Abstract
The maturation status of dendritic cells (DCs) is crucial for effective antigen presentation and initiation of the primary immune response. Maturation stimuli cause the adhesion of immature DCs to the extracellular matrix, which is accompanied by recruitment of the CD11b/CD18 [macrophage antigen-1 (Mac-1)] integrin receptor, cytoskeleton reorganization, and podosome formation. Cathepsin X, a cysteine protease expressed in DCs and other APCs, is involved in Mac-1 activation. We have shown that during maturation, cathepsin X translocates to the plasma membrane of maturing DCs, enabling Mac-1 activation and consequently, cell adhesion. In mature DCs, cathepsin X redistributes from the membrane to the perinuclear region, which coincides with the de-adhesion of DCs, formation of cell clusters, and acquisition of the mature phenotype. Inhibition of cathepsin X activity during DC differentiation and maturation resulted in an altered phenotype and function of mature DCs. It reduced surface expression of costimulatory molecules, increased expression of inhibitory Ig-like transcripts 3 and 4 (ILT3 and ILT4), almost completely abolished cytokine production, diminished migration, and reduced the capacity of DCs to stimulate T lymphocytes. These results stress the importance of cathepsin X in regulating DC adhesion, a crucial event for their maturation and T cell activation.
Keywords: carboxypeptidase, integrin, adhesion, antigen-presenting cell
INTRODUCTION
Dendritic cells (DCs) play a critical role in the initiation of adaptive immunity against pathogens and cancer cells as well as in immune dysfunctions, such as autoimmune diseases and cancer immune evasion [1]. Apart from their ability to activate T cells, DCs can also induce immune tolerance, depending on the status of their differentiation. Immature DCs capture and process antigens and in response to appropriate stimuli, migrate into lymphoid organs, where they mature with the ability to activate T cells. Mature DCs express more antigen-presenting and costimulatory molecules than immature DCs. Mature DCs are mainly responsible for activating naive T cells, whereas immature DCs predominantly account for mediating T cell tolerance [2].
Although many different activating signals of DC maturation have been identified, the mechanism of maturation is not well characterized. Maturation is accompanied by a range of morphological and cytoskeleton structure changes. In response to maturation stimuli in vitro, DCs adhere rapidly, develop polarity, and assemble actin-rich structures at the leading edge, known as podosomes [3]. The intracellular, cytoskeletal components of podosomes are linked to the extracellular matrix through the integrin receptor CD11b/CD18 [macrophage antigen-1 (Mac-1)], which is recruited specifically to the podosomes [3, 4]. In vivo peripheral blood DCs transmigrate through lymphatic endothelium, which expresses junction adhesion molecule-2 [5], a ligand for CD11b/CD18. The normal recruitment of CD11b/CD18 to DC podosomes is critical for adhesion and therefore, for transmigration through endothelial barriers such as those in afferent lymphatics and in the high endothelial venules of lymph nodes [6]. As maturation proceeds, DCs in vitro again de-adhere and become devoid of podosomes [3]. By this time, they are functionally primed for optimal interaction with T cells [7].
Proteases have been shown to regulate podosome disassembly [6]. For example, the cysteine protease calpain is capable of cleaving the intracellular domains of CD18, talin, proline-rich tyrosine kinase 2, and Wiskott-Aldrich syndrome protein, thereby regulating the podosome dynamics required for DC motility [6]. Cathepsin X, another cysteine protease, is also a candidate for regulating podosome function. Cathepsin X is involved in activation of the integrin receptor CD11b/CD18 (Mac-1) in differentiated U-937 cells [8], as well as CD11a/CD18 (LFA-1) in lymphocytes [9, 10]. Whereas LFA-1 predominantly enables cell–cell interactions and homotypic aggregation via LFA-1–ICAM-1 interactions, Mac-1 binds to plastic surfaces, fibrinogen, and ICAM-1 among others. Cathepsin X activity promotes adhesion of macrophages to plastic and to fibrinogen, as well as their phagocytic function [8]. In promonocytic U-937 cells, cathepsin X is localized in the perinuclear region and translocated to the plasma membrane upon differentiation [8]. However, during the maturation of DCs, the localization of cathepsin X is different from that in macrophages, suggesting different functions in these two cell types [11].
In the present study, we have investigated the role of cathepsin X during the maturation of DCs. We showed that under the influence of maturation stimuli, cathepsin X is translocated to the plasma membrane, enabling Mac-1 integrin receptor activation and podosome formation. Further, by Mac-1 integrin receptor activation, cathepsin X provides an essential stimulus for the substrate adhesion that is required to achieve the mature DC phenotype, which is capable of T cell stimulation.
MATERIALS AND METHODS
Preparation and culture of DCs
Buffy coats from the venous blood of normal, healthy volunteers were obtained by the Blood Transfusion Center of Slovenia, according to institutional guidelines. The National Medical Ethics Committee of the Ministry of Health, Republic of Slovenia, approved the study, and written consent was obtained before specimens were collected.
PBMCs were isolated using Lympholyte®-H (Cedarlane Laboratories, Ontario, Canada). The cells were washed twice with Dulbecco’s PBS (DPBS), counted, and used as a source for immunomagnetic isolation of CD14-positive cells (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). These were cultured in RPMI-1640 (Cambrex Corp., East Rutherford, NJ, USA) medium supplemented with 10% FBS, gentamicin (50 μg/ml; Gibco, Paisley, UK), 500 U/ml recombinant human (rh)GM-CSF, and 400 U/ml rhIL-4 (both Gentaur, Paris, France). On Day 2, one-half of the medium was exchanged with starting quantities of rhGM-CSF (500 U/ml) and rhIL-4 (400 U/ml). After 5 days, nonadherent, immature DCs were harvested and characterized by flow cytometry as CD1ahi, CD83−, CD86low and HLA−DRlow (data not shown). Cells were counted and resuspended in the medium containing 500 U/ml rhGM-CSF and 20 ng/ml LPS and cultured for a further 2 days.
Specific cathepsin X inhibitor
A neutralizing mouse mAb against cathepsin X (2F12) was prepared from a mouse hybridoma cell line as described [11] and used at a concentration of 0.5 μM. Cathepsin X-specific epoxysuccinyl-based inhibitor AMS36 was used at a concentration of 2 μM. 2F12 mAb or AMS36 was added to the culture medium of CD14-positive monocytes, Day 2-differentiated, immature DCs, and Day 5-differentiated, immature DCs during maturation.
We validated the lack of toxicity of inhibitors used in our study by FACS using propidium iodide (PI) exclusion.
Characterization of DCs
Stimulation markers
The levels of membrane markers were determined by flow cytometry using fluorescence-labeled antibodies. On Day 7, nonadherent cells were harvested and collected by centrifugation. Antibody (see below) was added, and the cells were incubated at room temperature for 15 min in the dark. The cells were then washed twice with DPBS and resuspended in 2% paraformaldehyde. The following mAb were used: anti-CD1a, anti-CD14, anti-CD80, and anti-CD4 (all from BioLegend, San Diego, CA, USA), labeled with FITC, FITC-labeled anti-CD83 (IQ Products, The Netherlands), FITC-labeled anti-CD86 (DakoCytomation, Denmark), FITC-labeled anti-Ig-like transcript 4 (ILT4), R-PE-labeled anti-ILT3 (both R&D Systems, Minneapolis, MN, USA), and R-PE-labeled anti-HLA-DR (Exalpha Biologicals, Watertown, MA, USA). A FITC-IgG1 and R-PE-IgG2a cocktail was used for isotype control (BioLegend). Results are expressed as mean fluorescence intensity (MFI) values after subtraction of the MFI obtained with the control antibody.
Cathepsin X and β2 integrins
mAb labeled with PE or Alexa-488 (BD Immunocytometry System, Becton Dickinson, San Jose, CA, USA) in conjunction with PI were used for three-color flow cytometry. The following antibodies were used for labeling: anti-CD11a-R-PE, anti-CD11b-R-PE, and anticathepsin X 2F12. A Simultest control (mouse IgG1-R-PE and mouse IgG1-Alexa-488) was used as background control. From a plot of forward side-scatter versus PI, the population of live cells (PI−) was gated for further analysis. Two-color flow cytometry was performed on a FACSCalibur (Becton Dickinson).
Confocal immunofluorescence microscopy
Immature DCs (1×105) on Day 5 of differentiation and mature DCs, matured for 2 days with 500 U/ml rhGM-CSF and 20 ng/ml LPS, were centrifuged with cytospin (Cytofuge) for 6 min at 1000 rpm onto glass cover slides. Maturing, adherent DCs were labeled by seeding 5 × 105/ml immature DCs onto glass coverslips in 24-well plates and allowing adherence for 20 h. Before labeling, cells were allowed to recover for 15 min and then fixed with 4% paraformaldehyde for 45 min and permeabilized by 0.1% Triton X-100 in PBS, pH 7.4, for 10 min. Nonspecific staining was blocked with 3% BSA in PBS, pH 7.4, for 1 h.
Actin was labeled with phalloidin-tetramethylrhodamine B isothiocyanate conjugate (Fluka, Germany; 500 ng/ml) for 30 min at room temperature and afterward, washed with PBS. Coverslips were mounted on glass slides with the ProLong antifade kit (Molecular Probes, Carlsbad, CA, USA). Controls were run in the absence of primary antibodies.
Cathepsin X was labeled with Alexa Fluor 488 (Molecular Probes)-labeled mouse 2F12 mAb that recognizes the mature, active form. For CD11b labeling, the primary antibodies were goat anti-human integrin αM from Santa Cruz Biotechnology (Santa Cruz, CA, USA). For labeling of active β2 integrin, the primary antibody was mAb 24, provided by Dr. Nancy Hogg (Macrophage Laboratory, Imperial Cancer Research Fund, Lincoln’s Inn Fields, London, UK). After 2 h incubation with primary antibodies, cells were washed with PBS and treated with Alexa Fluor 633-labeled donkey anti-goat or Alexa Fluor 488-labeled rabbit anti-mouse secondary antibodies (Molecular Probes) for 1 h. After a final wash with PBS, the ProLong antifade kit (Molecular Probes) was used to mount coverslips on glass slides. Controls were run in the absence of primary antibodies.
Fluorescence microscopy was performed using a Carl Zeiss LSM 510 confocal microscope. Alexa Fluor 488, phalloidin tetramethylrhodamine B isothiocyanate conjugate, or Alexa Fluor 633 was excited with an argon (488 nm) or He/Ne (543 nm, 633 nm) laser, and emission was filtered using narrow-band 505–530 nm, LP 560 nm, and LP 650 nm filters, respectively. Images were analyzed using Carl Zeiss LSM image software 3.0.
Quantitation of cytokine production
DCs were differentiated and subsequently activated with LPS in the presence or absence of cathepsin X inhibitor (2F12 mAb). Cell-free supernatants were harvested 48 h after cell activation. Cytokines were measured by sandwich ELISA using matched-pair antibodies. ELISA sets to human IL-10, IL-12p70, and TNF-α were obtained from BioLegend. Assays were set up in duplicate and performed in accordance with recommendations of the manufacturer. The lower limit of detection was 2 pg for IL-10 and 4 pg for IL-12p70 and TNF-α.
Time-lapse recording and analysis of DC adhesion
Wells of a 96-well culture plate were precoated or not with 50 μl fibrinogen (50 μg/ml; Kabi Diagnostica, Atlanta, GA, USA) in carbonate buffer, pH 9.6, overnight at 4°C. Wells were then washed once with PBS and incubated with 1% BSA in PBS for 30 min at room temperature. Nonadherent, immature DCs on Day 5, treated with 0.5 μM-neutralizing 2F12 mAb, 2 μM-specific synthetic, cathepsin X inhibitor AMS36, and control, immature DCs, were harvested, washed with PBS, and resuspended in the medium containing 500 U/ml rhGM-CSF and 20 ng/ml LPS at a 5 × 105/ml concentration. For treated DC, 0.5 μM 2F12 mAb or 2.0 μM AMS36 was added to the medium. Immature DCs (50 μl) were added to wells of a 96-well culture plate (TPP, Trasadingen, Switzerland). Cells were allowed to attach for 15 min, 45 min, 1.5 h, 3 h, and 5 h. Wells were then washed twice with PBS, and 100 μl medium was added. Each experiment was done in quadruplicate for treated and control cells. To determine total immature DCs, one quadruplicate of wells was not washed with PBS, but only 50 μl medium was added. After the last incubation, CellTiter 96® One Solution cell proliferation assay (MTS reagent, Promega, Madison, WI, USA) was used to assess the adhesion of cells, which was determined by Equation 1, where Atx and Atotal DC are the absorbance of formazan determined for cells washed after different time-points and all DCs, respectively.
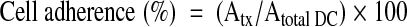 |
1 |
For time-lapse imaging, 150 μl control and 2F12 mAb-treated cells (2.5×105 /ml) were added to fibrinogen-precoated wells simultaneously, and mAb 2F12 was added to treated, immature DC at a final concentration of 0.5 μM. Cells were tracked at 30 s intervals for 270 min. Differential interference contrast images were taken by an Olympus IX 81 motorized, inverted microscope and Cell® software.
Migration assay
Transwells (Corning Costar, Corning, NY, USA) with 5 μm polycarbonate filters (5 μm pore size) were used. Fibronectin (Sigma Chemical Co., St. Louis, MO, USA; 10 μl 100 μg/ml) or fibrinogen (Kabi Diagnostica) was applied on the lower side of the filters and left for 1 h in a laminar hood to dry. Mature, nonadherent DCs, obtained after 7 days of culture and matured with LPS in the presence or absence of 0.5 μM 2F12 mAb, were washed with PBS and resuspended in RPMI-1640 medium. Cells (2×105) were added to each upper compartment in 200 μl serum-free medium. The lower compartments were filled with 700 μl serum-free medium. MCP-1 was added to the lower compartments (50 ng/ml), precoated with fibronectin. The control cells and pretreated cells were plated in the absence of the inhibitor. The plate was incubated for 7 h at 37°C and 5% CO2. Afterward, transwells were lifted, and media with migrated cells from the lower compartment were transferred separately to Eppendorf tubes and centrifuged at 2000 rpm for 5 min. Supernatants were discarded, and cells were resuspended in 100 μl medium and transferred to wells of a 96-well culture plate. MTS reagent (20 μl) was added to each well and incubated for an additional 2 h at 37°C and 5% CO2. The color intensity was measured at 592 nm. Migration was recorded as the percentage of cells that penetrated the filters.
Allogeneic T cell proliferation
DCs obtained after 7 days of culture in the presence or absence of 0.5 μM-neutralizing 2F12 mAb were washed twice in RPMI-1640 medium containing gentamycin and treated with mitomycin C (Sigma Chemical Co.). Purified, whole CD4+ T cells or PBMCs were used as responders. The assay was carried out in a 96-well V-bottomed plate with a total volume of 200 μl per well. Cells (1×104 and 1×105) were used for DCs and responder T cells, respectively. On Day 5, the wells were pulsed with 1 μCi/well 3H-thymidine (Perkin Elmer, Boston, MA, USA), and proliferation was measured by 3H-thymidine incorporation after 18 h by liquid scintillation counting.
β2-Cytoplasmic region synthesis and cleavage site studies
Recombinant cathepsin X was tested for its ability to degrade the carboxyl terminal of the β2-integrin cytoplasmic domain using a method described by Pfaff et al. [12]. The peptide KALIHLSDLREYRRFEKEKLKSQWNNDNPLFKSATTTVMNPKFAES769, corresponding to the entire β2-integrin cytoplasmic domain, was synthesized by Biosynthesis, Inc. (Lewisville, TX, USA). The peptide was digested at 120 μM with recombinant cathepsin X (0.75 μM) for 1 h at 37°C in 50 mM HEPES, 150 mM NaCl, 1 mM CaCl2, and 1 mM DTT, pH 5.5. For the inhibition of cathepsin X, 2F12 mAb (0.75 μM) was preincubated with recombinant cathepsin X for 10 min. Each sample was separated by reverse-phase HPLC using a Gemini C18 column (5 μm, 110 Å, 150×46 mm; Phenomenex®), and peak fractions were analyzed by Q-Tof Premier mass spectrometer (in electrospray ionization+ mode; Waters). Sequences were assigned to proteolytic fragments on the basis of the known sequence of the undigested peptide and the determined molecular masses of the fragments.
Statistical analysis
The SPSS software package (Release 13.0, SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The difference between the groups was evaluated using the nonparametric Mann-Whitney test. P < 0.05 was considered statistically significant.
RESULTS
DC cultivation
Normal, immature DCs in culture were nonadherent. Addition of maturing stimulus (20 ng/ml LPS) resulted in altered morphology and increased adhesion and polarization. With time, DCs rounded up and formed clumps. Therefore, maturation induced significant morphological and cytoskeletal changes in normal DCs as already reported [13,14,15].
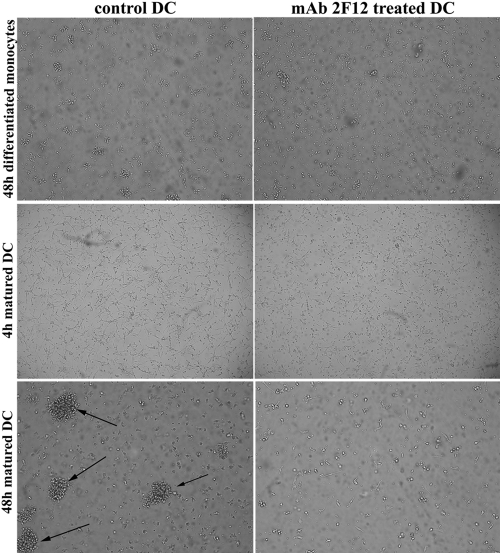
Inhibition of cathepsin X during differentiation of DCs from CD14+ monocytes and subsequent maturation with LPS resulted in marked phenotypic differences compared with untreated DCs (Fig. 1). Inhibition of cathepsin X completely abrogated the adhesion of DCs on addition of maturation stimulus and the development of prominent cell clusters, a striking feature of mature DCs. Treated, mature DCs remained as single, unattached cells floating in the medium.
Figure 1.
Inhibition of cathepsin X prevents maturation-induced changes in DC morphology. Monocyte-derived DCs were imaged in tissue-culture flasks using phase-contrast (at 10× original magnification) 48 h after inducing DC differentiation by the addition of rhGM-CSF (500 U/ml) and rhIL-4 (400 U/ml), as well as 4 h and 48 h after promoting DC maturation by the addition of rhGM-CSF (500 U/ml) and 20 ng/ml LPS in the presence or absence of the cathepsin X inhibitor (2F12 mAb). Differential interference contrast images of control and 2F12 mAb-treated DCs after the indicated time are shown. Arrows mark the loosely adherent clumps.
Characterization of mature DCs
Stimulation markers
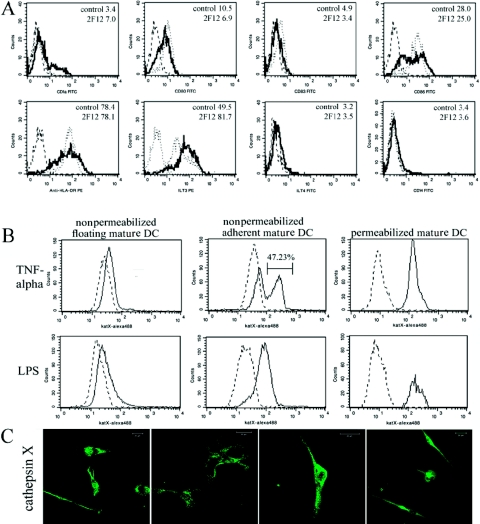
As the inhibition of cathepsin X markedly changed the phenotype of maturing DCs, we tested whether their maturation markers of DCs were affected. CD14, present on monocytes, CD1a, CD80, CD83, CD86, and HLA-DR, indicators of the maturation of DCs, as well as inhibitory ILT3 and ILT4, inhibitory receptors found on tolerogenic APCs, were all measured. All molecules were determined on mature DCs on Day 2 after LPS-triggered maturation in at least three different experiments of DCs, isolated each time from different individuals. Most importantly, CD14, a classic monocyte/macrophage marker, was not changed in cathepsin X-inhibited, mature DCs, indicating that the inhibition of cathepsin X does not subvert the program of differentiation to macrophages. Other maturation markers, apart from CD1a, were all reduced in cathepsin X-inhibited mature DCs, especially CD86 and HLA-DR. The tolerogenic receptors, ILT3 and ILT4, however, were increased in 2F12 mAb-treated mature DCs (Fig. 2A). Results clearly indicate that inhibition of cathepsin X during DC differentiation and maturation markedly reduces their maturation.
Figure 2.
Phenotypic characteristics of DC maturation. Surface marker expression was determined by FACS analysis of mature DCs stimulated for 48 h with LPS (20 ng/ml; A). Broken line shows staining with an isotype control, dotted line the staining of mature DCs, and solid line staining of DCs matured in the presence of the cathepsin X inhibitor. The results are representative of three independent experiments, and the average MFI for control and mAb 2F12-treated DCs is given in the right top corner in histograms. Surface expression of cathepsin X (solid line) was evaluated in adherent maturing and floating mature DCs (B and C). Immature DCs were stimulated with 20 ng/ml LPS for 48 h or TNF-α for 5 days and analyzed for cathepsin X (katX) membrane (nonpermeabilized, DC) or intracellular (permeabilized, DC) expression. Broken lines represent isotype controls (B). Confocal images of cathepsin X translocation to the plasma membrane in maturing, adherent DCs (C). Original scale bars represent 20 μm. Fluorescence microscopy was performed using a Carl Zeiss LSM 510 confocal microscope. Images were analyzed using Carl Zeiss LSM image software 3.0.
Cathepsin X and β2 integrins
Levels of cathepsin X were determined in immature and TNF-α-matured DCs (Days 1–5) by flow cytometry in our previous study [11] but only in nonadherent DCs. The results of the present study confirm the low membrane expression of cathepsin X in nonadherent, mature DCs. However, as shown in Figure 2B, the level of cathepsin X markedly increased in adherent DCs. The level of cathepsin X was the same in permeabilized and nonpermeabilized, adherent and nonadherent DCs, showing that the expression of cathepsin X did not change but only that it was translocated to the plasma membrane.
Confocal immunofluorescence microscopy
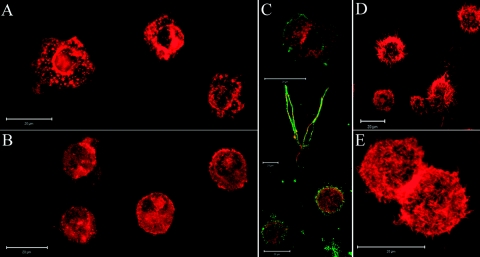
Alterations of the cytoskeleton were induced by the inhibition of cathepsin X, observed by rhodamine-phalloidin labeling of F-actin in immature DCs. Control, immature DCs had a round morphology, and a high proportion of cells formed podosomes (Fig. 3A). However, inhibition of cathepsin X prevented podosome formation in immature DCs (Fig. 3B). Active β2 integrin colocalized with actin only in maturing, adherent DCs but not in immature DCs or mature DCs (Fig. 3C). Mature DCs formed typical dendrites (Fig. 3D), and inhibition of cathepsin X did not affect actin reorganization in mature DCs (Fig. 3E).
Figure 3.
Cathepsin X enables podosome formation. Immature DCs on Day 5 of differentiation were centrifuged with cytospin (Cytofuge) for 6 min at 1000 rpm onto glass cover slides. Actin was labeled with phalloidin-tetramethylrhodamine B isothiocyanate conjugate (500 ng/ml) for 30 min at room temperature. Podosome formation, present in control, immature DCs (A), is prevented by inhibition of cathepsin X during DC differentiation (B). Adhesion of maturing DCs coincides with β2-integrin activation and colocalization with actin (C). Immature and mature DCs were labeled by centrifugation with cytospin (Cytofuge), whereas maturing, adherent DCs were labeled by seeding immature DCs onto glass coverslips in 24-well plates in the presence of 20 ng/ml LPS and allowing adherence for 20 h. The active form of β2 integrin was labeled with mAb 24 (green fluorescence) and colocalized with actin (red fluorescence) in adherent, mature DCs. Meanwhile, formation of typical dendrites in mature DCs (D) was not inhibited by cathepsin X inhibition (E). Original scale bars represent 20 μm. Fluorescence microscopy was performed using a Carl Zeiss LSM 510 confocal microscope. Images were analyzed using Carl Zeiss LSM image software 3.0.
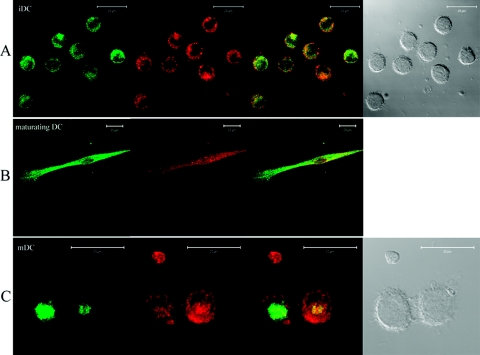
The localization of cathepsin X and the Mac-1 integrin receptor confirmed results from flow cytometry. In immature DCs, cathepsin X localized in the perinuclear cytoplasmic region, whereas Mac-1 clustered on the plasma membrane (Fig. 4A). Cathepsin X and the Mac-1 receptor colocalized in the cell poles and on the leading edges of maturing, adherent DCs, and with time, cathepsin X was seen to be translocated back to the perinuclear region (Fig. 4, A–C). In mature DCs, cathepsin X localized in a perinuclear, multivesicular bulb (Fig. 4C), as observed by Kos et al. [11], and the Mac-1 receptor remained on the plasma membrane, presumably in an inactive state [16].
Figure 4.
Cathepsin X colocalizes with the Mac-1 integrin receptor in maturing, adherent DCs. Cathepsin X was labeled with Alexa Fluor 488-labeled mouse 2F12 mAb that recognizes the mature, active form. Absence of colocalization of cathepsin X (green fluorescence) and Mac-1 integrin receptor (red fluorescence) in immature DC (iDC; A) and mature DCs (C) is shown. Differential interference contrast images are shown, respectively. Original bars, 20 μm. Maturing, adherent DCs were labeled by seeding immature DCs onto glass coverslips in 24-well plates in the presence of 20 ng/ml LPS and allowing adherence for 20 h. The translocation of cathepsin X in maturating, adherent DCs to the plasma membrane, where it colocalizes with the Mac-1 integrin receptor, is demonstrated (B). Original scale bars represent 20 μm. Fluorescence microscopy was performed using a Carl Zeiss LSM 510 confocal microscope. Images were analyzed using Carl Zeiss LSM image software 3.0.
Cytokine production
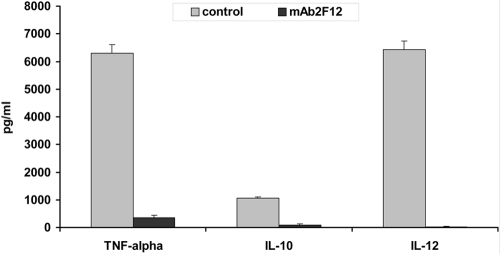
DCs are typically characterized by their ability to produce large amounts of predominantly T cell-modulatory cytokines [17]. A profound inhibition of IL-10, IL-12p70, and TNF-α production was observed in cells that were differentiated and subsequently matured in the presence of the cathepsin X inhibitor (2F12 mAb; Fig. 5). Mean cytokine levels (±sd) in stimulated control cells were 6300 pg/ml (TNF-α), 1050 pg/ml (IL-10), and 6420 pg/ml (IL-12). In stimulated, 2F12 mAb-pretreated cells, mean cytokine levels (±sd) were 350 pg/ml (TNF-α), 98 pg/ml (IL-10), and 28 pg/ml (IL-12).
Figure 5.
Cathepsin X inhibitor abrogates cytokine production in DCs, which were differentiated and subsequently activated (20 ng/ml LPS) in the presence or absence of the cathepsin X inhibitor. Cell-free supernatants were collected after 48 h and after the addition of LPS and then analyzed using ELISA. Student’s t-tests were calculated for control DCs versus mAb 2F12-treated DCs. Mean control DC responses from three independent experiments are shown.
Time-lapse recording and analysis of DC adhesion
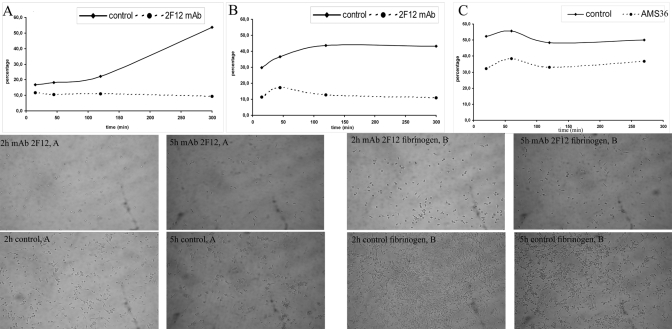
The adhesion of DCs after the addition of a maturation stimulus was estimated by plating immature DCs in 96-well plates in the presence of LPS. Nonadherent cells were removed at specific times, and adherent cells were evaluated with the MTS assay. Immature DCs were plated onto a plastic- or fibrinogen-coated surface. Maturing, control DCs gradually adhered to the plastic surface. In contrast, adhesion to fibrinogen, recruiting the Mac-1 receptor, was much more rapid and was most pronounced after 120 min. Inhibition of cathepsin X diminished adhesion to the plastic surface and to fibrinogen. The inhibiton of DC adhesion by 2F12 mAb ranged in time between 52.7% and 74.8% on fibrinogen and 31.0% and 82.7% on the plastic surface (Fig. 6). The similar results (26.4–38.3% of inhibition of DC adhesion) were obtained by the specific inhibitor of cathepsin X, AMS36, provided by Matthew Bogyo (Stanford University, Stanford, CA, USA; Fig. 6). Non-neutralizing mAb 3B10 (0.5 μM), recognizing the mature and pro-form of cathepsin X, did not influence the adhesion of DCs (data not shown).
Figure 6.
Cathepsin X enables adhesion of immature DCs upon addition of maturation stimulus. Immature DCs were matured with 20 ng/ml LPS on a plastic (A)- or fibrinogen (B and C)-coated surface in the absence (A) or presence of cathepsin X inhibitor mAb 2F12 (B) and AMS36 (C). The percentage of adherent DCs was estimated with the MTS assay. Data are as diagrams showing the time course of DC adhesion to a plastic- or fibrinogen-coated surface, respectively (see also Supplementary Videos 1 and 2 for adhesion in the presence or absence of the cathepsin X inhibitor 2F12 mAb to a fibrinogen-coated surface). (Lower) Photomicrographs of adherent DCs on a plastic (A)- or fibrinogen-coated surface (B) after the indicated time (2 h or 5 h) of LPS stimulation are shown for control and mAb 2F12-treated cells.
The same system was used for time-lapse, live cell imaging. Addition of LPS resulted in an alteration in the morphology of normal, immature DCs. By 270 min, cells had become adherent and well-polarized, forming large, leading-edge protrusions and migrating on a fibrinogen substratum (Supplemental Video 1). However, inhibition of cathepsin X reduced adhesion of immature DCs, thereby disabling their maturation (Supplemental Video 2). These data further support a specific role for cathepsin X in podosome formation, cell attachment, and migration, which is restricted to an early phase of maturation.
Cell migration
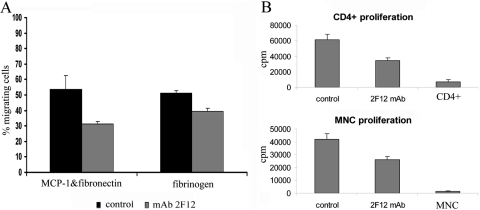
As shown previously, the migration of DCs correlates with their maturation status. Inhibition of podosome formation or inhibition of Mac-1 impairs migratory capacity of mature DCs [6]. Full migratory capacity is achieved after 24 h of stimulation [18]. Migration of DCs was reduced significantly in mature DCs differentiated and matured for 48 h in the presence of the cathepsin X inhibitor, although the inhibitor was not present during the migration assay (Fig. 7A).
Figure 7.
Mature DCs, differentiated and matured in the presence of the cathepsin X inhibitor, exhibit reduced migration and T cell-stimulatory capacity. The capacity of mature DCs to migrate was evaluated in 5 μm transwell plates. The chemotactic response of mature DCs to fibrinogen or fibronectin + MCP-1 (50 ng/ml) is presented as the percentage of transmigrated DCs in the bottom compartment of a transwell plate. Mature DCs, differentiated and matured in the presence of the cathepsin X inhibitor, 0.5 μM, show reduced migration ability compared with untreated, mature DCs, irrespective of chemotactic stimulus (A). The cathepsin X inhibitor was not present in the migration assay. Bars represent mean percentage of migrated DCs ± sd. Mature DCs were pretreated with mitomycin C and subsequently cocultured with purified, allogeneic T cells or mononuclear cells (MNC; B). Samples were treated in quadruplicate. Results are the means ± sd of at least three independent assays.
Allogeneic T cell proliferation
Exposure of DCs to the cathepsin X inhibitor during differentiation and maturation led to an inhibition of immune responsiveness, evident as reduced proliferation of CD4+ lymphocytes and MNC stimulated with mature DCs (Fig. 7B) in a MLR. Meanwhile, inhibition of cathepsin X in the proliferation reaction increased the proliferation of MNC (data not shown), as reported previously for macrophage-stimulated proliferation of MNC [9].
β2-Cytoplasmic region synthesis and cleavage site studies
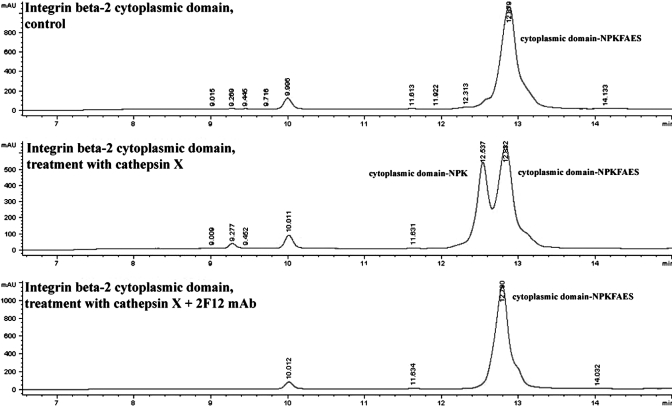
Incubation of the β2-integrin cytoplasmic domain peptide with recombinant cathepsin X resulted in a digested C-terminal end of the peptide, lacking terminal 4 aa (Fig. 8), as determined with mass spectrometry. However, preincubation of cathepsin X with 2F12-neutralizing mAb prevented the proteolytic activity of cathepsin X and cleavage of C-terminal amino acids (Fig. 8).
Figure 8.
HPLC analysis of the β2-integrin cytoplasmic domain cleaved with recombinant cathepsin X. The noncleaved cytoplasmic domain (cytoplasmic domain NPKFAES769) and corresponding cleaved cytoplasmic domain (cytoplasmic domain NPK765), detected after treatment with recombinant cathepsin X, were identified with electron-mass spectrometry in the fraction eluted between 8.6 and 9.5 min. Other peaks did not contain any cleaved peptides. Inhibition with 2F12 mAb completely abolished cathepsin X proteolytic activity, and the peak was identical to that of noncleaved peptide.
DISCUSSION
The presented data demonstrate that cathepsin X associates with the Mac-1 integrin receptor in maturing DCs and promotes Mac-1 integrin receptor activation (observed as adhesion of DCs in vitro) essential for effective DC maturation. Immature DCs are highly specialized in antigen capture, but to present the processed antigens to T lymphocytes, they need a stimulus to differentiate into a mature cell phenotype. Differentiation of nonadherent myeloid precursor cells along the monocytic/macrophage pathway is accompanied by cell-to-cell or cell-to-substrate adhesion [19]. Like other leukocytes, DCs need to attach to endothelium, achieving a firm adhesion, and finally extravasate and migrate within the tissue and secondary lymphoid organs through a process that involves the CD18 integrin receptor. When derived in vitro from monocytes, immature DCs are nonadherent and float in clumps. Upon addition of maturation stimuli, DCs, like macrophages, form the extensions, podosomes, which enhance their attachment to the surface [3]. It has been demonstrated that failure to form podosomes or selective inhibition of the CD18 receptor on the podosomes decreases the adhesion and subsequent differentiation [20].
As shown by confocal microscopy and flow cytometry, the maturation stimuli induce cathepsin X translocation to the plasma membrane of maturing, adherent DCs, where cathepsin X may activate the Mac-1 receptor and promote the adhesion to the plastic surface or fibrinogen, a substrate for the Mac-1 receptor. Cathepsin X binds cell-surface heparan sulfate proteoglycans [21], which are also involved in integrin regulation [22]. Mac-1 coprecipitates with cathepsin X [8], and the proteolytic cleavage of the C-terminal amino acids of the Mac-1 β2 chain by cathepsin X may trigger its activation. Cathepsin X gradually cleaves terminal amino acids F766, A767, E768, and S769 until P764 is present at the P2 position, as determined on 15 aa synthetic peptides (unpublished results). This study shows that the entire β2-cytoplasmic domain is similarly shortened by four C-terminal amino acids after cleavage with cathepsin X in vitro. As well as LPS, TNF-α, alone or in combination with IL-1β and IL-6, also induced cathepsin X translocation in DCs and their adhesion, showing that the effect was not restricted to the type of stimulation but linked with the maturation process itself. Inhibition of cathepsin X by 2F12 mAb or the specific synthetic cathepsin X inhibitor AMS36 significantly reduced the adhesion. Our results show that in addition to preventing adhesion, inhibition of cathepsin X prevented podosome formation, presumably via interaction with the Mac-1 receptor. This is consistent with the study of Burns et al. [3], which indicates that integrin subunit CD18 mediates DC-substratum contacts, namely podosomes, and that DCs from leukocyte adhesion deficiency-1 patients (with homozygous mutations of CD18) display abnormal morphology, lacking cellular projections [23]. Also, Calle et al. [6] have demonstrated that the inhibition of cathepsins results in the disassembly of podosomes in DCs. The temporal regulation of podosome assembly during DC maturation and its subsequent disassembly during the maturation process underline the importance of this cell projection in early DC movement [3]. Podosome biogenesis has been linked to lysosome exocytosis, and late endosomal/lysosomal proteins have been localized to podosomes (p61Hck, CD63) [24, 25]. Therefore, it has been suggested that late endosomal vesicles fusing with the plasma membrane contribute to podosome formation [25]. We demonstrate that cathepsin X, primarily a lysosomal cysteine protease, upon translocation during the maturation process, contributes to podosome formation.
Further, Burns et al. [3] demonstrated that DC maturation results in the loss of podosomes, as after 4–24 h, depending on maturation stimuli (TNF-α, IL-β, IL-6, or LPS), the cells round up and again form clumps. Whereas the effects of LPS stimulation on podosome disassembly appear to be indirect and involve a long lag time, the effect of TNF-α is immediate but only partial as a result of the lack of TNF-α receptor expression in some of the DCs. In our study, the stimulation with TNF-α resulted in two different populations of adherent, maturing DCs in regard to cathepsin X membrane expression, revealing its association with podosome disassembly. Along with the progressed maturation (demonstrated by increased expression of CD86 and HLA-DR), an increasing proportion of DCs detaches and assumes mature DC morphology. During podosome dissolution, increased vesicle transport occurs, and cathepsin X translocates back to the perinuclear region and condenses into a bulb consisting of multivesicular organelles as shown for mature DCs in our previous study [10]. This coincides with the de-adhesion of DCs and formation of cell clusters.
In mature DCs, the Mac-1 receptor is not constitutively active [16] but is redistributed during maturation. Whereas in immature DCs, CD18 predominantly localizes in podosomes, in mature DCs, it colocalizes with actin in ruffles throughout the cell surface [3]. This may reflect the change in adhesive phenotype to favor cell–cell interaction and immune synapse formation. Mac-1-mediated adhesion competes or interferes with LFA-1-dependent, intercellular adhesion. Hence, the attachment and spreading via Mac-1 greatly impair the formation of the LFA-1-mediated, homotypic aggregates on addition of maturation stimuli [26]. In mature DCs, inactive Mac-1 no longer competes with LFA-1, resulting in typical, large, homotypic aggregates of mature DCs. As a result of inactive Mac-1, mature DCs are the most potent “professional” APCs, with high ability to initiate the primary immune response [16], as is not the case with macrophages, which remain attached once they are differentiated. This can be attributed to the constitutive activity of Mac-1, which, however, inhibits T cell proliferation. Thus, the regulation of cathepsin X translocation in DCs and subsequent diversion of Mac-1 activity is crucial for an adequate immune stimulation by mature DCs [9].
Mature DCs possess immunostimulatory properties because of high expression of their antigen-presenting and costimulatory molecules and increased production of proinflammatory cytokines, including IL-12 and TNF-α [27]. As shown in this study, immature DCs were determined to be CD1a+, CD40+, HLA-DR+, and CD14−. During maturation, surface expression of CD80 and CD86 costimulatory molecules, together with CD83 and HLA-DR, was highly up-regulated, confirming the mature phenotype. However, when cathepsin X was inhibited, mature DCs expressed lower levels of CD80, CD83, CD86, and HLA-DR molecules. The production of proinflammatory cytokines IL-12 and TNF-α, as well as suppressive cytokine IL-10, was also strongly reduced, indicating the anergy of DCs. On the other hand, surface expression of the ILT3 and ILT4, markers of the tolerogenic capacity of DCs [28, 29], was increased, showing that cathepsin X inhibition impairs DC maturation into the immunostimulatory phenotype. The effect of cathepsin X inhibition was similar to that of the active metabolite of leflunomide, a potent, antirheumatic drug, which interferes with DC function during their differentiation and maturation and markedly decreases the profile of critical, costimulatory molecules as well as the production of TNF-α, IL-12, and IL-10 [27]. Interestingly, leflunomide has also been shown to impair integrin activation [30], as we suggest for cathepsin X inhibitors.
Inhibition of cathepsin X during DC maturation markedly inhibited their migratory capacity. During maturation, DCs become increasingly able to migrate [31]. Immature DCs show adhesive, low-speed, migratory behavior, whereas mature DCs can be defined as high-speed migration cells [32]. As monocyte-derived DCs selectively migrate in response to MCP-1 [33], the impaired, migratory capacity of DCs matured in the presence of mAb 2F12 may reflect their inadequate maturation status. Podosome turnover is required for effective cell adhesion and migration [6, 18], and integrin receptors should be involved in these processes. CD18−/− DCs, for example, display impaired adhesion and reduced migration [23, 34]. Active Mac-1 is therefore the prerequisite for maturation of DCs, providing the essential maturation stimulus. Its activators, such as cathepsin X, may promote this process and enable appropriate differentiation and maturation of DCs into a phenotype competent for potent immune stimulation.
In conclusion, our findings identify cathepsin X activity as a crucial factor in the regulation of DC maturation. We provide evidence that in immature DCs, cathepsin X translocates to the plasma membrane in response to maturation stimuli. This enables Mac-1 receptor activation and podosome formation, a process that is required for in vitro cell adhesion during maturation. However, in the next stage, mature DCs become devoid of podosomes and express an inactive Mac-1 receptor, and cathepsin X is redistributed from the cell membrane to the perinuclear region. In the absence of the temporary Mac-1 activation by cathepsin X, DCs fail to up-regulate costimulatory molecules and cytokine production and cannot migrate and stimulate allogeneic T lymphocytes but promote anergy and an inappropriate immune response.
Supplementary Material
Acknowledgments
This work was supported by the Research Agency of the Republic of Slovenia (grant T4-0127, J. K.) and partially by the 6th European Union Framework Integrated Project CancerDegradome. We thank Urša Pečar Fonovič, M.Sc., for the generous gift of active recombinant cathepsin X and Dr. Nancy Hogg for the generous gift of mAb 24. Many thanks to Dušan Žigon and Bogdan Kralj for help in mass spectrometry analysis, Bojan Doljak for help in cleavage site study, and Aimir Masoud Sadaghiani for the preparation of the synthetic inhibitor AMS36. The authors acknowledge Prof. Roger Pain for critical reading of the manuscript.
References
- Lipscomb M. F., Masten B. J. Dendritic cells: immune regulators in health and disease. Physiol Rev. 2002;82:97–130. doi: 10.1152/physrev.00023.2001. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Hawiger D., Liu K., Bonifaz L., Bonnyay D., Mahnke K., Iyoda T., Ravetch J., Dhodapkar M., Inaba K. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann N Y Acad Sci. 2003;987:15–25. doi: 10.1111/j.1749-6632.2003.tb06029.x. [DOI] [PubMed] [Google Scholar]
- Burns S., Hardy S. J., Buddle J., Yong K. L., Jones G. E., Trasher A. J. Maturation of DC is associated with changes in motile characteristics and adherence. Cell Motil Cytoskeleton. 2004;57:118–132. doi: 10.1002/cm.10163. [DOI] [PubMed] [Google Scholar]
- Gaidano G., Bergui L., Schena M., Gaboli M., Cremona O., Marchisio P. C., Caligaris-Cappio F. Integrin distribution and cytoskeleton organization in normal and malignant monocytes. Leukemia. 1990;4:682–687. [PubMed] [Google Scholar]
- Ueki T., Iwasawa K., Ishikawa H., Sawa Y. Expression of junctional adhesion molecules on the human lymphatic endothelium. Microvasc Res. 2008;75:269–278. doi: 10.1016/j.mvr.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Calle Y., Carragher N. O., Thrasher A. J., Jones G. E. Inhibition of calpain stabilizes podosomes and impairs dendritic cell motility. J Cell Sci. 2006;119:2375–2385. doi: 10.1242/jcs.02939. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. Presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermajer N., Premzl A., Zavasnik-Bergant T., Turk B., Kos J. Carboxypeptidase cathepsin X mediates β2-integrin-dependent adhesion of differentiated U-937 cells. Exp Cell Res. 2006;312:2515–2527. doi: 10.1016/j.yexcr.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Obermajer N., Repnik U., Jevnikar Z., Turk B., Kreft M., Kos J. Cysteine protease cathepsin X modulates immune response via activation of β-2 integrins. Immunology. 2008;124:76–88. doi: 10.1111/j.1365-2567.2007.02740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevnikar Z., Obermajer N., Bogyo M., Kos J. The role of cathepsin X in aggregation, migration and invasion of T lymphocytes. J Cell Sci. 2008 doi: 10.1242/jcs.023721. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Kos J., Sekirnik A., Premzl A., Zavasnik Bergant V., Langerholc T., Turk B., Werle B., Golouh R., Repnik U., Jeras M., Turk V. Carboxypeptidases cathepsins X and B display distinct protein profile in human cells and tissues. Exp Cell Res. 2005;306:103–113. doi: 10.1016/j.yexcr.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Pfaff M., Du X., Ginsberg M. H. Calpain cleavage of integrin β cytoplasmic domains. FEBS Lett. 1999;460:17–22. doi: 10.1016/s0014-5793(99)01250-8. [DOI] [PubMed] [Google Scholar]
- Winzler C., Rovere P., Rescigno M., Granucci F., Penna G., Adorini L., Zimmermann V. S., Davoust J., Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutt D. C., Daniels K. J., Carolan E. J., Hill A. C., Soll D. R. Changes in the motility, morphology, and F-actin architecture of human dendritic cells in an in vitro model of dendritic cell development. Cell Motil Cytoskeleton. 2000;46:200–221. doi: 10.1002/1097-0169(200007)46:3<200::AID-CM5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Burns S., Trasher A. Dentritic cells: the bare bones of immunity. Curr Biol. 2004;14:R965–R967. doi: 10.1016/j.cub.2004.10.044. [DOI] [PubMed] [Google Scholar]
- Varga G., Balkow S., Wild M. K., Stadtbaeumer A., Krummen M., Rothoeft T., Higuchi T., Beissert S., Wethmar K., Scharffetter-Kochanek K., Vestweber D., Grabbe S. Active MAC-1 (CD11b/CD18) on DCs inhibits full T-cell activation. Blood. 2007;109:661–669. doi: 10.1182/blood-2005-12-023044. [DOI] [PubMed] [Google Scholar]
- Everson M. P., McDuffie D. S., Lemak D. G., Koopman W. J., McGhee J. R., Beagley K. W. Dendritic cells from different tissues induce production of different T cell cytokine profiles. J Leukoc Biol. 1996;59:494–498. doi: 10.1002/jlb.59.4.494. [DOI] [PubMed] [Google Scholar]
- Van Helden S. F. G., Krooshoop D. J. E. H., Broers K. C. M., Raymakers R. A. P., Figdor C. G., van Leeuwen F. N. A critical role for prostaglandin E2 in podosome dissolution and induction of high-speed migration during dendritic cell maturation. J Immunol. 2006;177:1567–1574. doi: 10.4049/jimmunol.177.3.1567. [DOI] [PubMed] [Google Scholar]
- Tushinski R. J., Oliver I. T., Guilbert L. J., Tynan P. W., Warner J. R., Stanley E. R. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982;28:71–81. doi: 10.1016/0092-8674(82)90376-2. [DOI] [PubMed] [Google Scholar]
- Prudovsky I., Popov K., Akimov S., Serov S., Zelenin A., Meinhardt G., Baier P., Sohn C., Hass R. Antisense CD11b integrin inhibits the development of a differentiated monocyte/macrophage phenotype in human leukemia cells. Eur J Cell Biol. 2002;81:36–42. doi: 10.1078/0171-9335-00219. [DOI] [PubMed] [Google Scholar]
- Nascimento F. D., Rizzi C. C., Nantes I. L., Stefe I., Turk B., Carmona A. K., Nader H. B., Juliano L., Tersariol I. L. Cathepsin X binds to cell surface heparan sulfate proteoglycans. Arch Biochem Biophys. 2005;436:323–332. doi: 10.1016/j.abb.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Beauvais D. M., Burbach B. J., Rapraeger A. C. The sydecan-1 ectodomain regulates αVβ3 integrin activity in human mammary carcinoma cells. J Cell Biol. 2004;167:171–181. doi: 10.1083/jcb.200404171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorini M., Vermi W., Facchetti F., Moratto D., Alessandri G., Notarangelo L., Caruso A., Grigolato P., Ugazio A. G., Notarangelo L. G., Badolato R. Defective migration of monocyte-derived dendritic cells in LAD-1 immunodeficiency. J Leukoc Biol. 2002;72:650–656. [PubMed] [Google Scholar]
- Toyomura T., Murata Y., Yamamoto Y., Oka T., Sun-Wada G. H., Wada Y., Futai M. From lysosomes to the plasma membrane: localization of vacuolar-type H-ATPase with a3 isoform during osteoclast differentation. J Biol Chem. 2003;278:22023–22030. doi: 10.1074/jbc.M302436200. [DOI] [PubMed] [Google Scholar]
- Cougoule C., Carréno S., Castandet J., Labrousse A., Astarie-Dequeker C., Poincloux R., le Cabec V., Maridonneau-Parini I. Activation of the lysosome-associated p61Hck isoform triggers the biogenesis of podosomes. Traffic. 2005;6:682–694. doi: 10.1111/j.1600-0854.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- Nueda A., Lopez-Rodriguez C., Rubio M. A., Sotillos M., Postigo A., del Pozo M. A., Vega M. A., Corbi A. L. Hematopoietic cell-type-dependent regulation of leukocyte integrin functional activity: CD11b and CD11c expression inhibits LFA-1 dependent aggregation of differentiated U937 cells. Cell Immunol. 1995;164:163–169. doi: 10.1006/cimm.1995.1157. [DOI] [PubMed] [Google Scholar]
- Kirsch B. M., Zeyda M., Stuhlmeier K., Grisar J., Smolen J. S., Watschinger B., Stulnig T. M., Horl W. H., Zlabinger G. J., Saemann M. D. The active metabolite of leflunomide, A77 1726, interferes with dendritic cell function. Arthritis Res Ther. 2005;7:R694–R703. doi: 10.1186/ar1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad G., Liu G., Zhang Q. Y., Cortesini R., Suciu-Foca N. Immunosuppressive activity of recombinant ILT3. Int Immunopharmacol. 2006;6:1889–1894. doi: 10.1016/j.intimp.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Manavalan J. S., Rossi P. C., Vlad G., Piazza F., Yarilina A., Cortesini R., Mancini D., Sociu-Foca N. High expression of IL3 and Ilt4 is a general feature of tolerogenic dendritic cells. Transpl Immunol. 2003;11:245–258. doi: 10.1016/S0966-3274(03)00058-3. [DOI] [PubMed] [Google Scholar]
- Zeyda M., Poglitsch M., Geyeregger R., Smolen J. S., Zlabinger G. J., Hörl W. H., Waldhäusl W., Stulnig T. M., Säemann M. D. Disruption of the interaction of T cells with antigen-presenting cells by the active leflunomide metabolite teriflunomide: involvement of impaired integrin activation and immunologic synapse formation. Arthritis Rheum. 2005;52:2730–2739. doi: 10.1002/art.21255. [DOI] [PubMed] [Google Scholar]
- De Vries I. J., Krooshoop D. J., Scharenborg N. M., Lesterhuis W. J., Diepstra J. H., van Muijen G. N., Strijk S. P., Ruers T. J., Boerman O. C., Oyen W. J. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63:12–17. [PubMed] [Google Scholar]
- Friedl P., Wolf K. Proteolytic and non-proteolytic migration of tumor cells and leukocytes. Biochem Soc Symp. 2003;70:277–285. doi: 10.1042/bss0700277. [DOI] [PubMed] [Google Scholar]
- Zhu K., Shen Q., Ulrich M., Zheng M. Human monocyte-derived dendritic cells expressing both chemotactic cytokines IL-8, MCP-1, RANTES and their receptors, and their selective migration to these chemokines. Chin Med J (Engl) 2000;113:1124–1128. [PubMed] [Google Scholar]
- Schneeberger E. E., Vu Q., LeBlanc B. W., Doerschuk C. M. The accumulation of dendritic cells in the lung is impaired in CD18−/− but not in ICAM-1−/− mutant mice. J Immunol. 2000;164:2472–2478. doi: 10.4049/jimmunol.164.5.2472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.