Abstract
Aim: To construct and examine models of the vascular networks using the technique of vascular corrosion casting in placentas collected from normal pregnancies and from pregnancies complicated by fetal growth restriction (FGR). Methods: Twenty placentas were collected from normal term pregnancies (Group NP) and an equal number from pregnancies with idiopathic term FGR (Group FGR) and placental vascular network models constructed by perfusing an acrylic-based solution separately into the umbilical vein and arteries. Placental blood volumes and blood vessel characteristics (number of branches, diameter, and morphology) were then examined and compared. Results: In placentas from Group NP, the veins branched five to seven times with a peripheral artery-to-vein ratio ranging from 1:2 to 1:3. In placentas from Group FGR, the veins branched only four to five times with an artery-to-vein ratio of 1:1 to 2:1 and increased evidence of nodularity and pitting of the vessel walls. The two groups showed significant differences in placental blood volume and in the mean diameters of umbilical veins and arteries. In Group FGR, significant positive correlations could be found between birth weight and placental volume, venous diameters, and select arterial diameters. Conclusion: Vascular network models can be constructed from term placentas. Such modeling may provide novel insights and improve our understanding of the placental vascular system in both health and disease.
Key words: Placenta, Vascular corrosion casting, Fetal growth restriction, Pregnancy
The placenta is important for the maintenance of pregnancy and for the promotion of fetal growth. A thorough knowledge of the placental vasculature is thus critical to understanding normal fetal growth and development as well as a variety of pregnancy-related diseases. Indeed, as our understanding of pregnancy-related diseases such as preeclampsia and fetal growth restriction (FGR) has increased, more and more attention has focused on the role of the placental vasculature and placental function. Most of these studies used gross observation and immunohistochemistry to study the placental blood vessels in normal and abnormal pregnancies.1,2 However, these methods do not permit clear visualization of the large placental blood vessels, let alone the delicate network of vessels hidden within the body of the placenta.
Vascular corrosion casting provides an effective way to define small blood vessels, thereby allowing a more detailed and sophisticated rendering of the vascular network.3–6 In 1966, La Torretta and Cobellis attempted to infuse the placenta with a liquid rubber solution, 7 but this technique failed to adequately define all of the blood vessels. Since that time, several researchers have been able to observe the normal term placental vascular network as well as the presence of arteriovenous anastomoses in monochorionic placentas using vascular corrosion casting.8,9
Fetal growth restriction is a common obstetric complication and is associated with significant fetal morbidity and mortality. Although several causes of FGR exist (including fetal aneuploidy, infection, damage from drugs and alcohol, and structural anomalies), the majority of cases of idiopathic FGR result from uteroplacental insufficiency due to as yet poorly defined lesions of the placental vascular bed. Despite this observation and the fact that placental blood flow appears to be decreased about fourfold in placentas collected from pregnancies complicated with FGR compared with healthy gestational age-matched controls,1,10 pathologic examination of placentas from FGR pregnancies typically fail to identify any abnormalities. Until now, no placental vascular network models were available for FGR. The current study was designed to address this deficiency by constructing placental vascular network models for idiopathic term FGR placentas using vascular corrosion casting and comparing these models with those prepared from normal term pregnancies.
Materials and Methods
Sample Collection
Fresh term placentas with approximately 3 cm of attached umbilical cord were collected at the time of either vaginal or cesarean delivery from normal term pregnancies with well-grown fetuses (n = 20 [Group NP]) and an equal number from pregnancies complicated by idiopathic term FGR (Group FGR). Fetal growth restriction was defined as an estimated fetal weight below the 10th percentile for gestational age using population-specific ultrasound growth curves. Only pregnancies with idiopathic FGR were included; patients with FGR due to chromosomal abnormalities or congenital disorders were excluded from the study. Gestational age dating was confirmed by a firm last menstrual period consistent with a second trimester ultrasound. All pregnancies were free of underlying medical conditions (such as preeclampsia and diabetes) or placental abnormalities (placenta previa or accrete). All placentas were collected at Nanfang Hospital of Southern Medical University and Houjie People’s Hospital, Guangdong Province, China, between October 2008 and October 2009 under an Internal Review Board-approved protocol.
Processing of Placentas
At the time of delivery, the placentas were weighed and measured (length × width × height were used to calculate the placental area). All placentas were confirmed to have three vessel cords. Neonatal birth weight was recorded. The placentas were milked of excess blood and bathed in normal saline containing heparin. The umbilical vein and two umbilical arteries were cannulated and the proximal ends of the cannulas were secured to the vessel wall. The vessels were washed by passing normal saline through the cannulas at a constant perfusion pressure (40–45 cm H2O) as previously described.6 The specimens were then fixed in 95% alcohol and prepared for corrosion casting.
Initial experimentation carried out at this same institution as part of the Virtual Chinese Human (VCH) project4 demonstrated that self-curing denture base materials in the perfusate were best able to maintain vascular morphology and were associated with minimal supplement perfusion and less vascular shrinkage. Self-curing denture base materials consist of a mixture of self-curing denture acrylic and self-curing denture base water. The perfusate chosen for these studies consisted of dibutyl phthalate and a red or blue water-based dye. The red-dyed perfusate was inserted into the umbilical veins (which carry oxygenated blood) and the blue-dyed perfusate was used in the umbilical arteries (which carry deoxygenated blood). In brief, 20 mL syringes were used to inject perfusate simultaneously through the plastic cannulas and into the umbilical vein and arteries under standard pressure (40–45 cm H2O). The cannulas were then removed and the umbilical vessels ligated to prevent perfusate leakage. The placentas were maintained at room temperature for 2 days to allow the perfusate to solidify. Once hardened, the specimens were placed in 37% hydrochloric acid for 3 days to corrode away the placental tissues. The specimens were then washed in water, air-dried, and stored in plastic containers containing desiccant and preservatives.
During the perfusion process, the total volume of perfusate needed to fill each placental vascular network was recorded as the placental blood volume. After corrosion casting, the models were examined in a standardized and systematic manner by a single investigator blinded to the clinical data. The following features were recorded: (a) the morphology of the placental blood vessels; (b) the artery-to-vein ratio; (c) the number of branches with the placental veins and arteries; and (d) the diameters of the different grades of blood vessels, which were measured using a vernier caliper. A standardized grading system was used in all cases based on the number of branches of the major umbilical vessels. The umbilical vein and arteries were defined as Grade 1 blood vessels. The diameter reported for the Grade 1 artery was calculated as the average of the diameters measured for the two umbilical arteries. The diameters reported for Grade 2 and 3 blood vessels (ie, blood vessels produced after 1 and 2 branches, respectively) represent the mean measurements obtained from four randomly selected Grade 2 blood vessels and six randomly selected Grade 3 blood vessels, respectively. For every individual blood vessel examined, the diameters were measured at the proximal, middle, and distal ends of the vessel and the average diameter calculated and reported.
Statistical Analysis
All data are presented as mean ± standard deviation (SD). An independent samples t-test was used to compare measurements between the two groups. Pearson correlation analysis was used to examine the relationships between birth weight and placental measurements. P <.05 was used to designate statistical significance. SPSS (version 13.0) was used for all statistical analyses.
Results
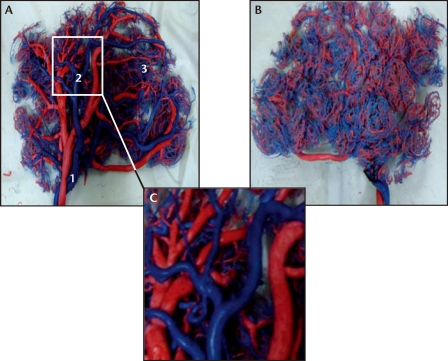
The distribution and morphology of different grades of placental blood vessels and their peripheral branches can be shown with great clarity in the placental vascular network models created with vascular corrosion casting, which cannot be appreciated by simply gross examination of the placenta alone. Such models have outstanding resolution and provide an accurate representation of the vessel network without distortion (Figure 1).
Figure 1.
Images of the fetal (A) and maternal (B) aspects of the placental vascular network in a representative placenta collected from a healthy normotensive pregnancy delivered at 38 weeks of gestation. Original magnification, ×1 . A separate image of the fetal aspect is shown under ×3 magnification (C). The umbilical arteries contain deoxygenated blood and are shown in blue; the umbilical veins contain oxygenated blood and are shown in red. 1, Grade 1 artery (umbilical artery); 2, Grade 2 artery; 3, Grade 3 artery.
Placental Weight and Volume
As expected, the weight and area of the placentas in Group FGR were significantly less than that of Group FNP (401 ± 28 g vs 504 ± 56 g [P < .001] and 276.3 ± 76.8 cm2 vs 346.0 ± 43.7 cm2 [P < .05], respectively). Similar differences were noted in mean placental volume as measured by the volume of perfusate needed to be injected to fill the vascular network in each individual placenta (108.4 ± 18.2 mL for Group FGR vs 152.1 ± 25.7 mL for Group NP [P< .001]).
Morphologic Characteristics of the Placental Vascular Network
In placental vascular models of Group NP (Figure 1), it was clear that the placental venous system was divided into five to seven grades, progressing from coarse to fine. The umbilical vein (designated as the Grade 1 vein of the placental blood vessels) branched into Grade 2 veins upon entering the placenta. As the Grade 2 veins traveled through the placenta, they further subdivided to form Grade 3 to 7 venous branches, the diameters of which decreased uniformly, ending in a dense and abundant peripheral network of placental capillaries. The mean diameter of the Grade 1 vein was 8.9 ± 1.2 mm. The diameters of the Grade 2 and 3 veins were 4.6 ± 0.5 mm and 3.5 ± 0.5 mm, respectively (Table 1). The two umbilical arteries (designated Grade 1 placental arteries) communicated with each other at the foot of umbilical cord and formed a vascular sinus, which then divided into a series of subordinate arterial branches that were one to two grades lower than those of the venous system, thereby forming a sparser peripheral arterial network. The mean diameters of Grade 1, 2, and 3 arteries were 3.8 ± 0.6 mm, 3.2 ± 0.5 mm, and 2.0 ± 0.3 mm, respectively. Interestingly, the ratio of peripheral arteries to veins in the placentas from Group NP ranged from 1:2 to 1:3. As regards the anatomic location of the blood vessels, Grade 2 blood vessels were present in the chronic plate, whereas Grade 2 and 3 blood vessels were evident in the area of the chorionic stem villi and intermediate villi. Careful examination showed the vascular walls to be smooth and regular, without significant nodularity or depressions (Figure 1C).
Table 1.
Comparison of Placental Volume and Diameters of Placental Blood Vessels Between Group FGR and Group NP
| Group | Placental Volume | Diameter of Placental Veins | Diameter of Placental Arteries | ||||
| (mL) | (mm) | (mm) | |||||
| Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | ||
| FGR | 108.4 ± 18.2 | 7.2 ± 0.6 | 3.8 ± 0.4 | 3.1 ± 0.4 | 3.2 ± 0.4 | 2.7 ± 0.3 | 1.7 ± 0.2 |
| NP | 152.1 ± 25.7 | 8.9 ± 1.2 | 4.6 ± 0.5 | 3.5 ± 0.5 | 3.8 ± 0.6 | 3.2 ± 0.5 | 2.0 ± 0.3 |
| T | −6.208 | −5.633 | −13.167 | −6.961 | −5.509 | −7.926 | −10.725 |
| P Value | < .001 | < .001 | < .001 | < .001 | < .001 | < .001 | < .001 |
Data are presented as mean ± SD of 20 samples in each group.
FGR, fetal growth restriction; NP, normal pregnancy.
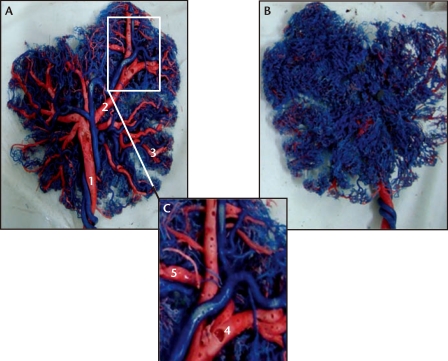
The vascular network models of FGR placentas (Figure 2) demonstrated that the placental venous system was divided into four to five grades, progressing from coarse to fine, which was one to two degrees fewer than in placentas from normal pregnancies. Detailed observation of the Grade 2 to 4 placental veins revealed that the surface of the blood vessels was irregular with widespread evidence of nodularity and depressions (Figure 2C). In some areas, the vascular walls became extremely thin and appeared transparent. Like Group NP, the placental arterial system in FGR placentas still contained four to five degrees of blood vessels, but the nodular and vacuolar changes persisted. The number of peripheral arterial branches in FGR placentas was higher than the number of peripheral vein branches, with a ratio of 1:1 to 2:1. This is evident when comparing the large number of peripheral arteries (in blue) relative to peripheral veins (in red) in the vascular network models of FGR placentas compared with normal placentas. This is seen most clearly on the maternal aspect of the placenta (compare Figure 2B and Figure 1B). A comparison of the placental blood vessel diameters demonstrates that the diameters of the Grade 1, 2, and 3 placental veins and arteries in FGR placentas are significantly smaller than those in normal placentas (Table 1).
Figure 2.
Images of the fetal (A) and maternal (B) aspects of the placental vascular network in a representative placenta collected from a pregnancy complicated by fetal growth restriction (FGR) and delivered at 37 weeks of gestation. Original magnification, ×1. A separate image of the fetal aspect is shown under 3× magnification (C). The umbilical arteries contain deoxygenated blood and are shown in blue; the umbilical veins contain oxygenated blood and are shown in red. Note that there appear to be many fewer veins in this FGR placenta compared with a normal placenta (Figure 1). 1, Grade 1 vein (umbilical vein); 2, Grade 2 vein; 3, Grade 3 vein; 4, vacuolated changes; 5, nodular changes.
Correlation Between Birth Weight and Placental Morphology in FGR Pregnancies
The average birth weight for neonates born in Group FGR was 2170 ± 200 g compared with Group NP (3537±287 g). The results of the Pearson correlation analysis for Group FGR demonstrated significant positive correlations between birth weight and placental volume, the diameters of all three grades of placental veins, and the diameters of Grade 2 (but not Grade 1 or 3) placental arteries (Table 2). Interestingly, the correlation coefficients between birth weight and placental vein diameters were larger than those for the corresponding grade of arteries (Table 2).
Table 2.
Correlation Between Birth Weight and Select Placental Measurements in Group FGR
| Placental | Diameter of Placental Veins | Diameter of Placental Arteries | |||||
| Volume | Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | |
| Coefficient | 0.662 | 0.643 | 0.554 | 0.679 | 0.158 | 0.515 | 0.205 |
| P Value | .001 | .002 | .011 | .001 | .505 | .020 | .386 |
All correlation coefficients and P values reflect the association between neonatal birth weight and the designated placental measurement.
FGR, fetal growth restriction.
Discussion
Fetal growth restriction remains a major cause of perinatal mortality and morbidity. Its impact extends well beyond intrauterine life to affect the physical and mental development of the offspring throughout their childhood and adolescence. The placenta, the organ that is most intimately associated with both the mother and the fetus, is critical to normal fetal growth and development. Prior studies based on gross placental morphology alone have shown that placental weight, diameter, and volume are strongly correlated with fetal growth.1,11 However, gross morphologic examination of the placenta is relatively crude and is unable to examine the placental vascular network in any level of detail. This is important because recent reports have suggested that many pregnancy-related disorders (including preeclampsia and FGR) result from shallow implantation of the placenta and a failure of the trophoblast to remodel the maternal spiral arteries and establish the definitive uteroplacental circulation. Arroyo and Winn, for example, revealed that FGR is associated with shallow vascular invasion of the placenta,10 which leads in turn to insufficient uteroplacental blood flow. To date, however, there has been no effective technique to directly examine the morphology of the placental blood vessels.
Vascular corrosion casting has been shown to be a valuable tool for modeling the vascular network of the whole body or individual organ systems.12,13 The cast clearly displays the pattern of the vascular structures, including tiny vascular branches. The Department of Anatomy at Nanfang Hospital of Southern Medical University has previously used this technique to delineate the vascular supply of the uterus.5,6 In this study, we constructed vascular network models of placentas from normal pregnancies and pregnancies complicated by FGR in an effort to better understand the pathogenesis of FGR. The three-dimensional reconstruction of the placental vascular network provided unique insight into the structure of the placental blood vessels, which can enhance clinical teaching and investigations of placental function.
In our study, examination of the placental vascular network models created from normal term pregnancies showed that the venous system (which transports oxygenated blood from the placenta to the fetus) was divided into five to seven grades, progressing from coarse to fine vessels, with diameters that decrease uniformly ending in a dense and abundant peripheral venous network within the substance of the placenta. The arterial system branched as progressively as the venous system, but with 1 to 2 degrees of branching less than that of the venous system, giving a 1:2 to 1:3 ratio of peripheral arteries to veins (Figure 1). This arrangement of the placental vessels likely allows for maximum exchange of oxygen and nutrients between the fetus and its mother.
In contrast, the placental vascular network models created from placentas collected from term idiopathic FGR pregnancies showed that both the placental volume and the diameters of the different grades of blood vessels were significantly lower than those for normal pregnancies. Furthermore, the branches of the venous system were fewer and the peripheral veins were sparser in FGR placentas, giving a 1:1 to 2:1 ratio of peripheral arteries to veins. However, the placental arterial system still contained 4 to 5 degrees of branching blood vessels. This aberrant placental vascular distribution greatly decreases the surface area available for oxygen, nutrient, and waste exchange. Whether this is the cause or consequence of FGR, however, remains to be determined. Additionally, the FGR placental vascular network models show significant nodularity and depressed changes (vacuolations or pitting) within the walls of the blood vessels, which were not evident in the placentas from non-FGR pregnancies. Several investigators have suggested that FGR may be associated with an aberrant immune response within the placenta.14–16 It is tempting to suggest, therefore, that these vascular irregularities may represent deposits of immunity complexes. Although attractive, this hypothesis remains to be confirmed.
In this study, positive correlations were demonstrated between birth weight and placental volume, the diameters of various grades of placental veins, and the diameter of Grade 2 (but not Grade 1 or 3) placental arteries. Even more interesting, the correlation coefficients for birth weight and umbilical vein diameters were all larger than the corresponding measurements for birth weight and umbilical arteries (Table 2). These data suggest that the morphology and capacity of the placental venous system have a larger impact on fetal growth than the arterial system.
Placental vascular network modeling can be used to accurately display the morphology and characteristics of the placental blood vessels and their distribution in both normal pregnancies and pregnancies complicated by FGR. Such modeling may contribute significantly to our understanding of the pathogenesis of FGR.
Main Points.
Twenty placentas were collected from normal term pregnancies (Group NP) and an equal number from pregnancies with idiopathic term FGR (Group FGR) and placental vascular network models constructed by perfusing an acrylic-based solution separately into the umbilical vein and arteries. Placental blood volumes and blood vessel characteristics (number of branches, diameter, and morphology) were then examined and compared.
In placentas from Group NP, the veins branched five to seven times with a peripheral artery-to-vein ratio ranging from 1:2 to 1:3. In placentas from Group FGR, the veins branched only four to five times with an artery-to-vein ratio of 1:1 to 2:1 and increased evidence of nodularity and pitting of the vessel walls. The two groups showed significant differences in placental blood volume and in the mean diameters of umbilical veins and arteries. In Group FGR, significant positive correlations could be found between birth weight and placental volume, venous diameters, and select arterial diameters.
Vascular network models can be constructed from term placentas. Such modeling may provide novel insights and improve our understanding of the placental vascular system in both health and disease.
Footnotes
The authors thank the Anatomy Department of Southern Medical University, China, for providing technical support, and Houjie People’s Hospital, Guangdong Province, China, for providing the placental materials for this study.
References
- 1.Biswas S, Ghosh SK. Gross morphological changes of placentas associated with intrauterine growth restriction of fetuses: a case control study. Early Hum Dev. 2008;84:357–362. doi: 10.1016/j.earlhumdev.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Sağol S, Ozkinay E, Oztekin K, Ozdemir N. The comparison of uterine artery Doppler velocimetry with the histopathology of the placental bed. Aust N Z J Obstet Gynaecol. 1999;39:324–329. doi: 10.1111/j.1479-828x.1999.tb03407.x. [DOI] [PubMed] [Google Scholar]
- 3.Verli FD, Rossi-Schneider TR, Schneider FL, et al. Vascular corrosion casting technique steps. Scanning. 2007;29:128–132. doi: 10.1002/sca.20051. [DOI] [PubMed] [Google Scholar]
- 4.Tang L, Chung MS, Liu Q, Shin DS. Advanced features of whole body sectioned images: Virtual Chinese Human. Clin Anat. 2010;23:523–529. doi: 10.1002/ca.20975. [DOI] [PubMed] [Google Scholar]
- 5.Chen CL, Guo HX, Liu P, et al. Three-dimensional reconstruction of the uterine vascular supply through vascular casting and thin slice computed tomography scanning. Minim Invasive Ther Allied Technol. 2009;18:98–102. doi: 10.1080/13645700902720324. [DOI] [PubMed] [Google Scholar]
- 6.Chen C, Huang R, Liu P, et al. Construction and clinical significance of normal uterine arterial vascular network models. Gynecol Obstet Invest. 2010;69:14–19. doi: 10.1159/000245941. [DOI] [PubMed] [Google Scholar]
- 7.La Torretta G, Cobellis G. Vascular aspects of the monochorial placenta in neoprene casts. Arch Ostet Ginecol. 1966;71:357–362. [Article in Italian.] [PubMed] [Google Scholar]
- 8.Habashi S, Burton GJ, Steven DH. Morphological study of the fetal vasculature of the human term placenta: scanning electron microscopy of corrosion casts. Placenta. 1983;4:41–56. doi: 10.1016/s0143-4004(83)80016-2. [DOI] [PubMed] [Google Scholar]
- 9.Wee LY, Taylor M, Watkins N, et al. Characterisation of deep arterio-venous anastomoses within monochorionic placentae by vascular casting. Placenta. 2005;26:19–24. doi: 10.1016/j.placenta.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Arroyo JA, Winn VD. Vasculogenesis and angiogenesis in the IUGR placenta. Semin Perinatol. 2008;32:172–177. doi: 10.1053/j.semperi.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Molteni RA. Placental growth and fetal placental weight (F/P) ratios throughout gestation-their relationship to patterns of fetal growth. Semin Perinatol. 1984;8:94–100. [PubMed] [Google Scholar]
- 12.Yuan Y, Qi L, Luo S. The reconstruction and application of virtual Chinese human female. Comput Methods Programs Biomed. 2008;92:249–256. doi: 10.1016/j.cmpb.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Bhutto IA, Amemiya T. Microvascular architecture of the rat choroids: corrosion cast study. Anat Rec. 2001;264:63–71. doi: 10.1002/ar.1102. [DOI] [PubMed] [Google Scholar]
- 14.Han VK, Bassett N, Walton J, Challis JR. The expression of insulin-like growth factor (IGF) and IGF-binding protein (IGFBP) genes in the human placenta and membranes: evidence for IGF-IGFBP interactions at the feto-maternal interface. J Clin Endocrinol Metab. 1996;81:2680–2693. doi: 10.1210/jcem.81.7.8675597. [DOI] [PubMed] [Google Scholar]
- 15.Khong TY. Placenta vascular development and neonatal outcome. Semin Neonatol. 2004;9:255–263. doi: 10.1016/j.siny.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta. 1980;1:3–19. doi: 10.1016/s0143-4004(80)80012-9. [DOI] [PubMed] [Google Scholar]