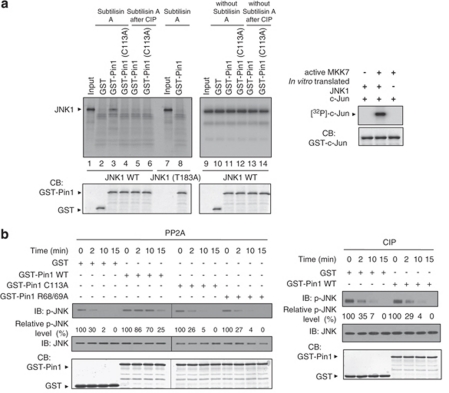
To investigate whether Pin1 induces conformational changes in JNK1, we performed partial proteolytic cleavage assays on in vitro-translated and purified JNK1 phosphorylated at Thr-183 by the JNK1 upstream activator MAPK kinase 7 (MKK7; Figure 4a, left panel). The in vitro-translated and -purified JNK1 phosphorylated by MKK7 was functionally active in in vitro kinase assays, suggesting that the in vitro-translated JNK1 was correctly folded (Figure 4a, right panel). Pin1 WT protected phosphorylated JNK1 WT from subtilisin-catalyzed proteolysis but did not protect CIP-treated JNK1 WT or the CIP-untreated JNK1 (T183A) mutant. Phosphorylated JNK1 WT in the absence of Pin1 was not protected from proteolysis, suggesting that no protein other than Pin1 affects proteolysis. Moreover, proteolytic cleavage assays with Pin1 (C113A) mutant demonstrated that the protection of JNK1 by Pin1 is not due to the steric hindrance caused by Pin1 binding. These results demonstrate that Pin1 promotes JNK1 activity by inducing conformational changes in JNK1 via binding to the pThr-Pro motif.
Figure 4.
Pin1-activated JNK1 undergoes conformational changes and is resistant to dephosphorylation by the trans-pSer/Thr-Pro isomer-specific phosphatase PP2A. (a) The purified 35S-labeled His-JNK1 WT and T183A were treated with active recombinant MKK7α1. Subtilisin cleavage was performed on His-JNK1 WT or T183A after incubation with GST (left panel, lane 2), GST-Pin1 (lanes 3 and 8) or GST-Pin1 C113A (lane 4), or after JNK1 dephosphorylation (lanes 5 and 6). The reaction products were analyzed by SDS-PAGE and autoradiography. To confirm that the in vitro-translated His-JNK1 WT was properly folded, His-JNK1 WT was phosphorylated by active MKK7α1 and used for in vitro kinase assays with GST-c-Jun (right panel). (b) GST, GST-Pin1, GST-Pin1 (C113A), or GST-Pin1 (R68/69A) proteins were pulled down with GS beads and then each bead complex was incubated with active JNK1 at 30 °C for 30 min. After centrifugation to remove GST fusion proteins, PP2A (0.1 U) or CIP (10 U) was added to the supernatants at 30 °C for the time periods indicated. Following incubation, reactions were stopped by the addition of SDS sample buffer, followed by SDS-PAGE and immunoblotting analysis with antibodies as indicated. The data quantified as a percentage of phospho-JNK1 were shown in Supplementary Figure 4
Given that Pin1 WT induces conformational changes in JNK1, it was important to examine whether Pin1-induced isomerization activity led to the cis/trans-isomerization of JNK1. The pThr-Pro-pTyr peptide that mimics the JNK phosphorylation loop could not be cleaved by the isomer-specific chymotrypsin and therefore was a poor substrate for conventional PPIase-induced isomerization assays.18 To address this question in an alternative way, we used PP2A to measure dephosphorylation of phospho-JNK1 for the following reasons. First, Pro-directed phosphatase PP2A specifically dephosphorylates the trans-pSer/Thr-Pro isomer.19 Second, JNK1 is a potential substrate for PP2A.20, 21 Third, Pin1 does not affect PP2A activity.19 We examined the effects of Pin1 on dephosphorylation of JNK1 by PP2A (Figure 4b and Supplementary Figure 4). Active JNK1 was preincubated with Pin1 WT, Pin1 (C113A), or Pin1 (R68/69A) before addition of PP2A. Dephosphorylation of JNK1 was determined by immunoblot analysis using an anti-phospho-JNK1 antibody. CIP, a nonspecific phosphatase, was used as a control. Pin1 significantly reduced dephosphorylation of phospho-JNK1 by PP2A, whereas Pin1 showed no effect on the dephosphorylation of JNK1 mediated by CIP. In addition, the catalytically inactive Pin1 (C113A) or Pin1 (R68/69A) mutant had no effect on the PP2A-mediated dephosphorylation of phospho-JNK1. As PP2A specifically dephosphorylates the trans-pSer/Thr-Pro isomer and Pin1 has no regulatory effect on PP2A activity, these results indicate that Pin1 isomerizes the trans-conformation of pThr-Pro motif of JNK1 to the cis-conformation.
On the basis of results from Figure 4b, we might predict that phospho level of JNK in Pin1−/− MEFs might be lower than that in Pin1+/+ MEFs, as PP2A dephosphorylates more p-JNK in the absence of Pin1. However, no significant difference in JNK phosphorylation between Pin1−/− and Pin1+/+ MEFs was observed (Figure 1c), which is somewhat contradictory to prediction from Figure 4b. This might be because of the presence of many other MAPK phosphatases that dephosphorylate p-JNK.22
Interaction between JNK1 and its substrates is enhanced by Pin1, and Pin1 stabilizes JNK1 activity
As Pin1 induces conformational changes in JNK1, we investigated the extent to which Pin1 influences the interaction between JNK1 and its substrates. In vitro binding analyses revealed that JNK1 interacts with c-Jun more strongly in the presence of co-expressed Pin1 WT than in the absence of Pin1 WT or in the presence of Pin1 mutants (Figure 5a). The Pin1 mutants, Pin1 (W34A) and Pin1 (R68/69A), did not show an increase in JNK1 binding to substrate, implying that both the JNK1 binding and isomerase activities of Pin1 are required for enhanced JNK1 binding to substrate. The in vivo assay results also demonstrated that the interaction between endogenous JNK and c-Jun or ATF-2 was greatly induced by treatment with H2O2 in Pin1+/+ MEFs, whereas the interaction was minimal in Pin1−/− MEFs (Figure 5b). Pin1-mediated conformational changes in JNK1 might be spontaneously reversible; Pin1-induced JNK1 activity might decline to basal levels in the absence of Pin1. We therefore performed in vitro kinase assays using c-Jun as a substrate to investigate the stability of Pin1-activated JNK1 after Pin1 depletion (Supplementary Figure 5). Treatment with Pin1 WT sustained JNK1 activity at maximal levels for at least 20 min, whereas the Pin1 (C113A) mutant had no effect on the stability of JNK1 activity. These results show that Pin1-induced phospho-JNK1 activity declines to basal phospho-JNK1 activity slowly. As Pin1 had no chance to associate c-Jun in these assays, the results also confirmed that Pin1 has distinguishable effects on JNK1.
Figure 5.
Pin1 induces JNK1 activity by enhancing the interaction between JNK1 and its substrates. (a) Pin1−/− MEFs were transfected with expression plasmids for FLAG-JNK1 alone or both JNK1 and Pin1 WT or various point mutants of Pin1. Cell extracts were immunoprecipitated with FLAG M2 agarose beads. The beads were washed twice with the lysis buffer and then incubated with 1 μg of GST-c-Jun. Protein complexes bound to the beads were subjected to SDS-PAGE, and then immunoblotted with an anti-GST antibody. The binding affinity relative to the amount of JNK1 is shown as the fold increase. (b) Lysates from H2O2-treated or -untreated Pin1−/− and Pin1+/+ MEFs were analyzed by immunoprecipitation and then immunoblotting as described in Materials and Methods
Pin1 enhances JNK1-induced apoptosis and TCFβ1 transactivation activity
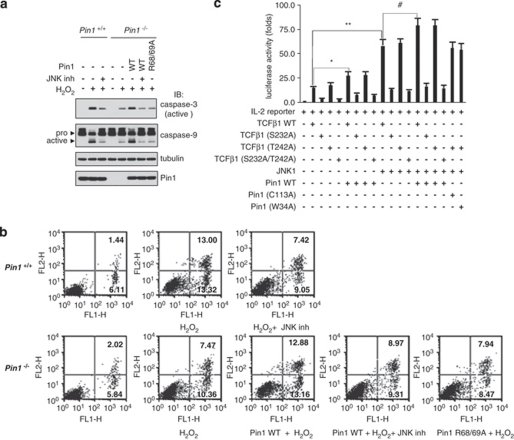
As Pin1 interacts with JNK1 and regulates JNK1 activity, we further examined a possible linkage between apoptosis and the JNK/Pin1 signaling cascade by measuring the processing of procaspases. On treatment with H2O2, Pin1+/+ cells displayed the enhanced caspase-3 and -9 processing, which was blocked by JNK inhibitor VIII that shows high selectivity over 74 other kinases (Figure 6a).23 Although Pin1−/− cells had no effect on the caspase-3/9 processing, the ectopic expression of Pin1 WT, but not Pin1 (R68/69A) mutant, restored a stimulatory effect toward caspase-3/9 processing. We confirmed that Pin1 affects JNK-dependent apoptosis with fluorescence-activated cell sorting analysis using annenix V as a specific apoptotic marker (Figure 6b).
Figure 6.
Pin1 enhances JNK-dependent apoptosis and JNK1/TCFβ1-mediated IL-2 production. (a) Pin1−/− and Pin1+/+ MEFs were untransfected or transfected with empty, HA-Pin1 WT, or HA-Pin1 (R68/69A) expression plasmids. Transfected MEFs untreated or pretreated for 1 h with JNK inhibitor VIII before exposure to H2O2 for 16 h were lysed and cleaved caspase-3 and caspase-9 were detected by immunoblotting using an anti-cleaved caspase-3 or anti-caspase-9 antibody. Pro, full-length caspase-9; active, cleaved caspase-9. (b) Transfected cells were exposed to H2O2 for 16 h and then were investigated for the percentage of apoptotic cells as described under Materials and Methods. The representative profile from flow cytometric analysis is shown, and apoptotic cells were evaluated by annexin V/PI and annexin V staining. Percentage of cells in each quadrant is indicated. Lower left quadrant: viable cells; lower right quadrant: early apoptotic cells; upper right quadrant: late apoptotic cells; and upper left quadrant: necrotic cells. (c) Jurkat T-cells were transfected with 1 μg pIL-2-Luc reporter, together with other plasmids. pCMV/β-gal (0.5 μg) was added to all transfections. Cells were activated with PMA (5 ng/ml) plus ionomycin (1 μM) for 16 h, harvested, and assayed for luciferase activity as described in Materials and Methods. The relative fold induction of luciferase activity was determined using an assay system (Promega, Madison, WI, USA) with a luminometer and normalized to β-galactosidase activity. Experiments were repeated three times. Error bars indicate±S.D. Statistical significance was determined by the Student t-test. *P<0.05 and **P<0.01 compared with control (TCFβ1 WT alone). #P<0.05 compared with control (TCFβ1 WT+JNK1)
We also examined the effect of Pin1 on the JNK signaling cascade by measuring TCFβ1-mediated interleukin-2 (IL-2) production. Phosphorylation of TCFβ1 by JNK has been shown to enhance its ability to bind to IL-2 promoter and to induce IL-2 production.17 Unlike most JNK targets, TCFβ1 is not a Pin1 target, as discussed above. TCFβ1 transactivation assays were performed using an IL-2-luciferase reporter after transfection of several expression plasmids into human Jurkat T-cells and phorbol myristate acetate (PMA)/ionomycin stimulation (Figure 6c). When the transactivation activities of TCFβ1 (T242A), TCFβ1 (S232A), and TCFβ1 (S232A, T242A) were compared with those of TCFβ1 WT, TCFβ1 (T242A) was as active as TCFβ1 WT, whereas TCFβ1 (S232A) and TCFβ1 (S232A, T242A) showed no transactivation activity. Pin1 WT cooperated with JNK1 to activate the IL-2 promoter, whereas Pin1 mutants (W34A and C113A) or TCFβ1 (S232A, T242A) failed to activate the promoter. We also measured IL-2 production in Jurkat T-cells after transfection and obtained similar results (Supplementary Figure 6). Taken together, our results indicate that Pin1 increases JNK1-mediated IL-2 production only if both the JNK1 binding and isomerase activities of Pin1 are not defective.
Discussion
We have shown that Pin1 directly targets and promotes JNK1 activity by modulating phosphorylated JNK1. Phosphorylation at Thr-183 in the Thr-Pro motif of the JNK1 phosphorylation loop plays a critical role in the activation of JNK1. Replacement of Ser/Thr in the phosphorylation loop of other kinases with acidic amino acids, such as Asp or Glu, has been demonstrated to mimic phosphorylation and result in constitutively active kinase mutants.24, 25 However, substitution of Thr-183 in JNK1 with Glu yielded a catalytically inactive mutant, suggesting that additional processes might be required for activation of JNK1 after phosphorylation. In addition, mutation of Pro-184 in the Thr-Pro motif to Gly or Glu to mimic p38 or ERK, respectively, resulted in no JNK1 activity, suggesting the importance of Pro for JNK1 activity. Because regulation of JNK1 by Pin1 is dependent on the phosphorylation status of the Thr-Pro motif, and because mutation of the JNK1 binding or isomerization domain of Pin1 abrogates JNK1 activation, it is likely that Pin1 induces conformational changes in JNK1 on binding to phosphorylated JNK1. Subtilisin proteolysis assay and trans-isomer-specific PP2A phosphatase assay data also suggested that Pin1 induces conformational changes in JNK1 from trans- to cis-form. Such regulation might be pathologically significant, as overexpression of Pin1 in breast cancer cell lines correlates with JNK activity.
As there is no structural information available for phospho-JNK and the phosphorylation loop, we propose a model based on the data presented here (Supplementary Figure 7). In our model, phosphorylated but partially active JNK1 becomes fully active through conformational changes induced by Pin1. In this model, phosphorylation of Thr-183 in the phosphorylation loop of JNK1 induces conformational changes in JNK1, making it partially active by partial opening of the docking domain and the catalytic pocket located near the phosphorylation loop.26 Binding of Pin1 to the pThr-183-Pro motif induces a further conformational change in JNK1. This additional conformational change facilitates flipping out of the phosphorylation loop in JNK1 and subsequent opening of the docking domain, which is structurally located near the loop,27 for substrate binding, thereby fully activating JNK1. Phospho-Tyr-185 in JNK1 might function to promote binding to Pin1 (Supplementary Figure 1a) or to facilitate conformational changes to the active form of JNK1 on binding of Pin1.
However, we cannot rule out the possibility that phosphorylated JNK1 might be in either an active or inactive form, depending on its cis- or trans-conformation, and, on binding to Pin1, the inactive phospho-JNK1 is converted into the active form of JNK1. It is not clear whether the pThr-Pro-motif of active JNK is in a cis- or trans-conformation because no structural information concerning the phosphorylation loop of active JNK is available. Even though the trans-conformation is energetically more favorable, the cis-conformation occurs naturally due to spontaneous equilibration, though at a slow rate.28 It is estimated that 10–20% of pSer/Thr-Pro-motifs are in the cis-conformation.10 This may explain why phospho-JNK1 is partially active in the absence of Pin1. Given the presence of both cis- and trans-forms of Pin1 targets in cells, JNK1 might be active in one form only. Although we could not identify whether the active conformation is in cis or trans due to technical problems with an isomer-specific protease-based assays, we speculate that the cis-conformation of JNK1 may be the active form because it has been reported that the Pin1 WW domain binds only the trans-conformation of pSer/Thr-Pro-motifs.11 As JNK1 has only one Pin1-binding site (Figure 2d), phospho-JNK1 might be inactive in the trans-conformation and, on binding to Pin1, phospho-JNK1 might be isomerized into the active cis-conformation. The data shown in Figure 4b and Supplementary Figure 4 further support this possibility, because Pin1 protected phospho-JNK1 from the trans-pSer/Thr-Pro isomer-specific PP2A. However, it is not clear whether this conformational change alters the active site of JNK1, thereby increasing the catalytic activity of JNK1.
Pin1 regulates other pro- or anti-apoptotic factors, such as tumor suppressor p53, c-Jun, and Bcl-2, all of which are phosphorylated by JNK.13, 15, 16, 29 Thus, Pin1 contributes to the regulation of apoptosis by targeting both JNK1 and its downstream pro- and anti-apoptotic factors. As most JNK substrates are also Pin1 targets, it is almost impossible to study in vivo effect of Pin1 on JNK by knocking out all of Pin1 target genes. In addition, it is impossible to create an activatable JNK mutant that lacks binding activity to Pin1 or a Pin1 mutant that specifically disrupts binding to JNK1 but not to other substrates. Currently, it is not clear why Pin1 targets both JNK1 and its downstream factors. One possibility is that Pin1 targeting of single substrates in signal cascades may not be enough to induce biological effects because the fold induction is relatively low. It is also possible that Pin1 may not bind to all JNK1 downstream targets, because Pin1 targets a pSer/Thr-Pro motif with flanking sequence specificity;30 Pin1 may therefore selectively amplify JNK1 downstream factors. For example, although TCFβ1 used in this study is a JNK1 substrate, it is not a Pin1 substrate, which is the case for other JNK1 substrates that are not regulated by Pin1. Further genetic and structural studies are needed to determine the precise roles of Pin1 in JNK1 activation.
Materials and Methods
Cell culture and transfection
HEK 293 cells, HeLa cells, JNK1/2−/− cells (a gift from Roger J Davis), MCF-7 cells, T47D cells, and MEFs from Pin1+/+ or Pin1−/− mice were maintained at 37 °C in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen) and penicillin/streptomycin in the presence of 5% CO2. Cells (184B5) were cultured in MEBM with hydrocortisone (0.5 μg/ml), human recombinant EGF (10 ng/ml), and bovine insulin (5.0 μg/ml). Human Jurkat T-cells and HCC-1937 cells were maintained in complete culture medium consisting of RPMI 1640 medium supplemented with 10% FBS. For transient transfection, 1.4 × 106 cells were plated in 60-mm cell culture plates, grown overnight, and then transfected with DNA using LipofectAMINE (Invitrogen). Both Pin1+/+ and Pin1−/− MEFs used for transfection were from passages from 15 to 20. The 3′-untranslated region region of Pin1 (Pin1 siRNA, 5′-GGGCCGAAUUGUUUCUAGU dTdT-3′) was obtained from Bioneer (South Korea) and transfected into T47D cells using Fugene HD Transfection Reagent (Roche, Mannheim, Germany) for knockdown experiments.
Plasmid constructions
HA-JNK1α, HA-JNK1α (T183A), HA-JNK1α (Y185F), HA-JNK1α (T93A/T243A/S377A), HA-JNK1α (T93A/T183A/T243A/S377A), HA-FLAG-JNK1α, HA-Pin1, HA-Pin1 (W34A), HA-Pin1 (C113A), FLAG-TCFβ1, FLAG-TCFβ1 (S232A), FLAG-TCFβ1 (T242A), FLAG-TCFβ1 (S232A/T242A), FLAG-Pin1, FLAG-Pin1 (W34A), FLAG-Pin1 (R68/69A), FLAG-Pin1 (C113A), FLAG-Pin1 WW, and FLAG-Pin1 PPIase expression plasmids were generated by polymerase chain reactions and subcloning into pcDNA3.1 (Invitrogen). Glutathione S-transferase (GST-Pin1), GST-Pin1 (C113A), and GST-c-Jun (1–79) were constructed in the pGEX-6P-1 (Amersham Biosciences, Little Chalfont, UK) plasmid for protein expressions in Escherichia coli. His-JNK1α and His-JNK1α (T183A) expression plasmids were used for in vitro coupled transcription/translation (Promega, San Luis Obispo, CA, USA). His-c-Jun (1–135), His-TCFβ1, His-TCFβ1 (T242A), and His-TCFβ1 (S232A/T242A) for in vitro kinase assays were constructed in pET-28a (Novagen, Darmstadt, Germany). All of the constructs were confirmed by DNA sequencing.
Reagents and antibodies
Polyclonal anti-JNK, anti-phospho-JNK (Thr-183/Tyr-185), anti-c-Jun, anti-phospho-c-Jun (Ser-63), anti-ATF-2, anti-phospho-ATF-2 (Thr-69/71), anti-cleaved caspase-3 (Asp175), anti-caspase-9, and anti-Pin1 antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). Anti-HA antibody and JNK inhibitor VIII were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The anti-FLAG M2 antibody, anti-FLAG M2 agarose beads, polyclonal rabbit anti-GST antibody, anti-tubulin antibody, PMA, and ionomycin were purchased from Sigma-Aldrich (St. Louis, MO, USA). TNF-α was obtained from Calbiochem (San Diego, CA, USA). Active MKK7α1, His-tagged active JNK1 (JNK1α1), and PP2A were provided by Upstate Biotechnology (Lake Placid, NY, USA). Ni-NTA agarose was obtained from Qiagen (Hilden, Germany). Glutathione-Sepharose was purchased from Amersham Biosciences and the PreScission Protease was from Invitrogen.
In vivo and in vitro binding assays
Cells were co-transfected with HA-JNK1 and FLAG-Pin1 expression plasmids. After 48 h of transfection, cells were washed twice with phosphate-buffered saline and extracted in a lysis buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 10 mM NaF, 1% Triton X-100, 0.5% deoxycholate, 1 mM DTT, 1 mM sodium orthovanadate, 1 mM PMSF, and 1 μg/ml aprotinin. Cell extracts were clarified by centrifugation and immunoprecipitated as previously described.31 The bound proteins were eluted with the SDS-PAGE sample buffer, separated by SDS-PAGE, and then immunoblotted with an anti-HA antibody. The protein bands were visualized using the ECL detection system (Pierce, Rockford, IL, USA).
To examine interactions between endogenous proteins, cell lysates were prepared from H2O2-treated or -untreated Pin1−/− and Pin1+/+ MEFs as described above and subjected to immunoprecipitation with rabbit anti-JNK antibody followed by incubation with protein A/G agarose for 16 h at 4 °C. The bound proteins were analyzed by immunoblots probed with anti-Pin1 or appropriate antibodies.
For in vitro phosphatase treatment and GST pull-down assays, cell lysates prepared in the absence of phosphatase inhibitors as described above were incubated with CIP (0.2 U/μl) at 30 °C for 30 min. GST pull-down experiments were performed by incubating cell lysates for 2 h at 4 °C with 20 μl Glutathione-Sepharose beads loaded with GST–Pin1 or control GST. Pulled-down complexes were subjected to SDS-PAGE and then immunoblotted with an anti-HA antibody.
In vitro kinase assays
For the immune complex kinase assay, cells were treated with 1 mM H2O2 for 1 h and then lysed in lysis buffer. Cell extracts were clarified by centrifugation, and the supernatants were immunoprecipitated with mouse anti-JNK antibody followed by incubation with protein A/G agarose for 16 h at 4 °C. The beads were washed once with lysis buffer, twice with a solution containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 2 mM dithiothreitol, and 1 mM PMSF, and then once with a solution containing 20 mM Tris-HCl (pH 7.5) and 20 mM MgCl2. The beads were then resuspended in 20 μl of kinase reaction buffer (20 mM Tris-HCl (pH 7.5), 20 mM MgCl2, 0.1 mM sodium orthovanadate, 1 mM dithiothreitol) containing 20 μM ATP and 0.3 μCi of [γ-32P] ATP with 1 μg of His-c-Jun (residues 1–135) or GST-c-Jun (residues 1–79) for 30 min at 30 °C. The products of kinase reactions were separated by SDS-PAGE. The gels were dried and exposed to film.
For kinase assays with active recombinant JNK1, JNK1 (20 ng/20 μl reaction) was incubated in the absence or presence of GST-Pin1 (1 μg) for 1 h at room temperature before addition of GST-c-Jun (residues 1–79, 1 μg) or His-TCFβ1 (T242A; 1 μg) as substrates.
In vitro Pin1 depletion assays
GST, GST-Pin1 WT, or Pin1 (C113A) mutant proteins were pulled down with Glutathione-Sepharose beads and then each bead complex was incubated with active JNK1 for 1 h at room temperature. Following incubation, GST, GST-Pin1 WT, or Pin1 (C113A) proteins were removed by centrifugation. After centrifugation, supernatants were sampled at various times (0, 20, 40, or 60 min) and used for JNK1 kinase assays or subjected to SDS-PAGE and then immunoblotted with appropriate antibodies.
Subtilisin proteolysis
35S-labeled His-JNK1 WT and T183A were expressed using in vitro coupled transcription/translation system and purified on a Ni-NTA column. Purified proteins bound to Ni-NTA beads were phosphorylated by active MKK7α1, and incubated with 1 μg of GST-Pin1, GST-Pin1 (C113A), or GST in the following buffer: 50 mM HEPES (pH 7.5), 100 mM NaCl, 1 mM MgCl2, and 1 mM dithiothreitol supplemented with phosphatase inhibitors. After 1 h incubation at 30 °C, subtilisin (25 ng/20 μl reaction, Sigma-Aldrich) was added for a further 4 min at 4 °C. The reaction was stopped by the addition of SDS-PAGE sample buffer and the reaction products were analyzed by 10% SDS-PAGE and autoradiography. For CIP treatment, phosphorylated JNK1 was incubated with CIP (10 U) at 30 °C for 30 min in the absence of phosphatase inhibitors before the addition of GST fusion proteins.
Effect of Pin1 on PP2A-mediated dephosphorylation of phospho-JNK1
Active His-JNK1 (20 ng) was incubated with 1 μg of GST-Pin1 WT or Pin1 (C113A) mutant bound to Glutathione-Sepharose beads in 20 μl of reaction buffer containing 20 mM HEPES (pH 7.0), 1 mM DTT, 1 mM MgCl2, 100 μg/ml BSA at 30 °C for 30 min, followed by centrifugation to remove GST fusion proteins. The supernatants were incubated with PP2A (0.1 U) or CIP (10 U) at 30 °C for times indicated in Figure 4b and Supplementary Figure 4. After incubation, reactions were stopped by the addition of SDS sample buffer, followed by SDS-PAGE and immunoblot analysis. GST was used as a control. Phospho-JNK1 level was normalized to total JNK1 protein level. The experiments were repeated at least three times with similar results.
Luciferase reporter assay
Jurkat T-cells (2 × 106 cells/well) were transfected with 1 μg each of pIL-2-Luc (a gift from Insug Kang) and 0.5 μg of pCMV/β-gal plasmid using Cell Line Nucleofector Kit V (Amaxa biosystems, Cologne, Germany). After stimulation with PMA (5 ng/ml) plus ionomycin (1 μM) for 16 h, the cells were washed, lysed, and analyzed using a luciferase assay system (Promega) with a luminometer. Luciferase activity was normalized to β-galactosidase activity. All transfections were repeated at least three times.
IL-2 ELISA
IL-2 protein concentrations were determined by standard sandwich ELISA using antibodies and standards obtained from BD Biosciences (San Diego, CA, USA) and used according to the manufacturer's instructions. Assays were performed on neat or diluted samples in duplicate on 96-well plates. Absorbance was measured by a microplate reader at 450 nm, and the concentrations were determined by comparison with a standard curve. All transfections were repeated at least three times.
Annexin V-fluorescein isothiocyanate (FITC) assay
The induction of apoptosis was assessed using flow cytometric dual parameter analysis of annexin V-FITC/propidium iodide (PI) in the Annexin-V-FLUOS staining kit (Roche). Briefly, 1 × 106 cells were plated in a six-well plate and allowed to attach overnight. Cell pellets were redisolved in annexin V-FITC and PI, and incubated at room temperature for 15 min according to the manufacturer's protocol. Using a FACSscan (Becton Dickinson, Sunnyvale, CA, USA), 10 000 cells were analyzed.
Acknowledgments
We thank Roger Davis, Insug Kang, Jong-Tae Kim, and Seung-Chul Choi for generously providing reagents or technical assistance. This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST; no. 2011-0003139) and by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A01-0385-A70604-07M7-00040B).
Glossary
- JNK
c-Jun N-terminal kinase
- Pin1
peptidyl-prolyl cis/trans-isomerase
- MEF
mouse embryonic fibroblast
- TCFβ1
T-cell factor β1
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by L Greene
Supplementary Material
References
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Waetzig V. AP-1 proteins in the adult brain: facts and fiction about effectors of neuroprotection and neurodegeneration. Oncogene. 2001;20:2424–2437. doi: 10.1038/sj.onc.1204387. [DOI] [PubMed] [Google Scholar]
- Rincon M, Flavell RA, Davis RJ. Signal transduction by MAP kinases in T lymphocytes. Oncogene. 2001;20:2490–2497. doi: 10.1038/sj.onc.1204382. [DOI] [PubMed] [Google Scholar]
- Waetzig V, Herdegen T. The concerted signaling of ERK1/2 and JNKs is essential for PC12 cell neuritogenesis and converges at the level of target proteins. Mol Cell Neurosci. 2003;24:238–249. doi: 10.1016/s1044-7431(03)00126-x. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev. 2006;70:1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KP, Hanes SD, Hunter T. A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature. 1996;380:544–547. doi: 10.1038/380544a0. [DOI] [PubMed] [Google Scholar]
- Lu PJ, Zhou XZ, Shen M, Lu KP. Function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science. 1999;283:1325–1328. doi: 10.1126/science.283.5406.1325. [DOI] [PubMed] [Google Scholar]
- Schutkowski M, Bernhardt A, Zhou XZ, Shen M, Reimer U, Rahfeld JU, et al. Role of phosphorylation in determining the backbone dynamics of the serine/threonine-proline motif and Pin1 substrate recognition. Biochemistry. 1998;37:5566–5575. doi: 10.1021/bi973060z. [DOI] [PubMed] [Google Scholar]
- Verdecia MA, Bowman ME, Lu KP, Hunter T, Noel JP. Structural basis for phosphoserine-proline recognition by group IV WW domains. Nat Struct Biol. 2000;7:639–643. doi: 10.1038/77929. [DOI] [PubMed] [Google Scholar]
- Stukenberg PT, Kirschner MW. Pin1 acts catalytically to promote a conformational change in Cdc25. Mol Cell. 2001;7:1071–1083. doi: 10.1016/s1097-2765(01)00245-3. [DOI] [PubMed] [Google Scholar]
- Wulf GM, Ryo A, Wulf GG, Lee SW, Niu T, Petkova V, et al. Pin1 is overexpressed in breast cancer and cooperates with Ras signaling in increasing the transcriptional activity of c-Jun towards cyclin D1. EMBO J. 2001;20:3459–3472. doi: 10.1093/emboj/20.13.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf GM, Liou YC, Ryo A, Lee SW, Lu KP. Role of Pin1 in the regulation of p53 stability and p21 transactivation, and cell cycle checkpoints in response to DNA damage. J Biol Chem. 2002;277:47976–47979. doi: 10.1074/jbc.C200538200. [DOI] [PubMed] [Google Scholar]
- Zacchi P, Gostissa M, Uchida T, Salvagno C, Avolio F, Volinia S, et al. The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults. Nature. 2002;419:853–857. doi: 10.1038/nature01120. [DOI] [PubMed] [Google Scholar]
- Zheng H, You H, Zhou XZ, Murray SA, Uchida T, Wulf G, et al. The prolyl isomerase Pin1 is a regulator of p53 in genotoxic response. Nature. 2002;419:849–853. doi: 10.1038/nature01116. [DOI] [PubMed] [Google Scholar]
- Kasibhatla S, Tailor P, Bonefoy-Berard N, Mustelin T, Altman A, Fotedar A. Jun kinase phosphorylates and regulates the DNA binding activity of an octamer binding protein, T-cell factor beta1. Mol Cell Biol. 1999;19:2021–2031. doi: 10.1128/mcb.19.3.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikata M, Suzuki K, Yoshimura Y, Deyama Y, Matsumoto A. A phosphotyrosine-containing quenched fluorogenic peptide as a novel substrate for protein tyrosine phosphatases. Biochem J. 1999;343 (Pt 2:385–391. [PMC free article] [PubMed] [Google Scholar]
- Zhou XZ, Kops O, Werner A, Lu PJ, Shen M, Stoller G, et al. Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol Cell. 2000;6:873–883. doi: 10.1016/s1097-2765(05)00083-3. [DOI] [PubMed] [Google Scholar]
- Kins S, Kurosinski P, Nitsch RM, Gotz J. Activation of the ERK and JNK signaling pathways caused by neuron-specific inhibition of PP2A in transgenic mice. Am J Pathol. 2003;163:833–843. doi: 10.1016/S0002-9440(10)63444-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley TP, Vasi N, Denenberg A, Wong HR. The serine/threonine phosphatase, PP2A: endogenous regulator of inflammatory cell signaling. J Immunol. 2001;166:966–972. doi: 10.4049/jimmunol.166.2.966. [DOI] [PubMed] [Google Scholar]
- Patterson KI, Brummer T, O′Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418:475–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- Szczepankiewicz BG, Kosogof C, Nelson LT, Liu G, Liu B, Zhao H, et al. Aminopyridine-based c-Jun N-terminal kinase inhibitors with cellular activity and minimal cross-kinase activity. J Med Chem. 2006;49:3563–3580. doi: 10.1021/jm060199b. [DOI] [PubMed] [Google Scholar]
- Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Huang S, Jiang Y, Li Z, Nishida E, Mathias P, Lin S, et al. Apoptosis signaling pathway in T cells is composed of ICE/Ced-3 family proteases and MAP kinase kinase 6b. Immunity. 1997;6:739–749. doi: 10.1016/s1074-7613(00)80449-5. [DOI] [PubMed] [Google Scholar]
- Kallunki T, Deng T, Hibi M, Karin M. c-Jun can recruit JNK to phosphorylate dimerization partners via specific docking interactions. Cell. 1996;87:929–939. doi: 10.1016/s0092-8674(00)81999-6. [DOI] [PubMed] [Google Scholar]
- Mooney LM, Whitmarsh AJ. Docking interactions in the c-Jun N-terminal kinase pathway. J Biol Chem. 2004;279:11843–11852. doi: 10.1074/jbc.M311841200. [DOI] [PubMed] [Google Scholar]
- Fischer G, Aumuller T. Regulation of peptide bond cis/trans isomerization by enzyme catalysis and its implication in physiological processes. Rev Physiol Biochem Pharmacol. 2003;148:105–150. doi: 10.1007/s10254-003-0011-3. [DOI] [PubMed] [Google Scholar]
- Basu A, You SA, Haldar S. Regulation of Bcl2 phosphorylation by stress response kinase pathway. Int J Oncol. 2000;16:497–500. doi: 10.3892/ijo.16.3.497. [DOI] [PubMed] [Google Scholar]
- Yaffe MB, Schutkowski M, Shen M, Zhou XZ, Stukenberg PT, Rahfeld JU, et al. Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science. 1997;278:1957–1960. doi: 10.1126/science.278.5345.1957. [DOI] [PubMed] [Google Scholar]
- Park JE, Park BC, Kim HA, Song M, Park SG, Lee DH, et al. Positive regulation of apoptosis signal-regulating kinase 1 by dual-specificity phosphatase 13A. Cell Mol Life Sci. 2010;67:2619–2629. doi: 10.1007/s00018-010-0353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.